Abstract
Population studies indicate that astigmatism decreases from the corneal center toward the periphery. A standard toric intraocular lens (IOL) with a constant cylinder power cannot correct uniformly across this gradient. We built an astigmatic eye model based on corneal topography data. A progressive-toric lens with gradually decreasing cylinder power was compared with an identically designed lens but featuring conventional astigmatism correction. Residual astigmatism did not differ significantly (P=0.06) at 3 mm, and the Strehl ratio was identical for both lenses (0.51 ±0.15, P=0.88). At 5 mm, the progressive IOL yielded significantly lower residual astigmatism by 0.10 D (P<0.001). The Strehl ratio was 0.30 ±0.08 with the progressive and 0.29 ±0.08 with the standard lens (P<0.001). At 3 mm, the optical performance was comparable for both IOLs. However, at 5 mm, the progressive-toric was more effective in correcting astigmatism, and it yielded reduced residual astigmatism compared to a standard toric lens.
1. Introduction
Although the first toric intraocular lens (IOL) was implanted in the 1990s, [1] only in recent years has it become routinely used in the correction of corneal-astigmatism in cataract patients. While it is a well-established and safe procedure, [1–13] using toric technology may still pose challenges [14–16]. Various causes of refractive surprise have been identified, arising from incorrect IOL orientation [15–17] or from the posterior corneal surface's contribution to astigmatism [18]. The progressive change of corneal-astigmatism related to pupil size is a less-known factor, but it too may adversely affect the postoperative refraction result for toric-IOL patients [5].
In a large-population study, our group recently analyzed the topography data of 641 patients to evaluate the extent of change of astigmatism in relation to pupil size [19]. The analysis confirmed that the corneal-astigmatism is heterogeneous and that, on average, its magnitude decreases from the center to the periphery [19]. In addition, we found that the level of astigmatism reduction from 3 mm to 6 mm in our European population was 0.29 D [19]. A larger difference, however, was found in Japanese patients who had an average decrease of 0.56 D [20]. The reduction of corneal-astigmatism is a consequence of gradual flattening of the cornea towards the periphery [21]. However, this change does not evenly affect the two principal meridians, which is more pronounced in the steepest than in the flattest meridian, resulting in the lowering of peripheral astigmatism [21]. The corneal inhomogeneity may, potentially, cause a refractive surprise in patients with larger pupils. And this, in turn, may affect visual function, as shown by Visser et al. [5]. Thus, when designing a toric IOL, giving some consideration to the cornea's peripheral flattening should prove particularly advantageous in patients who have different astigmatism in the corneal center compared to the periphery [19–21].
Current astigmatism-correcting implants feature a constant cylinder power throughout the lens optic. Recently, a new toric-IOL was introduced that provides a progressive decrease in cylinder power that can correct corneal-astigmatism as it changes with pupil size. This design is derived from the observation in population studies of corneal-shape change [19–21]. The impact of the progressive toric lens on the eye's optical performance has yet to be assessed, though. Since the progressive-toric has a unique geometry, this evaluation cannot be made using a standard (stigmatic) model cornea [22,23]. Instead, a model derived from corneal topography data of astigmatic patients should be used. The application of a customized model incorporating the individual corneal shape may mimic a clinical situation and provide a better understanding of how the progressive IOL performs in real eyes.
We set out to build an eye model with an astigmatic cornea based on topography data to simulate the peripheral corneal flattening reported in clinical studies. Secondly, the customized model was implemented in optical design software to evaluate the progressive-toric IOLs’ impact on the eye's imaging quality, which was compared against a standard-toric IOL correction.
2. Materials and methods
2.1. Ray-tracing model
A virtual eye was built in OpticStudio (Radiant Zemax LLC, USA) based on an anatomical model, which has been described by Liou and Brennan [22]. An average cornea proposed in that publication was, however, replaced by a customized lens based on corneal topography obtained with a Pentacam HR (Oculus Optikgeräte GmbH, Germany). The tenets of the Declaration of Helsinki were followed, and approval was obtained from the ethics committee of the University of Heidelberg. The corneal data had been routinely collected during patients’ examinations and screened by a clinician for corneal abnormality [19]. Thus, we ensured that none of the included cases had corneal pathology or a history of corneal surgery. Patients with irregular astigmatism, often indicating corneal abnormality, were excluded from the analysis as they might be deemed not suitable for toric-IOL implantation due to reduced predictability of the postoperative refractive outcome [24,25]. The study eyes were purposely selected to mimic the distribution of the peripheral corneal change in the normal population reported earlier [19]. In that study, the majority of patients showed a decrease of corneal-astigmatism with pupil size. However, we did include cases with no change or increase of the cylinder power towards the periphery (Fig. 1).
Fig. 1.
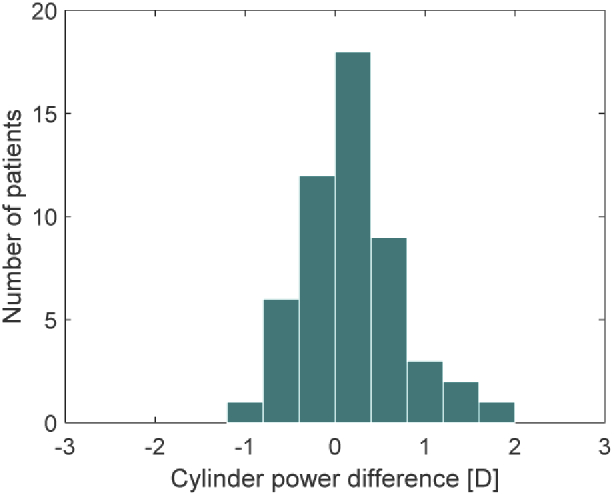
The distribution of the differences between the central and mid-peripheral corneal-astigmatism in the study population. The values represent the subtraction of the cylinder magnitude at 3 mm from that at 6 mm. The positive sign indicates the decrease of corneal-astigmatism with pupil size.
We used the following protocol to process topography results. Once we selected a case, we exported the corneal anterior and posterior surface elevation maps from the Pentacam device to fit them with Zernike functions using MATLAB (MathWorks, Inc, USA). We calculated Zernike modes up the 6th order over a 6-mm circular area centered at the corneal apex. The computed Zernike coefficients were used to simulate the anterior and posterior cornea in a Zemax model of the eye using a Zernike Standard Sag surface. The distance between the anterior and posterior corneal surfaces was set according to the central corneal thickness measured with the Pentacam HR. In each case, the model’s natural lens was replaced with a study IOL that had a nominal power of 20 D. Although the selected model corneas differed in their optical powers, we compensated for these differences by adjusting the axial length based on a minimum root-mean-square (RMS) wavefront error criterion. All optical simulations were performed in monochromatic green light (550 nm) with 3-mm (photopic) and 5-mm (scotopic) pupils [26].
2.2. IOLs study
We assessed five Avansee 1P Toric IOLs (Kowa Co. Ltd. Japan) - progressive-toric IOLs with cylinder powers at the IOL plane of 1.50D (T3), 2.25D (T4), 3.00D (T5), 3.75D (T6), and 4.50D (T7). The design files of the study IOLs were made available by Kowa Co. Ltd under the provisions of a non-disclosure agreement. Using the manufacturer’s files, we could simulate the implantation of intraocular lenses and their optical results in an astigmatic eye model.
The progressive toric IOLs in most respects follow the design features of their monofocal counterpart, the Avansee One-piece (1P), which is made of glistening-free hydrophobic-acrylic material with a refractive index of 1.519 [27]. The IOLs have an aspheric anterior surface that produces −0.04 µm of spherical aberration (SA) at a 5.15-mm pupil according to an ISO 11979-2 eye model, thus only slightly affecting the eye's intrinsic aberrations. In the toric IOL, the posterior surface has a torus and can be numerically defined by polynomial functions. The use of a freeform surface enables a gradual decrease in cylinder power from the IOL center to its periphery, which is intended to correct the human cornea’s different degrees of astigmatism between the center and the corneal periphery. The cylinder gradient differs between the IOL models, which was designed to mimic the changes observed in the Japanese population, i.e., the cylinder-power difference increases with the magnitude of corneal-astigmatism [20]. Thus, the largest difference was implemented in the T7 model.
We compared the new progressive design with conventional astigmatism-correcting IOLs, which we designed using files of the Avansee, with the only modification being the use of a standard posterior-toric surface to create a power difference between two meridians. Thus, the correction of primary SA was identical in the two types of astigmatic correction. The cylinder power of the conventional toric lenses was matched with the range of the progressive-toric IOLs (from T3 to T7).
2.3. Image quality metrics
The IOLs were compared according to their image quality metrics evaluated by means of a modulation transfer function (MTF) and higher-order aberrations (HOAs). The MTF was calculated for sagittal and tangential planes and averaged. We then used the MTF Strehl ratio, a multi-frequency parameter calculated over 100 lp/mm, to assess the retinal-image quality in the two simulated conditions [28,29]. Individual HOAs and their RMS were also computed and analyzed up to the 6th order. Zernike modes were presented graphically with a default Noll indexing method implemented in OpticStudio. Residual astigmatism was calculated for both apertures using Zernike coefficients converted to power vectors, as described by Thibos et al. [30].
2.4. Statistical analysis
Data analysis and visualization were performed using MATLAB Statistics and Machine Learning Toolbox (MathWorks, Inc., USA). After confirming normality, the optical-metrics were statistically compared with a paired-sample t-test. The differences were deemed statistically significant if the p-value was <0.05.
3. Results
We built 52 ray-tracing eye models based on patients’ topography data. The mean age of the population was 69.7 ± 5.7 years, and their corneal-astigmatism was 2.00 ± 0.75 D (range: 0.8 to 3.8 D). In seven cases, we corrected astigmatism using T3; in twelve, we used T4; in thirteen, we used T5, and twenty required T6 and T7 (ten each).
At 3 mm, astigmatism correction using the standard and progressive IOL yielded comparable residual astigmatism with the mean value of −0.36 ± 0.26 D and −0.38 ± 0.25 D, respectively. The difference did not reach the significance level (P=0.06). At 5 mm, the increase of residual astigmatism was observed in both groups. However, in the progressive model, astigmatism was significantly lower (P<0.001) with a value of −0.57 ± 0.30 D versus −0.67 ± 0.33 D found in the group with the standard toric correction.
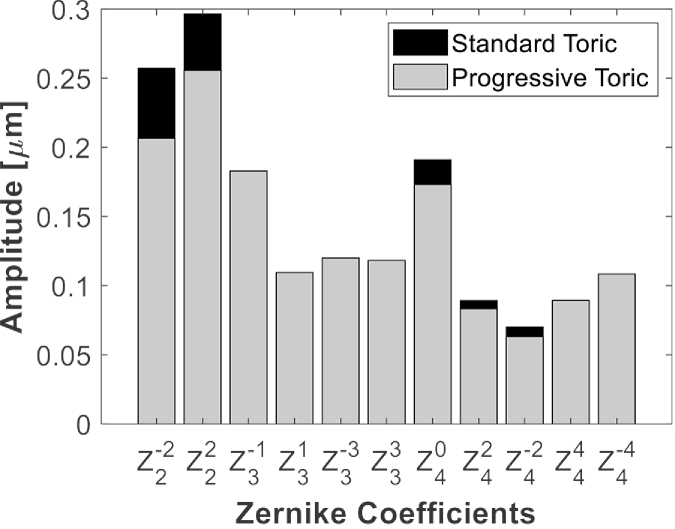
The RMS of HOAs in the ray-tracing models at 5 mm was 0.49 ± 0.21 µm with the progressive-toric and 0.50 ± 0.20 µm with the standard-toric (P<0.001). Figure 2 summarizes Zernike modes (up to the 4th order) of the studied eyes and compares the two types of astigmatism correction. The comparison of the HOAs shows that the new toric design lowers primary and secondary astigmatism and primary SA more effectively than the standard correction, but coma and trefoil aberrations did not differ.
Fig. 2.
The absolute mean of the Zernike coefficients in the analyzed set. The following terms were compared: =oblique astigmatism; =vertical astigmatism; =vertical coma; =horizontal coma; =vertical trefoil; =oblique trefoil; =primary SA; =vertical secondary astigmatism; =oblique secondary astigmatism; =vertical quadrafoil; =oblique quadrafoil.
The optical quality was comparable between the progressive and standard toric IOLs at 3 mm, with the Strehl ratio of 0.51 ± 0.15 for both conditions (P=.88). At 5 mm, the Strehl ratio was 0.30 ± 0.08 for the progressive and 0.29 ± 0.08 for the standard lens, which yielded minimal but statistically significant difference (P<.001). The comparison of the individual Strehl-ratio values obtained at the 5-mm aperture is presented in Fig. 3.
Fig. 3.
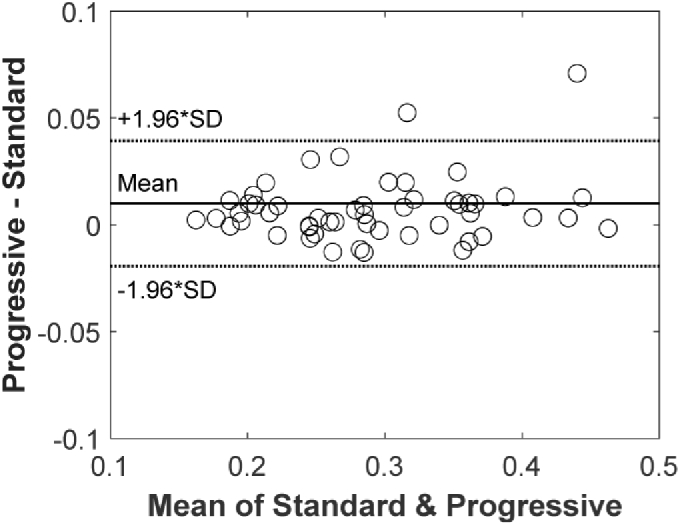
The Bland-Altman plot comparing the progressive-toric and standard-toric designs in their effect on the Strehl ratio.
4. Discussion
We demonstrated that the impact of the corneal-astigmatism increase with pupil size could be lowered if a progressive- rather than standard-toric IOL is used. Residual astigmatism of the two IOL types was comparable at 3 mm. However, it was reduced with the new design at 5 mm, and that, in turn, resulted in an improvement of the optical-quality metrics.
The effect of pupil size on postoperative astigmatism in pseudophakic patients has rarely been discussed in the literature. Visser et al. presented two cases with increased residual astigmatism after the uneventful implantation of toric IOLs [5]. Among others, the impact of pupil size was mentioned as a potential explanation for their observation. In one of the described cases, a large pupil with a diameter over 6 mm was found in a patient who was aged 58 years at the time of examination [5]. Visser’s group measured wavefront aberrations, and they found a decrease of corneal-astigmatism from −2.21 D x 171° at 4 mm to −1.40 D x 174° at 6 mm. In that case, residual astigmatism indeed affected the patient's vision, as uncorrected visual acuity (VA) was 20/30, which improved to 20/22 after a full astigmatic correction [5]. In our study, we also observed two cases with a more substantial improvement of the optical quality at the 5-mm pupil, which was above the average level, as shown by Fig. 3. Although we found only a small increase of the Strehl ratio, on average, the correction of astigmatism's peripheral change may prove particularly advantageous in individual cases, such as those described by Visser et al. [5].
A recent European-population analysis of corneal topography revealed that the corneal profile does not change up to 6 mm in only 8% of patients aged 60 years or older, [19] which in the Japanese population amounted to 4% [20]. In those cases, a standard toric IOL may provide an optimal refractive outcome. However, most Japanese (79%) and Caucasian corneas (65%) showed a decrease in astigmatism with the cornea zone, [19,20] indicating that a new approach is desirable to better correct astigmatism in patients with larger pupils or for all patients’ vision under low-light conditions. Indeed, we demonstrated that the progressive design lowers residual astigmatism for a scotopic pupil compared to the standard one in the Caucasian population. However, still, more than half a diopter of astigmatism remained uncorrected. Given that the data obtained by Kawamorita et al. were used to design the first progressive toric IOL, [20] one may speculate that the effectiveness of the proposed correction is higher in the Japanese than European cohort. However, more research is needed to confirm this assumption.
The assessment of simulated residual astigmatism at the 5-mm pupil demonstrated statistically significantly lower values with the progressive IOL model. However, the Strehl ratio results show that the impact of a 0.10 D difference on the visual quality might be minimal. In a study by Blendowske, [31] a simple model was proposed that predicts the loss of VA due to the presence of the refractive error. Based on this model, a spherical component equals zero, a cylindrical one equals −0.67, and this yields a loss of 0.09 logMAR. This improves by one letter (0.02 logMAR) with the progressive design (i.e., −0.57 D). Such a small difference falls below the measurement error of a standard VA assessment; hence, it might not be detected in a clinical evaluation. Besides, cofounding factors, such as IOL tilt and decentration, as well as corneal incision, that may affect postoperative outcomes pose another challenge for a future clinical study [3,32]. Thus, ray-tracing simulation appears most suited to objectively compare the two types of toric-IOL correction. Although the difference in simulated postoperative astigmatism might seem unimportant, in two cases, it reached 0.41 D. When we apply Blendowske's model in a case with −1 D of astigmatism and no sphere, a loss of 0.18 logMAR is predicted due to uncorrected astigmatism. But with a compensation of 0.41 D, more than one line (0.11 logMAR) can be gained with the progressive correction. Thus, in patients who present a substantial difference between central and peripheral astigmatism, the correction of the cornea's peripheral flattening may prove advantageous.
This may be particularly important in eyes with larger (photopic) pupils or in low-light-level situations. Scotopic pupil size may vary significantly in healthy subjects of different ages. Winn et al. reported a linear relationship between pupil size and age, showing that at 9 luminance and the age of 70 years, the average diameter is 5.0 mm [26]. The same value was used in our simulations. However, the pupil size increases in younger subjects as the measurements showed, on average, 5.5 mm and 5.9 mm at 60 and 50 years, respectively [26]. Westin et al. studied the demographics of refractive lens exchange (RLE) patients and reported the mean age of 51.1 ± 7.7 years, compared to 73.8 ± 9.3 in their cataract cohort [33]. Given that at larger pupils, the astigmatic difference is expected to increase, [19–21] the progressive technology might (potentially) reduce residual astigmatism after RLE procedures.
Managing corneal-astigmatism through toric-IOL implantation has become a standard in modern cataract surgery. Although toricity was first introduced on a monofocal platform, [1] it currently features on presbyopia-correcting IOLs, such as bifocal, [4] trifocal, [9] or extended-depth-of-focus lenses [11]. Various toric designs have been proposed, such as bitoric optic [12] where both anterior and posterior surfaces are cylindrical or a transitional conic toric with enlarged toric meridian to compensate for axis rotation [10]. The latter design indeed demonstrated median astigmatism of 0.25 D despite axis misalignment [10]. Thomas et al. reported that even in eyes with the largest deviation of alignment (from 11° to 16°), residual astigmatism did not change, and they attributed this finding to the transitional design [10]. Miyake et al. reported a value of −0.67 D after a standard monofocal toric-IOL implantation [8]. Two years after cataract surgery, the measured axis deviation was 4.1° ±3.0° [8]. In our simulations, the mean astigmatism at 3 mm was 0.37 D despite a precise alignment of the IOL in the eye model. This small residual astigmatism results from the range of available cylinder powers, which are rarely perfectly matched with the patient's cornea. In the selection process, a model that offers the lowest residual astigmatism is typically chosen, which can decrease further due to corneal incisions in a clinical situation [3]. In our simulation, we did not account for the impact of the corneal incision. Thus, the level of 0.37 D may be considered an optical limit of how effectively, on average, corneal-astigmatism can be corrected without a customized cylinder power. The expected impact of such low astigmatism on VA is minimal (about 0.03 logMAR), [31] which may still provide excellent visual outcomes and a high satisfaction rate of toric-IOL patients [1–12]. Thus, the fixed toric power range reflects the tradeoff between the minimum acceptable deviation and a cost-effective and large-scale production process.
Mencucci et al. studied higher-order aberrations in 120 astigmatic and control patients scheduled for cataract surgery [6]. This population was divided into three groups based on the corneal cylinder and the type of implanted IOL. In astigmatic patients with more than 1.50 D corneal-astigmatism, low order astigmatism (, ) was 0.36 ± 0.21 µm after toric IOL implantation at 4-mm pupils, which was slightly higher than that of subjects without astigmatism and a monofocal lens, and that was 0.25 ± 0.13 µm [6]. In a group with >1.5 D astigmatism and a non-toric lens, the primary astigmatism was 0.62 ± 0.13 µm, indicating a clear advantage of toric IOLs [6]. In our study, the mean of and was lower, i.e., 0.23 ± 0.17 µm and 0.28 ± 0.19 µm in the progressive and standard toric correction, respectively, despite using a larger 5-mm pupil. This, however, should be expected as optical simulations represent an ideal case, which is difficult to achieve clinically.
In another study, Toto et al. assessed wavefront aberrations in astigmatic patients after implanting toric IOLs [7]. They found the higher-order RMS of 0.43 ± 0.21 µm at 5 mm measured 180 days postoperatively [7]. In our study, we recorded slightly higher values for both types of correction. However, the difference lies with the standard deviation range and represents a normal variation of HOAs in the population. The coma-like aberrations level was higher than in the current study, but ocular SA was comparable, 0.14 ± 0.04 µm [7]. Positive SAs of the cornea can be compensated by an aspheric IOL with the opposite sign [34,35]. Although it is not clear whether an aspheric IOL was used in the Toto et al. study, the corneal SA level in that study was 0.11 ± 0.03 µm indicating an increase of net aberration [7]. Kasper et al. studied the wavefront errors in patients implanted with an SA-correcting IOL in one eye and a standard spherical IOL in the fellow eye [2]. They found a significant reduction of primary SA in eyes with the aspheric IOL compared to the spherical one with the values of (about) 0.04 µm and 0.18 µm at 5 mm, respectively [2]. In our study, the mean absolute SA ranged from 0.17 ± 0.09 µm (progressive toric) to 0.19 ± 0.10 µm (standard toric) indicating a low correction level of the Avansee models. Indeed, the aspheric profile of the IOL used in our study yields a mere −0.04 µm reduction of SA. Thus, SA is only minimally affected by the IOL.
Interestingly, the progressive toric designs enhance the IOL's asphericity, which leads to further reduction of SA. In laboratory studies, IOLs with a low level of SA correction have shown a better tolerance to tilt and decentration, [34,36] misalignments that are often seen in a clinical situation [37,38]. On the other hand, a comparison between SA-correcting and spherical IOLs shows a decreased optical quality in model corneas with positive SA [34–36]. Clinically, however, the SA-correcting IOLs fail to improve VA, but they may yield better mesopic contrast sensitivity than spherical IOLs according to the systematic review by Schuster et al. [39]. A broad range of SA levels in the population poses a challenge in the selection of SA-correcting IOLs. In a paper by Wang et al., SA values ranged from 0.055 to 0.544 µm, [40] showing the limitations of aspheric IOLs with a fixed level of SA correction, which on an individual level may not be optimally matched with patients’ aberrations. Variability in the change of corneal-astigmatism with pupil size may pose a similar challenge in selecting a toric IOL. For that reason, in the current study, we replicated the distribution of the difference between the magnitude of astigmatism at 3 mm and 6 mm (Fig. 1). And our analysis confirmed that, on average, the progressive correction performs better than the standard one in the general population.
5. Conclusions
We present the first report on the optical effects of a progressive-toric IOL - one with cylinder power gradually decreasing from the IOL’s center to its periphery. We found that the new and standard toric yield an equivalent optical quality at 3 mm. However, at 5 mm, the progressive IOLs significantly reduce residual astigmatism compared to the standard ones. Although on average, the Strehl ratio differed only minimally, in individual cases, a more substantial impact on the visual function may be expected, particularly in patients with a pronounced decrease of corneal-astigmatism with pupil size. Thus, this new technology may contribute to the continuous effort to improve toric-IOL patients’ visual quality.
Acknowledgments
Donald J. Munro contributed to reviewing and proofreading of the manuscript.
Funding
Klaus Tschira Stiftung10.13039/501100007316; Kowa Pharmaceutical Europe10.13039/100011027 Co. Ltd.
Disclosures
The authors have no proprietary or commercial interest in any materials discussed in this article. GU Auffarth and R Khoramnia report grants, personal fees and non-financial support from Alcon, grants, personal fees and non-financial support from Kowa, grants from Carl Zeiss Meditec, grants, personal fees and non-financial support from Hoya, grants and personal fees from Ophtec, grants and personal fees from Physiol, grants, personal fees and non-financial support from Rayner, grants, personal fees and non-financial support from SIFI, grants, personal fees and non-financial support from Johnson&Johnson, grants from Acufocus, non-financial support from Polytech, grants, personal fees and non-financial support from Oculentis, outside the submitted work. G Łabuz and D Varadi have nothing to disclose. The sponsors had no role in the design, execution, interpretation, or writing of the manuscript.
References
- 1.Shimizu K., Misawa A., Suzuki Y., “Toric intraocular lenses: correcting astigmatism while controlling axis shift,” J. Cataract Refractive Surg. 20(5), 523–526 (1994). 10.1016/S0886-3350(13)80232-5 [DOI] [PubMed] [Google Scholar]
- 2.Kasper T., Bühren J., Kohnen T., “Intraindividual comparison of higher-order aberrations after implantation of aspherical and spherical intraocular lenses as a function of pupil diameter,” J. Cataract Refractive Surg. 32(1), 78–84 (2006). 10.1016/j.jcrs.2005.11.018 [DOI] [PubMed] [Google Scholar]
- 3.Mingo-Botín D., Muñoz-Negrete F. J., Kim H. R. W., Morcillo-Laiz R., Rebolleda G., Oblanca N., “Comparison of toric intraocular lenses and peripheral corneal relaxing incisions to treat astigmatism during cataract surgery,” J. Cataract Refractive Surg. 36(10), 1700–1708 (2010). 10.1016/j.jcrs.2010.04.043 [DOI] [PubMed] [Google Scholar]
- 4.Khoramnia R., Auffarth G. U., Rabsilber T. M., Holzer M. P., “Implantation of a multifocal toric intraocular lens with a surface-embedded near segment after repeated LASIK treatments,” J. Cataract Refractive Surg. 38(11), 2049–2052 (2012). 10.1016/j.jcrs.2012.08.042 [DOI] [PubMed] [Google Scholar]
- 5.Visser N., Bauer N. J., Nuijts R. M., “Residual astigmatism following toric intraocular lens implantation related to pupil size,” J. Refractive Surg. 28(10), 729–732 (2012). 10.3928/1081597X-20120911-02 [DOI] [PubMed] [Google Scholar]
- 6.Mencucci R., Giordano C., Favuzza E., Gicquel J.-J., Spadea L., Menchini U., “Astigmatism correction with toric intraocular lenses: wavefront aberrometry and quality of life,” Br. J. Ophthalmol. 97(5), 578–582 (2013). 10.1136/bjophthalmol-2013-303094 [DOI] [PubMed] [Google Scholar]
- 7.Toto L., Vecchiarino L., Cardone D., Mastropasqua A., Mastropasqua R., Di Nicola M., D’Ugo E., “Astigmatism correction with toric IOL: analysis of visual performance, position, and wavefront error,” J. Refractive Surg. 29(7), 476–483 (2013). 10.3928/1081597X-20130617-06 [DOI] [PubMed] [Google Scholar]
- 8.Miyake T., Kamiya K., Amano R., Iida Y., Tsunehiro S., Shimizu K., “Long-term clinical outcomes of toric intraocular lens implantation in cataract cases with preexisting astigmatism,” J. Cataract Refractive Surg. 40(10), 1654–1660 (2014). 10.1016/j.jcrs.2014.01.044 [DOI] [PubMed] [Google Scholar]
- 9.Kretz F. T., Breyer D., Klabe K., Hagen P., Kaymak H., Koss M. J., Gerl M., Mueller M., Gerl R. H., Auffarth G. U., “Clinical outcomes after implantation of a trifocal toric intraocular lens,” J. Refractive Surg. 31(8), 504–510 (2015). 10.3928/1081597X-20150622-01 [DOI] [PubMed] [Google Scholar]
- 10.Thomas B. C., Khoramnia R., Auffarth G. U., Holzer M. P., “Clinical outcomes after implantation of a toric intraocular lens with a transitional conic toric surface,” Br. J. Ophthalmol. 102(3), 313–316 (2018). 10.1136/bjophthalmol-2017-310386 [DOI] [PubMed] [Google Scholar]
- 11.Sandoval H. P., Lane S., Slade S., Potvin R., Donnenfeld E. D., Solomon K. D., “Extended depth-of-focus toric intraocular lens targeted for binocular emmetropia or slight myopia in the nondominant eye: Visual and refractive clinical outcomes,” J. Cataract Refractive Surg. 45(10), 1398–1403 (2019). 10.1016/j.jcrs.2019.05.019 [DOI] [PubMed] [Google Scholar]
- 12.Moon J., Yoon C. H., Kim M. K., “Comparative effects of various types of toric intraocular lenses on astigmatism correction,” BMC Ophthalmol. 20(1), 1–9 (2020). 10.1186/s12886-019-1277-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khoramnia R., Fitting A., Rabsilber T., Auffarth G., Holzer M., “Postoperative results after implantation of a toric, aspheric intraocular lens,” Klin. Monatsbl. Augenheilkd. 232(07), 867–873 (2015). 10.1055/s-0034-1396179 [DOI] [PubMed] [Google Scholar]
- 14.Jin H., Limberger I.-J., Ehmer A., Guo H., Auffarth G. U., “Impact of axis misalignment of toric intraocular lenses on refractive outcomes after cataract surgery,” J. Cataract Refractive Surg. 36(12), 2061–2072 (2010). 10.1016/j.jcrs.2010.06.066 [DOI] [PubMed] [Google Scholar]
- 15.Alpins N., Ong J. K., Stamatelatos G., “Refractive surprise after toric intraocular lens implantation: graph analysis,” J. Cataract Refractive Surg. 40(2), 283–294 (2014). 10.1016/j.jcrs.2013.06.029 [DOI] [PubMed] [Google Scholar]
- 16.Giers B. C., Khoramnia R., Weber L. F., Tandogan T., Auffarth G. U., “Rotation and decentration of an undersized plate-haptic trifocal toric intraocular lens in an eye with moderate myopia,” J. Cataract Refractive Surg. 42(3), 489–493 (2016). 10.1016/j.jcrs.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 17.Kramer B. A., Berdahl J. P., Hardten D. R., Potvin R., “Residual astigmatism after toric intraocular lens implantation: Analysis of data from an online toric intraocular lens back-calculator,” J. Cataract Refractive Surg. 42(11), 1595–1601 (2016). 10.1016/j.jcrs.2016.09.017 [DOI] [PubMed] [Google Scholar]
- 18.Koch D. D., Ali S. F., Weikert M. P., Shirayama M., Jenkins R., Wang L., “Contribution of posterior corneal astigmatism to total corneal astigmatism,” J. Cataract Refractive Surg. 38(12), 2080–2087 (2012). 10.1016/j.jcrs.2012.08.036 [DOI] [PubMed] [Google Scholar]
- 19.Łabuz G., Varadi D., Khoramnia R., Auffarth G. U., “Central and mid-peripheral corneal astigmatism in an elderly population: a retrospective analysis of Scheimpflug topography results,” Sci Rep. In press (2021). [DOI] [PMC free article] [PubMed]
- 20.Kawamorita T., Shimizu K., Hoshikawa R., Kamiya K., Shoji N., “Relationship between central and peripheral corneal astigmatism in elderly patients,” Opt. Rev. 25(3), 336–339 (2018). 10.1007/s10043-018-0427-2 [DOI] [Google Scholar]
- 21.Read S. A., Collins M. J., Carney L. G., Franklin R. J., “The topography of the central and peripheral cornea,” Invest. Ophthalmol. Vis. Sci. 47(4), 1404–1415 (2006). 10.1167/iovs.05-1181 [DOI] [PubMed] [Google Scholar]
- 22.Liou H.-L., Brennan N. A., “Anatomically accurate, finite model eye for optical modeling,” J. Opt. Soc. Am. A 14(8), 1684–1695 (1997). 10.1364/JOSAA.14.001684 [DOI] [PubMed] [Google Scholar]
- 23.Atchison D. A., Thibos L. N., “Optical models of the human eye,” Clin. Exp. Optom. 99(2), 99–106 (2016). 10.1111/cxo.12352 [DOI] [PubMed] [Google Scholar]
- 24.Febbraro J.-L., Khan H. N., Koch D. D., Surgical Correction of Astigmatism (Springer, 2018). [Google Scholar]
- 25.Scharf D., Yildirim T. M., Auffarth G. U., Mayer C. S., Choi C. Y., Khoramnia R., “Implantation of a Phakic Posterior Chamber Lens in Eyes with Keratoconus,” Klin. Monatsbl. Augenheilkd. 237(09), 1102–1106 (2020). 10.1055/a-1217-0651 [DOI] [PubMed] [Google Scholar]
- 26.Winn B., Whitaker D., Elliott D. B., Phillips N. J., “Factors affecting light-adapted pupil size in normal human subjects,” Invest. Ophthalmol. Vis. Sci. 35(3), 1132–1137 (1994). [PubMed] [Google Scholar]
- 27.Łabuz G., Knebel D., Auffarth G. U., Fang H., Van den Berg T. J., Yildirim T. M., Son H.-S., Khoramnia R., “Glistening formation and light scattering in six hydrophobic-acrylic intraocular lenses,” Am. J. Ophthalmol. 196, 112–120 (2018). 10.1016/j.ajo.2018.08.032 [DOI] [PubMed] [Google Scholar]
- 28.Marsack J. D., Thibos L. N., Applegate R. A., “Metrics of optical quality derived from wave aberrations predict visual performance,” J. Vis. 4(4), 8 (2004). 10.1167/4.4.8 [DOI] [PubMed] [Google Scholar]
- 29.Łabuz G., Yildirim T. M., van den Berg T. J., Khoramnia R., Auffarth G. U., “Assessment of straylight and the modulation transfer function of intraocular lenses with centrally localized opacification associated with the intraocular injection of gas,” J. Cataract Refractive Surg. 44(5), 615–622 (2018). 10.1016/j.jcrs.2018.01.033 [DOI] [PubMed] [Google Scholar]
- 30.Thibos L. N., Hong X., Bradley A., Cheng X., “Statistical variation of aberration structure and image quality in a normal population of healthy eyes,” J. Opt. Soc. Am. A 19(12), 2329–2348 (2002). 10.1364/JOSAA.19.002329 [DOI] [PubMed] [Google Scholar]
- 31.Blendowske R., “Unaided visual acuity and blur: a simple model,” Optom. Vis. Sci. 92(6), e121–e125 (2015). 10.1097/OPX.0000000000000592 [DOI] [PubMed] [Google Scholar]
- 32.Weikert M. P., Golla A., Wang L., “Astigmatism induced by intraocular lens tilt evaluated via ray tracing,” J. Cataract Refractive Surg. 44(6), 745–749 (2018). 10.1016/j.jcrs.2018.04.035 [DOI] [PubMed] [Google Scholar]
- 33.Westin O., Koskela T., Behndig A., “Epidemiology and outcomes in refractive lens exchange surgery,” Acta Ophthalmol. 93(1), 41–45 (2015). 10.1111/aos.12460 [DOI] [PubMed] [Google Scholar]
- 34.Lee Y., Labuz G., Son H.-S., Yildirim T. M., Khoramnia R., Auffarth G. U., “Assessment of the image quality of extended depth-of-focus intraocular lens models in polychromatic light,” J. Cataract Refractive Surg. 46(1), 108–115 (2020). 10.1097/j.jcrs.0000000000000037 [DOI] [PubMed] [Google Scholar]
- 35.Tandogan T., Auffarth G. U., Choi C. Y., Liebing S., Mayer C., Khoramnia R., “In vitro comparative optical bench analysis of a spherical and aspheric optic design of the same IOL model,” BMC Ophthalmol. 17(1), 9 (2017). 10.1186/s12886-017-0407-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujikado T., Saika M., “Evaluation of actual retinal images produced by misaligned aspheric intraocular lenses in a model eye,” Clin. Ophthalmol. 8, 2415 (2014). 10.2147/OPTH.S72053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phillips P., Rosskothen H. D., Pérez-Emmanuelli J., Koester C. J., “Measurement of intraocular lens decentration and tilt in vivo,” J. Cataract Refractive Surg. 14(2), 129–135 (1988). 10.1016/S0886-3350(88)80086-5 [DOI] [PubMed] [Google Scholar]
- 38.Baumeister M., Bühren J., Kohnen T., “Tilt and decentration of spherical and aspheric intraocular lenses: effect on higher-order aberrations,” J. Cataract Refractive Surg. 35(6), 1006–1012 (2009). 10.1016/j.jcrs.2009.01.023 [DOI] [PubMed] [Google Scholar]
- 39.Schuster A. K., Tesarz J., Vossmerbaeumer U., “The impact on vision of aspheric to spherical monofocal intraocular lenses in cataract surgery: a systematic review with meta-analysis,” Ophthalmology 120(11), 2166–2175 (2013). 10.1016/j.ophtha.2013.04.011 [DOI] [PubMed] [Google Scholar]
- 40.Wang L., Dai E., Koch D. D., Nathoo A., “Optical aberrations of the human anterior cornea,” J. Cataract Refractive Surg. 29(8), 1514–1521 (2003). 10.1016/S0886-3350(03)00467-X [DOI] [PubMed] [Google Scholar]