Abstract
Background
Direct-to-implant (DTI) breast reconstruction provides high-quality aesthetic results in appropriate candidates. Most commonly, implants are placed in the subpectoral space which can lead to pain and breast animation. Surgical and technological advances have allowed for successful prepectoral implant placement which may eliminate these trade-offs.
Objectives
Here we present early outcomes from 153 reconstructions in 94 patients who underwent prepectoral DTI. We sought to determine whether these patients have less postoperative pain and narcotic use than subpectoral implant or expander placement.
Methods
A retrospective review was performed for all prepectoral DTI reconstructions at our institution from 2015 to 2016. Data were collected on postoperative pain and narcotic use while in hospital.
Results
The average follow-up time was 8.5 months (range, 3–17 months) and the overall complication rate was 27% (n = 41) with the most common complications being skin necrosis (9%, n = 13) and infection (7%, n = 11). No statistically significant difference in complications was found in patients who underwent postmastectomy radiation therapy. Patients who underwent prepectoral DTI reconstruction did not have a statistically significant difference in postoperative pain and narcotic use while in-hospital compared with other techniques.
Conclusion
Prepectoral DTI reconstruction provides good results with similar complication rates to subpectoral techniques. Prepectoral DTI eliminates the problem of breast animation. Although our series did not reach statistical significance in pain scores or requirement for postoperative narcotics, we believe that it is an important preliminary result and with larger numbers we anticipate a more definitive conclusion.
Level of Evidence: 4

Implant-based breast reconstruction is the most common reconstructive technique performed in women with breast cancer.1 Over the past decade, direct-to-implant (DTI) breast reconstruction has increased in popularity and has several benefits over expander-based reconstruction, including avoidance of repeated expansions, shorter time to completion of reconstruction, psychological relief from the immediate return to a normal body image, improved sexual well-being, and decreased cost.2-7 Commonly, DTI reconstruction is partially submuscular: the implant is positioned with pectoralis major muscle coverage superiorly and acellular dermal matrix (ADM) coverage of the lower pole. Drawbacks to subpectoral implant placement include breast animation deformity with activation of the pectoralis major muscle and an increase in discomfort after submuscular dissection.8-10 Recent surgical and technological advances have paved the way for a rebirth of prepectoral DTI breast reconstruction.11-16 Prepectoral DTI breast reconstruction may eliminate the disadvantages of subpectoral implant placement, and recent reports of this technique support a similar safety profile and outcomes to the submuscular approach.13,17 The purpose of this study was to present our experience with prepectoral DTI breast reconstruction as well as assess differences in postoperative pain between prepectoral and subpectoral techniques. We hypothesize that patients who undergo prepectoral DTI have less postoperative pain than those who undergo subpectoral DTI or expander placement. Here we present our outcomes from 153 prepectoral DTI breast reconstructions in 94 patients. We analyzed our patients’ postoperative pain and narcotic requirement to determine whether prepectoral DTI patients have less postoperative pain and require less narcotics compared with subpectoral techniques.
METHODS
We performed a retrospective chart review, approved by the University of North Carolina Institutional Review Board, of all prepectoral DTI breast reconstructions consecutively performed at our institution from January 2015 to December 2016. Only patients with at least 3 months of postoperative follow-up were included. Patient demographics, factors, and complications were recorded. Major infection was defined as infection requiring admission to the hospital for IV antibiotics and minor infection defined as infection treated with oral antibiotics alone.
To compare postoperative pain and narcotic requirements between prepectoral and subpectoral techniques, a subgroup of prepectoral DTI patients were compared with subpectoral DTI and expander patients. The prepectoral DTI subgroup excluded patients with a different simultaneous contralateral breast procedure and patients with previous breast augmentation. The subpectoral cohort received separate institutional review board approval and included all subpectoral DTI and expander breast reconstructions consecutively performed at our institution from 2014 to 2016. Early in the study period, our primary method of implant-based reconstruction was subpectoral DTI or expander which allowed for the collection of these cases for review. Our subpectoral technique included the placement of an Alloderm Regenerative Tissue Matrix (LifeCell Corporation, Allergan, Dublin, Ireland) ADM sling for inferior pole coverage. Patient demographics and factors were recorded for the subpectoral patients as well. Postoperative pain scores and narcotic intake were collected on all patients. During the study period, no patients were part of an enhanced recovery after surgery pathway and did not receive preoperative analgesics or nerve blocks. All patients received intradermal and subcutaneous injections of 1% lidocaine into the area of planned incision prior to initial incision. Local anesthetic injections were not repeated during or after surgery. Patients received short-acting intravenous narcotics during surgery but did not receive intravenous non-narcotic adjuncts. A baseline pain score was recorded prior to surgery and all postoperative pain-related data were collected from the time the patient left the operating room until the time of discharge. Pain scores were recorded using the Numerical Pain Rating Scale (NPRS) whereby patients rate their pain quantitatively on a Likert scale from 0 (no pain) to 10 (worst pain). Pain scores in the postanesthesia care unit were recorded every 15 min and on the floor, no more frequently than every 3 hr until the time of discharge from the hospital. Morphine milligram equivalents (MME) were determined based on conversions established by the Agency Medical Directors’ Group.18 Patients were excluded from the pain analysis if they had a different simultaneous contralateral procedure, had an implant in place from a prior augmentation, or if their preoperative pain score was above zero. All reconstructions were performed by one of the two attending surgeons at our institution.
Surgical Technique
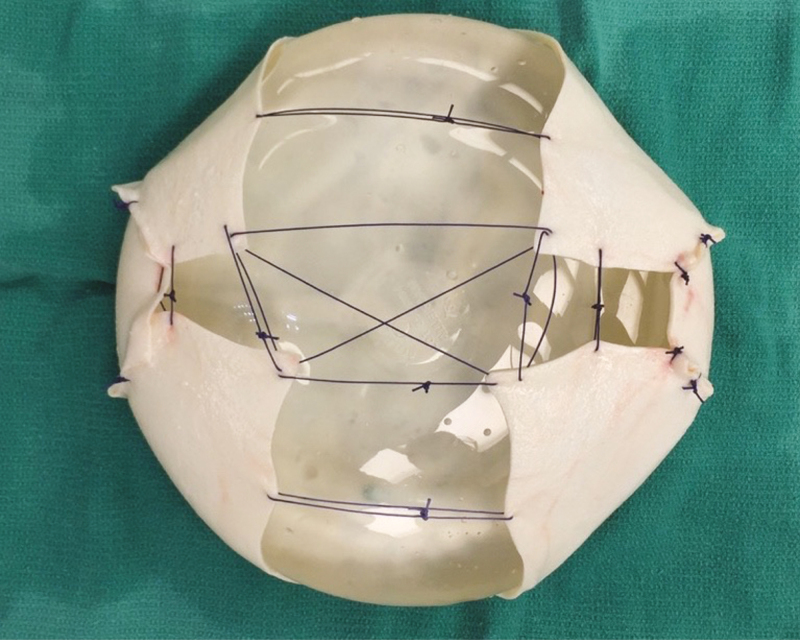
Our technique for prepectoral DTI reconstruction begins with intraoperative clinical assessment of the mastectomy skin flap. Skin color, temperature, capillary refill, dermal edge bleeding are assessed, and if the mastectomy skin flap appears well perfused, we proceed with prepectoral DTI. The lateral breast border of mastectomy skin is secured to the chest wall at the anterior axillary line with suture to prevent lateral displacement of the implant. This allows for appropriate positioning of the ADM wrapped implant without the need for securing the ADM itself to the chest wall. Implant sizers placed into the pocket until the optimal implant volume and profile chosen. A 16 × 20 cm piece of ADM is wrapped around the permanent implant to cover the entire anterior surface and as much of the posterior surface as possible with a single piece of ADM (Figure 1). The posterior edges are secured to each other with spanning sutures. The ADM-covered implant is placed into the pocket. A single drain is placed and the incision is closed. Nitroglycerine paste is placed on the NAC and/or mastectomy skin unless the anesthesia team has had difficulty with intraoperative hypotension. Several large pieces of Tegaderm (3M, Minneapolis, MN, USA) are placed over the breast and used to secure the nipple in its ideal location on the implant. Drains were typically removed 1 week postoperatively, as long as the output per drain was less than 30 cc in a 24-hr period for two consecutive days. Figures 2-4 demonstrate preoperative and postoperative photographs from 3 patients who underwent prepectoral DTI reconstruction.
Figure 1.
A smooth round silicone implant with an ADM wrap. The posterior surface of the implant is shown here, with spanning sutures to secure the ADM in place.
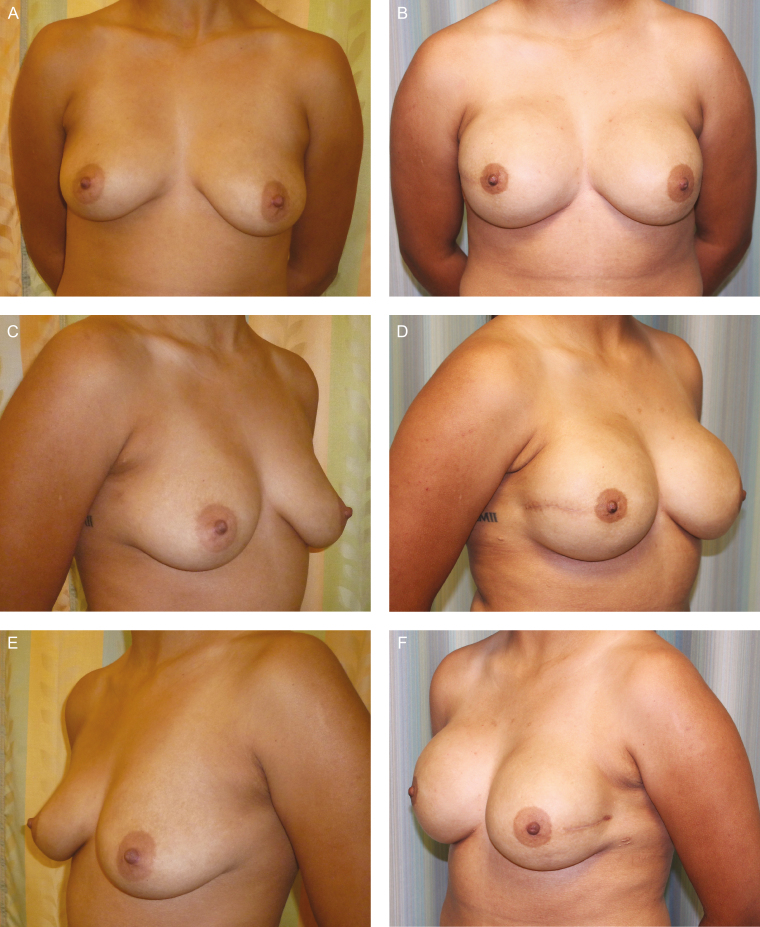
Figure 2.
A 25-year-old woman (BMI, 23.87 kg/m2), BRCA2 gene mutation, underwent bilateral prophylactic nipple-sparing mastectomies and immediate bilateral prepectoral DTI breast reconstruction. She has Natrelle Inspira SCX-700 cc implant (Allergan, Inc., Irvine, CA) in the right-side breast, SCX-580 implant in the left-side breast, and underwent fat grafting. (A, C, E) Preoperative and (B, D, F) postoperative views at 13 months postoperatively.
Figure 3.
A 56-year-old woman (BMI, 21.58 kg/m2), right breast cancer, underwent bilateral nipple-sparing mastectomies and immediate bilateral prepectoral DTI breast reconstruction. She has Natrelle Inspira SRF-385cc implants (Allergan, Inc., Irvine, CA) and underwent fat grafting. (A, C, E) Preoperative and (B, D, F) postoperative views at 16 months postoperatively.
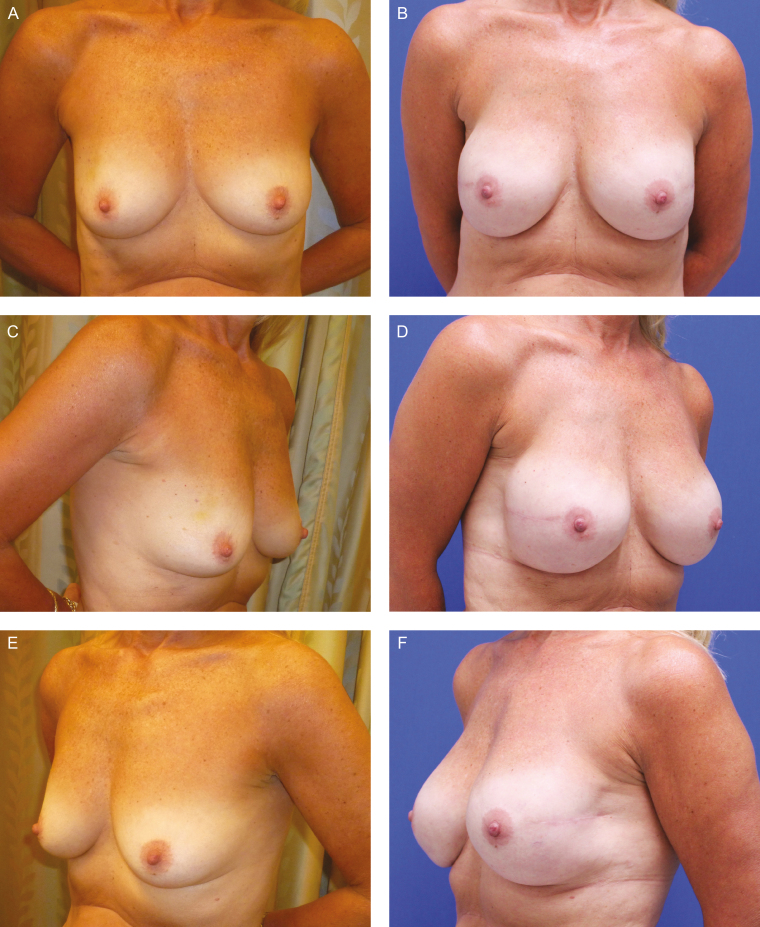
Figure 4.
A 42-year-old woman (BMI, 25.96 kg/m2), right breast cancer, underwent right skin-sparing mastectomy and immediate prepectoral DTI breast reconstruction. She received post-mastectomy radiation therapy. She has Natrelle Inspira SCX-800cc implants (Allergan, Inc., Irvine, CA). (A, C, E) Preoperative and (B, D, F) postoperative views at 8 months postoperatively without further surgery or evidence of capsular contracture.
Statistical Methods
Descriptive statistics for prepectoral DTI reconstructions were calculated for patient demographics and factors are listed in Table 1 and complications are listed in Table 2. Descriptive statistics for age, body mass index (BMI), and additional unplanned surgery were calculated per patient, whereas descriptive statistics for all other demographics, factors, and complications were calculated per breast. Two covariates of interest (radiation before and after surgery) were assessed for a univariate relationship with each outcome using Fisher’s exact test. Percentages were reported along with Clopper-Pearson exact 95% confidence intervals. The effects of covariates on each outcome were assessed using logistic models fit with generalized estimating equations to account for correlations between observations on the same patient. Descriptive statistics for subpectoral DTI and expander reconstructions were calculated for patient demographics and factors are listed in Table 3. Descriptive statistics for age and BMI were calculated per patient, whereas descriptive statistics for all other demographics were calculated per breast. T-tests were used to look for differences in demographics between prepectoral DTI and subpectoral DTI and subpectoral expander. Means and standard deviations were calculated per patient for average pain score, and MMEs for prepectoral DTI, subpectoral DTI, and subpectoral expander. Chi-square tests were used to look for any differences in pain scores and narcotic use among the three groups. A P-value of <0.05 was considered statistically significant. All analyses were performed using R software (R Core Team, 2016).19
Table 1.
Prepectoral DTI Demographics/Factors (n = 94 patients, 153 breasts)
| Value (%) or meana (standard deviation, range) | |
|---|---|
| Age (years) | 49.63 (±11.01, 24-81) |
| BMI (kg/m2) | 26.87 (±5.81, 19.11-47.91) |
| Prophylactic mastectomy | 70 (46%) |
| Stage 0b | 16 (19%) |
| Stage 1 | 43 (52%) |
| Stage 2 | 20 (24%) |
| Stage 3 | 4 (5%) |
| Nipple-sparing mastectomy | 92 (60%) |
| Skin-sparing mastectomy | 61 (40%) |
| Mastectomy weight (g) | 622.66 (±401.38, 115-2225) |
| Implant size (mL) | 586.16 (±157.76, 205-800) |
| Radiation before reconstruction | 4 (3%) |
| Radiation after reconstruction | 9 (6%) |
| Smoker | 3 (3%) |
| Textured shaped implant | 17 (11%) |
| Smooth round implant | 136 (89%) |
a Means for age and BMI calculated per patient, all other means and rates calculated per breast. b Determined by American Joint Committee on Cancer (AJCC) staging system, Eighth Edition; DTI, direct-to-implant.
Table 2.
Complicationsa (n = 94 patients, 153 breasts)
| Events | Incidence (%)b |
|---|---|
| Full thickness necrosis | 13 (9) |
| Major infection | 11 (7) |
| Partial thickness necrosis | 4 (3) |
| Seroma | 4 (2) |
| Implant malposition | 4 (3) |
| Wound dehiscence | 3 (2) |
| Minor infection | 2 (1) |
| Capsular contracture (Grade III/IV) | 0 (0) |
| Implant removed and not replaced (reconstructive failure) | 9 (6) |
| Implant removed and replaced | 7 (5) |
| Additional unplanned surgeryc | 24 (26) |
| Total complications | 41 (27) |
a Mean follow-up time 8.5 (±3.9) months.
b All rates per breast except additional unplanned surgery which is per patient.
c Unplanned surgery required to treat a complication.
Table 3.
Subpectoral Demographics/Factors
| Subpectoral DTI (n = 11 breasts, 7 patients) | Subpectoral expander (n = 15 breasts, 10 patients) | |
|---|---|---|
| Value (%) or meana (standard deviation, range) | ||
| Age (years) | 45.00 (±8.35, 33–57) | 41.90 (±12.55, 22–66) |
| BMI (kg/m2) | 23.83 (±4.92, 21.24–33.77) | 24.25 (±4.43, 19.18–29.97) |
| Prophylactic mastectomy | 2 (18%) | 6 (40%) |
| Nipple-sparing mastectomy | 7 (64%) | 6 (40%) |
| Skin-sparing mastectomy | 3 (27%) | 9 (60%) |
| Stage 0 | 3 (27%) | 4 (27%) |
| Stage 1 | 3 (27%) | 1 (7%) |
| Stage 2 | 2 (18%) | 4 (27%) |
| Stage 3 | 0 | 0 |
| Mastectomy weight (g) | 262.2 (±88.80, 135–325)b | 373.33 (± 281.06, 155–1095)b |
a Means for age and BMI calculated per patient, all other means and rates calculated per breast. b S tatistically significant difference between these values when compared to prepectoral DTI (P < 0.05). DTI, direct-to-implant.
RESULTS
During the study period, 153 prepectoral DTI reconstructions were performed on 94 patients (Table 1). The mean follow-up time was 8.5 months (range, 3–17 months). The mean age of patients was 50 years (range, 24–81 years) with a mean BMI of 26.87 kg/m2 (range, 19.11–47.91 kg/m2). Seventy reconstructions (46%) were performed after prophylactic mastectomy. Ninety-two reconstructions (60%) were performed after nipple-sparing mastectomy and 61 (40%) were performed after skin-sparing mastectomy. Of the 92 nipple-sparing mastectomies, a lateral radial incision was performed in 54 breasts (59%), and an inframammary fold (IMF) incision was performed in 38 breasts (41%). The mean mastectomy specimen weight was 622.66 (±401.38) g, and the mean implant volume placed was 586.16 (± 157.76) cc.
The overall complication rate was 27% with the most common complications being full-thickness mastectomy skin necrosis in 13 breasts (9%) and major infection in 11 breasts (7%) (Table 2). Due to complications, 9 implants (6%) were removed and not replaced (reconstructive failure) and 7 implants (5%) were removed and replaced. Twenty-four patients (26%) required unplanned surgery to manage a complication. Of the 13 breasts that developed full-thickness mastectomy skin necrosis, 10 had undergone nipple-sparing mastectomies and 3 had undergone skin-sparing mastectomies (odds ratio 2.3; 95% CI 0.61-8.8; P = 0.22). Among the 10 nipple-sparing mastectomies that developed full-thickness skin necrosis, 3 breasts had a radial incision and 7 breasts had an IMF incision (odds ratio 3.8; 95% CI 0.92-15.9, P = 0.06).
Four patients underwent radiation prior to reconstruction and one had a major infection necessitating implant removal. Eight patients received postmastectomy radiation therapy (PMRT) with complications of one (11%) major infection requiring implant removal and one (11%) minor infection. When compared with non-radiated patients, PMRT was not associated with a significant increased risk of any complication (Table 4).
Table 4.
Prepectoral Implants Radiated After Reconstructiona
| Events (n = 9 breasts, 8 patients) | Incidence (%)b |
|---|---|
| Major infection | 1 (11) |
| Minor infection | 1 (11) |
| Implant removed and replaced | 1 (11) |
| Additional unplanned surgeryc | 1 (13) |
a No significant differences were found in any outcomes between those who did and did not receive radiation after surgery (P > 0.05). No occurrence of skin necrosis, seroma, implant malposition, wound dehiscence, capsular contracture (Grade III/IV), or implants removed and not replaced.
b All rates per breast except additional unplanned surgery which is per patient.
c Unplanned surgery required to address a complication.
During the study period, 11 subpectoral DTI reconstructions were performed on 7 patients and 15 subpectoral expander reconstructions were performed on 10 patients. All subpectoral reconstructions were dual plane with ADM. Patients were not given muscle relaxants while hospitalized. The only significant difference in demographics between these groups and the prepectoral DTI patients was the mastectomy specimen weight, for which both subpectoral DTI and expander had lower mastectomy specimen weights (Table 3). Among prepectoral DTI reconstructions included in the postoperative pain and narcotic requirement analysis, during the patients’ hospital stay, the mean NPRS score was 4.12 (±1.56) which was similar to the pain score for subpectoral DTI (4.23 ± 2.41) and subpectoral expander reconstructions (4.63 ± 1.27) with no statistically significant difference found between them (P = 0.64). Regarding postoperative narcotics during the patients’ hospital stay, patients with prepectoral DTI required an average of 4.65 (± 5.91) MME/hr, subpectoral DTI required an average of 6.28 (±8.53) MME/hr, and subpectoral expander required an average of 9.83 (±15.06) MME/hr. These differences were not statistically significant (Table 5).
Table 5.
Postoperative Pain and Narcotic Requirement
| Reconstructive technique (patients) | Postoperative pain score in hospital, NPRS; mean (SD) | Postoperative narcotics administered per hour in hospital, MME/hr; mean (SD) |
|---|---|---|
| Prepectoral DTI (76) | 4.12 (1.56) | 4.65 (5.91) |
| Subpectoral DTI (7) | 4.23 (2.41) | 6.28 (8.53) |
| Subpectoral expander (10) | 4.63 (1.27) | 9.83 (15.06) |
| Comparison of postoperative pain scores; P-value | Comparison of postoperative narcotics; P-value | |
| Prepectoral DTI vs subpectoral DTI | 0.91 | 0.26 |
| Prepectoral DTI vs subpectoral expander | 0.27 | 0.30 |
| Subpectoral DTI vs subpectoral expander | 0.70 | 0.12 |
DTI, direct-to-implant; MME, morphine milligram equivalent; NPRS, numerical pain rating scale; SD, standard deviation.
DISCUSSION
In the 1960s, breast reconstruction was performed with implants in the subcutaneous space.20 Complications were common, including implant rippling, step-offs, skin necrosis, implant exposure, and capsular contracture.21,22 To mitigate these complications, the reconstructive technique was modified to provide additional soft tissue coverage and the implant was moved to a submuscular position.23
Submuscular implant placement is performed with either total muscular coverage or a dual-plane approach. In the latter, the pectoralis major was allowed to windowshade up and the inferior border was secured to the mastectomy skin flap. Both techniques have limitations. Total submuscular coverage did not frequently result in a natural-appearing breast, and sole reliance on the mastectomy flap in the inferior pole risked many of the complications that submuscular placement sought to avoid, namely mastectomy skin necrosis and capsular contracture.
A turning point in implant-based reconstruction came with the introduction of ADM to the market. By utilizing ADM for lower pole coverage, total submuscular coverage could be avoided, and an additional layer of tissue would exist between the implant and mastectomy flap at the lower pole. This technique allowed for safe and successful DTI breast reconstruction and has arguably become the standard of care for DTI reconstruction.24
DTI breast reconstruction in the subpectoral dual plane has been shown to provide excellent aesthetic results; however, animation and pain continue to be problematic.8-11,17 Additionally, implant size may be limited to the inherent subpectoral space.25 Although not a limiting factor for smaller breasted women, larger breasted women may not be able to return to their pre-mastectomy size, despite having an adequate skin envelope.
The sine qua non of subcutaneous implant reconstruction is that its success is reliant upon the viability of the overlying mastectomy skin flap. These flaps were often thin which put the skin at risk for ischemia, breakdown, and infection. The skin provided little camouflage over the structure of the implant which can result in unacceptable implant visibility and rippling. Over the past decade, advances in surgical technique and technologies have allowed for each of these issues to be addressed and re-consideration of the benefits of prepectoral implant placement.
At our institution, consistently well-perfused mastectomy skin flaps have been a crucial factor in enabling successful placement of implants in the prepectoral space. The mastectomy skin flap is always carefully assessed clinically, assessing skin color, temperature, capillary refill, dermal edge bleeding, prior to selection of reconstructive technique. Additionally, after the implant is placed, the mastectomy flap is clinically assessed again to ensure that the skin flap remains well perfused with the implant in place. Many studies have reported on the efficacy of laser-assisted indocyanine green angiography in detecting mastectomy flap ischemia and preventing necrosis; however, as a recent cost analysis showed, the use of laser-assisted indocyanine green angiography is not cost-effective when used indiscriminately but can be cost-effective when used for certain high-risk patients.26 We do not routinely use tissue perfusion technology given its unavailability at our outpatient surgery center where most of our reconstructions are performed. We apply topical nitroglycerin to the nipple-areolar complex (NAC) and surrounding skin and frequently to the peri-incisional area of SSMs as well. Nevertheless, full thickness mastectomy skin necrosis occurred after 9% (13) of reconstructions, making it the most common complication in our series. This is, however, very consistent with recent literature rates of skin flap necrosis (10%-14%) after subpectoral DTI and expander breast reconstructions.27
Over the past decade, implant manufacturers have developed more highly cohesive silicone implants that cause less rippling and feel more natural than earlier generation implants. With these attributes, they require less soft tissue coverage to mask their inherent deficiencies. Nevertheless, thinner patients and thin flaps will still have some rippling with prepectoral implant placement. This is easily addressed with fat grafting to the upper pole of the mastectomy skin.
Fat grafting is now widely accepted in revisional breast reconstruction with no known impact on cancer detection or recurrence.28-30 In addition to masking rippling, fat grafting also improves the transition from chest wall to implant, creating a more natural slope, and increasing upper pole fullness. In our series, in patients who had at least 12 months of follow-up (n = 12), 38% of patients did not undergo any fat grafting, 52% of patients underwent a single fat grafting procedure, and 10% of patients underwent two rounds of fat grafting. We have found that with highly cohesive implants, rippling is less of a problem and patients require fat grafting less frequently than with earlier generations of silicone implants.
By placing the implant above the pectoralis major muscle, the skin envelope can be filled without any volume restriction by overlying muscle. With subpectoral DTI, a smaller implant may need to be placed to fit under the muscle despite a voluminous skin envelope. In prepectoral DTI reconstructions, the skin envelope can be fully filled and the breast size can often be augmented without additional stretch on the skin. The mean mastectomy weight and implant size in this series (613 g, 583 cc) is larger than in published series using subpectoral DTI (419 g, 485 cc).31 Excluding patients with mastectomy weights above 800 g (as the largest implant size available is 800 cc, these patients cannot be augmented with implants alone), the average mastectomy weight in this series was 429 g and the average implant size in those patients was 527 cc, reflecting the ability to fully fill and potentially augment breasts in patients with small- to moderately-sized pre-mastectomy breasts.
We believe ADM to be essential to the success of prepectoral DTI.32 ADM provides an additional layer of tissue between implant and skin which may reduce many of the complications seen when implants were placed directly under the skin. We believe the ADM to function as a barrier between the mastectomy skin and the implant. In 50% of cases (8/16) where we have seen ADM exposure from either mastectomy skin necrosis or incisional dehiscence, we have been able to close the mastectomy skin secondarily without changing the underlying device. In 1 year of follow-up, we have yet to see any Baker grade three or four capsular contracture. We hypothesize that placing an implant under ADM is protective against capsular contracture, a finding that is supported in several previously published studies.31-34 Long-term follow-up will be essential in determining whether the rate of capsular contracture differs in prepectoral versus subpectoral breast implants. Of note, ADM is approved by the US Food and Drug Administration (FDA) to provide structural support of an implant for breast reconstruction and therefore, wrapping the implant with ADM constitutes off-label usage, which must be discussed with patients during the preoperative consultation.
In an era of soaring healthcare costs, it is essential to consider the cost-effectiveness of different breast reconstruction techniques. A recent cost-utility analysis showed that subpectoral DTI was more cost-effective than expander-based reconstruction, with a margin of $44,336.59.27 Factors in the prepectoral technique used in this series that could result in additional costs include the cost of a larger, 16 × 20 cm piece of ADM, as well as additional fat grafting procedures. Nevertheless, given that the prepectoral technique has similar outcomes and complication rate to subpectoral reconstruction, and considering the large cost margin by which subpectoral DTI remains cost-effective over expander-based reconstruction, prepectoral DTI would likely remain cost-effective when compared with expander-based reconstruction. A thorough cost-utility analysis will need to be performed to quantify this, and assess whether prepectoral DTI is cost-effective compared with subpectoral DTI.
Radiation therapy delivered after mastectomy before reconstruction has been shown to increase the risk of complication in delayed implant-based reconstruction.35 The effect of radiation on an implant in the setting of prior breast conservation therapy (BCT) is more poorly understood but may have an overall complication rate similar to nonradiated breasts.36 In our series, there were 5 patients who had undergone prior BCT. They were counseled that they may have an increased risk of certain complications. Among this small group of patients, one complication occurred which was an infection eventually necessitating the removal of the implant. An analysis of a larger cohort of patients who underwent BCT prior to prepectoral reconstruction is necessary to determine whether these patients are truly at an increased risk of complications.
PMRT is an integral part of the oncological management of breast cancer and more patients are receiving PMRT than previously.37-39 PMRT may have an adverse effect on the outcomes of implant-based reconstruction with studies showing a higher incidence of capsular contracture and need for unplanned revisions.35,37,41 In our series, 12 patients required PMRT after reconstruction (Table 4). Of these, one developed a major infection and required implant removal and replacement and another developed a minor infection that improved with oral antibiotics. None of these 12 patients developed symptomatic capsular contracture or reconstructive failure. PMRT was not significantly associated with an increased risk of any complication when compared with patients who did not receive PMRT. These preliminary data suggest that radiating a prepectoral-placed implant does not significantly increase the risk of capsular contracture or need for major revision. This may be a result of not placing the implant under a radiated and fibrotic muscle as well as a the presence of ADM, which may protect against the development of capsular contracture.32,34 Our results reflect only early outcomes, and larger studies over longer periods of time will prove essential in elucidating if PMRT after prepectoral reconstruction has a lower rate of complication compared with other reconstructive techniques.
Our initial consultation for patients anticipating mastectomy involves an in-depth discussion of all methods of breast reconstruction. We consider prepectoral implant placement for all patients regardless of mastectomy indication (in situ or invasive disease and genetic predisposition). When a nipple-sparing mastectomy is determined to be oncologically safe, the breast size and shape prior to mastectomy determine the feasibility of a nipple-sparing approach.
In our experience, a relative contraindication to prepectoral DTI has been the lack of fat grafting donor site. We tend to reconstruct these very thin women as a dual plane with ADM. However, these same patients who are typically the most active may not tolerate visible animation deformity and thus it remains ever important to review the trade-offs of the two planes with the patient. Furthermore, if there is a preexisting subpectoral augmentation, we utilize the space available and commonly stay subpectoral with the reconstruction. If the mastectomy flaps are found to be too thin or poorly perfused in the operating room, a tissue expander is placed or more commonly a delayed approach is utilized with a 2-week delay prior to expander or implant placement.
Early in our experience with prepectoral techniques, we observed patients required less postoperative narcotics than patients with subpectoral implant or expander placement. Comparison between patients’ mean postoperative pain score and narcotic intake among the different reconstructive techniques did not show a statistically significant difference, however (Table 5). It has also been our observation that after hospital discharge, patients with prepectoral reconstructions had less pain and required less narcotics; however, we did not have these data available for retrospective review. In the current US opioid epidemic, the ability to perform breast reconstruction in a manner that limits their need for narcotic medication is significant. Many of our immediate prepectoral DTI reconstructions are now performed on an outpatient basis with discharge the same day as surgery. Patients’ postoperative pain is a complex symptom that is difficult to measure and affected by numerous and sometimes unmeasurable variables with much patient variability, narcotic side effects etc. We sought consistency by using the same pain scale and intervals for all of the techniques that were compared. In our analysis, we included all of the subpectoral DTI and expanders performed during the study period, although we performed less of these reconstructions than the prepectoral reconstructions, so these cohorts were small. The small sample size in the subpectoral cohorts limited the statistical power when comparing these groups with the prepectoral cohort. Therefore, we were not able to support our initial hypothesis with statistically significant data. Further investigation with a more highly powered comparison between reconstructive techniques will be useful to understand differences between these techniques. Furthermore, the retrospective and nonrandomized nature of this study allows for the possibility of sampling bias.
Several additional limitations to our review exist. Most importantly, these are preliminary results, with a mean follow-up of 8.5 months. The incidence of late complications in these patients is not known. Studies with a long-term follow-up of a larger cohort of prepectoral DTI patients and comparisons to similarly sized cohorts of subpectoral techniques are needed to fully understand outcomes and how they compare to other techniques. Additionally, analysis of patients’ satisfaction and quality of life after prepectoral DTI reconstruction is important to understand the impact and effectiveness of this technique compared with alternative techniques.
CONCLUSION
In summary, prepectoral DTI provides reliable early results with similar complication rates to other implant-based reconstructive techniques. Prepectoral DTI eliminates implant animation; yet, in this small series, it does not significantly decrease pain or narcotic intake after surgery. Our findings are encouraging and support our continued practice of prepectoral DTI breast reconstruction in the appropriate patient.
Disclosures
Dr Roughton was previously a consultant for Lifecell. The other authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
REFERENCES
- 1. American Society of Plastic Surgeons. 2016. Plastic Surgery Statistics Report. https://d2wirczt3b6wjm.cloudfront.net/News/Statistics/2016/plastic-surgery-statistics-full-report-2016.pdf. Accessed June 1, 2017.
- 2. Dauplat J, Kwiatkowski F, Rouanet P, et al. ; STIC-RMI Working Group . Quality of life after mastectomy with or without immediate breast reconstruction. Br J Surg. 2017;104(9):1197-1206. [DOI] [PubMed] [Google Scholar]
- 3. Ng WKY, Chesney A, Farrokhyar F, Hodgson N, Dal Cin A. One stage placement of permanent implant compared to two stage tissue expander reconstruction. J Plast Surg Hand Surg. 2016;27:1–7. [DOI] [PubMed] [Google Scholar]
- 4. Teo I, Reece GP, Christie IC, et al. Body image and quality of life of breast cancer patients: Influence of timing and stage of breast reconstruction. Psychooncology. 2016;25(9):1106-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Singletary SE. Skin-sparing mastectomy with immediate breast reconstruction: the M.D. Anderson Cancer Center experience. Ann Surg Oncol. 1996;3(4):411-416. [DOI] [PubMed] [Google Scholar]
- 6. Susarla SM, Ganske I, Helliwell L, Morris D, Eriksson E, Chun YS. Comparison of clinical outcomes and patient satisfaction in immediate single-stage versus two-stage implant-based breast reconstruction. Plast Reconstr Surg. 2015;135(1):1e-8e. [DOI] [PubMed] [Google Scholar]
- 7. Malata CM, McIntosh SA, Purushotham AD. Immediate breast reconstruction after mastectomy for cancer. Br J Surg. 2000;87(11):1455-1472. [DOI] [PubMed] [Google Scholar]
- 8. Spear SL, Schwartz J, Dayan JH, Clemens MW. Outcome assessment of breast distortion following submuscular breast augmentation. Aesthetic Plast Surg. 2009;33(1):44-48. [DOI] [PubMed] [Google Scholar]
- 9. Weichman KE, Hamill JB, Kim HM, Chen X, Wilkins EG, Pusic AL. Understanding the recovery phase of breast reconstructions: patient-reported outcomes correlated to the type and timing of reconstruction. J Plast Reconstr Aesthet Surg. 2015;68(10):1370-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Becker H, Fregosi N. The impact of animation deformity on quality of life in post-mastectomy reconstruction patients. Aesthet Surg J. 2017;37(5):531-536. [DOI] [PubMed] [Google Scholar]
- 11. Sbitany H, Piper M, Lentz R. Prepectoral breast reconstruction: a safe alternative to submuscular prosthetic reconstruction following nipple-sparing mastectomy. Plast Reconstr Surg. 2017;140(3):432-443. [DOI] [PubMed] [Google Scholar]
- 12. Vidya R, Iqbal FM. A guide to prepectoral breast reconstruction: a new dimension to implant-based breast reconstruction. Clin Breast Cancer. 2017;17(4):266-271. [DOI] [PubMed] [Google Scholar]
- 13. Sigalove S, Maxwell GP, Sigalove NM, et al. Prepectoral implant-based breast reconstruction: rationale, indications, and preliminary results. Plast Reconstr Surg. 2017;139(2):287-294. [DOI] [PubMed] [Google Scholar]
- 14. Caputo GG, Marchetti A, Dalla Pozza E, et al. Skin-reduction breast reconstructions with prepectoral implant. Plast Reconstr Surg. 2016;137(6):1702-1705. [DOI] [PubMed] [Google Scholar]
- 15. Woo A, Harless C, Jacobson SR. Revisiting an old place: Single-surgeon experience on post-mastectomy subcutaneous implant-based breast reconstruction. Breast J. 2017;23(5):545-553. [DOI] [PubMed] [Google Scholar]
- 16. Nahabedian MY. Implant-based breast reconstruction: Strategies to achieve optimal outcomes and minimize complications. J Surg Oncol. 2016;113(8):895-905. [DOI] [PubMed] [Google Scholar]
- 17. Gabriel A, Sigalove S, Sigalove NM, et al. Prepectoral revision breast reconstruction for treatment of implant-associated animation deformity: A review of 102 reconstructions. Aesthet Surg J. 2018;38(5):519-526. [DOI] [PubMed] [Google Scholar]
- 18. Agency Medical Directors’ Group. Opioid Dose Calculator. http://www.agencymeddirectors.wa.gov/opioiddosing.asp. Accessed June 1, 2017.
- 19. R Core Team (2016) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 20. Fredericks S. A 10-year experience with subcutaneous mastectomy. Clin Plast Surg. 1975;2(3):347-357. [PubMed] [Google Scholar]
- 21. Radovan C. Breast reconstruction after mastectomy using the temporary expander. Plast Reconstr Surg. 1982;69(2):195-208. [DOI] [PubMed] [Google Scholar]
- 22. Prpic I. Reconstruction of the breast after mastectomy for carcinoma. Acta Chir Belg. 1980;79(2):103-104. [PubMed] [Google Scholar]
- 23. Gruber RP, Kahn RA, Lash H, Maser MR, Apfelberg DB, Laub DR. Breast reconstruction following mastectomy: A comparison of submuscular and subcutaneous techniques. Plast Reconstr Surg. 1981;67(3):312-317. [DOI] [PubMed] [Google Scholar]
- 24. Rodriguez-Feliz J, Codner MA. Embrace the change: incorporating single-stage implant breast reconstruction into your practice. Plast Reconstr Surg. 2015;136(2):221-231. [DOI] [PubMed] [Google Scholar]
- 25. Roostaeian J, Pavone L, Da Lio A, Lipa J, Festekjian J, Crisera C. Immediate placement of implants in breast reconstruction: Patient selection and outcomes. Plast Reconstr Surg. 2011;127(4):1407-1416. [DOI] [PubMed] [Google Scholar]
- 26. Kanuri A, Liu AS, Guo L. Whom should we SPY? A cost analysis of laser-assisted indocyanine green angiography in prevention of mastectomy skin flap necrosis during prosthesis-based breast reconstruction. Plast Reconstr Surg. 2014;133(4):448e-454e. [DOI] [PubMed] [Google Scholar]
- 27. Krishnan NM, Fischer JP, Basta MN, Nahabedian MY. Is single-stage prosthetic reconstruction cost effective? A cost-utility analysis for the use of direct-to-implant breast reconstruction relative to expander-implant reconstruction in postmastectomy patients. Plast Reconstr Surg. 2016;138(3):537-547. [DOI] [PubMed] [Google Scholar]
- 28. Kaoutzanis C, Xin M, Ballard NS, et al. Outcomes of autologous fat grafting following breast reconstruction in post-mastectomy patients. Plast Reconstr Surg. 2014;134:270-275. [Google Scholar]
- 29. Seth AK, Hirsch EM, Kim JY, Fine NA. Long-term outcomes following fat grafting in prosthetic breast reconstruction: A comparative analysis. Plast Reconstr Surg. 2012;130(5):984-990. [DOI] [PubMed] [Google Scholar]
- 30. Rigotti G, Marchi A, Galiè M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: A healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;119(5):1409-14 22; discussion 1423. [DOI] [PubMed] [Google Scholar]
- 31. Pittman TA, Fan KL, Knapp A, Frantz S, Spear SL. Comparison of different acellular dermal matrices in breast reconstruction: the 50/50 Study. Plast Reconstr Surg. 2017;139(3):521-528. [DOI] [PubMed] [Google Scholar]
- 32. Salzberg CA, Ashikari AY, Berry C, Hunsicker LM. Acellular dermal matrix-assisted direct-to-implant breast reconstruction and capsular contracture: A 13-year experience. Plast Reconstr Surg. 2016;138(2):329-337. [DOI] [PubMed] [Google Scholar]
- 33. Schmitz M, Bertram M, Kneser U, Keller AK, Horch RE. Experimental total wrapping of breast implants with acellular dermal matrix: A preventive tool against capsular contracture in breast surgery? J Plast Reconstr Aesthet Surg. 2013;66(10):1382-1389. [DOI] [PubMed] [Google Scholar]
- 34. Seth AK, Hirsch EM, Fine NA, Kim JY. Utility of acellular dermis-assisted breast reconstruction in the setting of radiation: A comparative analysis. Plast Reconstr Surg. 2012;130(4):750-758. [DOI] [PubMed] [Google Scholar]
- 35. Kronowitz SJ. Current status of implant-based breast reconstruction in patients receiving postmastectomy radiation therapy. Ann Plast Surg. 2011;66:444-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Khansa I, Colakoglu S, Curtis MS, et al. Postmastectomy breast reconstruction after previous lumpectomy and radiation therapy: Analysis of complications and satisfaction. Ann Plast Surg. 2011;66(5):444-451. [DOI] [PubMed] [Google Scholar]
- 37. Jassem J. Post-mastectomy radiation therapy after breast reconstruction: Indications, timing and results. Breast. 2017;34(Suppl 1):S95-S98. [DOI] [PubMed] [Google Scholar]
- 38. Kronowitz SJ, Lam C, Terefe W, et al. A multidisciplinary protocol for planned skin-preserving delayed breast reconstruction for patients with locally advanced breast cancer requiring postmastectomy radiation therapy: 3-year follow-up. Plast Reconstr Surg. 2011;127(6):2154-2166. [DOI] [PubMed] [Google Scholar]
- 39. Jethwa KR, Kahila MM, Whitaker TJ, et al. Immediate tissue expander or implant-based breast reconstruction does not compromise the oncologic delivery of post-mastectomy radiotherapy (PMRT). Breast Cancer Res Treat. 2017;164(1):237-244. [DOI] [PubMed] [Google Scholar]
- 40. Seth AK, Silver HR, Hirsch EM, Kim JY, Fine NA. Comparison of delayed and immediate tissue expander breast reconstruction in the setting of postmastectomy radiation therapy. Ann Plast Surg. 2015;75(5):503-507. [DOI] [PubMed] [Google Scholar]
- 41. Santosa KB, Chen X, Qi J, et al. Postmastectomy radiation therapy and two-stage implant-based breast reconstruction: Is there a better time to irradiate? Plast Reconstr Surg. 2016;138(4):761-769. [DOI] [PMC free article] [PubMed] [Google Scholar]