Abstract
Guidelines published by the National Comprehensive Cancer Network state that standard of care treatment for the majority of patients with breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL) is surgical resection. This cancer is generally indolent, and if confined to the capsule, curative treatment is usually surgery alone. An en bloc resection involves a total capsulectomy, explantation, complete excision of associated masses, and excision of any involved lymph node(s). Patients with surgical control of disease have favorable long-term overall and event-free survival. Oncologic principles should be applied when resecting BIA-ALCL, and a complete oncologic resection is essential to cure patients of the disease. Incomplete resections, partial capsulectomies, and positive margins are all associated with high rates of disease recurrence and have potential for progression of the disease. Routine sentinel lymph node biopsy is unnecessary and full axillary lymph node dissection is rarely indicated except in cases of proven involvement of multiple nodes. Lymphoma oncology consultation and disease staging by imaging is performed prior to surgery. Importantly, en bloc resection is indicated only for an established diagnosis of BIA-ALCL, and is not recommended for merely suspicious or prophylactic surgeries. The purpose of this article was to demonstrate a stepwise approach to surgical ablation of BIA-ALCL with an emphasis on oncologic considerations critical to disease prognosis.
Surgical management of breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL) has demonstrated improved event-free progression and overall survival in long-term follow-up compared with any other treatment modality.1,2 On the basis of this research, the National Comprehensive Cancer Network (NCCN) established consensus standard of care treatment guidelines for the surgical resection of BIA-ALCL.3 Surgical management of BIA-ALCL has now been recommended by the World Health Organization and the US Food and Drug Administration.4,5 BIA-ALCL is generally indolent, and if confined to the capsule, can usually be cured with surgery alone.6,7 An en bloc resection involves a total capsulectomy, explantation, complete excision of associated masses, and excision of any involved lymph node(s).
In general, plastic surgeons are most familiar with complete capsulectomy performed for capsular contracture associated with implants and they may not be accustomed to oncologic ablation of a lymphoma. Capsulectomy for benign conditions may only be partial, and resection is often performed in a piecemeal fashion. In contrast, oncologic principles should be applied when resecting BIA-ALCL and a complete oncologic resection is essential to curative treatment for the disease. Incomplete resections, partial capsulectomies, and positive margins are all associated with high rates of disease recurrence and, in rare cases, accelerated progression of disease. In this article, we describe a stepwise approach to surgical ablation of BIA-ALCL with an emphasis on oncologic considerations pertinent to a plastic surgery audience.
Institutional Experience
A prospective study of all institutional cases from 2013 to 2018 was performed for patients receiving surgical management of BIA-ALCL. Intraoperative techniques and outcomes were reviewed. The study was approved by institutional review board of MD Anderson Cancer Center and all patients submitted written consent. Twenty-six consecutive patients were identified receiving en bloc surgical resection and explantation by the authors. Perioperative complications included one case of pneumothorax (3.8%), treated with closure of the pleural defect over suction without further sequelae. Two disease recurrences (7.8%) were noted at an average of 5 months from surgery. All patients eventually achieved complete remission (100%) and no deaths were reported.
Patient Selection and Preoperative Evaluation
The surgical treatment of a patient with pathologically confirmed BIA-ALCL follows a standardized approach set forth by the NCCN. We have previously reviewed the approach to a suspected case and the pathologic workup of a delayed seroma which are outside the scope of this article.8 In brief, BIA-ALCL most commonly presents with a delayed (>1 year from implantation) periprosthetic fluid collection around a textured implant (Figure 1). Diagnosis of BIA-ALCL is established with: (1) large anaplastic morphology on cytology; (2) a single T-cell clone on flow cytometry; and (3) CD30+ confluent immunohistochemistry. CD30 IHC is a diagnostic test for BIA-ALCL, but by itself is not pathognomonic for the disease as lymphocytes can express trace CD30 in certain inflammatory states. When a patient is found to have BIA-ALCL, a complete evaluation with metastatic workup is indicated prior to surgical intervention. Preoperative evaluation and staging ideally includes a multidisciplinary approach involving a lymphoma oncologist, hematopathologist, plastic surgeon, and a surgical oncologist. A positron emission tomography (PET) computed tomography (CT) scan should be obtained preoperatively to determine the stage of disease and also for surgical planning. Following surgery, PET/CT is unreliable for the detection of disease for several months due to postsurgical inflammation. The preoperative PET/CT scan can serve as an intraoperative surgical roadmap for resection of disease to ensure any associated masses are resected with the specimen. Importantly, an en bloc resection is indicated only for an established diagnosis of BIA-ALCL, and is not recommended for merely suspicious or prophylactic surgeries.
Figure 1.
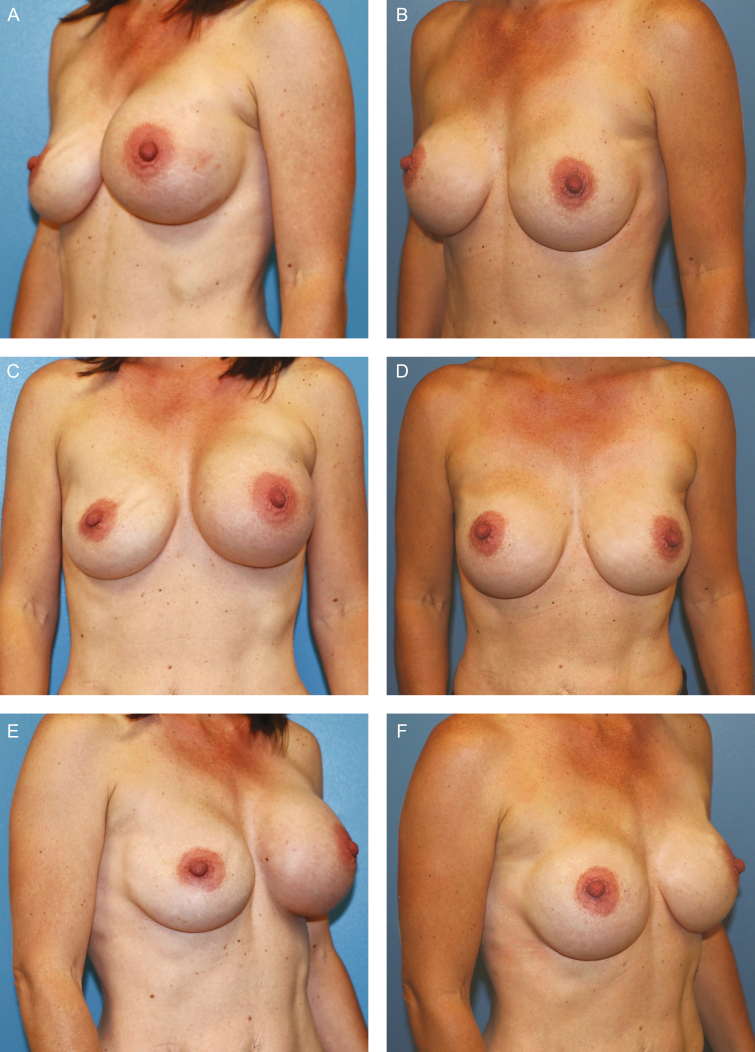
BIA-ALCL patient example. This 43-year-old woman had a past medical history of left breast cancer and bilateral nipple sparing mastectomies and reconstruction with textured (Allergan Corporation, Biocell 410 style, 475 ml) silicone implant reconstruction. Three years following implantation, she developed a left periprosthetic effusion. (A,C,E) Fine needle aspiration demonstrated BIA-ALCL and she underwent a single-stage en bloc resection with explantation, total capsulectomy, and immediate reconstruction with smooth silicone implants. (B,D,F) The patient is shown at 18-month follow-up and remains in complete remission.
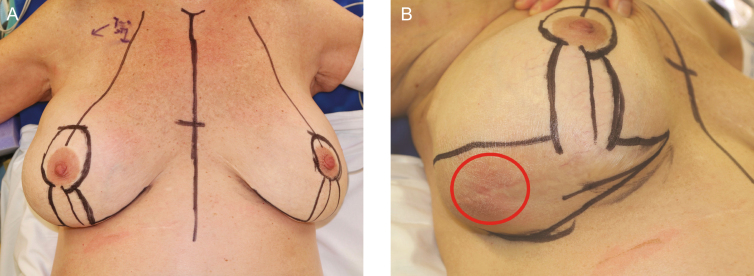
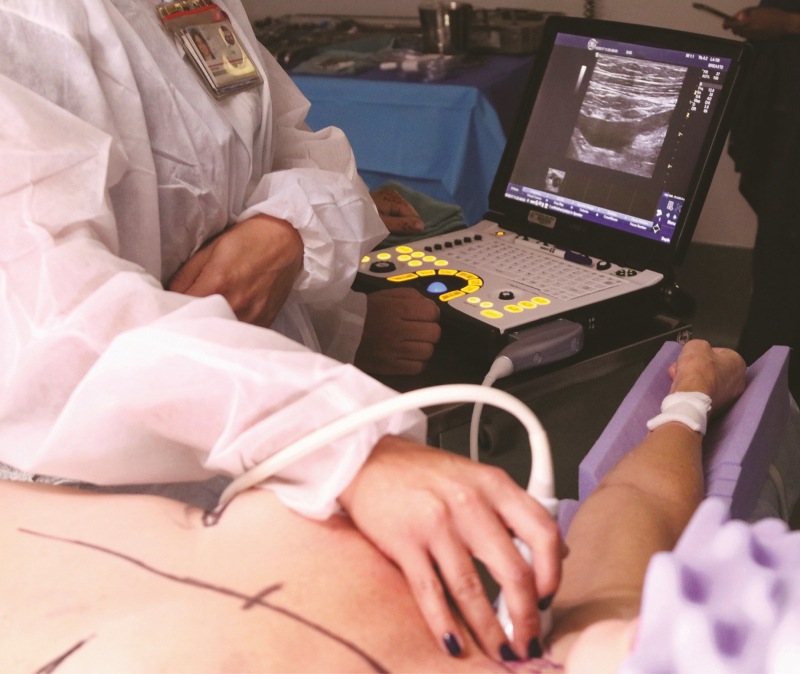
Preoperative markings are made with the patient in a standing position (Figure 2). Chest wall anatomy is highlighted, which includes inframammary folds and breast meridian bilaterally. Note that a large effusion around the breast can dissect tissue planes destroying the inframammary fold or lateral axillary line which may need to be addressed and corrected intraoperatively. NCCN guidelines on BIA-ALCL recommend consideration of contralateral explantation due to known cases of bilateral disease. We recommend en bloc resection of bilateral breast implants and surrounding capsules at the same time. Any previous incisions should be noted and utilized for access to the breast to preserve blood supply. Of note, many patients undergoing a large volume explantation will have significant redundant skin which can be resected in a mastopexy skin excision at the time of explantation (Figure 2B). Some patients may have a mass that involves overlying skin or is particularly close to the skin requiring skin excision. Ideally, skin invasion can also be incorporated into a mastopexy, Wise-pattern, or modified patterns to account for skin loss (Figure 2C). Prophylactic antibiotics are administered intravenously, and sequential compression devices are placed before the induction of anesthesia. Patients with active cancer are at higher risk to develop venous thromboembolism.9 Patients are positioned supine with the arms abducted on the operating table. Ultrasound evaluation in the operating room can help localize any associated mass or lymphadenopathy (Figure 3).10 Biopsy confirmed lymph node metastasis should be excised at the time of explantation if feasible.
Figure 2.
Patient positioning and preoperative marking. Patients are marked in an upright position prior to going to the OR. Markings should include normal anatomic boundaries and account for how an enlarged breast from an effusion may need to be reshaped for symmetry. Explantation without reimplantation may require a concomitant mastopexy to account for excess skin redundancy. (A) The decision to perform a mastopexy is based on the skin laxity, here demonstrated in a 64-year-old woman. (B) In rare situations, a BIA-ALCL mass may be near or invade overlying skin (red circle). In these cases where skin excision is required, a Wise-pattern type skin excision or modification can be employed to account for the skin loss while still maintaining the tear-drop shape of the breast.
Figure 3.
Intraoperative ultrasound. Ultrasound is a helpful intraoperative imaging tool for localizing extent of effusion pocket, masses, and metastatic lymph nodes. In this example, the patient had a single supraclavicular lymph node which was first appreciated by preoperative PET/CT scan, then localized intraoperatively with ultrasound to facilitate excisional biopsy.
Intraoperative Technique
The procedure for en bloc resection of BIA-ALCL is described in 18 steps and is presented in Table 1. A video that demonstrates the surgical process is available as Supplementary Material. An oncologic en bloc resection consists of explantation, total capsulectomy including the posterior wall along with any associated masses. The excision should be performed en bloc and every effort should be made to avoid contaminating the wound with tumor. There is no role for total mastectomy, sentinel lymph node biopsy, or routine axillary lymph node dissection in the treatment of BIA-ALCL. Any suspicious or biopsy-proven metastatic lymph nodes warrant excision.
Table 1.
En Bloc Resection of BIA-ALCL with Key Oncologic Principles
| Surgical management of BIA-ALCL with oncologic technique |
|---|
| • A preoperative PET/CT scan serves as a surgical road map for planning en bloc resection. |
| • Mark the patient preoperatively for breast anatomy as well as planned incisions for mastopexy if necessary, reconstruction, and need for skin excision. |
| • Intraoperative ultrasound or localization with radioactive or magnetic seeds can localize capsular masses or discrete lymph nodes to facilitate resection. |
| • For select patients, tumescence of skin flaps may facilitate ablation. |
| • Utilize existing breast incisions. |
| • Inframammary approach provides the best exposure for an en bloc resection. |
| • Establish dissection plane surrounding the capsule with elevation of overlying skin and parenchymal flaps. |
| • Complete capsulectomy should always include the posterior capsule. |
| • Tumescence hydrodissection aids in the elevation of the posterior capsule off of the chest wall. |
| • Excise suspicious and confirmed lymphadenopathy, avoid sentinel lymph node or full axillary dissection. |
| • Ensure meticulous hemostasis. |
| • Liberal drain placement. |
| • Change instruments for contralateral procedures. |
| • Specimen should be oriented for pathology evaluation. |
| • Surgical clips should be placed in the tumor bed particularly in an area where a mass is resected. |
| • Local anesthetic injection for intercostal blocks with lidocaine/Marcaine or Exparel. |
| • Reconstruction with smooth implants or autologous tissue based on stage of disease. |
| • Postoperative immobilization in surgical bra. |
For select patients, subcutaneous tumescence with solution containing lidocaine (0.05% or 0.1%), saline, and epinephrine (1:1,000,000) can help facilitate ablation while not compromising the blood supply of skin flaps (Figure 4).11,12 High-risk patients most amenable to tumescence technique include breast cancer reconstruction patients with previous mastopexy scars or patients with prepectoral subcutaneous reconstructions where the resection of the capsule might overly compromise the skin flap vascularity. Tumescence should be placed just into the subcutaneous plane and not within the breast parenchyma or directly into the capsule. The establishment of tissue planes by tumescence and hydrodissection is oncologically sound and does not disrupt margin status.
Figure 4.
Tumescence of skin flaps. For select patients, tumescence and hydrodissection of skin planes can facilitate ablative surgery. (A) A 58-year-old woman with previous mastopexy. Previous nipple sparing mastectomies, or prepectoral implant placement with thin skin flaps also benefit from hydrodissection (B) which allows for a sharp dissection of tissue and (C) minimal compromise of vascularity. Tumescence should be placed just into the subcutaneous plane and not within the breast parenchyma or directly into the capsule.
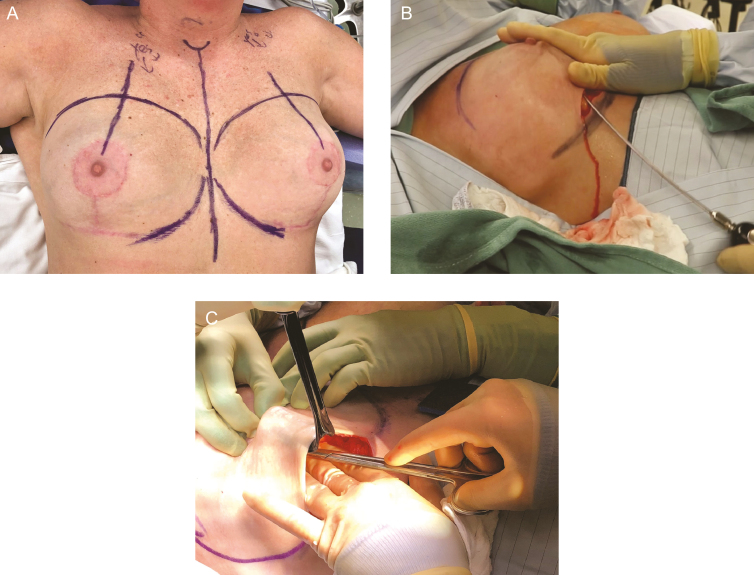
Skin incisions are made along previous scars such as along the inframammary fold (Figure 5). Previous transaxillary incisions and periareolar incisions may be too small and too remote to perform an en bloc resection, and therefore adequate access may necessitate an inframammary fold approach. Dissection proceeds through the skin and parenchyma down to, but not into the capsule. A small rim of healthy tissue should be kept around the capsule for evaluation of margins. Tissue flaps are created of skin, subcutaneous fat, and breast parenchyma first superficial and then deep to the capsule and implant. For patients with subpectoral implants, the plane of dissection needs to remain below the level of the muscle to prevent inadvertent resection of the muscle. Prepectoral implants are technically easier to remove off of the posterior chest wall, but may result in particularly thin skin flaps on the anterior surface. We find the PlasmaBlade (Medtronic, Minneapolis, MN) creates less thermal injury and more precise plane of dissection compared with standard electrocautery in these challenging cases.
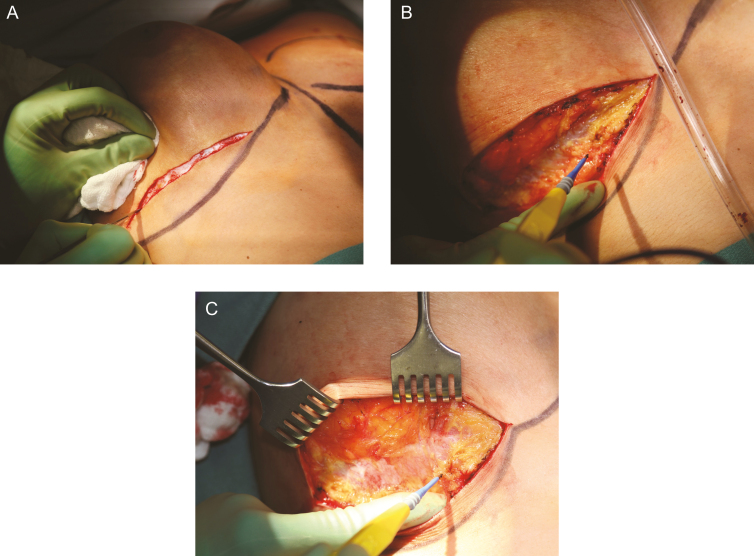
Figure 5.
Incision and establishment of dissection plane. A 36-year-old woman is shown. En bloc resection is technically easier through an inframammary fold incision (A) and may be more difficult through a periareolar or axillary incision. Incision should utilize previous scars and proceed down to but not entering the capsule. Skin and parenchymal flaps are elevated off of the capsule. (B,C) Care should be taken to stay on the capsule and not resect the pectoralis major muscle with the specimen. BIA-ALCL is not a disease of the breast parenchyma and therefore a mastectomy is not indicated. Counter traction with surgical rakes helps maintain plane of dissection.
During surgery, physicians should attempt to adhere to oncologic principles of tumor ablation. This includes changing instruments when moving to a different surgical site such as from one breast to another. An en bloc resection is ideally removed as one specimen with the implant, capsule, and peri-implant fluid collection intact. Care should be made to avoid spillage of the malignant effusion which may lead to spread of the disease along the chest wall. The chest wall is a frequent location of recurrence following partial capsulectomy that leaves the posterior capsule wall. For this reason, the entire capsule needs to be removed with the main specimen (Figure 6A, B). For implants placed in the submuscular position, the capsule may be densely adherent to the ribs and periosteum. Tumescence of the posterior wall can facilitate capsule elevation off of the chest wall as shown in Figure 6C. Care should be taken to avoid dissection into the intercostal muscles to minimize the risk of pneumothorax, the sole intraoperative complication we have encountered. Small rents in the pleura can be closed over suction through a red rubber tube, whereas a large pneumothorax would require a chest tube placement. If violation of the chest wall and lung pleura is in question during the dissection, a postoperative chest x-ray is recommended to rule out a pneumothorax. At the most distal aspect of resection, a lighted retractor, extended bovie tip, and skin counter traction will facilitate exposure and resection (Figure 7).
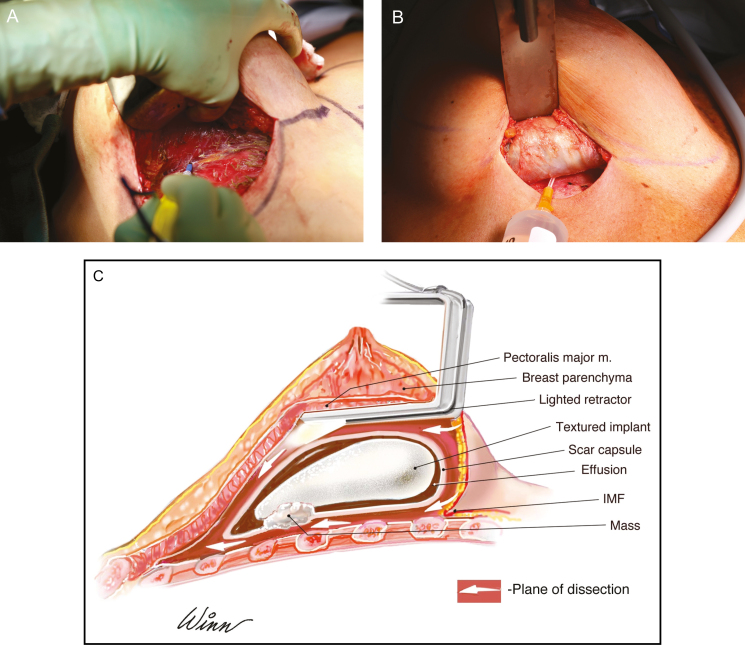
Figure 6.
Resection of posterior capsule. This 36-year-old woman is shown demonstrating removal of the posterior capsule off of the ribcage which is essential to performing a complete capsulectomy. (A) This tissue plane may be difficult to establish and frequently involves stripping of the periosteum of the ribs. (B) Injection of tumescence via a blunt tip needle can facilitate the establishment of a surgical plane and resection of the posterior wall. (C) Illustration demonstrates an en bloc resection which encompasses a total capsulectomy with explantation and resection of any associated masses. A breast augmentation patient with a retropectoral textured implant is shown and surgical approach is via an inframammary fold incision. Any suspicious or biopsy-proven lymph node metastasis also warrants excision. Complete surgical ablation is essential in the treatment of BIA-ALCL, and for many patients can be curative.
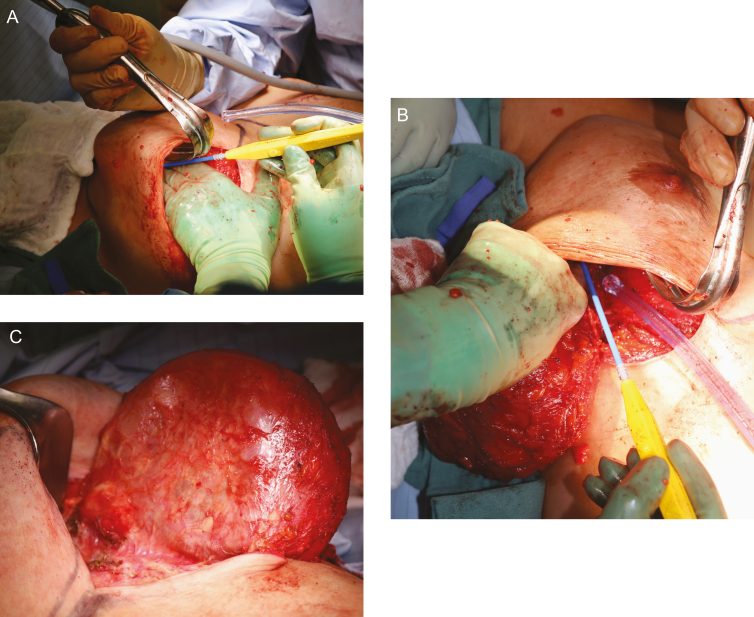
Figure 7.
Resection of specimen. The 36-year-old woman is again shown demonstrating that a lighted retractor, extended bovie device, and skin counter-traction all facilitate removal of the most distal aspects of the capsule. (A,B) Capsules around implants may be quite extensive extending up to the clavicle and deep into the axilla. When resecting a specimen, care should be taken to note any associated capsular mass and resect with a rim of normal tissue. (C) A mass on the posterior capsule that was excised at the time of en bloc resection.
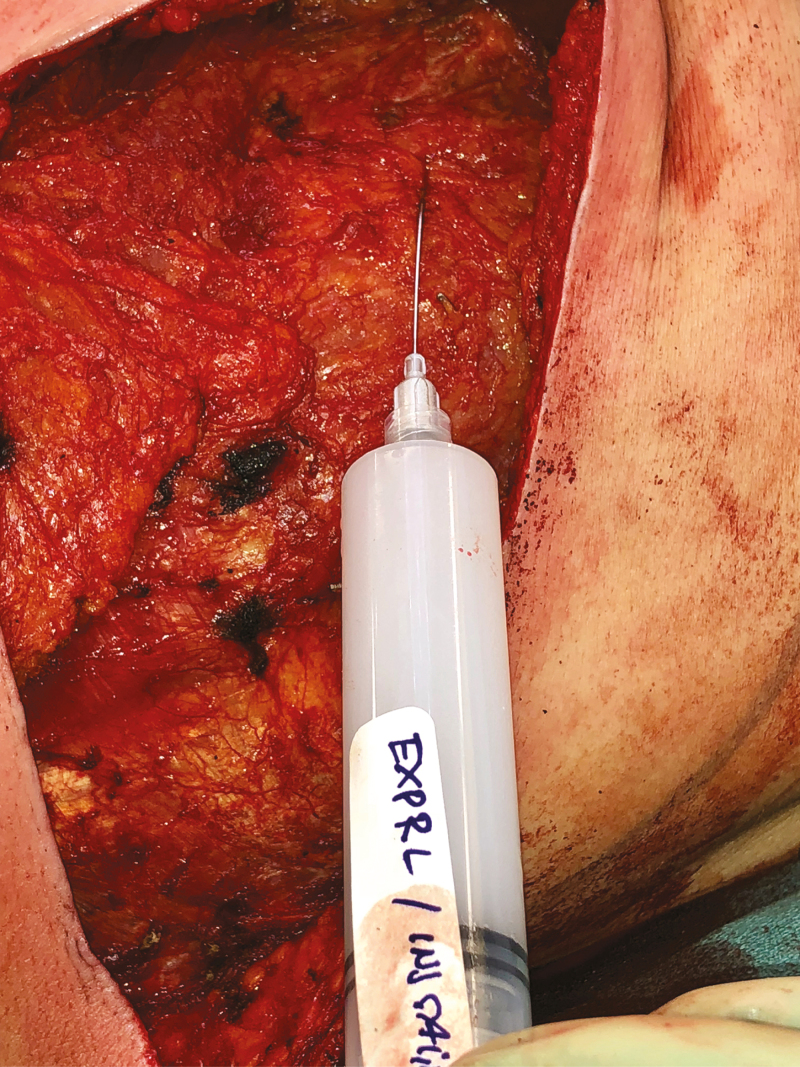
En bloc resection of an implant and capsule can lead to significant postoperative pain, particularly in cases involving periosteal stripping along the ribs. Therefore, local anesthetic should be administered intraoperatively to minimize postoperative narcotic requirements.13 Either a 1% lidocaine/0.5% marcaine mix or Exparel (bupivacaine liposome injectable suspension, Pacira Pharmaceuticals, Parsippany, NJ) injection can be administered with a blunt tip needle for an intercostal nerve block (Figure 8). Typically, intercostal nerves 4-5 are targeted (3 ml per rib), as these nerves provide the majority of sensation to the breast area.
Figure 8.
Local anesthetic. Intercostal nerve blocks decrease postoperative narcotic use and expedite recovery. Local anesthetic with lidocaine/Marcaine or Exparel is injected liberally surrounding the breast cavity and drain sites.
Immediate reconstruction, either autologous or smooth implant, may be performed as clinically indicated based on stage of disease. Note that when performing a smooth implant reconstruction, the pocket must be recreated as resection of the capsule will obliterate the boundaries of the implant. Re-establishment of the lateral fold and inframammary fold can be performed via tissue approximation using absorbable (3-0 polydioxanone [PDS] suture, Ethicon LLC, Somerville, NJ) (Figure 9). Alternatively, if recreation of the anatomic boundaries of the breast pocket is not possible with suture alone, acellular dermal matrix reinforcement of the pocket for soft tissue support may be utilized similar to a standard prosthetic breast reconstruction. The large cavity created by an en bloc resection may be prone to seroma formation particularly in the absence of reconstruction. Therefore, quilting sutures down to the chest wall, fibrin tissue sealant, liberal use of drains can help minimize this risk. For significant skin redundancy, an immediate mastopexy can be performed based on the size of the implant removed, intrinsic skin laxity, existence of previous scar lines, and amount of excess skin. For resectable disease, stage 1A-2A, we will offer the patient an immediate reconstruction. For invasive disease, questions regarding the completeness of resection, and metastasis, we will delay reconstruction 6 to 12 months to confirm complete remission. At the conclusion of the procedure, patients receive postoperative immobilization in a surgical bra (Figure 10).
Figure 9.
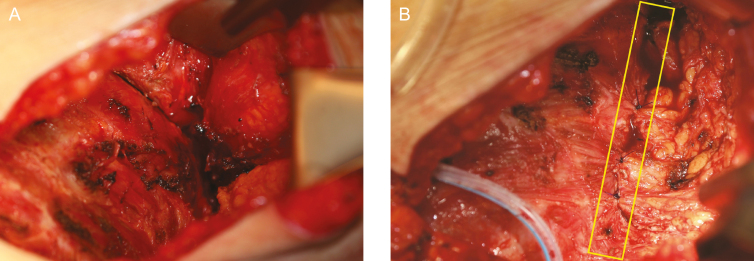
Recreation of anatomic boundaries. An en bloc resection with total capsulectomy can destroy the natural boundaries of the breast such as the lateral and inframammary fold. (A) Demonstrates obliteration of the lateral axillary line of the breast with a cavity well into the patient’s axilla. Immediate smooth implant reconstruction requires recreation of these anatomic boundaries by means of suture approximation of the soft tissue. (B) Demonstrates recreation of the lateral axillary line (yellow box) using an absorbable 3-0 polydioxanone (PDS) suture. Alternatively, acellular dermal matrix may be utilized for soft tissue support to replace the resected scar capsule.
Figure 10.
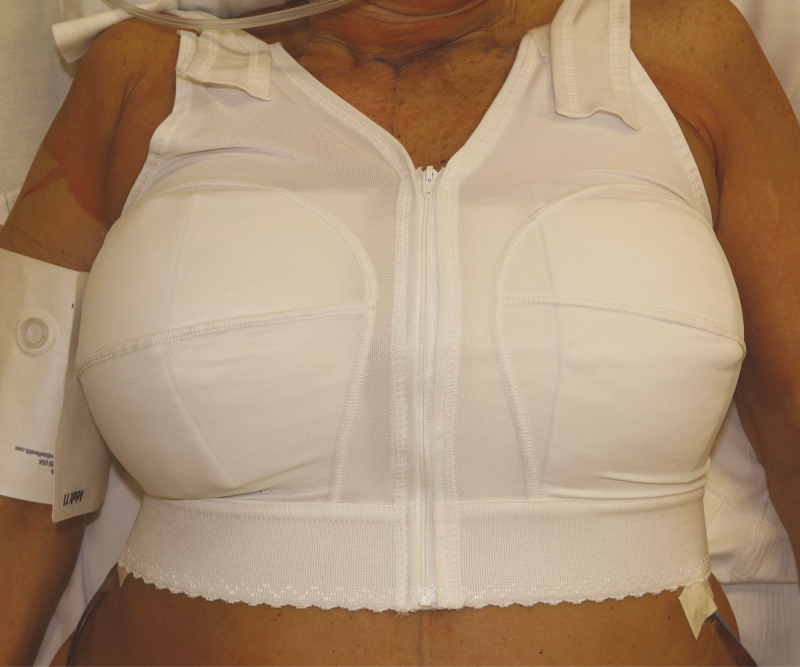
Postoperative support. At the conclusion of the case, patients are placed in a surgical bra for immobilization and to prevent seroma formation. A 64-year-old woman is demonstrated immediately following en bloc resection with smooth implant reconstruction. For immediate smooth implant reconstructions, a supportive bra is maintained 4 to 6 weeks to prevent displacement and device malposition.
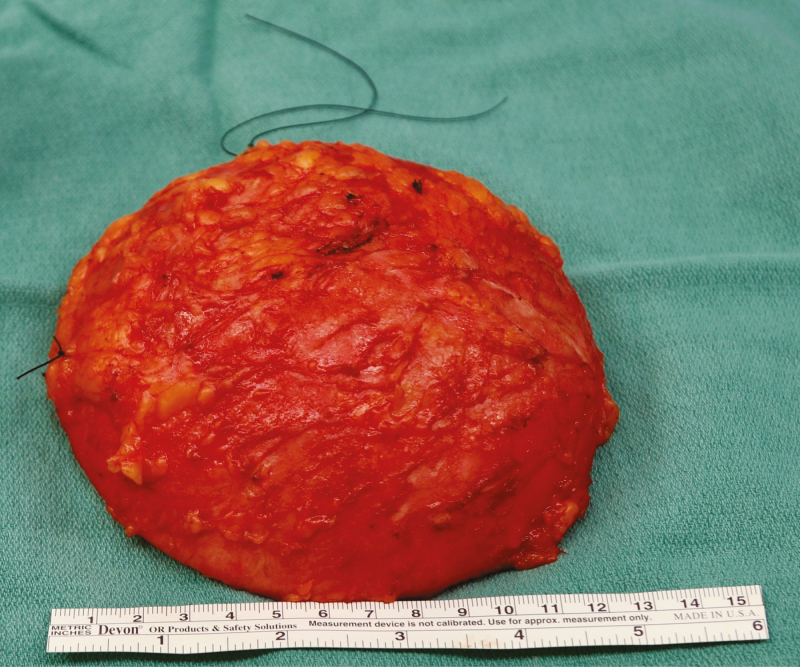
Specimens should be oriented for pathologic review to allow for future surgical or radiation therapy planning. Specimen orientation may consist of intraoperative marking of the specimen with ink or marking two margins (superior and lateral) with sutures (Figure 11).14,15 Medium-sized surgical clips should be placed in the capsulectomy defect, particularly in any areas directly adjacent to a resected mass, to ensure the location of the excision cavity can be identified on imaging for disease surveillance and the potential for salvage radiation therapy in the setting of recurrence.16 Consensus on margin adequacy has not been established. Positive margins with cancer cells at the inked edge of the tissue resection have demonstrated high local recurrence rates. At our institution, demonstration of margins with “no ink on tumor” is deemed sufficient.17,18 A pathologist may classify the margins as close, lymphoma cells near the edge of the resected tissue, but not right at the edge free of ink (Figure 12).
Figure 11.
Specimen orientation. Orientation of the specimen is critical in the event that the patient has an associated capsular mass with a positive margin. Orientation sutures are demonstrated superiorly (short) and laterally (long) which allows for a complete three-dimensional orientation of the specimen by pathology.
Figure 12.
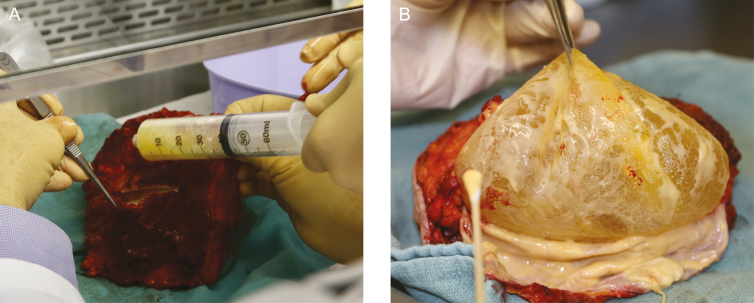
Pathology evaluation. An en bloc resection removed as a single specimen allows pathology to evaluate the effusion (A) and anatomic orientation of any associated masses to the implant and the capsule (B). The appearance of a malignant effusion which is sent for CD30 immunohistochemistry and flow cytometry. Microbiology culture swabs can be sent if there is a concern for infection.
Postoperative Considerations
Following en bloc resection, most patients will be discharged on the same day or with 23-hr observation in an outpatient setting. For smooth implant reconstruction, a supportive bra is maintained 4 to 6 weeks to prevent displacement and malposition. Patients treated with complete resection are followed with history and physical examination every 3 to 6 months for 2 years postoperatively. Drain tube removal generally occurs at 2 to 3 weeks. Patients with residual disease or who underwent incomplete resection require evaluation by a multidisciplinary team to discuss adjuvant chemotherapy and/or radiation therapy.
DISCUSSION
BIA-ALCL is typically indolent in nature and prognosis following complete resection is very good. An evaluation of 74 patients with breast implant ALCL who were treated with complete oncologic resection demonstrated event rates (local and distant) of 0% for stage T1 or T2 disease and 14.3% for T4 disease (P < 0.001).1 BIA-ALCL has been shown to metastasize to regional nodal basins, bone, small bowel, liver, and skin in rare cases, particularly following incomplete resections of disease. To date, 16 patient deaths have been reported in association with BIA-ALCL which emphasizes that BIA-ALCL is a lymphoma, a malignancy, and the ability to invade surrounding tissue demonstrates the need for prompt diagnosis and adequate treatment.6 Patients should ideally be evaluated by oncology preoperatively so that the patient may be followed up for disease surveillance. Clinical examination is recommended at intervals of 3 to 6 months with surveillance PET/CT scan at 6-month intervals for at least 2 years.
CONCLUSION
Complete en bloc surgical excision is essential for curative treatment of BIA-ALCL. A complete resection should include removal of the entire capsule as well as any associated masses and any suspicious lymph nodes. Oncologic principles such as specimen orientation and placing clips in the tumor cavity should be employed during oncologic ablation. An en bloc resection is indicated only for an established diagnosis of BIA-ALCL, and is not recommended for merely suspicious or prophylactic surgeries. When treated appropriately and in a timely fashion, BIA-ALCL has an excellent prognosis.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
Supplementary Material
REFERENCES
- 1. Clemens MW, Medeiros LJ, Butler CE, et al. Complete surgical excision is essential for the management of patients with breast implant-associated anaplastic large-cell lymphoma. J Clin Oncol. 2016;34(2):160-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brody GS. Anaplastic large cell lymphoma occurring in women with breast implants: analysis of 173 cases. Plast Reconstr Surg. 2015;136(4):553e-554e. [DOI] [PubMed] [Google Scholar]
- 3. Clemens MW, Jacobsen ED, Horwitz SM. 2019 NCCN Consensus Guidelines on the Diagnosis and Treatment of Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL). Aesthet Surg J. 2019;39(S1):S3-S13. [DOI] [PubMed] [Google Scholar]
- 4. Feldman AL, Harris NL, Stein H, et al. Breast implant-associated anaplastic large cell lymphoma. In: Swerdlow SH, Campo E, Harris NL, et al. , editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Revised 4th ed, World Health Organization Classification of Tumours. Lyon: International Agency for Research on Cancer; 2017. p 421-422. [Google Scholar]
- 5. U.S. Food and Drug Administration. Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL).https://www.fda.gov/medicaldevices/productsandmed icalprocedures/implantsandprosthetics/breastimplants/ucm239995.htm. Accessed September 1, 2018.
- 6. Clemens MW, Brody GS, Mahabir RC, Miranda RN. How to diagnose and treat breast implant-associated anaplastic large cell lymphoma. Plast Reconstr Surg. 2018;141(4):586e-599e. [DOI] [PubMed] [Google Scholar]
- 7. Leberfinger AN, Behar BJ, Williams NC, et al. Breast implant-associated anaplastic large cell lymphoma: a systematic review. JAMA Surg. 2017;152(12):1161-1168. [DOI] [PubMed] [Google Scholar]
- 8. Quesada AE, Medeiros LJ, Clemens MW, Ferrufino-Schmidt MC, Pina-Oviedo S, Miranda RN. Breast implant-associated anaplastic large cell lymphoma: a review. Mod Pathol. 2019;32(2):166-188. [DOI] [PubMed] [Google Scholar]
- 9. Stein PD, Beemath A, Meyers FA, Skaf E, Sanchez J, Olson RE. Incidence of venous thromboembolism in patients hospitalized with cancer. Am J Med. 2006;119(1):60-68. [DOI] [PubMed] [Google Scholar]
- 10. Colakovic N, Zdravkovic D, Skuric Z, Mrda D, Gacic J, Ivanovic N. Intraoperative ultrasound in breast cancer surgery-from localization of non-palpable tumors to objectively measurable excision. World J Surg Oncol. 2018;16(1):184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abbott AM, Miller BT, Tuttle TM. Outcomes after tumescence technique versus electrocautery mastectomy. Ann Surg Oncol. 2012;19(8):2607-2611. [DOI] [PubMed] [Google Scholar]
- 12. Khater A, Mazy A, Gad M, Taha Abd Eldayem O, Hegazy M. Tumescent mastectomy: the current indications and operative tips and tricks. Breast Cancer (Dove Med Press). 2017;9:237-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shah A, Rowlands M, Krishnan N, Patel A, Ott-Young A. Thoracic intercostal nerve blocks reduce opioid consumption and length of stay in patients undergoing implant-based breast reconstruction. Plast Reconstr Surg. 2015;136(5):584e-591e. [DOI] [PubMed] [Google Scholar]
- 14. Guidi AJ, Connolly JL, Harris JR, Schnitt SJ. The relationship between shaved margin and inked margin status in breast excision specimens. Cancer. 1997;79(8):1568-1573. [PubMed] [Google Scholar]
- 15. Van Zee KJ, Subhedar P, Olcese C, Patil S, Morrow M. Relationship between margin width and recurrence of ductal carcinoma in situ: analysis of 2996 women treated with breast-conserving surgery for 30 years. Ann Surg. 2015;262(4):623-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krawczyk JJ, Engel B. The importance of surgical clips for adequate tangential beam planning in breast conserving surgery and irradiation. Int J Radiat Oncol Biol Phys. 1999;43(2):347-350. [DOI] [PubMed] [Google Scholar]
- 17. Moran MS, Schnitt SJ, Giuliano AE, et al. ; Society of Surgical Oncology; American Society for Radiation Oncology . Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. J Clin Oncol. 2014;32(14):1507-1515. [DOI] [PubMed] [Google Scholar]
- 18. Tevis SE, Neuman HB, Mittendorf EA, et al. Multidisciplinary intraoperative assessment of breast specimens reduces number of positive margins. Ann Surg Oncol. 2018;25(10):2932-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.