Pregnant patients with severe or critical coronavirus disease 2019 (COVID-19), but not those with mild or moderate COVID-19, were at increased risk for perinatal complications compared with asymptomatic patients.
OBJECTIVE:
To describe coronavirus disease 2019 (COVID-19) severity in pregnant patients and evaluate the association between disease severity and perinatal outcomes.
METHODS:
We conducted an observational cohort study of all pregnant patients with a singleton gestation and a positive test result for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) who delivered at 1 of 33 U.S. hospitals in 14 states from March 1 to July 31, 2020. Disease severity was classified by National Institutes of Health criteria. Maternal, fetal, and neonatal outcomes were abstracted by centrally trained and certified perinatal research staff. We evaluated trends in maternal characteristics and outcomes across COVID-19 severity classes and associations between severity and outcomes by multivariable modeling.
RESULTS:
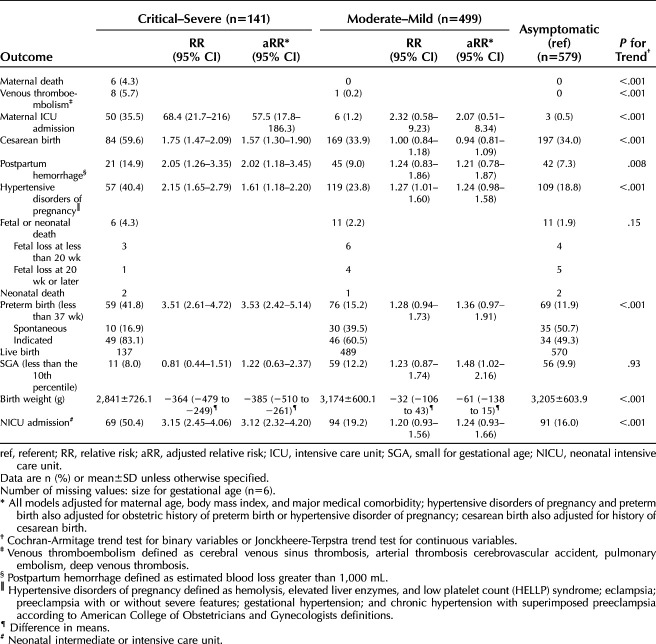
A total of 1,219 patients were included: 47% asymptomatic, 27% mild, 14% moderate, 8% severe, 4% critical. Overall, 53% were Hispanic; there was no trend in race–ethnicity distribution by disease severity. Those with more severe illness had older mean age, higher median body mass index, and pre-existing medical comorbidities. Four maternal deaths (0.3%) were attributed to COVID-19. Frequency of perinatal death or a positive neonatal SARS-CoV-2 test result did not differ by severity. Adverse perinatal outcomes were more frequent among patients with more severe illness, including 6% (95% CI 2–11%) incidence of venous thromboembolism among those with severe–critical illness compared with 0.2% in mild–moderate and 0% in asymptomatic (P<.001 for trend across severity). In adjusted analyses, severe–critical COVID-19 was associated with increased risk of cesarean birth (59.6% vs 34.0%, adjusted relative risk [aRR] 1.57, 95% CI 1.30–1.90), hypertensive disorders of pregnancy (40.4% vs 18.8%, aRR 1.61, 95% CI 1.18–2.20), and preterm birth (41.8% vs 11.9%, aRR 3.53, 95% CI 2.42–5.14) compared with asymptomatic patients. Mild–moderate COVID-19 was not associated with adverse perinatal outcomes compared with asymptomatic patients.
CONCLUSION:
Compared with pregnant patients with SARS-CoV-2 infection without symptoms, those with severe–critical COVID-19, but not those with mild–moderate COVID-19, were at increased risk of perinatal complications.
Existing reports of coronavirus disease 2019 (COVD-19) in pregnant patients are largely limited to single centers or geographic areas, registries requiring self-referral or health care practitioner referral, and meta-analyses of case series in the inpatient setting.1–4 A systematic review and meta-analysis of cohort studies with pregnant patients found an association between COVID-19 and both preterm birth and neonatal intensive care unit (NICU) admission.5 However, available data did not permit examination of other important perinatal outcomes, and the meta-analysis includes some studies in which COVID-19 was diagnosed based on clinical suspicion without testing. In addition, data from the United States are largely derived from those contained in the Morbidity and Mortality Weekly Report,6 which are administrative data provided by public health departments without details regarding clinical disease course.
Recently Adhikari et al7 performed a retrospective cohort study of pregnant patients delivering at a single academic center and found no association between COVID-19 and a composite adverse perinatal outcome of preterm birth, preeclampsia with severe features, or cesarean birth. However, 95% of the cohort had asymptomatic or mild disease, and it remains unknown whether perinatal outcomes differ by COVID-19 severity. The relationship between COVID-19 and perinatal outcomes among pregnant patients in the United States remains largely unknown.
The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network consists of 12 centers with more than 30 separate academic and community hospitals. Together these sites represent the demographic, racial-ethnic and socioeconomic diversity present in the United States. At each site, trained research staff have the capacity to identify all pregnant patients with a positive test result for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) delivering there and to perform detailed medical record abstraction using a standardized protocol. Therefore, we aimed to describe COVID-19 disease severity in a large, diverse cohort of pregnant patients. In addition, we aimed to compare perinatal outcomes among patients based on disease severity. We hypothesized that patients with more severe COVID-19 would have worse perinatal outcomes.
METHODS
This study was an observational cohort of all pregnant patients with a positive SARS-CoV-2 molecular or antigen test result who delivered at 1 of 33 NICHD MFMU sites (six community and 27 academic) in 14 states between March 1, 2020, and July 31, 2020. Patients were included if they had a positive test result at any point during pregnancy (inpatient or outpatient), and a singleton gestation. Both symptomatic patients and asymptomatic patients were included. During the study time period, some of the sites performed SARS-CoV-2 testing for all patients admitted for delivery regardless of symptoms or known exposures. Patients with positive antibody testing alone were not included. Owing to public health concern and potential selection bias, data were collected under a waiver of informed consent with institutional review board approval at each of the participating institutions. Data were analyzed by an independent data coordinating center.
Gestational age at the time of the positive SARS-CoV-2 test result was calculated based on best obstetric estimated date of delivery and the date of the first positive SARS-CoV-2 test result. Detailed electronic medical record abstraction was performed by local perinatal research teams at each MFMU site after centralized training and certification by the data coordinating center. Data quality checks were performed on an ongoing basis to ensure high-quality data across all participating sites.
Descriptive data regarding maternal COVID-19 disease severity were abstracted from the electronic medical record, including symptoms, vital signs, imaging results, laboratory values, treatments, complications, and high-intensity interventions including intubation, dialysis, and extracorporeal membrane oxygenation. Hospitalizations and intensive care unit (ICU) admissions during pregnancy through 42 days postpartum also were enumerated. Self-reported symptoms were recorded based on record review from the patient encounter at the time of a SARS-CoV-2 test that included a duration of onset on or 14 days before the test or diagnosis date.
Patients were classified as having asymptomatic, mild, moderate, severe, or critical illness based on National Institutes of Health guidelines for severity of clinical presentation (see Appendix 2, available online at http://links.lww.com/AOG/C219).8 Classification was based on self-reported symptoms as described above or the patient's worst clinical status from their presentation for SARS-CoV-2 testing through delivery discharge.
Maternal outcomes included death, ICU admission, venous thromboembolism, postpartum hemorrhage, hypertensive disorders of pregnancy, and cesarean birth. Venous thromboembolism was defined as cerebral venous sinus thrombosis, arterial thrombosis cerebrovascular accident, pulmonary embolism, or deep venous thrombosis. Postpartum hemorrhage was defined as estimated blood loss greater than 1,000 mL.9 Hypertensive disorders of pregnancy were defined according to American College of Obstetricians and Gynecologists criteria for hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome; eclampsia; preeclampsia with or without severe features; gestational hypertension; and chronic hypertension with superimposed preeclampsia.10 Medical records were reviewed for outpatient visits and re-admissions through 42 days postpartum for maternal outcomes.
Neonatal outcomes included perinatal death, positive molecular or antigen SARS-CoV-2 test result during birth admission, and preterm birth before 37 weeks of gestation. Among live births, additional outcomes included neonatal intermediate care unit or NICU admission, birth weight, 5-minute Apgar score 3 or less as a marker of adverse neonatal outcomes,11 and small-for-gestational-age birth weight less than the 10th percentile based on the Duryea et al12 nomogram. Neonates were followed only through the delivery hospitalization.
Summary statistics were calculated for baseline characteristics. Race and ethnicity data were abstracted from the medical record and were based on patient self-report at the time of clinical care. All patients identified as Hispanic ethnicity, regardless of race, were categorized as Hispanic. The “other race” category includes non-Hispanic Asian, Native Hawaiian or Pacific Islander, American Indian/Alaskan Native, unknown or more than one race. These categories were collapsed to make comparisons between groups with low frequency of patients in the other race categories. In addition, those with severe and critical illness were grouped together for statistical comparisons, as were those with mild and moderate illness. The Cochran-Armitage trend test for binary variables, score test from multinomial logistic regression for multinomial variables, or Jonckheere-Terpstra trend test for continuous variables were used to assess trends in baseline characteristics and perinatal outcomes across the severe–critical, mild–moderate, and asymptomatic categories.
Severe–critical COVID-19 and mild–moderate COVID-19 were each compared with asymptomatic patients using multivariable modeling. Multivariable modeling was not performed for outcomes with low frequencies including maternal death, stillbirth or neonatal death, maternal venous thromboembolism, or positive SARS-CoV-2 test results for the neonate. Covariates for modeling were selected based on clinical relevance and included maternal age, body mass index (BMI, calculated as weight in kilograms divided by height in meters squared) at first prenatal visit or (if that was not available) preconceptionally, and major medical comorbidity (any of the following: asthma of any severity or chronic obstructive pulmonary disease, chronic hypertension, or pregestational diabetes).
Models for hypertensive disorders of pregnancy and preterm birth also included a covariate for obstetric history (categorized as no prior pregnancy 20 weeks of gestation or longer, history of preterm birth or hypertensive disorder of pregnancy, or prior pregnancy without preterm birth or hypertensive disorder of pregnancy). The model for cesarean birth included history of cesarean birth (categorized as no prior pregnancy 20 weeks of gestation or longer, history of only vaginal births, or any prior cesarean birth) in addition to the baseline demographic variables above. For continuous outcomes, generalized linear models were used to estimate the difference of means and 95% CIs. For categorical outcomes, modified Poisson regression models were used to estimate relative risks and 95% CIs.
A sensitivity analysis was performed in which missing BMI values were imputed based on a generalized linear model and adjusted models were re-run. The imputation modeled the natural-log scale BMI with linear, quadratic, and cubic natural-log scale BMI at delivery calculated from the most recent pregnancy weight before delivery. For patients with BMI at delivery and without prenatal (or preconceptional) BMI, imputed BMI values were the back-transformed predicted values based on the model.
Analyses were performed with SAS 9.4. Adjustments were not made for multiple comparisons.
RESULTS
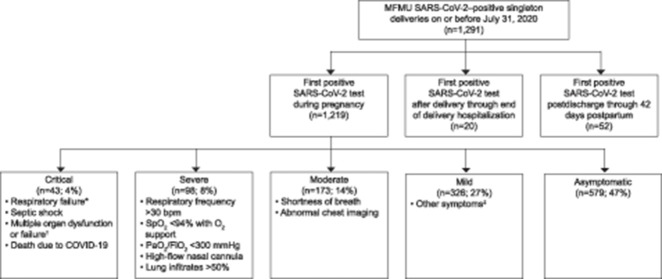
Of 1,291 pregnant patients with a positive SARS-CoV-2 test result over the study time period, 1,219 (94%) had a singleton gestation and tested positive during pregnancy, and were included in the analysis (Fig. 1). Of these, 579 (47%) were asymptomatic, 326 (27%) had mild illness, 173 (14%) had moderate illness, 98 (8%) had severe illness, and 43 (4%) had critical illness (Fig. 1). Among the patients with severe–critical illness, 11% were transported from another health care facility.
Fig. 1. Study cohort. First positive test result (molecular or antigen) for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); coronavirus disease 2019 (COVID-19) classification based on data through delivery hospitalization. *Respiratory failure is use of any of the following: extracorporeal membrane oxygenation, continuous positive airway pressure, bi-level positive airway pressure, ventilation. †Multiple organ dysfunction or failure is two or more of the following: cardiac arrest, impaired liver function, renal insufficiency (serum creatinine greater than 1.10 mg/dL), renal failure requiring dialysis, encephalopathy, any need for pressor support, thrombocytopenia (platelets less than 100,000). ‡Other symptoms include fever, cough, sore throat, fatigue, muscle pain, chills, back pain, nausea, vomiting, joint pain, nasal stuffiness, conjunctivitis, confusion, loss of smell or taste, diarrhea, or other. MFMU, Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units.
Metz. COVID-19 Severity and Perinatal Outcomes. Obstet Gynecol 2021.
The median (interquartile range) gestational age at the time of first positive SARS-CoV-2 test result was 37.7 weeks of gestation (33.7–39.1). The majority of SARS-CoV-2 tests were performed in the inpatient setting (67%). The number of days from the first positive SARS-CoV-2 test result to delivery varied by disease severity, with a median of 4 days (1–28) among those with severe–critical illness, 18 days (1–47) among those with mild–moderate illness, and 1 day (0–2) for asymptomatic individuals. Of those who were asymptomatic with a positive SARS-CoV-2 test result, 97% were tested in the context of universal screening at the time of delivery admission.
The majority of critically ill patients were classified as such owing to respiratory failure (Table 1). Overall, the most common patient-reported symptoms were cough (34%), dyspnea (19%), and myalgias (19%). Those classified as severe most frequently had tachypnea or hypoxia. Although the majority of patients with severe and critical illness had abnormal results on chest radiologic imaging (chest X-ray or computed tomography scan), only a small proportion had more than 50% lung involvement (1% and 5%, respectively).
Table 1.
Coronavirus Disease 2019 (COVID-19) Disease Severity Classification for Study Cohort*
Tests of trend were significant for differences in age, median BMI, and insurance status across disease severity (Table 2). The majority (53%) of enrolled patients were of Hispanic ethnicity; however, there was no trend in race–ethnicity distribution by severity. Tests of trend were significant for differences in frequency of medical comorbidities including asthma or chronic obstructive pulmonary disease, chronic hypertension, prepregnancy diabetes, chronic liver disease, and seizure disorder across disease severity. Illness severity was not related to ABO blood type or presence of Rhesus factor (Table 2).
Table 2.
Baseline Demographic and Clinical Characteristics
Vital sign and laboratory findings by COVID-19 severity are described in Appendix 3, available online at http://links.lww.com/AOG/C219. Maternal and perinatal deaths are presented in Table 3. Four maternal deaths (0.3%) were thought to be due to COVID-19; autopsies were not performed in all cases. Two other maternal deaths occurred during the study time period and were not related to infection; both had severe COVID-19 before dying of other causes. The frequency of neonatal or fetal death by disease severity is described in Table 3.
Table 3.
Perinatal Outcomes by Coronavirus Disease 2019 (COVID-19) Severity
A thromboembolic event occurred in eight patients (6%, 95% CI 2–11%) in the severe–critical group; all of these events were deep venous thromboses or pulmonary emboli. In comparison, there was one (0.2%) thromboembolism in the mild–moderate group and none (0%) in the asymptomatic group (P<.001 for trend across severity) (Table 3). Of those with a venous thromboembolism, five received prophylactic anticoagulation before the event, all in the severe–critical group.
Overall, 6% of the cohort had an antepartum admission for treatment of COVID-19 separate from the delivery hospitalization. Fifty-nine (5%) were admitted to the ICU either during the pregnancy or within 42 days postpartum (Table 3). The majority of the ICU admissions (45/59, 76%) were for an indication of COVID-19. Among the patients initially classified as asymptomatic during the delivery hospitalization, one had a hospital readmission with ICU care for COVID-19 after delivery discharge but before 42 days postpartum. Other ICU admissions in asymptomatic patients were for indications unrelated to COVID-19.
Rates for cesarean birth, postpartum hemorrhage, hypertensive disorders of pregnancy and preterm birth were 36.9%, 8.9%, 23.4% and 16.7%, respectively. Adverse maternal and neonatal outcomes were more frequent in patients with severe COVID-19 illness (Table 3). In adjusted analyses, severe–critical COVID-19 was associated with a higher risk of cesarean birth (adjusted relative risk [aRR] 1.57, 95% CI 1.30–1.90), postpartum hemorrhage (aRR 2.02, 95% CI 1.18–3.45), hypertensive disorders of pregnancy (aRR 1.61, 95% CI 1.18–2.20), and preterm birth (aRR 3.53, 95% CI 2.42–5.14) compared with asymptomatic patients. In a sensitivity analysis in which BMI was imputed for those with missing values (n=159, 13%), the adjusted association between severe–critical and postpartum hemorrhage was no longer significant (aRR 1.68, 95% CI 1.00–2.84).
Preterm births among patients with severe–critical illness were indicated (rather than spontaneous) in 83% of cases compared with 61% in patients with mild–moderate illness and 49% in patients who were symptomatic (P<.001 for trend across severity for indicated delivery among preterm births). Of those induced preterm (n=67), COVID-19 was the primary indication for induction of labor in 3%. The most common indications for induction among the preterm births were hypertensive disorders of pregnancy (33%), stillbirth (16%), and preterm prelabor rupture of membranes (13%). Of those who underwent preterm cesarean birth (n=106), COVID-19 was the primary indication for cesarean in 22%. The other common indications for cesarean among those with preterm births were nonreassuring fetal status (29%), hypertensive disorders of pregnancy (15%), and abnormal presentation (11%).
Severe–critical maternal illness was associated with NICU admission and lower birth weight. Mild–moderate maternal illness was marginally associated with small-for-gestational-age birth weight, although this association was no longer significant when BMI was imputed for those with missing values. Among patients who were induced or had cesarean birth, COVID-19 was the indication for induction or cesarean in 32 of 112 (29%) of the patients with severe–critical illness, 12 of 330 (4%) with mild–moderate illness, and 16 of 352 (5%) who were asymptomatic.
In total, 1.0% (95% CI 0.5–1.8%) of neonates tested positive for SARS-CoV-2 before discharge. The rate of positive neonatal SARS-CoV-2 test results among live births was 1.5% in the severe–critical group, 0.6% in the mild–moderate group, and 1.2% in the asymptomatic group. The percentage of neonates with a 5-minute Apgar score of 3 or less was 2.9% in the severe–critical, 0.2% in the mild–moderate, and 0.7% in the asymptomatic group. The majority (900/1,190 with available data, 76%) of neonates in the cohort were breastfed or breast and bottle fed. Among patients in whom an offer of maternal–neonatal separation owing to SARS-CoV-2 infection was documented (n=448, 37%), 312 were actually separated (26% of total population): 59 of 64 (92%) with severe–critical illness, 77 of 126 (61%) with mild–moderate illness, and 176 of 258 (68%) who were in the asymptomatic group.
DISCUSSION
We found that 12% of pregnant patients with COVID-19 had severe or critical illness. Patients with severe or critical COVID-19 are at risk for a number of perinatal complications including cesarean birth, hypertensive disorders of pregnancy, and preterm birth. Those with severe or critical illness also had increased frequency of venous thromboembolism compared with those with less severe illness. In addition, severe or critical maternal illness was associated with higher risk of NICU admission and lower birth weight compared with neonates of asymptomatic patients.
Our findings regarding preterm birth and NICU admission are consistent with a previous report.5 However, in our cohort, the increased risk of preterm birth was driven by indicated, rather than spontaneous, preterm birth. Our work also expands what is known about perinatal outcomes in patients with COVID-19 by demonstrating that severe or critical illness is associated with a number of other adverse perinatal outcomes for both the patient and the neonate.
We observed a 6% VTE rate in the severe–critical group. National Institutes of Health treatment guidelines recommend prophylactic anticoagulation in pregnant hospitalized patients with COVID-19.8 Nonetheless, our data are consistent with those in nonpregnant patients in that critically ill patients with COVID-19 have a high rate of VTE even when receiving prophylactic anticoagulation.13 Ongoing randomized controlled trials are evaluating whether therapeutic anticoagulation reduces risk of VTE when compared with prophylactic anticoagulation. Further study is also needed to determine the need for anticoagulation in the setting of less severe COVID-19 illness during pregnancy as there was an isolated venous thromboembolic event in the mild–moderate group (1 in 499).
The rate of a positive neonatal SARS-CoV-2 test result was approximately 1% across sites. The majority of these neonates were breastfed or breast and bottle fed. We were limited in our capacity to evaluate for vertical transmission because only clinical testing was available, and, in many cases, this does not include serial testing of the neonate and evaluation of the placenta as required to confirm vertical transmission rather than horizontal transmission after birth.14 Nonetheless, despite a majority of neonates receiving at least some breastfeeding, positive viral testing in the neonates was infrequent.
Initial Morbidity and Mortality Weekly Report data demonstrated an increased risk for hospitalization among pregnant patients compared with females of reproductive age who were not pregnant; however, it was not possible to delineate whether the patient was simply admitted for delivery (rather than COVID-19 complications).15 Our data indicate that, overall, 6% of pregnant patients who tested positive for SARS-CoV-2 had a hospital admission for COVID-19 separate from their delivery hospitalization. Although this is similar to the 5.8% hospitalization rate published by the Centers for Disease Control and Prevention for nonpregnant reproductive-age females, direct comparisons cannot be made given differences in sampling strategy. In addition, patients with critical illness may have required delivery to improve maternal status during what was initially a COVID-19 hospitalization.
Centers for Disease Control and Prevention data6 demonstrate an increased risk of death from COVID-19 and ICU admission among pregnant patients compared with nonpregnant patients. Recent hospital-level administrative data16 also demonstrate increased risks of both of these outcomes among patients with COVID-19 compared with those without COVID-19. Our maternal death rate was 0.3% (3/1,000 patients with COVID-19), and the ICU admission rate was 4.8% (48/1,000). Both of these rates are higher than those previously published. A small proportion of the increase may reflect transports requiring critical care from other facilities to MFMU tertiary care centers. However, transports only comprised 11% of the severe–critical study population. Therefore, the higher rates may also reflect increased ascertainment of these outcomes through manual medical record abstraction rather than relying on administrative data.
Consistent with prior studies,5 risk factors for severe or critical COVID-19 included older age, increased BMI, and underlying medical comorbidities such as asthma, chronic hypertension, and pregestational diabetes. The majority of pregnant patients with COVID-19 in our cohort were of Hispanic ethnicity (53%); however, there was no trend in COVID-19 severity based on race–ethnicity. We found a higher frequency of current employment and private insurance among those with higher disease severity, which may in part reflect transport of patients with higher illness severity to tertiary care centers included in the MFMU.
Limitations of this analysis include the fact that not all sites were performing universal screening for SARS-CoV-2 infection during the entire study period. Overall, the mean gestational age at infection was late in the third trimester, which was at least partially attributable to the inclusion criterion for this analysis being delivery by July 31, 2020; COVID-19 was not widely prevalent in the United States until March 2020. Therefore, the association between COVID-19 and early pregnancy complications such as miscarriage or congenital anomalies could not be evaluated. Many of the NICHD MFMU sites are in urban locations which may limit generalizability to more rural settings; however, both academic and community-based hospitals were included. Results regarding hypertensive disorders of pregnancy must be interpreted in the context of overlapping signs (eg hypertension and lab abnormalities) and symptoms (eg headache) between preeclampsia with severe features and severe or critical COVID-19; however, standard American College of Obstetricians and Gynecologists’ diagnostic criteria were used for this endpoint to minimize subjectivity. In addition, owing to the clinical importance and descriptive nature of the data, analyses were not adjusted for multiple comparisons; therefore, some findings could be attributed to chance. Finally, treatment for COVID-19 was rapidly evolving during the study period, and the effect of current treatments on outcomes could not be evaluated.
Strengths of this study include the standardized collection methods of detailed medical record abstraction by trained and certified perinatal research staff. The study included 33 demographically and socioeconomically diverse sites, which makes these data more generalizable than single-center data. In addition, the size of the study population afforded by multiple sites allowed for a larger sample to evaluate differences in outcomes by disease severity. We included patients with positive test results in both the inpatient and outpatient setting, which expands knowledge related to COVID-19 in pregnancy, as the results are not biased by only including inpatients.
These results suggest that pregnant patients with higher levels of COVID-19 severity are at higher risk of perinatal complications. Clinicians should be aware of these risks and consider strategies to mitigate complications when possible.
Footnotes
This work is funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (UG1 HD087230, UG1 HD027869, UG1 HD027915, UG1 HD034208, UG1 HD040500, UG1 HD040485, UG1 HD053097, UG1 HD040544, UG1 HD040545, UG1 HD040560, UG1 HD040512, UG1 HD087192, U10 HD036801) and the National Center for Advancing Translational Sciences (UL1TR001873). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial Disclosure: Torri Metz is the site Principal Investigator (PI) for a Pfizer RSV vaccination study, a Novavax RSV vaccination study, and a Gestvision study of the validity of a point-of-care preeclampsia test. She also receives royalties for two UpToDate topics on vaginal birth after cesarean. Brenna Hughes disclosed receiving funds from Merck. Cynthia Gyamfi-Bannerman disclosed that money was paid to her institution from NICHD/NHLBI and AMAG/SMFM. She has received funds from Sera Prognostics as a Medical Advisory Board Member. Alan Tita reports money was paid to his institution from Pfizer. The other authors did not report any potential conflicts of interest.
Presented at the Society for Maternal-Fetal Medicine’s 41st Annual Pregnancy Meeting, held virtually, January 25–30, 2021.
A list of other members of the NICHD MFMU Network is available in the Appendix 1 online at http://links.lww.com/AOG/C219.
Dr. Rouse, Editor-in-Chief, and Dr. Metz, Associate Editor, Obstetrics, for Obstetrics & Gynecology, were not involved in the review or decision to publish this article.
Each author has confirmed compliance with the journal's requirements for authorship.
Published online ahead-of-print February 8, 2021.
Peer reviews and author correspondence are available at http://links.lww.com/AOG/C220.
Contributor Information
Rebecca G. Clifton, Email: rclifton@bsc.gwu.edu.
Brenna L. Hughes, Email: brenna.hughes@duke.edu.
Grecio Sandoval, Email: sandoval@bsc.gwu.edu.
George R. Saade, Email: gsaade@utmb.edu.
William A. Grobman, Email: w-grobman@northwestern.edu.
Tracy A. Manuck, Email: tmanuck@med.unc.edu.
Menachem Miodovnik, Email: menachem.miodovnik@nih.gov.
Amber Sowles, Email: amber.sowles@hsc.utah.edu.
Kelly Clark, Email: kelly_clark@med.unc.edu.
Cynthia Gyamfi-Bannerman, Email: cg2231@cumc.columbia.edu.
Hector Mendez-Figueroa, Email: hector.mendezfigueroa@uth.tmc.edu.
Harish M. Sehdev, Email: hsehdev2@pennmedicine.upenn.edu.
Dwight J. Rouse, Email: drouse@wihri.org.
Alan T.N. Tita, Email: atita@uabmc.edu.
Jennifer Bailit, Email: jbailit@metrohealth.org.
Maged M. Costantine, Email: maged.costantine@osumc.edu.
Hyagriv N. Simhan, Email: hsimhan@mwri.magee.edu.
George A. Macones, Email: george.macones@austin.utexas.edu.
Figure.
No available caption
REFERENCES
- 1.Khoury R, Bernstein PS, Debolt C, Stone J, Sutton DM, Simpson LL, et al. Characteristics and outcomes of 241 births to women with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection at five New York City medical centers. Obstet Gynecol 2020;136:273–82. doi: 10.1097/AOG.0000000000004025 [DOI] [PubMed] [Google Scholar]
- 2.Griffin I, Benarba F, Peters C, Oyelese Y, Murphy T, Contreras D, et al. The impact of COVID-19 infection on labor and delivery, newborn nursery, and neonatal intensive care unit: prospective observational data from a single hospital system. Am J Perinatol 2020;37:1022–30. doi: 10.1055/s-0040-1713416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afshar Y, Gaw SL, Flaherman VJ, Chambers BD, Krakow D, Berghella V, et al. Clinical presentation of coronavirus disease-2019 (COVID-19) in pregnant and recently pregnant people. Obstet Gynecol 2020;136:1117–25. doi: 10.1097/AOG.0000000000004178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prabhu M, Cagino K, Matthews KC, Friedlander RL, Glynn SM, Kubiak JM, et al. Pregnancy and postpartum outcomes in a universally tested population for SARS-CoV-2 in New York City: a prospective cohort study. BJOG 2020;127:1548–56. doi: 10.1111/1471-0528.16403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allotey J, Stallings E, Bonet M, Yap M, Chatterjee S, Kew T, et al. Clinical manifestations, risk factors and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ 2020;370:m3320. doi: 10.1136/bmj.m3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zambrano LD, Ellington S, Strid P, Galang RR, Oduyebo T, Tong VT, et al. Characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–October 3, 2020. Accessed January 12, 2020. https://www.cdc.gov/mmwr/volumes/69/wr/mm6944e3.htm [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adhikari EH, Moreno W, Zofkie AC, MacDonald L, McIntire DD, Collins RRJ, et al. Pregnancy outcomes among women with and without severe acute respiratory syndrome coronavirus 2 infection. JAMA Netw Open 2020;3:e2029256. doi: 10.1001/jamanetworkopen.2020.29256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Institutes of Health. Coronavirus disease 2019 (COVID-19) treatment guidelines. Accessed January 27, 2021. https://www.covid19treatmentguidelines.nih.gov [PubMed]
- 9.Postpartum hemorrhage. Practice Bulletin No. 183. American College of Obstetricians and Gynecologists. Obstet Gynecol 2017;130:e168–86. doi: 10.1097/AOG.0000000000002351 [DOI] [PubMed] [Google Scholar]
- 10.Gestational hypertension and preeclampsia. ACOG Practice Bulletin No. 222. American College of Obstetricians and Gynecologists. Obstet Gynecol 2020;135:e237–260. doi: 10.1097/AOG.0000000000003891 [DOI] [PubMed] [Google Scholar]
- 11.Cnattingius S, Johansson S, Razaz N. Apgar score and risk of neonatal death among preterm infants. N Engl J Med 2020;383:49–57. doi: 10.1056/NEJMoa1915075 [DOI] [PubMed] [Google Scholar]
- 12.Duryea EL, Hawkins JS, McIntire DD, Casey BM, Leveno KJ. A revised birth weight reference for the United States. Obstet Gynecol 2014;124:16–22. doi: 10.1097/AOG.0000000000000345 [DOI] [PubMed] [Google Scholar]
- 13.Spyropoulos AC Levy JH Ageno W Connors JM Hunt BJ Iba T, et al. Scientific and Standardization Committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost 2020;18:1859–65. doi: 10.1111/jth.14929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blumberg DA, Underwood MA, Hedriana HL, Lakshminrusimha S. Vertical transmission of SARS-CoV-2: what is the optimal definition? Am J Perinatol 2020;37:769–72. doi: 10.1055/s-0040-1712457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellington S, Strid P, Tong VT, Woodworth K, Galang RR, Zambrano LD, et al. Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status-United States, January 22 to June 7, 2020. MMWR Morb Mortal Wkly Rep 2020;69:769–75. doi: 10.15585/mmwr.mm6925a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jering KS, Claggett BL, Cunningham JW, Rosenthal N, Vardeny O, Greene MF, et al. Clinical characteristics and outcomes of hospitalized women giving birth with and without COVID-19. JAMA Int Med;2021 Jan 15. [Epub ahead of print]. doi: 10.1001/jamainternmed.2020.9241 [DOI] [PMC free article] [PubMed] [Google Scholar]