Purpose of review
We present recent developments in the field of small vessel disease (SVD)-related vascular cognitive impairment, including pathological mechanisms, updated diagnostic criteria, cognitive profile, neuroimaging markers and risk factors. We further address available management and therapeutic strategies.
Recent findings
Vascular and neurodegenerative pathologies often co-occur and share similar risk factors. The updated consensus criteria aim to standardize vascular cognitive impairment (VCI) diagnosis, relying strongly on cognitive profile and MRI findings. Aggressive blood pressure control and multidomain lifestyle interventions are associated with decreased risk of cognitive impairment, but disease-modifying treatments are still lacking. Recent research has led to a better understanding of mechanisms leading to SVD-related cognitive decline, such as blood-brain barrier dysfunction, reduced cerebrovascular reactivity and impaired perivascular clearance.
Summary
SVD is the leading cause of VCI and is associated with substantial morbidity. Tackling cardiovascular risk factors is currently the most effective approach to prevent cognitive decline in the elderly. Advanced imaging techniques provide tools for early diagnosis and may play an important role as surrogate markers for cognitive endpoints in clinical trials. Designing and testing disease-modifying interventions for VCI remains a key priority in healthcare.
Keywords: imaging, small vessel disease, vascular cognitive impairment, vascular dementia, vascular risk factors
INTRODUCTION
Vascular cognitive impairment (VCI) refers to conditions in which cerebrovascular diseases contribute to decline in mental abilities [1,2▪▪]. Although these diseases can independently lead to cognitive deficits and account for 15–30% of dementia cases, second only to Alzheimer's disease, they rarely occur in isolation [2▪▪,3▪]. Importantly, age-related cognitive impairment is typically driven by co-occurring vascular and neurodegenerative pathologies [4]. In a recent clinical–pathologic populational study, the majority of participants (∼78%) had at least two concomitant neuropathologies at the time of death, most commonly neurodegenerative and vascular diseases [3▪].
Among the multiple mechanisms involved in VCI, cerebral small vessel disease (SVD) is arguably the most prevalent one [5], contributing to cognitive impairment irrespective of stroke [2▪▪]. SVD is characterized by abnormalities that affect the structure and function of small vessels of the brain, with multiple neuroimaging and neurological manifestations, including cognitive decline [6▪▪]. Rather than a homogeneous disorder, SVD encompasses different sporadic and inherited diseases, resulting from a complex mix of genetic and vascular risk factors. The prevalence of SVD increases with age, and the two most common sporadic types are arteriolosclerosis, also referred to as hypertensive arteriopathy or deep perforator arteriopathy, and cerebral amyloid angiopathy (CAA) [7]. Arteriolosclerosis has been traditionally linked to hypertension and type II diabetes [8]. In pathology, arteriosclerosis is characterized by abnormal thickening of arteriolar walls, preferentially located in the deep grey nuclei and deep white matter, observed in >80% of individuals over 80 years of age, according to autopsy studies [8]. CAA is defined by pathological deposition of amyloid-β in the walls of cortical and leptomeningeal arterioles and capillaries. Moreover, CAA is known to commonly co-occur with Alzheimer's disease [9▪▪].
In this narrative review, we focus on the latest advances in the management of sporadic SVD-related VCI, with an update on diagnostic criteria, neuroimaging markers and cognitive profile. We further address the current state of prevention and therapeutic approaches.
Box 1.

no caption available
POTENTIAL PATHOLOGICAL MECHANISMS LINKING SMALL VESSEL DISEASE TO COGNITIVE IMPAIRMENT
Interactions between ageing [10], environmental risk factors [11▪▪] and genetic variants [12,13] are thought to contribute to the development of sporadic SVD (Fig. 1a). In the early pathogenic stages, damage to functional units composed of neurons, endothelial cells, astrocytes and pericytes (neurovascular units) may lead to impaired regulation of blood flow, vascular permeability, immune trafficking and waste clearance [6▪▪,16]. A cascade of events may arise, including blood--brain barrier (BBB) leakage, deficient cerebrovascular reactivity (CVR), inflammation, vessel wall thickening and remodelling, as well as luminal narrowing (Fig. 1b) [6▪▪,8]. Together, these processes contribute to the broad spectrum of SVD-related brain parenchymal injury, including haemorrhagic and nonhaemorrhagic (presumably ischemic) lesions (Fig. 1c). Through impaired vasomotion and reduced vasoreactivity, it has been hypothesized that SVD might compromise the drainage of solutes along the vessels [9▪▪,17,18▪▪], potentially facilitating interstitial and perivascular accumulation of proteins, including amyloid-β. Such aggregation, could contribute to secondary neuronal degeneration and loss of vascular smooth muscle cells [9▪▪]. This potential interactive pathway is thought to play a critical role in comorbid CAA and Alzheimer's disease [9▪▪] and may lead to a self-reinforcing cycle in which neurodegenerative and vascular pathologies intensify and aggravate each other [9▪▪]. Furthermore, sleep has been found to act as an important modulator of perivascular clearance and may also play a relevant role in age-related cognitive decline [18▪▪,19].
FIGURE 1.
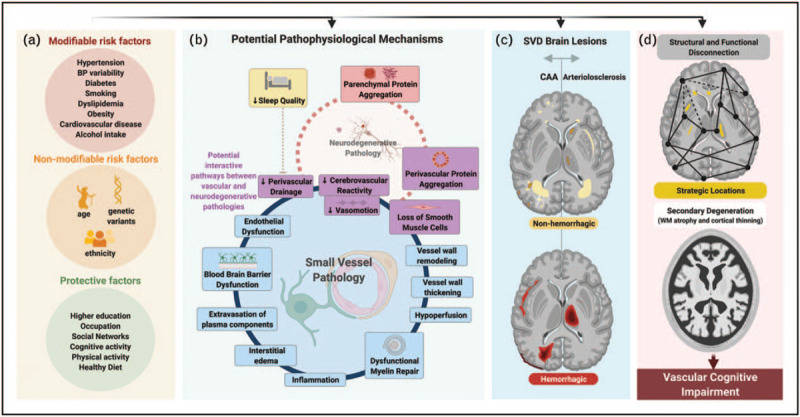
Schematic overview of potential mechanisms leading to vascular cognitive impairment. (a) Risk factors associated with SVD and related cognitive decline. (b) Potential pathophysiological mechanisms of SVD. Dysfunctional NVUs have an important role in early SVD pathology. Several effects are described around the blue circle, the order of which is not yet established. Combined, these effects are thought to contribute to exacerbate tissue injury. (c) Typical brain lesions associated with sporadic SVD: CAA (left) and arteriolosclerosis (right) patterns. The hemorrhagic lesions (bottom figure) include: CMB, cSS, SAH and ICH. The non-hemorrhagic lesions (upper figure) include WMH, lacunes, PVS, small acute subcortical infarcts and cortical CMI. (d) Potential mechanisms involved in SVD-related cognitive decline: impairment of structural and functional connectivity (upper figure) and secondary degeneration (lower figure). AD, Alzheimer's disease; CAA, cerebral amyloid angiopathy; CMB, cerebral microbleed; CMI, cerebral microinfarcts; cSS, cortical superficial siderosis; ICH, intracerebral hemorrhage; NVU, neurovascular unit; PVS, perivascular spaces; SAH, subarachnoid hemorrhage; SVD, small vessel disease; WMH, white matter hyperintensity. Adapted from [5,11▪▪,14,15]. Created with BioRender.com.
Evidence suggests that SVD-related brain lesions affect cognition also by disrupting structural and functional networks, causing a disconnection syndrome [14]. The strategic anatomical locations of some lesions play an important role and help explain VCI's heterogeneous neuropsychological manifestations [14,20,21]. In addition, subcortical lesions may further contribute to functional decline by triggering secondary degeneration of affected white matter tracts, leading to remote abnormalities such as white matter atrophy and cortical thinning (Fig. 1d) [6▪▪,14].
CLINICAL CLASSIFICATION
Several classification systems have been proposed over the years to guide clinical diagnosis of VCI, reflecting methodological and diagnostic challenges [5,22–27]. When using these classifications, it is important to be aware of the broad overlap between neurodegenerative and vascular diseases in clinical [28▪] and pathological levels [3▪], acknowledging the great heterogeneity in the cognitive impact of such diseases at an individual level [3▪].
The clinical diagnosis of VCI relies strongly on cognitive profile and neuroimaging findings. According to the most recent diagnostic guidelines, from the Vascular Impairment of Cognition Classification Consensus Study (VICCCS), VCI's definition aligns with the terminology of DSM-V and encompasses a broad clinical spectrum that ranges from mild to major VCI, and incorporates mixed-disease cases (Table 1) [24,25]. Subcortical ischemic vascular dementia (SIVaD) refers to cases in which SVD is the primary mechanism underlying cognitive decline, and the most common brain lesions are white matter hyperintensities (WMH) and lacunar infarcts [24,25]. Importantly, according to VICCCS guidelines, individuals with neuroimaging signs of SVD may qualify for SIVaD, post-stroke dementia or mixed-dementia, depending on temporal associations and comorbidities.
Table 1.
Summary of the Vascular Impairment of Cognition Classification Consensus Study criteria
| Definition | VCI is defined as impairment in at least one cognitive domain and in IADL/ADLs independent of the motor/sensory sequelae of the vascular event: Mild VCI: at least one cognitive domain affected and mild to no impairment in IADL/ADLs. Major VCI (vascular dementia): clinically significant deficits of sufficient severity in at least one cognitive domain and severe disruption of IADL/ADLs. |
| Evaluation | Cognitive assessment should include five core domains: executive function and processing speed, attention, memory, language, and visuospatial domains. The full-length protocol takes 60 min to complete but can be shortened to 30 or even 5 min using Montreal Cognitive Assessment (MoCA) [22]. |
| Imaging | MRI is considered the ‘gold-standard’ imaging method for the clinical diagnosis of VCI. |
| Certainty of evidence | Probable VCI: if (1) only CT imaging is available or (2) aphasia is present after vascular event, but normal cognition was documented (e.g. annual cognitive evaluations) before the clinical event. Possible VCI: if neither MRI nor CT is available, but VCI is suspected clinically. |
| Major VCI subtypes | Post-stroke dementia: a clear temporal relationship (within 6 months) of irreversible cognitive decline following the vascular event. Subcortical ischemic vascular dementia (SIVaD): small vessel disease is the main vascular cause, including lacunar infarcts and white matter hyperintensities are the main lesions. Multi-infarct (cortical) dementia: large cortical infarcts contributing to dementia. Mixed pathology: VCI-AD, AD-VCI or VCI-DLB, VCI-∗ depending on probable contribution. |
| Exclusion criteria | Drug/alcohol abuse/dependence within the last 3 months, other causes of sustained impairment (e.g. depression, vitamin D deficiency, other vitamin or hormonal deficiencies). |
(VICCCS) diagnosis guidelines. AD, Alzheimer's disease; ADL, activities of daily living; CT, computed tomography; DLB, dementia with Lewy bodies; IADL, instrumental activities of daily living; VCI, vascular cognitive impairment.
Other possible disease.
Adapted from Vascular Impairment of Cognition Classification Consensus Study [24].
Cognitive profile
Cognitive decline linked to cerebrovascular diseases, including SVD, is thought to typically present in a stepwise and gradual pattern, progressing slowly and affecting processing speed, complex attention and frontal-executive functions [5,26]. Disturbances in the frontal-executive domain are considered more likely to be present in mild VCI than in Alzheimer's disease related mild cognitive impairment (MCI), in which decline in episodic memory is the most prominent feature [29].
This observed predilection for impairment of frontal-executive functions is thought to result from the disruptive effect of SVD lesions on the brain's structural and functional connectivity [14]. Neuroimaging studies have shown that the degree of structural network disruption is associated with the burden and extent of SVD lesions [30,31] and, at least in part, mediates their association with cognitive decline [32,33]. Also, functional networks associated with attention and executive functions have been found to be predominantly affected in SVD patients [14].
Traditionally, a history of early onset of memory deficit and worsening of cortical functions (aphasia, apraxia, agnosia), in the absence of corresponding vascular brain imaging lesions, can be suggestive of Alzheimer's disease as primary diagnosis [26]. Nonetheless, episodic and semantic memory can also be affected in cognitive impairment of presumably vascular origin [34–36].
Importantly, there is much overlap in cognitive profiles across dementia types [37], possibly related to the multifactorial nature of age-related cognitive decline combined with patient-specific factors related to cognitive reserve and spatial distribution of vascular lesions.
Accordingly, recent findings support a much more heterogeneous spectrum of cognitive impairment related to SVD. This suggests that multiple domains can be affected, owing not only to overlapping diseases but also to close interdependence of executive function and processing speed to perform fluid cognitive tasks [38]. Interestingly, in severe CAA cases [39], visuospatial dysfunction has also been reported [40], hypothesized to relate to a posterior predominance of amyloid disease.
Psychiatric, behavioural and other manifestations
Additional features of VCI, depending on lesion localization and severity, may include personality and mood alterations (apathy, depression, emotional incontinence) [41,42], disturbed sleep [42], motor and gait disturbances (frequent falls, small-step parkinsonian gait) [43], early urinary incontinence and pseudobulbar palsy due to lacunes in basal ganglia or pons [26,27]. VCI is often associated with psychiatric and behavioural symptoms underlined by lesions in thalamocortical, striatocortical and prefrontal-basal ganglia pathways [44] and are often amenable to therapy (reviewed in detail elsewhere [45▪▪]). Although depression often manifests with a reversible decline in cognitive function, late-life occurrence can also be an early sign of dementia [46].
A distinct form of SVD is CAA-related inflammation (CAA-ri), characterized by an autoimmune reaction to cerebrovascular amyloid-β deposits [47], and clinical presentation may include subacute cognitive dysfunction [47,48]. Early identification is critical, as neurological deficits can be reversible with early immunosuppressive treatment [49▪].
NEUROIMAGING EVALUATION
In the context of cognitive impairment, detection of underlying microvascular disease relies strongly on neuroimaging [26], for which MRI is considered the ‘gold-standard’ [24,50▪▪]. Many SVD features are visible and detectable almost exclusively through MRI, but none are considered pathognomonic and, hence, must be interpreted in light of clinical findings [26]. Neuroimaging also helps to distinguish etiological subtypes of SVD. The modified Boston criteria [51] and the Edinburgh criteria [52] enable in-vivo diagnosis of CAA, with the former providing high positive predictive values even in the absence of intracerebral haemorrhage (ICH), thus facilitating CAA diagnosis in memory-clinic patients [53]. The likelihood of underlying arteriolosclerosis or CAA can be further inferred based on the distribution pattern of several MRI-visible lesions (Fig. 1c; Fig. 2) [54–57]. Arteriolosclerosis has been found to more commonly present with deep cerebral and cerebellar microbleeds (CMB) [56], deep lacunes [57], perivascular spaces (PVS) visible in the basal ganglia [55], deep ICH and peri-basal ganglia WMH [54] (Fig. 1c, Fig. 2). In contrast, evidence suggests that CAA more typically presents with cortical CMB [53,56], cortical cerebral microinfarcts (CMI) [58], lobar lacunes [57], PVS visible in the centrum semiovale [55], cortical superficial siderosis (cSS) [51], convexity subarachnoid hemorrhage (cSAH) [59,60], lobar ICH and WMH foci distributed as multiple subcortical spots [54] (Fig. 1c, Fig. 2). This distinction is particularly important because CAA frequently overlaps with Alzheimer's disease, and confers higher risk of haemorrhagic complications, influencing decision-making on antithrombotic treatment [61▪▪]. Importantly, detection of SVD MRI lesions in young individuals without significant risk factors should prompt investigation of monogenic SVD [62].
FIGURE 2.
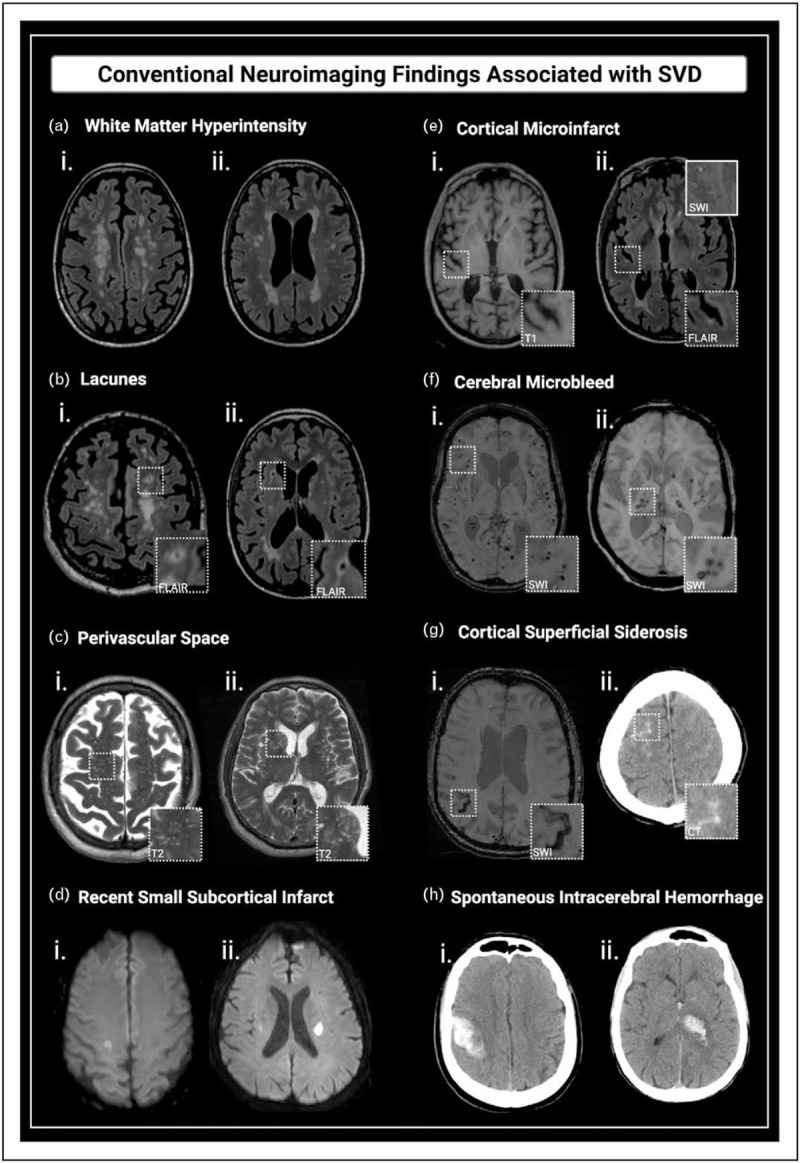
Conventional neuroimaging findings associated with SVD. (a) WMH: confluent hyperintensity foci visible on FLAIR (i and ii). (b) Lacunes: fluid-filled subcortical cavities, 3–15 mm, isointense to CSF, often with hyperintense rims on FLAIR (i - lobar lacune; ii - deep lacune). (c) PVS: linear, ovoid or round-shaped fluid-filled spaces, following the course of vessels (i - predominating in the centrum semiovale; ii - affecting the basal ganglia). (d) Recent small subcortical infarcts: hyperintense foci on DWI (i and ii). (e) Cortical CMIs: intracortical lesions ≤ 4 mm, hypointense on T1 (i) and hyper or isointense on FLAIR (ii). (f) CMBs: foci of hemosiderin deposition, with very low signal intensity on SWI (lobar CMBs (i), and deep CMBs (ii)). (g) cSS: linear hypointense foci with gyriform pattern over the cerebral cortex on SWI (i). The acute form of superficial bleeding is cSAH, seen as linear hyperdensities on CT (ii) or as hyperintensity on FLAIR. (h) Spontaneous ICH: nontraumatic lobar (i) and deep (ii) hemorrhages, depicted as focal hyperdense lesions on CT. CAA, cerebral amyloid angiopathy; CMB, cerebral microbleed; CMI, cerebral microinfarcts; cSAH, convexity subarachnoid hemorrhage; CSF, cerebrospinal fluid; cSS, cortical superficial siderosis; CT, computed tomography; DWI, diffusion weighted image; FLAIR, Fluid-attenuated inversion recovery; ICH, intracerebral hemorrhage; PVS, perivascular space; SVD, small vessel disease; SWI, susceptibility weighted imaging; WMH, white matter hyperintensity. Adapted from [14]. Created with BioRender.com.
Conventional neuroimaging markers in small vessel disease-related cognitive impairment
MRI-visible lesions represent only a small portion of the spectrum of brain injury related to SVD and probably reflect late and irreversible steps in this pathological process [15]. Established SVD markers include WMH, lacunes, PVS, recent small subcortical infarcts, CMB, cSS, ICH and atrophy (Fig. 2). More recently, CMI and cSAH have been associated with SVD, especially with CAA (Fig. 2) [58–60]. Even though these markers are useful for the diagnosis of SVD, their relevance as predictors of cognitive impairment and dementia is less evident.
CMB's occurrence, number and topographical distribution reflect the presence, severity and cause of the underlying SVD and correlate with increased mortality and a higher risk of haemorrhagic and ischemic stroke [63▪▪,64]. However, although there is some evidence linking CMB to cognitive impairment, studies have yielded conflicting results and small effect sizes [63▪▪,64,65], possibly because CMB do not cause significant disruption of adjacent tissues [65]. Similarly, cSS contributes to in-vivo diagnosis of CAA and is strongly associated with recurrent lobar ICH [66], but there are insufficient data supporting an independent association with cognition [67].
In contrast, nonhaemorrhagic markers, in general, are more strongly associated with cognition [65]. WMH is one of the earliest and most established markers of SVD, associated with increased risk of stroke and mortality [63▪▪]. Recent meta-analyses support a strong link between WMH and VCI and indicate that extensive baseline burden, progression and periventricular distribution of WMH are associated with an increased risk of dementia [63▪▪,68]. Likewise, incident lacunes have been associated with dementia, worse executive function and psychomotor speed [69]. Although the burden of cortical CMI is underestimated on MRI, they are considered the most widespread form of brain infarct, associated with CAA and highly prevalent in cognitively impaired individuals [58]. In contrast with CMB, they are strongly associated with cognitive endpoints [70,71▪], affecting performance potentially by disrupting adjacent tracts, with secondary perilesional and remote degeneration [58]. Although brain atrophy has been frequently associated with SVD, it reflects the final converging effects of aging and several other pathologies, including neurodegenerative diseases [72▪,73]. Several studies link smaller brain volumes with cognitive impairment, yet none of them controlled for co-occurring Alzheimer's disease, limiting causal inferences [74]. Increased visibility of PVS has also been associated with worse cognitive performance [72▪], but overall results are still conflicting, and its usefulness as a biomarker for cognition remains largely unknown [75].
Finally, in an attempt, to capture the overall burden of SVD, sum scores have been developed based on visual ratings of several aforementioned MRI-visible lesions [76,77]. In population-based and patient cohorts, higher scores were associated with cognitive decline and increased risk of dementia [78–81].
Advanced neuroimaging markers in small vessel disease-related cognitive impairment
Although conventional MRI markers are appealing for being widely available and easily evaluated, advanced MRI techniques offer stronger cognitive associations in general, likely as a result of their sensitivity to microstructural abnormalities and disruption of network connections.
Diffusion tensor imaging (DTI) is considered one of the most promising MRI techniques in the fields of VCI and SVD. Diffusion properties of the water molecules reflect microstructural integrity and correlate with relevant histopathological changes [82]. DTI markers are sensitive to early and widespread abnormalities that go undetected on conventional MRI [14] and outperform MRI-visible lesions [83,84▪] by explaining more cognitive variance [14,85]. Furthermore, diffusion changes seem to be predominantly driven by SVD in comparison to Alzheimer's disease pathology [86▪]. To overcome challenges imposed by highly complex and time-consuming postprocessing techniques, novel automated DTI-based markers, such as peak width of skeletonized mean diffusivity (PSMD), have been developed. PSMD reflects the heterogeneity of diffusivity across the main white matter tracts [85]. It shows consistent cognitive associations in SVD and ageing populations [85,87–91] and is considered a promising biomarker to be applied in future clinical trials in the field of VCI. Furthermore, through the combination of tractography and graph-theory analysis, valuable metrics of structural connectivity can be derived from diffusion images and have been found to predict cognitive decline [84▪,92], conversion to dementia [93] and even all-cause mortality [92] in SVD populations.
Other advanced imaging techniques evaluating functional connectivity status [14,94] and pathological changes in perfusion, vascular permeability and vasoreactivity [95,96] are still under investigation.
Despite their relevance in the research field, the aforementioned modalities require further validation before they can be applied in clinical practice.
MANAGEMENT OF VASCULAR COGNITIVE IMPAIRMENT
Currently, management of VCI is centred on preventing and controlling vascular risk factors such as hypertension, obesity, smoking and diabetes (Fig. 1) [45▪▪,97,98]. Together, they account for 25–40% of dementia cases [2▪▪,45▪▪]. Better control of these factors is partly responsible for the decreasing incidence of dementia observed in high-income countries [45▪▪,99], mostly driven by lower rates of vascular dementia, considered the most preventable component of age-related cognitive decline [4,97,98]. Known protective factors include markers of increased cognitive reserve, such as higher education, occupation, social networks, cognitive and physical activity [11▪▪,100].
Vascular risk factor control
High blood pressure (BP) represents the primary modifiable risk factor involved in SVD progression and VCI [50▪▪]. Hypertension affects more than 75% of individuals over 65 years, of whom nearly 53% are inadequately controlled [101]. Although both mid-life and late-life hypertension are associated with WMH progression, lower brain volume in later life [102] and disruption of white matter microstructure [103,104], mid-life hypertension is more strongly linked to dementia, as BP begins to fall 5 years before diagnosis [105]. Likewise, systolic BP (SBP) of more than 130 mmHg at age 50, but not later in life, was associated with an increased risk of dementia [106]. Adding to the complexity, decline in BP in late life was associated with cerebral (micro)infarcts, with hypoperfusion being a potential culprit [107]. Interestingly, the effect of elevated BP on cognitive decline appears to be mediated by both vascular pathology as well as neuritic plaques and neurofibrillary tangles, suggesting that managing BP can alleviate both vascular and neurodegenerative pathways [107–109]. Beyond elevated mean BP, fluctuations of BP over a period of hours, days and years have been increasingly found to influence brain health [110,111▪,112▪▪]. Emerging evidence suggests a link between BP variability, SVD progression and dementia risk [111▪,113]. More insights are needed to define this complex relationship between BP profile in ageing and cognitive impairment.
Findings from clinical trials support that interventions may lower the risk of VCI progression. In the SPRINT-MIND study, middle-aged individuals (>50 years) and the elderly (mean age 68 years) with an increased vascular risk submitted to intensive BP management (SBP goal <120 mmHg) showed reduced WMH progression [114▪] and smaller incidence of MCI in comparison to the control group (SBP goal <140 mmHg) [115▪▪]. The ACCORD-MIND substudy and the INFINITY study also supported that intensive BP control might help slow down WMH progression, with varying effects on cognition [116,117]. Of importance, after ICH, inadequate BP control is associated with increased risk of lobar and nonlobar ICH recurrence [118].
For patients with VCI, expert's consensus advocates that antihypertensive therapy should be initiated when BP is ≥140/90 mmHg and should aim to achieve a BP treatment target of <130/80 mmHg (<140/80 mmHg in elderly patients) [50▪▪,119]. New evidence suggest, that in eligible middle-aged and in the elderly with vascular risk factors, targeting SBP less than 120 mmHg with careful side effect monitoring may prevent development of MCI when compared with standard therapy [50▪▪,115▪▪] There is a lack of convincing data, on the choice of antihypertensive medications, but despite limited evidence [120], calcium-channel blockers and angiotensin receptor blockers may be preferred treatments [50▪▪,121].
Diabetes is an established risk factor for future dementia [122▪] and has been associated with poorer processing speed and executive function, likely by contributing to SVD-related disruption of structural and functional connectivity [103,122▪]. There is conflicting evidence regarding whether intensive glycaemic control in diabetic individuals could reduce micro/macrovascular complications [123,124], and no evidence insofar has been found for a protective effect on SVD progression or cognitive decline [116,125,126]. The focus should be on preventing hyperglycaemia and repetitive hypoglycaemia, as both have been linked to dementia [45▪▪,125].
Although the effects of hypertension and diabetes on cognition appear to be driven mainly by vascular disease, evidence suggests that dyslipidaemia contributes more to Alzheimer's disease related degeneration [103,127]. For instance, midlife dyslipidaemia has been associated with amyloid and tau deposition later in life [127,128]. Although these findings imply a potential benefit of lipid-lowering therapy on cognition, clinical trials have not yet shown encouraging results [129,130]. Moreover, a large prospective study, including 96 043 participants, suggested that lowering LDL cholesterol below 70 mg/dl may increase the risk of ICH [131▪]. Further research should evaluate the benefit/risk ratio of lipid-lowering therapy in patients at a higher risk of haemorrhagic complications, such as CAA cases [61▪▪].
Mid-life obesity is another emerging risk factor for dementia in later life [132]. Weight-loss of at least 2 kg was associated with improved attention and memory [133]. Not surprisingly, heavy midlife smoking has also been linked to increased risk of cognitive decline [45▪▪]. Smoking appears to contribute in a dose-dependent way to increase WMH burden and to disrupt microstructural integrity, effects that may be partly reversible after cessation [134,135].
As expected, in addition to SVD, other cerebrovascular diseases play a major role in the pathogenesis of vascular cognitive impairment. Stroke itself is a powerful risk factor for dementia, increasing the risk two-fold [136]. The postevent conversion rate to dementia at 1 year is estimated at 34.4% in patients with severe stroke, 8.2% in minor stroke and 5.2% in those with TIA [137▪▪]. The variability in the incidence and temporality of poststroke dementia suggests that other factors, such as SVD, may influence poststroke outcomes [138]. Accordingly, atrial fibrillation is considered a potent risk factor for cognitive decline [139]. In a large registry study, incident dementia risk was reduced by 48% in patients with atrial fibrillation on oral anticoagulation vs. no anticoagulation [139]. Lastly, haemorrhagic stroke, accounting for highest burden of stroke-related morbidity and mortality, confer an increased risk of dementia, particularly high in lobar ICH cases, in which CAA disease plays an important role [61▪▪,140]. All things considered, strategies to prevent ischaemic and haemorrhagic stroke hold the potential to significantly reduce the burden of cognitive impairment in the population.
Symptomatic treatment
Acetylsalicylic acid is considered a reasonable therapy in patients with MCI or dementia presenting covert brain infarcts on imaging, yet confirmatory trials are still missing [50▪▪]. In contrast, aspirin is not recommended for patients presenting with VCI attributable to confluent WMH only, without other evidence-based indications [50▪▪]. Of note, the recent ASPREE trial did not show aspirin treatment to be beneficial in terms of cognitive outcomes in the general elderly population (>70 years) [141]. The question still remains if prophylactic antithrombotic treatment can benefit patients with underlying moderate to severe vascular disease.
Cholinesterase inhibitors (donepezil, galantamine, rivastigmine) and N-methyl D-aspartate antagonist memantine may be considered for symptomatic treatment in selected patients with dementia and SVD [23,50▪▪]. Only donepezil has shown a modest clinically appreciable effect on cognition in trials that evaluated demented patients with vascular component [142]. Galantamine can be considered for treatment in patients with mixed neurodegenerative and SVD pathology [23]. Because cholinesterase inhibitors and memantine in VCI are considered off-label by FDA, the decision to administer these drugs should be taken with caution and discussed with patients in light of the risk of adverse events. Finally, extracts of ginkgo biloba (EGb761) were reported to have some effect on cognition and ADLs [143].
Protective lifestyle factors
Maintaining cognitive activity in late-life plays a major role in improving and maintaining brain structure and function [100,144]. Evidence suggests that low levels of education contribute to cognitive impairment [38], whereas physical activity in the elderly decreases the risk of developing dementia [145,146]. Interestingly, the preventive effect of exercise and cultural activities on cognition is enhanced when conducted in company, reinforcing the importance of social networks [147]. Furthermore, Mediterranean diet is generally recommended to reduce the risk of cognitive decline [148,149], and both Mediterranean and vegetarian diets were shown to be associated with stroke risk reduction [150,151▪].
Combining different interventions is a promising approach. The Finish FINGER trial found improved cognitive performance in at-risk elderly individuals receiving a multidomain lifestyle intervention that included nutritional guidance, exercise, cognitive training and management of vascular risk factors [152].
Novel insights into sleep and cognition [18▪▪,19] suggest that short night sleep (<5 h), poor quality sleep and hypnotics use are associated with an increased risk of dementia in healthy adults [153,154]. Accordingly, moderate to severe sleep apnoea is linked to WMH and silent brain infarctions [155,156▪]. Recent findings on the influence of non-REM-sleep in perivascular clearance of metabolites from the brain, with potential impact on neurodegenerative and vascular pathways [19], raise questions as to whether interventions focused on improving sleep quality could prevent or reverse age-related cognitive decline. Further research is required to clarify associations between sleep quality and cognitive decline.
CONCLUSION AND FUTURE DIRECTIONS
Recent research has taught us that cognitive decline in the elderly is driven by interacting neurodegenerative and vascular pathways, with significant contribution from SVD. Reports of declining incidence of dementia in high-income countries, together with recent positive trials on multifactorial interventions and BP control, are encouraging and highlight the importance of further investigating the impact of vascular risk-factors on cognition. Although there is a challenging path ahead in the quest for disease-modifying interventions, a better understanding of pathological mechanisms underlying SVD could lead to identifying new potential therapeutic targets. Novel neuroimaging markers are promising tools for clinical trials in the field and may act as surrogate markers for cognitive endpoints [72▪,157]. As vascular disease is considered the most preventable component of cognitive decline in the elderly, tackling cardiovascular risk-factors remains the cornerstone therapeutic approach.
Acknowledgements
None.
Financial support and sponsorship
L.S. was supported by a scholarship from the Swiss National Science Foundation [P2GEP3_191584].
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Flier WM, van der, Skoog I, et al. Vascular cognitive impairment. Nat Rev Dis Primers 2018; 4:18003. [DOI] [PubMed] [Google Scholar]
- 2▪▪.Wolters FJ, Ikram MA. Epidemiology of vascular dementia. Arterioscler Thromb Vasc Biol 2019; 39:1542–1549. [DOI] [PubMed] [Google Scholar]; This review highlights the multifactorial nature of cognitive decline in the elderly, and suggests that definitions and classifications in the field of dementia should acknowledge the potential contributions from different pathways.
- 3▪.Boyle PA, Yu L, Wilson RS, et al. Person-specific contribution of neuropathologies to cognitive loss in old age. Ann Neurol 2018; 83:74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]; A recent clinical–pathologic populational study on the prevalence and co-occurrence of multiple neuropathologies in the elderly, quantifying their contributions to cognitive loss at the individual level.
- 4.Viswanathan A, Rocca WA, Tzourio C. Vascular risk factors and dementia. Neurology 2009; 72:368–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dichgans M, Leys D. Vascular cognitive impairment. Circ Res 2017; 120:573–591. [DOI] [PubMed] [Google Scholar]
- 6▪▪.Wardlaw JM, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol 2019; 18:684–696. [DOI] [PubMed] [Google Scholar]; Detailed discussion on the pathophysiology underlying SVD and how its clinical presentation and course are influenced by multiple factors.
- 7.Cannistraro RJ, Badi M, Eidelman BH, et al. CNS small vessel disease: a clinical review. Neurology 2019; 92:1146–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blevins BL, Vinters HV, Love S, et al. Brain arteriolosclerosis. Acta Neuropathol 2020; 141:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9▪▪.Greenberg SM, Bacskai BJ, Hernandez-Guillamon M, et al. Cerebral amyloid angiopathy and Alzheimer disease: one peptide, two pathways. Nat Rev Neurol 2020; 16:30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]; Detailed discussion on the similarities and differences in the pathogenesis of CAA and Alzheimer disease, and potential interactive pathways.
- 10.Li T, Huang Y, Cai W, et al. Age-related cerebral small vessel disease and inflammaging. Cell Death Dis 2020; 11:932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11▪▪.Iadecola C, Duering M, Hachinski V, et al. Vascular cognitive impairment and dementia. J Am Coll Cardiol 2019; 73:3326–3344. [DOI] [PMC free article] [PubMed] [Google Scholar]; This scientific expert panel review provides a comprehensive overview of recent advances in the field of vascular cognitive impairment and dementia.
- 12.Marini S, Anderson CD, Rosand J. Genetics of cerebral small vessel disease. Stroke 2019; 51:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rutten-Jacobs LCA, Rost NS. Emerging insights from the genetics of cerebral small-vessel disease. Ann Ny Acad Sci 2020; 1471:5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Telgte A, ter Leijsen EMC, et al. Cerebral small vessel disease: from a focal to a global perspective. Nat Rev Neurol 2018; 14:387–398. [DOI] [PubMed] [Google Scholar]
- 15.Charidimou A, Boulouis G, Gurol ME, et al. Emerging concepts in sporadic cerebral amyloid angiopathy. Brain 2017; 140:1829–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iadecola C. The pathobiology of vascular dementia. Neuron 2013; 80:844–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veluw SJ, van Hou SS, et al. Vasomotion as a driving force for paravascular clearance in the awake mouse brain. Neuron 2020; 105:549–561. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18▪▪.Nedergaard M, Goldman SA. Glymphatic failure as a final common pathway to dementia. Science 2020; 370:50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]; An updated review on the pathophysiology of the glymphatic system and its relation with sleep and neurodegeneration.
- 19.Fultz NE, Bonmassar G, Setsompop K, et al. Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science 2019; 366:628–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duering M, Gesierich B, Seiler S, et al. Strategic white matter tracts for processing speed deficits in age-related small vessel disease. Neurology 2014; 82:1946–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao L, Biesbroek JM, Shi L, et al. Strategic infarct location for poststroke cognitive impairment: a multivariate lesion-symptom mapping study. J Cereb Blood Flow Metab 2017; 38:1299–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hachinski V, Iadecola C, Petersen RC, et al. National Institute of Neurological Disorders and Stroke: Canadian Stroke Network Vascular Cognitive Impairment Harmonization Standards. Stroke 2006; 37:2220–2241. [DOI] [PubMed] [Google Scholar]
- 23.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia. Stroke 2011; 42:2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skrobot OA, Black SE, Chen C, et al. Progress toward standardized diagnosis of vascular cognitive impairment: guidelines from the Vascular Impairment of Cognition Classification Consensus Study. Alzheimers Dement 2018; 14:280–292. [DOI] [PubMed] [Google Scholar]
- 25.Skrobot OA, O’Brien J, Black S, et al. The vascular impairment of Cognition Classification Consensus Study. Alzheimers Dement 2017; 13:624–633. [DOI] [PubMed] [Google Scholar]
- 26.Sachdev P, Kalaria R, O’Brien J, et al. Diagnostic criteria for vascular cognitive disorders. Alz Dis Assoc Dis 2014; 28:206–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies: report of the NINDS-AIREN International Workshop. Neurology 1993; 43:250–1250. [DOI] [PubMed] [Google Scholar]
- 28▪.Boyle PA, Yu L, Leurgans SE, et al. Attributable risk of Alzheimer's dementia attributed to age-related neuropathologies. Ann Neurol 2019; 85:114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]; The attributable risk of Alzheimer's disease was calculated for nine neuropathologies in two longitudinal clinical-pathological studies.
- 29.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011; 7:270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frey BM, Petersen M, Schlemm E, et al. White matter integrity and structural brain network topology in cerebral small vessel disease: the Hamburg city health study. Hum Brain Mapp 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valenti R, Reijmer YD, Charidimou A, et al. Total small vessel disease burden and brain network efficiency in cerebral amyloid angiopathy. J Neurol Sci 2017; 382:10–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du J, Wang Y, Zhi N, et al. Structural brain network measures are superior to vascular burden scores in predicting early cognitive impairment in post stroke patients with small vessel disease. Neuroimage Clin 2019; 22:101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lawrence AJ, Chung AW, Morris RG, et al. Structural network efficiency is associated with cognitive impairment in small-vessel disease. Neurology 2014; 83:304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arvanitakis Z, Leurgans SE, Wang Z, et al. Cerebral amyloid angiopathy pathology and cognitive domains in older persons. Ann Neurol 2011; 69:320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boyle PA, Yu L, Nag S, et al. Cerebral amyloid angiopathy and cognitive outcomes in community-based older persons. Neurology 2015; 85:1930–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiong L, Davidsdottir S, Reijmer YD, et al. Cognitive profile and its association with neuroimaging markers of non-demented cerebral amyloid angiopathy patients in a stroke unit. J Alzheimers Dis 2016; 52:171–178. [DOI] [PubMed] [Google Scholar]
- 37.Smits LL, Harten AC, van, et al. Trajectories of cognitive decline in different types of dementia. Psychol Med 2015; 45:1051–1059. [DOI] [PubMed] [Google Scholar]
- 38.Hamilton OKL, Backhouse EV, Janssen E, et al. Cognitive impairment in sporadic cerebral small vessel disease: a systematic review and meta-analysis. Alzheimers Dement 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thanprasertsuk S, Martinez-Ramirez S, Pontes-Neto OM, et al. Posterior white matter disease distribution as a predictor of amyloid angiopathy. Neurology 2014; 83:794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valenti R, Charidimou A, Xiong L, et al. Visuospatial functioning in cerebral amyloid angiopathy: a pilot study. J Alzheimers Dis 2017; 56:1223–1227. [DOI] [PubMed] [Google Scholar]
- 41.Jouvent E, Reyes S, Mangin J-F, et al. Apathy is related to cortex morphology in CADASIL. Neurology 2011; 76:1472–1477. [DOI] [PubMed] [Google Scholar]
- 42.Reyes S, Viswanathan A, Godin O, et al. Apathy. Neurology 2009; 72:905–910. [DOI] [PubMed] [Google Scholar]
- 43.Reijmer YD, Fotiadis P, Martinez-Ramirez S, et al. Structural network alterations and neurological dysfunction in cerebral amyloid angiopathy. Brain 2015; 138:179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Brien JT, Erkinjuntti T, Reisberg B, et al. Vascular cognitive impairment. Lancet Neurol 2003; 2:89–98. [DOI] [PubMed] [Google Scholar]
- 45▪▪.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020; 396:413–446. [DOI] [PMC free article] [PubMed] [Google Scholar]; An extensive recent review on prevention and management of dementia and related neuropsychiatric symptoms.
- 46.Li G, Wang LY, Shofer JB, et al. Temporal relationship between depression and dementia: findings from a large community-based 15-year follow-up study. Arch Gen Psychiat 2011; 68:970–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Auriel E, Charidimou A, Gurol ME, et al. Validation of clinicoradiological criteria for the diagnosis of cerebral amyloid angiopathy–related inflammation. JAMA Neurol 2016; 73:197–202. [DOI] [PubMed] [Google Scholar]
- 48.Bouthour W, Sveikata L, Vargas MI, et al. Clinical reasoning: rapid progression of reversible cognitive impairment in an 80-year-old man. Neurology 2018; 91:1109–1113. [DOI] [PubMed] [Google Scholar]
- 49▪.Regenhardt RW, Thon JM, Das AS, et al. Association between immunosuppressive treatment and outcomes of cerebral amyloid angiopathy–related inflammation. JAMA Neurol 2020; 77: [DOI] [PMC free article] [PubMed] [Google Scholar]; A retrospective analysis of a large cohort of rare CAA-related inflammation cases, evaluating the effectiveness of immunosuppressive therapy on recovery and recurrence.
- 50▪▪.Smith EE, Barber P, Field TS, et al. Canadian Consensus Conference on Diagnosis and Treatment of Dementia (CCCDTD)5: guidelines for management of vascular cognitive impairment. Alzheimers Dement (N Y) 2020; 6:e12056. [DOI] [PMC free article] [PubMed] [Google Scholar]; The most recent guidelines form the Canadian Consensus Conference on VCI management.
- 51.Linn J, Halpin A, Demaerel P, et al. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy (CME). Neurology 2010; 74:1346–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rodrigues MA, Samarasekera N, Lerpiniere C, et al. The Edinburgh CT and genetic diagnostic criteria for lobar intracerebral haemorrhage associated with cerebral amyloid angiopathy: model development and diagnostic test accuracy study. Lancet Neurol 2018; 17:232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martinez-Ramirez S, Romero J-R, Shoamanesh A, et al. Diagnostic value of lobar microbleeds in individuals without intracerebral hemorrhage. Alzheimers Dement 2015; 11:1480–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Charidimou A, Boulouis G, Haley K, et al. White matter hyperintensity patterns in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology 2016; 86:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Charidimou A, Boulouis G, Pasi M, et al. MRI-visible perivascular spaces in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology 2017; 88:1157–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pasi M, Pongpitakmetha T, Charidimou A, et al. Cerebellar microbleed distribution patterns and cerebral amyloid angiopathy. Stroke 2019; 50:1727–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pasi M, Boulouis G, Fotiadis P, et al. Distribution of lacunes in cerebral amyloid angiopathy and hypertensive small vessel disease. Neurology 2017; 88:2162–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Veluw SJ, van Shih AY, et al. Detection, risk factors, and functional consequences of cerebral microinfarcts. Lancet Neurol 2017; 16:730–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raposo N, Charidimou A, Roongpiboonsopit D, et al. Convexity subarachnoid hemorrhage in lobar intracerebral hemorrhage: a prognostic marker. Neurology 2020; 94:e968–e977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Q, Zotin MCZ, Warren AD, et al. CT-visible convexity subarachnoid hemorrhage is associated with cortical superficial siderosis and predicts recurrent ICH. Neurology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61▪▪.Kozberg MG, Perosa V, Gurol ME, et al. A practical approach to the management of cerebral amyloid angiopathy. Int J Stroke 2020; 174749302097446.[Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]; A comprehensive review on cerebral amyloid angiopathy management.
- 62.Mancuso M, Arnold M, Bersano A, et al. Monogenic cerebral small-vessel diseases: diagnosis and therapy. Consensus recommendations of the European Academy of Neurology. Eur J Neurol 2020; 27:909–927. [DOI] [PubMed] [Google Scholar]
- 63▪▪.Debette S, Schilling S, Duperron M-G, et al. Clinical significance of magnetic resonance imaging markers of vascular brain injury: a systematic review and meta-analysis. JAMA Neurol 2019; 76:81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]; A systematic review and meta-analysis of imaging markers of covert vascular brain injury, namely white matter hyperintensities, infarcts, cerebral microbleeds and perivascular spaces.
- 64.Moulin S, Cordonnier C. Role of cerebral microbleeds for intracerebral haemorrhage and dementia. Curr Neurol Neurosci 2019; 19:51. [DOI] [PubMed] [Google Scholar]
- 65.Reijmer YD, Veluw SJ, van Greenberg SM. Ischemic brain injury in cerebral amyloid angiopathy. J Cereb Blood Flow Metab 2015; 36:40–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Charidimou A, Boulouis G, Roongpiboonsopit D, et al. Cortical superficial siderosis and recurrent intracerebral hemorrhage risk in cerebral amyloid angiopathy: large prospective cohort and preliminary meta-analysis. Int J Stroke 2018; 14:723–733. [DOI] [PubMed] [Google Scholar]
- 67.Banerjee G, Wilson D, Jäger HR, Werring DJ. Novel imaging techniques in cerebral small vessel diseases and vascular cognitive impairment. Biochim Biophys Acta 2016; 1862:926–938. [DOI] [PubMed] [Google Scholar]
- 68.Hu H-Y, Ou Y-N, Shen X-N, et al. White matter hyperintensities and risks of cognitive impairment and dementia: a systematic review and meta-analysis of 36 prospective studies. Neurosci Biobehav Rev 2020; 120:16–27. [DOI] [PubMed] [Google Scholar]
- 69.Ling Y, Chabriat H. Incident cerebral lacunes: a review. J Cereb Blood Flow Metab 2020; 40:909–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cao L, Tan L, Wang H-F, et al. Cerebral microinfarcts and dementia: a systematic review and metaanalysis. Curr Alzheimer Res 2017; 14:802–808. [DOI] [PubMed] [Google Scholar]
- 71▪.Hilal S, Tan CS, Veluw SJ, et al. Cortical cerebral microinfarcts predict cognitive decline in memory clinic patients. J Cereb Blood Flow Metab 2019; 40:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]; A study on the association between cerebral microinfarcts and cognitive decline within 2 years of follow-up in memory-clinic individuals.
- 72▪.Smith EE, Biessels GJ, Guio FD, et al. Harmonizing brain magnetic resonance imaging methods for vascular contributions to neurodegeneration. Alzheimers Dement (Amst) 2019; 11:191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]; An expert's initiative providing a framework for developing and validating neuroimaging biomarkers in the field of VCI, aimed at harmonizing MRI methods for future studies.
- 73.Jouvent E, Viswanathan A, Chabriat H. Cerebral atrophy in cerebrovascular disorders. J Neuroimaging 2010; 20:213–218. [DOI] [PubMed] [Google Scholar]
- 74.Guio FD, Duering M, Fazekas F, et al. Brain atrophy in cerebral small vessel diseases: extent, consequences, technical limitations and perspectives: the HARNESS initiative. J Cereb Blood Flow Metab 2019; 40:231–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wardlaw JM, Benveniste H, Nedergaard M, et al. Perivascular spaces in the brain: anatomy, physiology and pathology. Nat Rev Neurol 2020; 16:137–153. [DOI] [PubMed] [Google Scholar]
- 76.Charidimou A, Martinez-Ramirez S, Reijmer YD, et al. Total magnetic resonance imaging burden of small vessel disease in cerebral amyloid angiopathy: an imaging-pathologic study of concept validation. JAMA Neurol 2016; 73:994–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Staals J, Booth T, Morris Z, et al. Total MRI load of cerebral small vessel disease and cognitive ability in older people. Neurobiol Aging 2015; 36:2806–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Olama AAA, Wason JMS, Tuladhar AM, et al. Simple MRI score aids prediction of dementia in cerebral small vessel disease. Neurology 2020; 94:e1294–e1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yilmaz P, Ikram MA, Ikram MK, et al. Application of an imaging-based sum score for cerebral amyloid angiopathy to the general population: risk of major neurological diseases and mortality. Front Neurol 2019; 10:1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yilmaz P, Ikram MK, Niessen WJ, et al. Practical small vessel disease score relates to stroke, dementia, and death: the Rotterdam Study. Stroke 2018; 49:2857–2865. [DOI] [PubMed] [Google Scholar]
- 81.Brutto VJD, Ortiz JG, Brutto OHD, et al. Total cerebral small vessel disease score and cognitive performance in community-dwelling older adults. Results from the Atahualpa Project. Int J Geriatr Psych 2018; 33:325–331. [DOI] [PubMed] [Google Scholar]
- 82.Veluw SJ, van Reijmer YD, et al. Histopathology of diffusion imaging abnormalities in cerebral amyloid angiopathy. Neurology 2019; 92:e933–e943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Williams OA, Zeestraten EA, Benjamin P, et al. Diffusion tensor image segmentation of the cerebrum provides a single measure of cerebral small vessel disease severity related to cognitive change. Neuroimage Clin 2017; 16:330–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84▪.Boot EM, Mc van Leijsen E, Bergkamp MI, et al. Structural network efficiency predicts cognitive decline in cerebral small vessel disease. Neuroimage Clin 2020; 27:102325. [DOI] [PMC free article] [PubMed] [Google Scholar]; Within the RUN-DMC study, the association of network efficiency measures and cognitive performance have been studied.
- 85.Baykara E, Gesierich B, Adam R, et al. A novel imaging marker for small vessel disease based on skeletonization of white matter tracts and diffusion histograms. Ann Neurol 2016; 80:581–592. [DOI] [PubMed] [Google Scholar]
- 86▪.Finsterwalder S, Vlegels N, Gesierich B, et al. Small vessel disease more than Alzheimer's disease determines diffusion MRI alterations in memory clinic patients. Alzheimers Dement 2020; 16:1504–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]; Diffusion abnormalities in memory-clinic patients were found to be more likely driven by SVD than Alzheimer's disease components.
- 87.Wei N, Deng Y, Yao L, et al. A neuroimaging marker based on diffusion tensor imaging and cognitive impairment due to cerebral white matter lesions. Front Neurol 2019; 10:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Deary IJ, Ritchie SJ, Maniega SM, et al. Brain peak width of skeletonized mean diffusivity (PSMD) and cognitive function in later life. Front Psychiatry 2019; 10:524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Low A, Mak E, Stefaniak JD, et al. Peak width of skeletonized mean diffusivity as a marker of diffuse cerebrovascular damage. Front Neurosci 2020; 14:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lam BYK, Leung KT, Yiu B, et al. Peak width of skeletonized mean diffusivity and its association with age-related cognitive alterations and vascular risk factors. Alzheimers Dement Diagn Assess Dis Monit 2019; 11:721–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Raposo N, Zotin MCZ, Schoemaker D, et al. Peak width of skeletonized mean diffusivity as neuroimaging biomarker in cerebral amyloid angiopathy. Am J Neuroradiol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tuladhar AM, Tay J, Leijsen E, et al. Structural network changes in cerebral small vessel disease. J Neurol Neurosurg Psychiatry 2020; 91:196. [DOI] [PubMed] [Google Scholar]
- 93.Lawrence AJ, Zeestraten EA, Benjamin P, et al. Longitudinal decline in structural networks predicts dementia in cerebral small vessel disease. Neurology 2018; 90:e1898–e1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang R, Liu N, Tao Y-Y, et al. The application of rs-fMRI in vascular cognitive impairment. Front Neurol 2020; 11:951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magnet Reson Med 2015; 73:102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Thrippleton MJ, Backes WH, Sourbron S, et al. Quantifying blood-brain barrier leakage in small vessel disease: review and consensus recommendations. Alzheimers Dement 2019; 15:840–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Satizabal CL, Beiser AS, Chouraki V, et al. Incidence of dementia over three decades in the Framingham Heart Study. N Engl J Med 2016; 374:523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hachinski V, Einhäupl K, Ganten D, et al. Preventing dementia by preventing stroke: the Berlin Manifesto. Alzheimers Dement 2019; 15:961–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu Y-T, Beiser AS, Breteler MMB, et al. The changing prevalence and incidence of dementia over time: current evidence. Nat Rev Neurol 2017; 13:327–339. [DOI] [PubMed] [Google Scholar]
- 100.Stern Y. Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurol 2012; 11:1006–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Muntner P, Carey RM, Gidding S, et al. Potential US population impact of the 2017 ACC/AHA High Blood Pressure Guideline. Circulation 2018; 137:109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lane CA, Barnes J, Nicholas JM, et al. Associations between blood pressure across adulthood and late-life brain structure and pathology in the neuroscience substudy of the 1946 British birth cohort (Insight 46): an epidemiological study. Lancet Neurology 2019; 18:942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Power MC, Tingle JV, Reid RI, et al. Midlife and late-life vascular risk factors and white matter microstructural integrity: the Atherosclerosis Risk in Communities Neurocognitive Study. J Am Heart Assoc 2017; 6:e005608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Amier RP, Marcks N, Hooghiemstra AM, et al. Hypertensive exposure markers by MRI in relation to cerebral small vessel disease and cognitive impairment. JACC Cardiovasc Imaging 2020; 14:176–185. [DOI] [PubMed] [Google Scholar]
- 105.Peters R, Peters J, Booth A, Anstey KJ. Trajectory of blood pressure, body mass index, cholesterol and incident dementia: systematic review. Br J Psychiatry 2020; 216:16–28. [DOI] [PubMed] [Google Scholar]
- 106.Abell JG, Kivimäki M, Dugravot A, et al. Association between systolic blood pressure and dementia in the Whitehall II cohort study: role of age, duration, and threshold used to define hypertension. Eur Heart J 2018; 39:3119–3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Arvanitakis Z, Capuano AW, Lamar M, et al. Late-life blood pressure association with cerebrovascular and Alzheimer disease pathology. Neurology 2018; 91:e517–e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Petrovitch H, White LR, Izmirilian G, et al. Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: the HAAS. Neurobiol Aging 2000; 21:57–62. [DOI] [PubMed] [Google Scholar]
- 109.Shah NS, Vidal J-S, Masaki K, et al. Midlife blood pressure, plasma β-amyloid, and the risk for Alzheimer disease. Hypertension 2012; 59:780–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rothwell PM. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet Lond Engl 2010; 375:938–948. [DOI] [PubMed] [Google Scholar]
- 111▪.Ma Y, Tully PJ, Hofman A, Tzourio C. Blood pressure variability and dementia: a state-of-the-art review. Am J Hypertens 2020. [DOI] [PubMed] [Google Scholar]; A state-of-the-art review on association between blood pressure variability and cognitive decline.
- 112▪▪.Ma Y, Yilmaz P, Bos D, et al. Blood pressure variation and subclinical brain disease. J Am Coll Cardiol 2020; 75:2387–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]; A population-based study linking blood pressure variability with progression of subclinical small-vessel disease in the general population.
- 113.Ma Y, Wolters FJ, Chibnik LB, et al. Variation in blood pressure and long-term risk of dementia: a population-based cohort study. PLoS Med 2019; 16:e1002933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114▪.Nasrallah IM, Pajewski NM, Chelune G, et al. Association of intensive vs standard blood pressure control with cerebral white matter lesions. JAMA 2019; 322:524. [DOI] [PMC free article] [PubMed] [Google Scholar]; SPRINT-MIND substudy showing association of intensive BP therapy and reduced WMH and brain atrophy progression.
- 115▪▪.Williamson JD, Pajewski NM, Auchus AP, et al. Effect of intensive vs standard blood pressure control on probable dementia. JAMA 2019; 321:553. [DOI] [PMC free article] [PubMed] [Google Scholar]; First clinical trial to suggest MCI risk reduction with intensive BP therapy.
- 116.Havenon A de, Majersik JJ, Tirschwell DL, et al. Blood pressure, glycemic control, and white matter hyperintensity progression in type 2 diabetics. Neurology 2019; 92:e1168–e1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.White WB, Wakefield DB, Moscufo N, et al. Effects of intensive versus standard ambulatory blood pressure control on cerebrovascular outcomes in older people (INFINITY). Circulation 2019; 140:1626–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Biffi A, Anderson CD, Battey TWK, et al. Association between blood pressure control and risk of recurrent intracerebral hemorrhage. JAMA 2015; 314:904–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension 2020; 75:1334–1357. [DOI] [PubMed] [Google Scholar]
- 120.Peters R, Yasar S, Anderson CS, et al. Investigation of antihypertensive class, dementia, and cognitive decline: a meta-analysis. Neurology 2019; 94:e267–e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Forette F, Seux M-L, Staessen JA, et al. The prevention of dementia with antihypertensive treatment: new evidence from the Systolic Hypertension in Europe (Syst-Eur) Study. Arch Intern Med 2002; 162:2046–2052. [DOI] [PubMed] [Google Scholar]
- 122▪.Sloten TT, van Sedaghat S, et al. Cerebral microvascular complications of type 2 diabetes: stroke, cognitive dysfunction, and depression. Lancet Diabetes Endocrinol 2020; 8:325–336. [DOI] [PMC free article] [PubMed] [Google Scholar]; A detailed review on the associations between diabetes, microvascular dysfunction and cognition.
- 123.Reaven PD, Emanuele NV, Wiitala WL, et al. Intensive glucose control in patients with Type 2 diabetes: 15-year follow-up. N Engl J Med 2019; 380:2215–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Group AS, Gerstein HC, Miller ME, et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med 2011; 364:818–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Biessels GJ, Despa F. Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat Rev Endocrinol 2018; 14:591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Launer LJ, Miller ME, Williamson JD, et al. Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): a randomised open-label substudy. Lancet Neurol 2011; 10:969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cheng Y-W, Chiu M-J, Chen Y-F, et al. The contribution of vascular risk factors in neurodegenerative disorders: from mild cognitive impairment to Alzheimer's disease. Alzheimers Res Ther 2020; 12:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nägga K, Gustavsson A-M, Stomrud E, et al. Increased midlife triglycerides predict brain β-amyloid and tau pathology 20 years later. Neurology 2017; 90:e73–e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Trompet S, Vliet P, van, et al. Pravastatin and cognitive function in the elderly. Results of the PROSPER study. J Neurol 2009; 257:85. [DOI] [PubMed] [Google Scholar]
- 130.Biessels GJ, Verhagen C, Janssen J, et al. Effect of linagliptin on cognitive performance in patients with Type 2 diabetes and cardiorenal comorbidities: the CARMELINA randomized trial. Diabetes Care 2019; 42:1930–1938. [DOI] [PubMed] [Google Scholar]
- 131▪.Ma C, Gurol ME, Huang Z, et al. Low-density lipoprotein cholesterol and risk of intracerebral hemorrhage: a prospective study. Neurology 2019; 93:e445–e457. [DOI] [PMC free article] [PubMed] [Google Scholar]; This large community-based study showed an association of intensive LDL-cholesterol lowering targets with increased risk of ICH.
- 132.Albanese E, Launer LJ, Egger M, et al. Body mass index in midlife and dementia: systematic review and meta-regression analysis of 589,649 men and women followed in longitudinal studies. Alzheimers Dement Diagn Assess Dis Monit 2017; 8:165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Veronese N, Facchini S, Stubbs B, et al. Weight loss is associated with improvements in cognitive function among overweight and obese people: a systematic review and meta-analysis. Neurosci Biobehav Rev 2017; 72:87–94. [DOI] [PubMed] [Google Scholar]
- 134.Gons RAR, Norden AGW, van, et al. Cigarette smoking is associated with reduced microstructural integrity of cerebral white matter. Brain 2011; 134:2116–2124. [DOI] [PubMed] [Google Scholar]
- 135.Power MC, Deal JA, Sharrett AR, et al. Smoking and white matter hyperintensity progression. Neurology 2015; 84:841–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kuźma E, Lourida I, Moore SF, et al. Stroke and dementia risk: a systematic review and meta-analysis. Alzheimers Dement 2018; 14:1416–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137▪▪.Pendlebury ST, Rothwell PM, Study OV. Incidence and prevalence of dementia associated with transient ischaemic attack and stroke: analysis of the population-based Oxford Vascular Study. Lancet Neurol 2019; 18:248–258. [DOI] [PMC free article] [PubMed] [Google Scholar]; A large population-based study on poststroke dementia risk.
- 138.Mok VCT, Lam BYK, Wong A, et al. Early-onset and delayed-onset poststroke dementia: revisiting the mechanisms. Nat Rev Neurol 2017; 13:148–159. [DOI] [PubMed] [Google Scholar]
- 139.Friberg L, Rosenqvist M. Less dementia with oral anticoagulation in atrial fibrillation. Eur Heart J 2017; 39:453–460. [DOI] [PubMed] [Google Scholar]
- 140.Biffi A, Bailey D, Anderson CD, et al. Risk factors associated with early vs delayed dementia after intracerebral hemorrhage. JAMA Neurol 2016; 73:969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ryan J, Storey E, Murray AM, et al. Randomized placebo-controlled trial of the effects of aspirin on dementia and cognitive decline. Neurology 2020; 95:e320–e331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kavirajan H, Schneider LS. Efficacy and adverse effects of cholinesterase inhibitors and memantine in vascular dementia: a meta-analysis of randomised controlled trials. Lancet Neurol 2007; 6:782–792. [DOI] [PubMed] [Google Scholar]
- 143.Gunten A, von, Schlaefke S, Überla K. Efficacy of Ginkgo biloba extract EGb 761 in dementia with behavioural and psychological symptoms: a systematic review. World J Biol Psychiatry 2015; 17:1–12. [DOI] [PubMed] [Google Scholar]
- 144.Sajeev G, Weuve J, Jackson JW, et al. Late-life cognitive activity and dementia. Epidemiology 2016; 27:732–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ravaglia G, Forti P, Lucicesare A, et al. Physical activity and dementia risk in the elderly. Neurology 2008; 70:1786–1794. [DOI] [PubMed] [Google Scholar]
- 146.Buchman AS, Boyle PA, Yu L, et al. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology 2012; 78:1323–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Monma T, Takeda F, Noguchi H, et al. The impact of leisure and social activities on activities of daily living of middle-aged adults: evidence from a National Longitudinal Survey in Japan. PLoS One 2016; 11:e0165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Scarmeas N, Stern Y, Mayeux R, et al. Mediterranean diet and mild cognitive impairment. Arch Neurol 2009; 66:216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Keenan TD, Agrón E, Mares JA, et al. Adherence to a Mediterranean diet and cognitive function in the Age-Related Eye Disease Studies 1 & 2. Alzheimers Dement 2020; 16:831–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Estruch R, Ros E, Salas-Salvadó J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med 2018; 378:e34. [DOI] [PubMed] [Google Scholar]
- 151▪.Chiu THT, Chang H-R, Wang L-Y, et al. Vegetarian diet and incidence of total, ischemic, and hemorrhagic stroke in 2 cohorts in Taiwan. Neurology 2020; 94:e1112–e1121. [DOI] [PMC free article] [PubMed] [Google Scholar]; A study in two large population cohorts linking vegetarian diet with decreased ischemic and haemorrhagic stroke risk.
- 152.Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 2015; 385:2255–2263. [DOI] [PubMed] [Google Scholar]
- 153.Ohara T, Honda T, Hata J, et al. Association between daily sleep duration and risk of dementia and mortality in a Japanese community. J Am Geriatr Soc 2018; 66:1911–1918. [DOI] [PubMed] [Google Scholar]
- 154.Sindi S, Kåreholt I, Johansson L, et al. Sleep disturbances and dementia risk: a multicenter study. Alzheimers Dement 2018; 14:1235–1242. [DOI] [PubMed] [Google Scholar]
- 155.Huang Y, Yang C, Yuan R, et al. Association of obstructive sleep apnea and cerebral small vessel disease: a systematic review and meta-analysis. Sleep 2019; 43:1–10. [DOI] [PubMed] [Google Scholar]
- 156▪.Chokesuwattanaskul A, Lertjitbanjong P, Thongprayoon C, et al. Impact of obstructive sleep apnea on silent cerebral small vessel disease: a systematic review and meta-analysis. Sleep Med 2020; 68:80–88. [DOI] [PubMed] [Google Scholar]; A meta-analysis suggesting an association between OSA and SVD markers.
- 157.Leurent C, Goodman JA, Zhang Y, et al. Immunotherapy with ponezumab for probable cerebral amyloid angiopathy. Ann Clin Transl Neur 2019; 6:795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]


