Abstract
Background
Routine viral testing strategies for SARS-CoV-2 infection might facilitate safe airline travel during the COVID-19 pandemic and mitigate global spread of the virus. However, the effectiveness of these test-and-travel strategies to reduce passenger risk of SARS-CoV-2 infection and population-level transmission remains unknown.
Methods
In this simulation study, we developed a microsimulation of SARS-CoV-2 transmission in a cohort of 100 000 US domestic airline travellers using publicly available data on COVID-19 clinical cases and published natural history parameters to assign individuals one of five health states of susceptible to infection, latent period, early infection, late infection, or recovered. We estimated a per-day risk of infection with SARS-CoV-2 corresponding to a daily incidence of 150 infections per 100 000 people. We assessed five testing strategies: (1) anterior nasal PCR test within 3 days of departure, (2) PCR within 3 days of departure and 5 days after arrival, (3) rapid antigen test on the day of travel (assuming 90% of the sensitivity of PCR during active infection), (4) rapid antigen test on the day of travel and PCR test 5 days after arrival, and (5) PCR test 5 days after arrival. Strategies 2 and 4 included a 5-day quarantine after arrival. The travel period was defined as 3 days before travel to 2 weeks after travel. Under each scenario, individuals who tested positive before travel were not permitted to travel. The primary study outcome was cumulative number of infectious days in the cohort over the travel period without isolation or quarantine (population-level transmission risk), and the key secondary outcome was the number of infectious people detected on the day of travel (passenger risk of infection).
Findings
We estimated that in a cohort of 100 000 airline travellers, in a scenario with no testing or screening, there would be 8357 (95% uncertainty interval 6144–12831) infectious days with 649 (505–950) actively infectious passengers on the day of travel. The pre-travel PCR test reduced the number of infectious days from 8357 to 5401 (3917–8677), a reduction of 36% (29–41) compared with the base case, and identified 569 (88% [76–92]) of 649 actively infectious travellers on the day of flight; the addition of post-travel quarantine and PCR reduced the number of infectious days to 1474 (1087–2342), a reduction of 82% (80–84) compared with the base case. The rapid antigen test on the day of travel reduced the number of infectious days to 5674 (4126–9081), a reduction of 32% (26–38) compared with the base case, and identified 560 (86% [83–89]) actively infectious travellers; the addition of post-travel quarantine and PCR reduced the number of infectious days to 2518 (1935–3821), a reduction of 70% (67–72) compared with the base case. The post-travel PCR alone reduced the number of infectious days to 4851 (3714–7679), a reduction of 42% (35–49) compared with the base case.
Interpretation
Routine asymptomatic testing for SARS-CoV-2 before travel can be an effective strategy to reduce passenger risk of infection during travel, although abbreviated quarantine with post-travel testing is probably needed to reduce population-level transmission due to importation of infection when travelling from a high to low incidence setting.
Funding
University of California, San Francisco.
Introduction
The COVID-19 pandemic has substantially changed daily life for people and reduced global travel. Since the first report of a cluster of cases of pneumonia, later identified as a novel coronavirus, in Wuhan, China, on Dec 31, 2019, the causative virus SARS-CoV-2 has spread globally with an unprecedented number of cases and deaths.1 The principal public health strategies have been the implementation of universal use of facemasks, physical distancing interventions aimed at reducing the number of social interactions, and test-and-isolate strategies to slow the spread of the virus.2 During the pandemic period of 2020 to early 2021, domestic and international airline travel has been reduced globally by over 80%, as estimated by the US Transportation Security Administration.3 The decrease in airline travel is due to multiple causes, including personal choice to minimise risk of infection, governmental and employer policies enforcing travel restrictions or quarantine requirements, cancellation of professional and social events requiring travel, and other motivations for reducing travel.3
Research in context.
Evidence before this study
We searched PubMed for relevant articles in English, published since database inception, on Jan 23, 2021, using the search terms (“air”[Title/Abstract] OR “travel”[Title/Abstract]) AND(“COVID-19”[Title/Abstract] OR “SARS-CoV-2”[Title/Abstract])AND (“screen”[Title/Abstract] OR “test*”[Title/Abstract])AND (“strategy”[Title/Abstract] OR “model*”[Title/Abstract] OR “simulate”[Title/Abstract]). We identified 116 articles. We found one article that discussed risk of international importation of infections during the early COVID-19 pandemic. We also identified modelling work on testing and quarantine strategies that found shorter quarantine periods with testing to have similar effectiveness to standard 14 day quarantines, and variable benefit of different approaches of pre-travel testing.
Added value of this study
We analysed routine viral testing strategies to reduce individual-level and population-level risk of COVID-19 associated with airline travel. We used a large-scale computer simulation to provide a comprehensive comparison of possible SARS-CoV-2 testing and quarantine strategies to facilitate safe airline travel. We found that pre-travel testing strategies, including same-day rapid antigen testing and pre-travel PCR testing (within 3 days before departure) reduced the risk of infection and transmission of SARS-CoV-2 associated with travelling. Notably, the strategy of a rapid antigen test on the day of travel gave similar results to the pre-travel PCR test strategy (when assuming performance characteristics for the rapid antigen test have 90% of the sensitivity of PCR during the active infection period), which is different from current national guidance in the USA. We examined the addition of PCR testing 5 days after arrival and found that this reduced the overall number of infectious days during the travel period, providing a larger population-level effect through reduced importation of infections. This post-travel quarantine period is shorter than most policy recommendation. The relative benefit of post-travel testing was related to the incidence of the destination city, with largest benefit when travelling from high to low incidence settings.
Implications of all evidence available
Our findings suggest that test-and-travel strategies for SARS-CoV-2 infection will probably improve the safety of airline travel and could be a public health tool to reduce importation of infections into low incidence settings. Governmental policies and airlines should consider inclusion of rapid antigen tests for pre-travel testing and abbreviated 5-day quarantine periods with PCR testing to balance effectiveness of test-and-travel strategies with logistical considerations. All testing strategies were imperfect and should be viewed as a tool to be used alongside physical distancing, universal wearing of facemasks, and other infection control measures during travel.
Asymptomatic viral testing strategies for SARS-CoV-2 could facilitate safe airline travel through reduction of passenger risk of infection and population-level risk from importation of infection due to travel. An estimated 30–40% of people infected with SARS-CoV-2 are asymptomatic and do not know about their infection, and this population contributes to a large proportion of new cases and transmissions.4, 5 A strategy of routine viral testing during travel has two possible applications: reduction in passenger risk of infection in the airport or on aeroplanes by detecting passengers who are infected and preventing their travel, and reduction in the number of importations of infections to a new city, hence reducing the effect of travel on population-level transmission risk. As of January, 2021, the mainstay strategy in most countries has been to avoid travelling altogether, although this strategy is likely to change over time, especially as vaccination programmes become more prevalent. Some travellers might elect for testing before travel, as has started to be offered by some airlines,6 whereas others might prefer to be tested upon arrival at their destination. In other situations, travellers might elect or be required to quarantine upon arrival in the absence of testing. In late 2020, the US Centers for Disease Control and Prevention (CDC) released recommendations for asymptomatic testing for SARS-CoV-2 including getting tested 1–3 days before a flight, and getting tested 3–5 days after travel with a post-travel quarantine period of 7–10 days.7
To design routine testing strategies to minimise passenger and population risk of SARS-CoV-2 infection associated with travel, many factors must be taken into consideration. Testing strategies designed to minimise passenger risk of infection will focus on pre-travel testing, whereas strategies designed to reduce population-level transmission will include both pre-travel and post-travel testing. The choices of test could include PCR tests, the current diagnostic gold-standard, which have very high sensitivity but slow turnaround time; or rapid tests (either antigen or nucleic acid based) that have a fast turnaround time (<30 min) and have been shown to have good sensitivity to detect infection during the most infectious period, although this sensitivity is variable between manufacturers. Testing strategies might also require enforcement by airlines and governmental policy.
Domestic and international airline travel is likely to increase over time compared with the low numbers seen during 2020, and the emergence of new and more transmissible lineages of SARS-CoV-2, such as B.1.1.7, all motivate investigation into how routine testing could minimise the risk of infection with or transmission of SARS-CoV-2 during airline travel.8 The goal of this study was to estimate the effectiveness of test-and-travel strategies.
Methods
Study design and model design
In this simulation study, we developed a microsimulation model of SARS-CoV-2 transmission to identify the optimal testing strategy to detect airline travellers who are infectious before travel or soon after arrival at their destination with similar structure to previous models of SARS-CoV-2 transmission.9 We simulated a population of 100 000 individual US domestic airline travellers over their travel period, which was defined as the 3 days before travel (when the earliest testing would take place), the day of travel, and 2 weeks after arrival at their final destination. This time horizon was chosen to fully capture the effect of all testing strategies on both individual passenger risk and population-level risk of travel.
Each traveller was assigned a single health state at a given point in time from one of five states that included: susceptible to infection (non-immune), latent period, early infectious period (the pre-symptomatic infectious period), late infectious period (symptomatic infectious period, if symptomatic), or recovered (with immunity). The early and late infectious states were further separated into those experiencing subclinical infection or clinical disease. We assumed a mean infectious duration of 5 days.5, 10 We used a static infection model, meaning each individual had a fixed probability of being infected on each day, with an increased risk during the day of travel.3, 11 We estimated a per-day risk of infection with SARS-CoV-2 corresponding to a daily incidence of 150 infections per 100 000 people, which is representative of the incidence observed in many US states as of Jan 31, 2021 (appendix p 8). We simulated new infections starting 2 months before travel to allow the model to reach equilibrium; we counted the number of infectious days over the travel period to include any infections related to travel. We assumed a two-times increased risk of infection on the day of travel on the basis of the number of social interactions in the airport or on the aeroplane and in the published literature, including with airport employees without daily testing.3, 11 We did not explicitly account for differences in duration of airflight travel given the paucity of scientific literature to inform differential risk, and modelled only one-way travel for an average flight.
We accounted for a number of unique features of the natural history of SARS-CoV-2 infection and COVID-19 disease, including the latent and infectious periods, proportion of infections that are subclinical (which are often asymptomatic), presymptomatic transmission, and severity of illness (appendix p 5).4, 10, 12, 13, 14 We included a modest baseline seropositive (and immune) fraction in the population in the model on the basis of estimates in the general US population (appendix p 7).15 More details on the model structure and parameters are in the appendix (pp 3–9), published code, and previous publication.9 The simulation analysis was programmed in R (version 4.0.2).
Simulation of testing strategies
We assessed five viral testing strategies of airline travellers around the time of travel: (1) anterior nasal PCR test within 3 days of departure, meaning travellers were tested 2–3 days before the day of travel; (2) PCR test within 3 days of departure and PCR test on day 5 after arrival, with 5 days of quarantine upon arrival; (3) rapid antigen test on the day of travel; (4) rapid antigen test on the day of travel and PCR test on day 5 after arrival, with 5 days of quarantine upon arrival; and (5) PCR test 5 days after arrival. We did a base case analysis with no testing or quarantine for comparison with these strategies. These strategies were chosen on the basis of informal consultations with experts, guidance from the CDC,6 and US state policies. Notably, the testing strategies differentially affect the passenger risk of infection while travelling (strategies 1–4) versus decreasing population-level transmission risk from importation of new infections to a destination city (strategies 2, 4, and 5).
We assumed some non-adherence to public health guidance, including that 20% of people with symptoms compatible with COVID-19 would attempt to travel and that only 80% of people would complete the recommended quarantine period.16 We assumed that travellers who tested positive did not travel, because this would be enforceable. In all strategies, people who tested positive for SARS-CoV-2 were isolated following national guidance. We did not include any self-quarantine requirements for pre-travel-only testing strategies (strategies 1 and 3).
We used published scientific literature on the sensitivity and specificity of rapid antigen tests and PCR tests for SARS-CoV-2, incorporating time-varying estimates of sensitivity based on time since exposure.17 On the basis of published data from two studies on PCR sensitivity over time, we assumed PCR tests to have sensitivity of 80–95% during the first 2 weeks after exposure, with a peak in sensitivity by day 7 (following the viral kinetics over time within individuals; a curve of test sensitivity over time is in the appendix [p 9]), and a specificity of 99·5–100% (with a mean of 99·8%).17, 18 We assumed a 1-day turnaround time for PCR tests. People were assumed to be detectable by PCR for up to 2 weeks after no longer being infectious.17 For rapid antigen tests, wide variation exists in the sensitivity and specificity of assays. We assumed the rapid antigen tests had the sensitivity and specificity of the Abbot BinaxNOW COVID-19 Ag Card on the basis of available literature to support a sensitivity of 90% relative to PCR tests (during periods of high viral loads compatible with infectiousness) and specificity of 99·5–100% (with a mean of 99·8%).19 Other rapid testing platforms have variable sensitivity and specificity (including some with <50% sensitivity compared with PCR tests).19, 20, 21, 22
We ran each strategy simulation 3000 times, and calculated the mean and 95% uncertainty interval (UI) for each outcome. 95% UIs are the 2·5th to 97·5th percentile values across all simulations.
Outcomes
The primary outcome for each testing strategy was the cumulative number of infectious days in the cohort over the travel period without isolation or quarantine, referred to as cumulative infectious days.10 This outcome is most relevant for population-level risk of transmission and was selected on the basis of the public health goal of reducing overall transmission. In strategies with pre-travel and post-travel testing, we estimated the attributable effect of each test separately on this outcome.
The key secondary outcomes was number of infectious travellers with SARS-CoV-2 detected on the day of travel, which is most relevant for passenger risk of infection during travel and for the airlines whose goal is to minimise transmission at the airport and during airflight travel. The other secondary outcome was the ratio of false positives to true positives for each testing strategy and infection incidence.
Scenario and sensitivity analyses
We did several scenario analyses to further test our strategies. We examined pre-travel PCR testing 2, 5, and 7 days before travel for strategies 1 and 2 and also post-travel quarantine extended to 7 and 14 days with testing on day 5 for strategies 2 and 4. We also examined study outcomes in relation to the SARS-CoV-2 infection incidence in the destination city. To examine the effect of each testing strategy when travelling from a high to relatively lower incidence city, we calculated the ratio of cumulative infectious days in an origin city relative to a destination city under different SARS-CoV-2 infection incidence and under different testing strategies. We estimated the ratio of false positive to true positive test results under different baseline SARS-CoV-2 infection incidence settings.
We did sensitivity analyses to measure the effect of varying individual and multiple model parameters on the study findings. We varied individual model inputs including natural history parameters for SARS-CoV-2 such as duration of infectiousness, subclinical fraction, day-of-travel relative risk of infection, test sensitivity and specificity, daily infection incidence, adherence to testing and quarantine. Specifically, we did a sensitivity analysis in which the daily risk of infection was varied from five to 500 daily infections per 100 000 people. Because uncertainty and variation in the risk of SARS-CoV-2 infection occurs during travel, we varied this relative risk widely in sensitivity analysis, from a scenario with no increased risk (due to pre-departure testing, adequate ventilation, physical distancing, and the wearing of facemasks) to a ten-times higher risk.3, 23 We also modelled risk of infection on the day of travel that depended on whether pre-departure testing was done, which would reduce the number of infectious people in the airport and affect transmission dynamics.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
In a cohort of 100 000 airline travellers with a baseline daily incidence of 150 infections per 100 000 population, the model predicted 649 (95% UI 505–950; 0·6%) people would be actively infectious on the day of travel in the absence of testing or any symptom screening. An estimated 195 (141–284; 30%) of 649 infectious individuals would have subclinical infections. Over the travel period, under this no testing scenario, we estimated a total of 8357 (6144–12 831) infectious days.
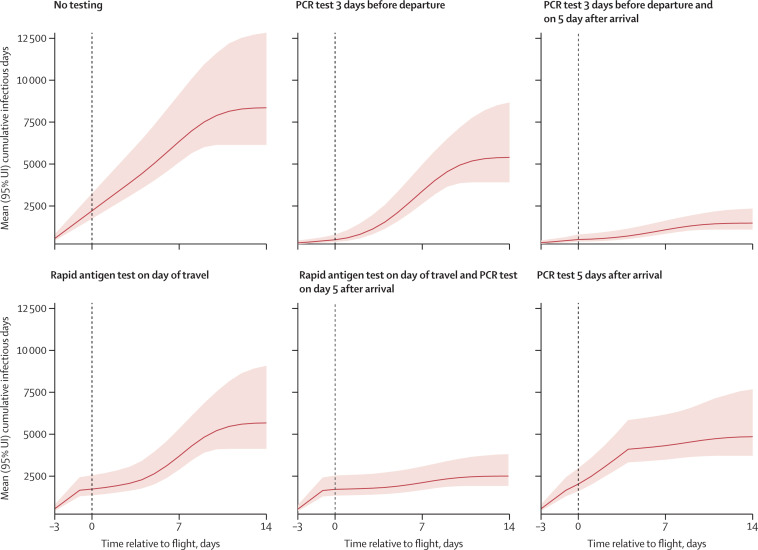
For our first strategy, we estimated that anterior nasal PCR testing within 3 days of departure would reduce the total number of infectious days in the cohort by 36% (29–41) to 5401 (3917–8677) infectious days over the travel period (figure 1 ), and 2460 positive travellers (2247–2687; defined as having any positive test) would be identified in our cohort. In this strategy, the number of actively infectious individuals identified on the day of travel would be 569 (95% UI 459–749), corresponding to identification of 88% (76–92) of all infectious travellers (table ). At the assumed incidence of 150 daily infections per 100 000 people and test specificity of 99·8%, there were 205 (31–397) false positives.
Figure 1.
Predicted number of cumulative SARS-CoV-2 infectious days over the travel period under different test-and-travel strategies
Estimated number of cumulative infectious days without quarantine or isolation (y axis) over time for each test-and-travel strategy. The x axis shows the time over the simulation (in days) relative to the day of travel (vertical dashed line). Solid lines show the mean and shaded areas the 95% UI across 3000 simulations. UI=uncertainty interval.
Table.
Effectiveness of test-and-travel strategies on study outcomes
|
Cumulative infectious days without isolation or quarantine |
Infectious people identified on day of travel (N=649) |
Ratio of false positives to true positives (base case incidence) | |||
|---|---|---|---|---|---|
| Absolute number | Relative reduction | Absolute number | Proportion | ||
| No testing, no screening | 8357 (6144–12 831) | NA | 0 | NA | NA |
| PCR test within 3 days of departure | 5401 (3917–8677) | 36% (29–41) | 569 (459–749) | 88% (76–92) | 0·09 (0·01–0·17) |
| PCR test within 3 days of departure and PCR test within 5 days after arrival* | 1474 (1087–2342) | 82% (80–84) | 569 (459–749) | 88% (76–92) | 0·13 (0·02–0·25) |
| Rapid antigen testing on day of travel | 5674 (4126–9081) | 32% (26–38) | 560 (444–806) | 86% (83–89) | 0·16 (0·02–0·32) |
| Rapid antigen testing on day of travel and PCR test within 5 days after arrival* | 2518 (1935–3821) | 70% (67–72) | 560 (444–806) | 86% (83–89) | 0·21 (0·03–0·42) |
| PCR test within 5 days after arrival† | 4851 (3714–7679) | 42% (35–49) | .. | .. | 0·11 (0·01–0·22) |
Data are mean with 95% uncertainty intervals in parentheses. All relative reductions are relative to the base case of no testing, no screening strategy. All testing strategies assume 80% of symptomatic passengers will not travel. NA=not applicable.
These strategies include a 5-day quarantine period upon arrival.
The strategy has only post-travel testing so does not detect any infected passengers before travelling.
For our second strategy of anterior nasal PCR testing within 3 days of departure and PCR testing 5 days after arrival with quarantine for 5 days, the total number of infectious days in the cohort was reduced by 82% (80–84) to 1474 (1087–2342) infectious days over the travel period (table). Approximately 42·9% of the reduction in infectious days was attributable to the pre-travel PCR and 57·1% was attributable to the post-travel PCR test and quarantine. This strategy identified 3455 positive travellers (3024–3927), and the number of actively infectious travellers identified on the day of travel was 569 (459–749), corresponding to identification of 88% (76–92) of all infectious travellers, which is the same as the pre-travel testing strategy. There were an estimated 410 (64–790) false positives.
For our third strategy, we estimated that the use of a rapid antigen test on the day of travel would reduce the total number of infectious days in the cohort by 32% (26–38) to 5674 (4126–9081) infectious days over the travel period (table). This strategy identified 1423 positive travellers (1227–1625), and the number of actively infectious travellers identified on the day of travel would be 560 (444–806), corresponding to identification of 86% (83–89) of all infectious travellers. There were an estimated 205 (31–401) false positives.
For our fourth strategy, we estimated that a rapid antigen test on the day of travel and PCR testing 5 days after arrival with quarantine for 5 days would reduce the total number of infectious days in the cohort by 70% (67–72) to 2518 (1935–3821) infectious days over the travel period (table), with approximately 45·9% attributable to the rapid antigen test and 54·1% attributable to the post-travel PCR test and quarantine. This strategy identified 2260 positive travellers (1839–2729). On the day of travel, the rapid antigen test identified 560 (444–806) actively infectious travellers, corresponding to identification of 86% (83–89) of all infectious traveller, which is the same as the pre-travel testing strategy. There were an estimated 410 (63–795) false positives.
For our fifth strategy, we estimated that anterior nasal PCR testing 5 days after arrival would reduce the total number of infectious days in the cohort by 42% (35–49) to 4851 (3714–7679) infectious days over the travel period (table). This strategy identified 1959 positive travellers (1727–2199). There were an estimated 205 (22–398) false positives.
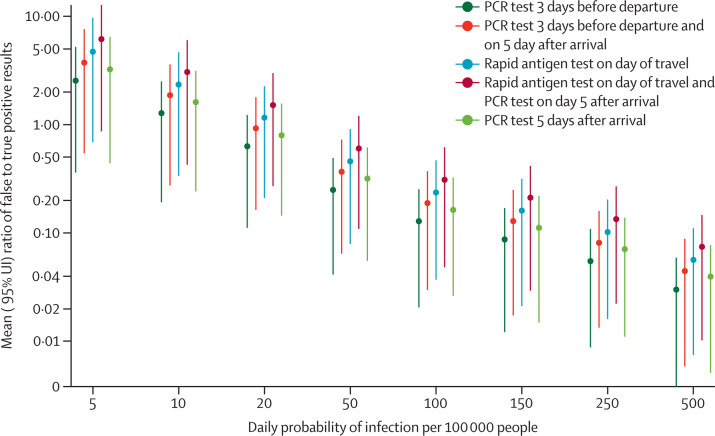
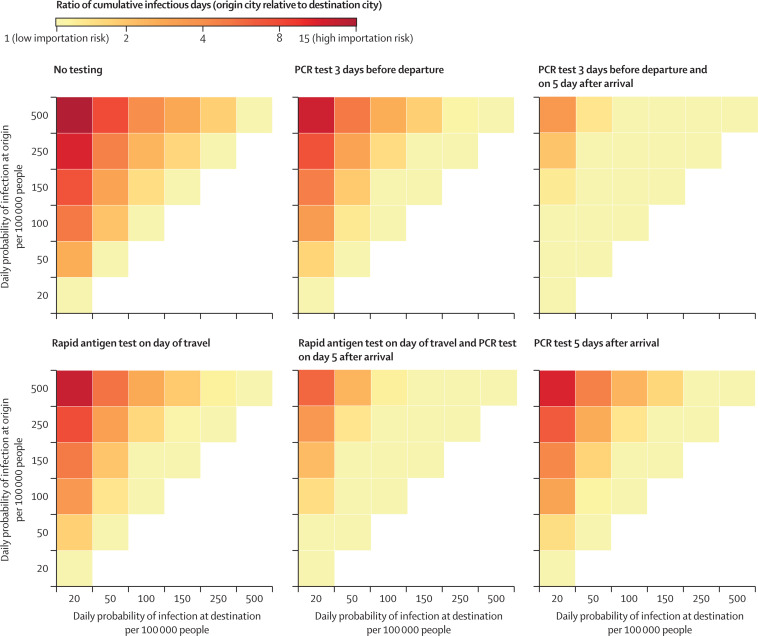
We did additional scenario analyses. The ratio of false positives to true positives for each strategy varied from 6 to 0·04 depending on the testing strategy and baseline infection incidence (figure 2 ). We simulated strategies 1 and 2 under varying pre-travel PCR testing schedules, and found that the number of actively infectious travellers on the day of travel was reduced by 91% (86–94) if the test was done 2 days before travel, 67% (48–80) if it was done 5 days before travel, and 39% (26–57) if it was done 7 days before travel (appendix p 19). We assessed extending the post-travel quarantine period from 5 days to 7–14 days in strategies 2 and 4, which provided little additional benefit (appendix p 20). In a destination city with a higher incidence (250 daily infections per 100 000 people), post-travel testing strategies provided moderate relative reductions in SARS-CoV-2 infections; by contrast, in a destination city with a low incidence (50 daily infections per 100 000 people), the post-travel testing strategies provided a large relative reduction in infections (figure 3 ).
Figure 2.
Ratio of false positive to true positive test results for test-and-travel strategies under different baseline SARS-CoV-2 infection incidence settings
Datapoints are mean and the vertical lines show 95% uncertainty intervals. The x axis shows SARS-CoV-2 infection incidence, including asymptomatic cases (daily cases per 100 000 people). The y axis shows the ratio of false positives to true positives, where higher numbers correspond to a higher number of false positives.
Figure 3.
Change in population-level transmission of SARS-CoV-2 between origin and destination cities for airline travellers at various infection incidences by test-and-travel strategy
For each strategy, we calculated the ratio of cumulative infectious days in an origin city relative to a destination city under different assumptions of SARS-CoV-2 infection incidence for both locations. The ratio is represented by the coloured boxes, where boxes in darker reds are high ratios (corresponding to higher importation risk) and yellow is lower ratios (corresponding to lower importation risk). The white boxes represent scenarios where the ratio is less than one, meaning travellers are moving from a low to high incidence city (corresponding to minimal relative importation risk). Test-and-travel strategies had the largest effect when they reduced the ratio of cumulative infectious days compared with base case (no testing), as shown by a shift from darker colour to lighter colour for a given incidence scenario.
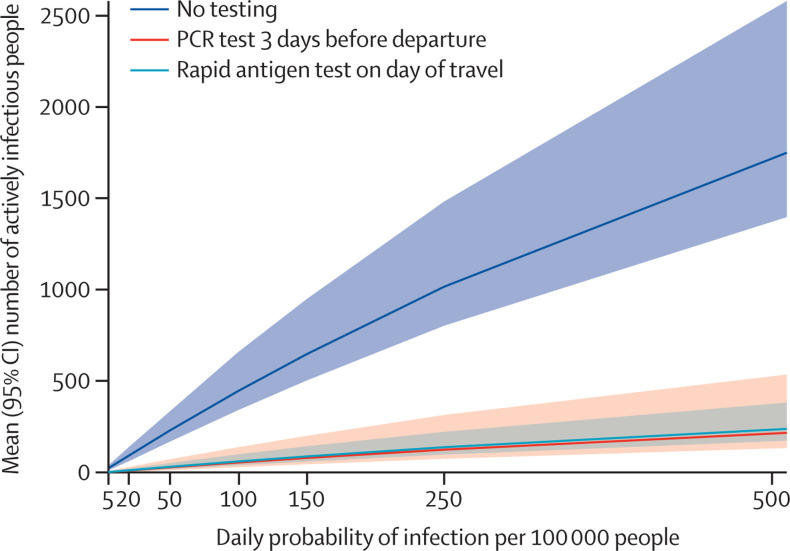
In sensitivity analysis, we varied single model inputs to determine their effect on study findings. Varying the proportion of people who had subclinical infection did not substantially change the study results (appendix p 18). Running the simulation assuming a lower sensitivity of tests reduced the proportion of infectious travellers identified and the number of infectious days averted, while lower specificity of tests increased the number of positive tests and proportion of false positives (appendix pp 13–14). A higher incidence of SARS-CoV-2 infections increased the absolute effectiveness of all testing strategies with a higher number of averted infectious days (figure 4 ). When modelling scenarios with varying relative risk of infection, from no increased risk up to a ten-times increased risk of infection during the day of travel, or modelling this risk as dependent on pre-travel testing, we found minimal changes across all relative risk scenarios, but a scenario with a ten-times increased risk of infection would slightly favour addition of a post-travel quarantine and test strategy (appendix pp 12, 21). Low adherence to the post-quarantine period decreased the reduction in cumulative infectious days compared with the base case analysis (appendix p 17).
Figure 4.
Effect of pre-travel testing strategies on the absolute number of travellers with active SARS-CoV-2 infection
Mean number of total actively infectious people on the day of travel in the cohort of 100 000 (y axis) under each pre-travel testing strategy. We varied the baseline SARS-CoV-2 infection incidence (x axis) from 5 to 500 daily infections per 100 000 people. The y axis represents the mean and 95% uncertainty interval across 3000 simulations.
Discussion
We found that test-and-travel strategies for SARS-CoV-2 infection that apply routine viral testing around airline travel can reduce both the passenger risk of infection and population-level transmission risk of SARS-CoV-2 during travel. We found that both pre-travel testing with a rapid antigen test on the day of travel or PCR testing within 3 days before departure could reduce the risk of SARS-CoV-2 transmission during travel, with the majority of benefit being seen in other travellers who might otherwise have become infected. We found that the addition of post-travel testing and abbreviated quarantine of 5 days could provide further benefit at the public health level by reducing importation and ongoing transmission in the destination city, especially if travelling from high to low incidence settings. Overall, our findings support that a test-and-travel strategy for SARS-CoV-2 infection will likely improve the safety of airline travel and could be incorporated into national policy as a public health tool during the COVID-19 pandemic, alongside physical distancing, universal wearing of facemasks, and other infection control measures during travel.
We found that all test-and-travel strategies had some benefit, and each had strengths and drawbacks. The rapid antigen test had the advantage of timing because it can be administered on the day of travel with immediate turnaround, meaning the test is optimally timed to detect an infectious individual before departure. Notably, rapid tests (antigen and nucleic acid based) have highly variable test sensitivity and specificity. In our study, we parameterised the model, focusing on the Abbott BinaxNOW based on available scientific literature or comparable tests that have reasonably high sensitivity (90% relative to PCR tests during the infectious period), with peak sensitivity based on the viral dynamics during the actively infectious period.19, 20, 21 Alternative rapid testing platforms, such as loop-mediated isothermal amplification assays, with similar test characteristics to rapid antigen tests, are also viable options if available as point-of-care tests.20 Use of a PCR test within 3 days of travel had the benefit of higher analytical sensitivity because PCR remains the gold standard diagnostic tool, but has the drawback of slower turnaround time than rapid antigen tests, can be less convenient, and detects previous SARS-CoV-2 infections that are no longer infectious. Because of the delay in turnaround, PCR tests are likely to be done in the days before travel, and could miss an individual who is not yet exposed to SARS-CoV-2 or who is exposed but has not yet become positive by viral testing, which usually occurs around 3–7 days after exposure.
In scenario analyses, we found that pre-travel testing completed within 2–3 days of travel reduced the number of actively infectious travellers on the day of travel with greater success than with longer lead times of 5–7 days. Both use of rapid antigen tests on the day of travel and of PCR tests 2–3 days before the day of departure appeared to have similar benefits for reducing the number of infectious travellers, although same-day rapid antigen tests are not recommended by CDC guidelines for pre-travel testing purposes.7 We also examined the addition of a post-travel testing strategy with an abbreviated 5-day quarantine period, which resulted in a greater reduction in overall infectious days associated with travel compared with the pre-travel and same-day testing strategies alone.
When interpreting the findings of our study, the public health perspective (eg, destination city of travel) would naturally focus on reducing population-level transmission with a goal of reducing importation of infectious individuals and ongoing transmission, and the primary outcome of reduction in infectious days would be most relevant. From this perspective, the testing strategies that include post-travel quarantine and PCR testing appear more favourable, especially when travelling from high to low incidence settings where importation of infection poses substantial public health risk and, to a lesser extent, those infected during travel. Notably, the strategy with only post-travel testing did not identify people as being infectious to prevent them from travelling. Strategies with only pre-travel testing did not reduce the number of total infectious days to as great an extent as the other strategies. By contrast, if the focus of airlines is to reduce passenger risk of infection during travel, as measured by the proportion of infectious travellers detected, the pre-travel testing strategies appear to be favourable and efficient. Some actively infected travellers were missed with these pre-travel testing strategies, which was mostly related to imperfect test sensitivity and people who were exposed during travel but not yet detectable at the time of testing (in the case of PCR testing 2–3 days before travel).
Although intuitively pre-travel testing should detect infectious people, the key purpose of this study was to provide estimates that could guide policy. Further refinements could be made with consideration of incidence of SARS-CoV-2 infection in the origin and destination cities to influence testing requirements, such as when travelling from a high to low incidence city. The population of travellers might also be younger on average than the general population, meaning a potentially larger proportion of subclinical infections, increasing the benefit of routine viral testing strategies.4, 24 Notably all strategies missed a sizable proportion of infected individuals, and test-and-travel strategies overall should be viewed as a risk mitigation control strategy that should be adopted in combination with physical distancing, universal wearing of facemasks, and other infection control measures when airline travel is essential.2
Although the test-and-travel strategies identified a large number of the actively infectious travellers in our simulations, the testing strategies also resulted in a large number of positive tests in travellers who were not infectious, including false positive results due to imperfect specificity of the test, and people who were previously infected and will test positive for up to 2–4 weeks after exposure but are no longer infectious (most relevant for PCR).10 The implication of this result means that some passengers will be unable to travel despite not being infectious or a risk to other passengers, and this finding is consistent with some international travel requirements.7
Our findings are supported by studies that have examined the effectiveness of asymptomatic testing strategies for COVID-19 to detect cases and reduce overall transmission.9, 25 These studies similarly found that a delayed turnaround time in testing substantially reduced the effectiveness of test-and-travel strategies. Modelling studies have found that post-travel quarantine periods with testing could potentially be shortened compared with the standard 14 days with similar effectiveness26, 27 and they identified variable benefits of different approaches of pre-travel testing.28, 29
Our findings should be interpreted within the context of the limitations of the data and model assumptions. Key uncertainties remain in the natural history of COVID-19, heterogeneity in transmission, and variation in diagnostic test accuracy and quality that we simplified in this modelling analysis. We assumed a high degree of participation in testing and quarantine programmes that would require enforcement by the airline or governmental policy (eg, verification before entry into the airport), and perfect self-isolation of those who test positive. The pre-travel testing at airports could potentially expose individuals to heightened risk of infection, but this was not included. We further assumed that most people who are symptomatic do not travel because they are following current guidance; if this assumption is not true, then routine testing strategies become more important. We included a higher risk of infection during the day of travel, although this risk is uncertain and probably varies substantially, including due to so-called superspreader events.3, 23 We did not include a dynamic transmission model. We assumed all flights had similar associated risks, despite variation in the number of passengers and duration of flights. Routine test-and-travel strategies for asymptomatic individuals would require considerable resources, coordination, guidelines, and ongoing participation of airlines and travellers. Airline travellers will likely bear the cost of routine testing (often US$100), although airlines or the government could provide subsidies; we did not account for costing in this analysis. Finally, our analysis did not address how vaccination affects testing strategies.
In summary, our findings support adoption of testing strategies for SARS-CoV-2 in asymptomatic airline passengers to reduce the risk of infection from travel during the pandemic.
This online publication has been corrected. The corrected version first appeared at thelancet.com/infection on September 15, 2021
Data sharing
The full analytical code and data in this analysis are publicly available on GitHub.
Acknowledgments
Acknowledgments
Some of the computing for this project was performed on the Nero platform at Stanford University (Stanford, CA, USA). We thank Stanford University, the Stanford Research Computing Center, and Stanford Medicine for providing computational resources and support that contributed to these research results. NCL is supported by the University of California, San Francisco (Department of Medicine). MVK is supported in part by the National Institute on Drug Abuse of the NIH (K99DA051534). BQH is supported in part by the National Library of Medicine of the NIH (T15LM007033). The content of this Article is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Contributors
NCL and MVK contributed to the study design. MVK, ETC, BQH, LACC, and NCL contributed to data analysis. All authors contributed to data interpretation. NCL wrote the first draft of the manuscript. All authors contributed intellectual material and approved the final draft. MVK and NCL had access to and verified the underlying study data. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Declaration of interests
NCL has received grants and personal fees from WHO and grants from California Department of Public Health outside of the submitted work. GWR has received grants from the San Francisco Department of Public Health and the California Department of Public Health for COVID-19-related work unrelated to the current study. DH reports grants from US National Institutes of Health (NIH) and non-financial support from Abbott outside of the submitted work. SB reports grants from NIH and US Centers for Disease Control and Prevention, and personal fees from PLOS Medicine, The New England Journal of Medicine, Collective Health, HealthRight 360, Kaiser Permanente Medical Group, and Research Triangle Institute, outside the submitted work. All other authors declare no competing interests.
Supplementary Material
References
- 1.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chu DK, Akl EA, Duda S, et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395:1973–1987. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi EM, Chu DKW, Cheng PKC, et al. In-flight transmission of SARS-CoV-2. Emerg Infect Dis. 2020;26:2713–2716. doi: 10.3201/eid2611.203254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buitrago-Garcia D, Egli-Gany D, Counotte MJ, et al. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: a living systematic review and meta-analysis. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li R, Pei S, Chen B, et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2) Science. 2020;368:489–493. doi: 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaper D. United Airlines says it will offer travelers coronavirus tests at the airport. NPR. Sept 24, 2020. https://www.npr.org/sections/coronavirus-live-updates/2020/09/24/916541587/united-airlines-says-it-will-offer-travelers-coronavirus-tests-at-the-airport
- 7.US Centers for Disease Control and Prevention Testing and international air travel. Feb 4, 2021. https://www.cdc.gov/coronavirus/2019-ncov/travelers/testing-air-travel.html
- 8.Volz E, Mishra S, Chand M, et al. Transmission of SARS-CoV-2 lineage B.1.1.7 in England: insights from linking epidemiological and genetic data. medRxiv. 2021 doi: 10.1101/2020.12.30.20249034. published online Jan 4. (preprint). [DOI] [Google Scholar]
- 9.Chin ET, Huynh BQ, Chapman LAC, Murrill M, Basu B, Lo NC. Frequency of routine testing for coronavirus disease 2019 (COVID-19) in high-risk healthcare environments to reduce outbreaks. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1383. published online Oct 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26:672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 11.Myers JF, Snyder RE, Porse CC, et al. Identification and monitoring of international travelers during the initial phase of an outbreak of COVID-19 - California, February 3-March 17, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:599–602. doi: 10.15585/mmwr.mm6919e4. [DOI] [PubMed] [Google Scholar]
- 12.Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection: a narrative review. Ann Intern Med. 2020;173:362–367. doi: 10.7326/M20-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun K, Wang W, Gao L, et al. Transmission heterogeneities, kinetics, and controllability of SARS-CoV-2. Science. 2021;371 doi: 10.1126/science.abe2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172:577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bajema KL, Wiegand RE, Cuffe K, et al. Estimated SARS-CoV-2 seroprevalence in the US as of September 2020. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.7976. published online Nov 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Webster RK, Brooks SK, Smith LE, Woodland L, Wessely S, Rubin GJ. How to improve adherence with quarantine: rapid review of the evidence. Public Health. 2020;182:163–169. doi: 10.1016/j.puhe.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kucirka LM, Lauer SA, Laeyendecker O, Boon D, Lessler J. Variation in false-negative rate of reverse transcriptase polymerase chain reaction-based SARS-CoV-2 tests by time since exposure. Ann Intern Med. 2020;173:262–267. doi: 10.7326/M20-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hay AH, Kennedy-Shaffer L, Kanjilal S, et al. Estimating epidemiologic dynamics from cross-sectional viral load distributions. medRxiv. 2021 doi: 10.1101/2020.10.08.20204222. published online Feb 13. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pilarowski G, Lebel P, Sunshine S, et al. Performance characteristics of a rapid severe acute respiratory syndrome coronavirus 2 antigen detection assay at a public plaza testing site in San Francisco. J Infect Dis. 2021 doi: 10.1093/infdis/jiaa802. published online Jan 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gibani MM, Toumazou C, Sohbati M, et al. Assessing a novel, lab-free, point-of-care test for SARS-CoV-2 (CovidNudge): a diagnostic accuracy study. Lancet Microbe. 2020;1:e300–e307. doi: 10.1016/S2666-5247(20)30121-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albert E, Torres I, Bueno F, et al. Field evaluation of a rapid antigen test (Panbio™ COVID-19 Ag Rapid Test Device) for the diagnosis of COVID-19 in primary healthcare centers. Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.11.004. published online Nov 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwob JM, Miauton A, Petrovic D, et al. Antigen rapid tests, nasopharyngeal PCR and saliva PCR to detect SARS-CoV-2: a prospective comparative clinical trial. medRxiv. 2020 doi: 10.1101/2020.11.23.20237057/. published online Nov 24. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swadi T, Geoghegan JL, Devine T, et al. Genomic evidence of in-flight transmission of SARS-CoV-2 despite predeparture testing. Emerg Infect Dis. 2021;27:687–693. doi: 10.3201/eid2703.204714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davies NG, Klepac P, Liu Y, Prem K, Jit M, Eggo RM. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020;26:1205–1211. doi: 10.1038/s41591-020-0962-9. [DOI] [PubMed] [Google Scholar]
- 25.Grassly NC, Pons-Salort M, Parker EPK, et al. Comparison of molecular testing strategies for COVID-19 control: a mathematical modelling study. Lancet Infect Dis. 2020;20:1381–1389. doi: 10.1016/S1473-3099(20)30630-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clifford S, Quilty BJ, Russell TW, et al. Strategies to reduce the risk of SARS-CoV-2 re-introduction from international travellers. medRxiv. 2020 doi: 10.1101/2020.07.24.20161281. published online July 25. (preprint). [DOI] [Google Scholar]
- 27.Wells CR, Townsend JP, Pandey A, et al. Optimal COVID-19 quarantine and testing strategies. Nat Commun. 2021;12:356. doi: 10.1038/s41467-020-20742-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dickens BL, Koo JR, Lim JT, et al. Strategies at points of entry to reduce importation risk of COVID-19 cases and reopen travel. J Travel Med. 2020;27 doi: 10.1093/jtm/taaa141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johansson MA, Wolford H, Paul P, et al. Reducing travel-related SARS-CoV-2 transmission with layered mitigation measures: symptom monitoring, quarantine, and testing. medRxiv. 2020 doi: 10.1101/2020.11.23.20237412. published online Nov 24. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The full analytical code and data in this analysis are publicly available on GitHub.