Abstract
Cell sheet technology using UpCell™ (Thermo Fisher Scientific, Roskilde, Denmark) plates is a modern tool that enables the rapid creation of single-layered cells without using extracellular matrix (ECM) enzymatic digestion. Although this technique has the advantage of maintaining a sheet of cells without needing artificial scaffolds, these cell sheets remain extremely fragile. Collagen, the most abundant ECM component, is an attractive candidate for modulating tissue mechanical properties given its tunable property. In this study, we demonstrated rapid mechanical property augmentation of human dermal fibroblast cell sheets after incubation with bovine type I collagen for 24 h on UpCell plates. We showed that treatment with collagen resulted in increased collagen I incorporation within the cell sheet without affecting cell morphology, cell type, or cell sheet quality. Atomic force microscopy measurements for controls, and cell sheets that received 50 and 100 μg/mL collagen I treatments revealed an average Young's modulus of their respective intercellular regions: 6.6 ± 1.0, 14.4 ± 6.6, and 19.8 ± 3.8 kPa during the loading condition, and 10.3 ± 4.7, 11.7 ± 2.2, and 18.1 ± 3.4 kPa during the unloading condition. This methodology of rapid mechanical property augmentation of a cell sheet has a potential impact on cell sheet technology by improving the ease of construct manipulation, enabling new translational tissue engineering applications.
Impact statement
We demonstrated that collagen I treatment can rapidly augment the mechanical properties of monolayer fibroblast cell sheets by increasing stiffness in a concentration-dependent manner without affecting cell morphology, cell type, or cell sheet quality. This methodology has a potential impact on cell sheet technology by providing an avenue for matrix stabilization. It can improve the ease of construct manipulation while allowing for rapid harvest of monolayer fibroblast cell sheets. Future investigations should evaluate mechanical property responses of cell sheets that consist of other cell types for broader translational tissue engineering applications.
Keywords: atomic force microscopy, Young's modulus, cell sheet
Introduction
Cell sheet engineering using UpCell™ (Thermo Fisher Scientific, Roskilde, Denmark) plates is a modern technology that enables the creation of a single layer of cells in a rapid manner without the use of extracellular matrix (ECM) enzymatic digestion.1 The UpCell plates are grafted with thermoresponsive cell culture surfaces made of poly(N-isopropylacrylamide) that changes from hydrophobic to hydrophilic when the temperature is lowered to allow cell detachment with unaltered cell-cell junctions and ECM deposition.2 This technology is influential in that it can rapidly generate a sheet of cells within 24 h without using artificial scaffolds and can be utilized for 3D construction with cell type control.1–3 In contrast, traditional cell sheets generated using standard tissue culture surfaces require several weeks of culture and consist of multiple layers of cells.4 Although this modern cell sheet technology has been adopted for a wide variety of applications,5–7 one of the major weaknesses of these cell sheets is their frailty and inability to tolerate manipulations required for translational applications.
The ECM has become a popular avenue for tissue mechanical property modifications.2 ECM proteins are highly biocompatible and biodegradable, and can rapidly aid in tissue remodeling in vivo.8 Among these, collagen is the most abundant ECM component, providing a scaffold that binds other ECM proteins and proteoglycans.9,10 Previous studies have utilized collagen and its mechanically tunable properties to generate ECMs of varying stiffnesses without altering cell topology or integrin engagement.9,11 Collagen can be useful for engineering cell environmental changes at the submicron scale.9,12
Atomic force microscopy (AFM) is a powerful tool that can quantitatively evaluate various materials' mechanical properties at micrometer to nanometer scales.13 It uses both a surface image tool and a force sensor in liquid at physiologic temperatures, which make it particularly suitable for biological applications.14,15 Flexible AFM cantilevers can be applied to a single cell, and the Young's modulus of the substrate can be extracted from the sample stiffness measured by AFM.16
In this study, we rapidly augmented the mechanical properties of cell sheets consisting of a monolayer of confluent human dermal fibroblasts (HDFa) by mixing bovine type I collagen (collagen I) in the cell culture media at the time of plating. We showed that a 24-h treatment of collagen I did not alter fibroblast morphology or cell type. To quantify changes in stiffness after collagen I treatment, we measured the Young's modulus of the cell sheets by AFM, indenting intercellular regions under both loading and unloading conditions. The results confirmed our hypothesis that cell sheet stiffness can be rapidly altered using collagen I treatment. This methodology has a potential impact on cell sheet technology by providing an avenue for the rapid cell matrix stabilization required in translational tissue engineering.
Materials and Methods
Cell culture maintenance
HDFa cells were purchased commercially (ThermoFisher Scientific) and cryopreserved as primary cultures. They were cultured in growth media: Medium 106 (ThermoFisher Scientific) containing the low serum growth supplement kit (ThermoFisher Scientific) with added 1% Penicillin/Streptomycin (ThermoFisher Scientific).
Cell sheet preparation
Three groups were generated: control (no treatment), treatment with 50 μg/mL bovine collagen I (ThermoFisher Scientific), and treatment with 100 μg/mL collagen I. Collagen I was added directly into the growth media and mixed thoroughly with the cells for the treatment groups. HDFa cells were then plated onto the 35-mm UpCell plates (ThermoFisher Scientific) at a concentration of 1.0 × 106 cells/plate. These cells were cultured in the growth media at 37°C and 5% CO2 for 24 h. The dishes were then transferred into a 20°C incubator, and the confluent cell sheets detached spontaneously from the plates. The recovered cell sheets were laid flat in the optimum cutting temperature compound (Fisher HealthCare) using 2-methylbutane on dry ice. The samples were stored at −80°C until use.
Histological and immunohistochemical staining
Ten micrometers standard cryosections in the cross-sectional direction were prepared and stained with the Shandon Rapid-Chrome Hematoxylin and Eosin (H&E) Frozen Section Staining Kit (Thermo Fisher Scientific). For immunohistochemistry staining, the cryosections were incubated with 4% PFA at room temperature, rinsed with 3% bovine serum albumin in PBS, and then incubated in 0.5% PBS-Tween. The sections were incubated with anti-fibroblast surface protein (FSP) antibody, anti-alpha smooth muscle actin (α-SMA) antibody, anti-collagen I antibody, anti-integrin beta 5 antibody, and anti-elastin antibody using an 1:100 dilution (Abcam), and then incubated at 37°C for 90 min in the dark with anti-mouse 488, anti-goat 594, anti-mouse 594, anti-goat 488, and anti-rabbit 647 secondary antibodies using an 1:200 dilution (Abcam), respectively. Finally, the sections were incubated with DAPI (NucBlue Fixed Cell ReadyProbes Reagent, Cat: R37606; ThermoFisher Scientific). The stained slides were kept at 4°C until imaging.
Microscopy imaging
Bright-field images of HDFa cells on the UpCell plates before harvesting were acquired using the EVOS XL Core Imaging System (ThermoFisher Scientific) at 10 × magnification. H&E-stained sections were imaged using the Leica modular DMi8 inverted microscope (Leica Microsystems) at 20 × magnification. Immunohistochemically stained sections were imaged using an inverted Zeiss LSM 780 multiphoton laser scanning confocal microscope (Carl Zeiss Microscopy) at 20 × magnification. Captured Z-stacked images were processed and analyzed using Zeiss Zen software. Further imaging processing was completed using ImageJ (National Institutes of Health).
AFM sample preparation
HDFa cells were plated onto the 50 mm tissue culture grade polystyrene glass bottom WillCo Wells Petri dishes (Ted Pella) at a concentration of 1.0 × 106 cells/plate. Three groups were generated: control, treatment with 50 μg/mL collagen I, and treatment with 100 μg/mL collagen I, and each group included three independent samples. Collagen I was added directly into the growth media, mixed thoroughly with the cells, and plated together with HDFa cells. The cells were incubated at 37°C and 5% CO2 for 24 h before AFM measurements.
AFM data acquisition
AFM measurements were performed using a BioScope Resolve AFM (Bruker) system mounted on an Axio Observer Z1 inverted optical microscope (Carl Zeiss Microscopy). The microscopy stage was preheated to 37°C before data acquisition, and the temperature was kept constant during the measurements. A silicon nitride cantilever with a precalibrated spring constant of 0.15 N/m terminated with borosilicate glass colloidal sphere with a radius of 15 μm (PT.GS; Novascan) was used for all measurements. The cantilever deflection sensitivity was calibrated before each experiment using the integrated “No Touch Calibration” software routine (Nanoscope 9.4; Bruker). Using a PeakForce tapping mode, Force Volume maps were collected over a randomly selected intercellular area on each cell sheet, while keeping the following parameters constant: ramp size = 848 nm, ramp rate = 1 Hz, and Trigger Force = 1.2 nN. For each AFM sample, one force volume map was obtained.
AFM Young's modulus analysis
Both the extension and the retraction curves were processed using the Nanoscope Analysis 1.9 software (Bruker). In brief, baseline correction was first performed for both the extend and the retract curve taken from the contact point within the intercellular region. The linear portion of the extend curve (10–80% of force fit boundary) and the last portion of the retract curve (10–40% of force fit boundary) were selected. Next, the force curves were fitted using the Hertzian model: Equation (1),17 where F is the force, E the Young's modulus, R the radius of the indenter, δ the indentation, and υ the Poisson's ratio, set as 0.5 for this study.17
| (1) |
Including the absolute minimum adhesion algorithm, the Young's modulus map was generated for both the loading and unloading conditions. For each force volume map, four to five areas of cytoplasm edges were randomly selected using the roughness analysis to calculate for the averaged Young's modulus for each sample.
Statistical analysis
Statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc., NC). Continuous variables were reported as mean ± standard error. Sample variance was assessed using F-test. Comparison between treatment groups was performed using student t-test. A p-value <0.05 was considered statistically significant.
Results
Cell morphology and cell sheet quality after collagen I treatment
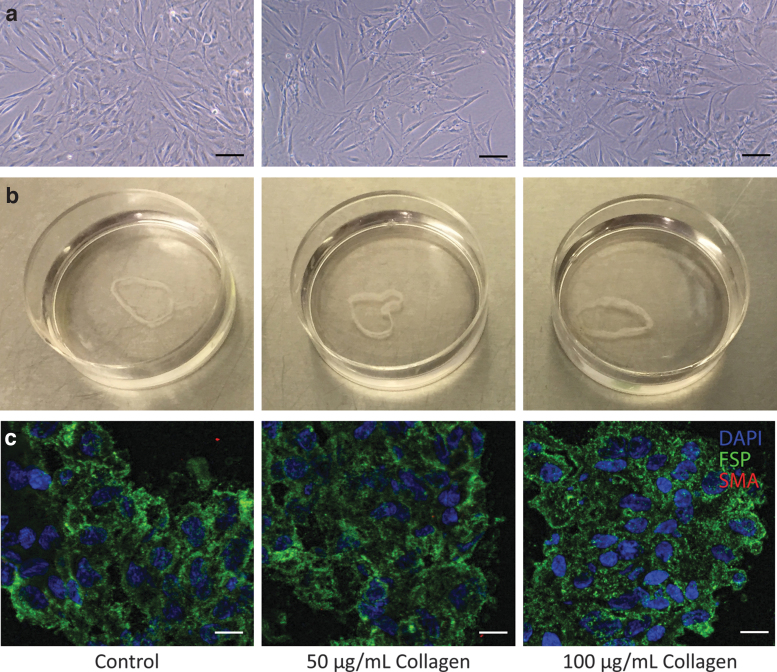
After a 24-h collagen treatment, bright-field images demonstrated that treated cells had similar morphology and size compared to those from the controls (Fig. 1a). All cell sheets were recovered intact maintaining sheet-like structures. The cell sheet that received 100 μg/mL collagen I treatment appeared to be the thickest, whereas the control cell sheet appeared to be the thinnest (Fig. 1b). All HDFa cell sheets demonstrated ubiquitous FSP signal surrounding DAPI-stained nuclei (Fig. 1c). No identifiable α-SMA signal was observed in any cell sheet (Fig. 1c).
FIG. 1.
Preservation of cell morphology, cell sheet quality, and cell type after collagen I treatment. (a) Bright-field images of HDFa cells with 50 or 100 μg/mL collagen I treatment compared to control demonstrated similar cell morphology and size. Scale bar: 100 μm. (b) Cell sheets harvested from 3.5 cm Nunc™ dishes with UpCell™ surface remained intact with collagen I treatment compared to that of control. The cell sheet that received 100 μg/mL collagen I treatment appeared to be the thickest, whereas the control cell sheet appeared to be the thinnest. (c) Cell sheet sections underwent immunofluorescence staining. FSP (green) signals were observed ubiquitously, and no α-SMA (red) signal was observed with or without collagen I treatment. Scale bar: 50 μm. α-SMA, alpha smooth muscle actin; FSP, fibroblast surface protein; HDFa, human dermal fibroblasts.
Cell sheet histology and collagen incorporation
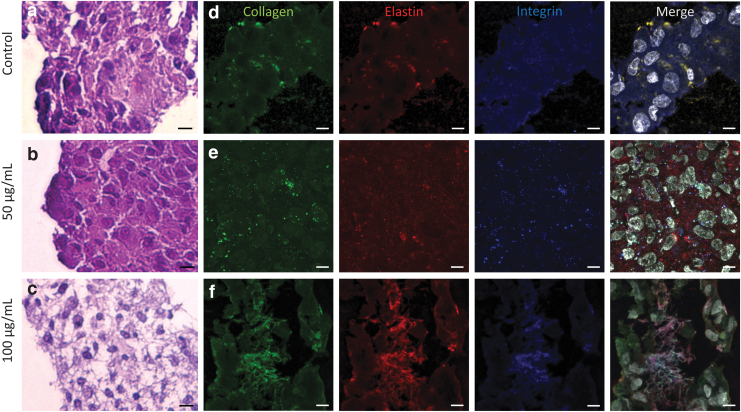
H&E staining showed that the control and the 50 μg/mL collagen I-treated cell sheet demonstrated tightly packed cells in an organized manner (Fig. 2a–c). The 100 μg/mL collagen I-treated cell sheet maintained an intact sheet structure and demonstrated filamentous material filling the gaps between the cells (Fig. 2c). Cell sheet sections were stained for collagen I, elastin, and integrin (Fig. 2d–f). The 100 μg/mL collagen I-treated cell sheets exhibited the greatest intensity of collagen I signal compared to the other groups. Co-localization of collagen I, elastin, and integrin signals was most prominent in the 100 μg/mL collagen I-treated cell sheets (Fig. 2f).
FIG. 2.
Cell sheet sections demonstrated increased collagen incorporation within the cell sheet after collagen I treatment. (a–c) H&E stain of cell sheet sections demonstrated increased cell sheet integrity with collagen I treatment, with increased concentration. One hundred microgram per milliliter collagen treatment resulted in an increased amount of filamentous material between cells. Scale bar: 200 μm. (d–f) Immunohistochemistry stain of cell sheet sections with 100 μg/mL collagen I treatment demonstrated a greater intensity of collagen I signal compared to that of controls and cell sheets that received 50 μg/mL collagen I treatment. Co-localization of collagen I (green), elastin (red), and integrin (blue) signals was most prominent in the 100 μg/mL collagen I-treated cell sheets. Scale bar: 40 μm. White: DAPI. H&E, hematoxylin and eosin.
Nanoindentation of cell sheets using AFM
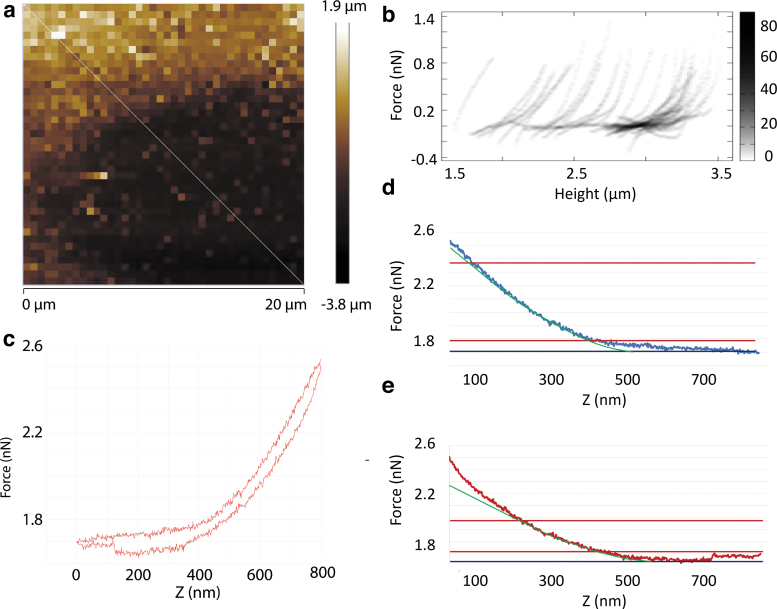
AFM nanoindentation was performed on confluent cell sheets for randomly chosen intercellular regions. The median selected region area was 412.1 μm2 with an interquartile range of 272.3 to 1004.9 μm2. Figure 3a represents a standard topography or height map of an intercellular region. A cross-section taken on the topography map generated a 2D histogram of force curves (Fig. 3b). A typical force curve is shown in Figure 3c. Hysteresis was observed in all samples. For reliable comparisons between samples, the linear portion of the indentation curves and the final portion of the retraction curves were each fitted with a linearized model after baseline correction (Fig. 3d, e) with an average R2 of 0.99 ± 0.01 and 0.91 ± 0.14 for the indentation and retraction curves, respectively.
FIG. 3.
Representative intercellular topography and force curves. (a) A standard topography or height map of an intercellular region. The taller area represents the intracellular region. The white line indicates a cross-section taken on the topography map. (b) A 2D histogram of multiple force curves from the cross-section taken from (a) demonstrated differences in the force curves taken from the intracellular versus extracellular region. The darkness of the pixel represents the number of force curves occurring at this pixel. (c) A typical force curve taken from the edge of the cytoplasm within the intracellular region. Note that hysteresis between the indentation and the retraction curve, and adhesive force in the retraction curve were observed. (d, e) After baseline correction, a linearized model (green) was fitted to the linear portion of the indentation curve (d) and the last portion of the retraction curve (e) just before the probe detached from the sample. The black horizontal lines indicate the baseline. The red horizontal lines indicate the interval within which the force curve data were taken for linearized model fitting.
AFM quantification of cell sheet mechanical properties
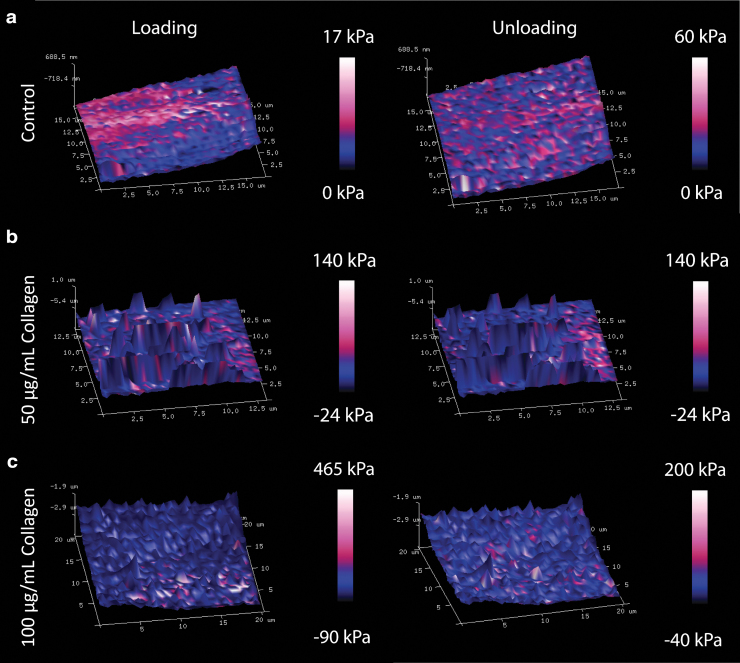
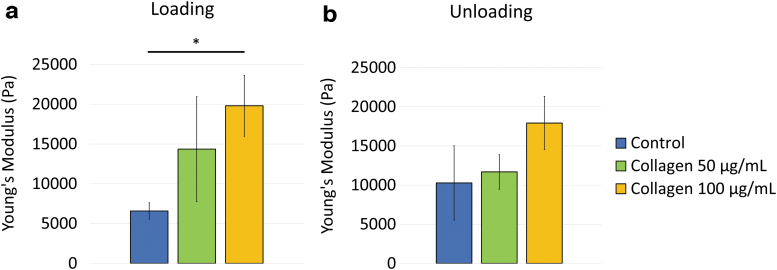
The representative 3D Young's modulus maps of the treated and control cell sheets are shown in Figure 4. Due to the hysteretic behavior observed, the loading maps were evaluated separately from the unloading maps for each sample. The Young's modulus results for each treatment group are summarized in Table 1. Compared to the control Young's modulus (6.6 ± 1.0 kPa), the loading Young's modulus increased with collagen I treatment for the 100 μg/mL samples (19.8 ± 3.8 kPa, p = 0.029, Fig. 5a). During unloading, samples that were treated with collagen I did not show any statistically significant stiffness change compared to that of the controls (Fig. 5b). The Young's modulus measured during loading compared to unloading was similar within each treatment group (Table 1).
FIG. 4.
3D Young's modulus maps illustrated different mechanical properties (a) without collagen I treatment, (b) with 50 μg/mL collagen I treatment, and (c) with 100 μg/mL collagen I treatment. Cell sheet samples demonstrated hysteretic behavior. Overall, Young's modulus increased with collagen I treatment and increased collagen concentration.
Table 1.
Young's Modulus of Each Treatment Group During the Loading and Unloading Conditions
| Loading Young's Modulus (kPa), mean ± standard error | p | Unloading Young's Modulus (kPa), mean ± standard error | p | p Value comparing loading and unloading for each group | |
|---|---|---|---|---|---|
| Control | 6.6 ± 1.0 | 0.28 | 10.3 ± 4.7 | 0.80 | 0.52 |
| 50 μg/mL collagen | 14.4 ± 6.6 | 0.029 | 11.7 ± 2.2 | 0.25 | 0.66 |
| 100 μg/mL collagen | 19.8 ± 3.8 | 0.46 | 18.1 ± 3.4 | 0.17 | 0.76 |
p value in bold denotes statistical significance (<0.05).
The data presented in Figure 5 are summarized in this table.
FIG. 5.
Young's modulus of the intercellular region increased with collagen I treatment. (a) Samples measured under the loading condition demonstrated increased Young's modulus with 100 μg/mL collagen I treatment compared to that of controls. (b) Samples demonstrated similar unloading Young's modulus. Data presented as mean ± standard error. Comparison was performed using two-sampled t-test. The data presented in this figure are summarized in Table 1. *Indicates p < 0.05.
Discussion
Traditionally, fibroblast cell sheets are harvested after culturing on standard tissue culture surface for 6 to 8 weeks.4 However, this long production time may hinder the clinical application of cell sheet-derived tissue engineering constructs in both the urgent and elective setting. The cell sheet technology using UpCell plates has made rapid, single-layer cell sheet harvesting feasible.2 Other thermoresponsive substrates, such as methylcellulose hydrogels, have also been created as temporary substrates for fibroblast cell sheet fabrication.18 Alternatively, carboxymethyl cellulose has been used to treat culture surfaces so that cell sheets can be harvested upon cellulase treatment.19 These results are encouraging as researchers continue to explore different ways of generating 2D sheet-like structures, which is a critical milestone for 3D tissue construct biofabrication.
One major drawback of the cell sheet technology is the cell sheet's fragility and inability to tolerate moderate manipulation, which is necessary for tissue reconstruction. To conquer this issue, artificial scaffolds are used to enhance tissue strength and aid in cell regeneration and geometrically specified growth.20,21 However, biodegradability, immunogenic response, and potential interference with native tissue's function present significant challenges and may affect long-term performance.2,5 Alternatively, ascorbic acid has been shown to affect collagen synthesis, which in turn provides tensile strength, regulates cell adhesion, supports chemotaxis and migration, and directs tissue development.9,10,22 Collagen I is the most prevalent type of collagen and is present during tissue repair and regeneration.23 Ascorbic acid has been shown to stimulate collagen production in mesenchymal stem cell sheets22; therefore, it should, in theory, enhance the mechanical properties of the cell sheets generated. Studies have also shown that collagen addition to HDFa cell culture can induce a higher synthesis of collagen I mRNA, possibly exerting a synergistic effect.23 It is also known that collagen I can induce cell differentiation into myofibroblasts characterized by increased expression of α-SMA.24,25 In our study, we did not observe any α-SMA signal from the HDFa cells that received collagen I treatment for 24 h. This is expected as the HDFa cell culture should not contain any mesenchymal cell, and 24 h of incubation time may not be sufficient to induce overexpression of α-SMA.
In this study, we selected AFM materials and modeling parameters that most accurately reflect the cell sheet mechanical properties. We elected to use colloidal probes because cells are extremely fragile and thus conical probes may easily penetrate the cells without causing adequate indentation. A colloidal probe can deform the spherical cell shape and allow for complete shape recovery upon release.26 In addition, to impute the mechanical properties of a specific intercellular region, our probe size, selected to be 15 μm, was sufficiently large with respect to the average cell size of 10–15 μm.27 Moreover, we used an appropriate probe spring constant, which was important to match with the sample stiffness.21,26 Using a cantilever that is too stiff will not generate enough deflection to be detected and can damage the cell, whereas using a cantilever that is too soft will not indent the cell sufficiently to obtain reliable force curves against thermal vibrations.21,26 From the force curves obtained for our samples, we confirmed the viscoelastic properties of the cell sheets.27 Although adhesion was observed from the force curve when the probe was retracted, it remained minimal across different treatment groups and was subsequently accounted for during the data analysis.
Nevertheless, our study is constrained by AFM limitations. To more accurately assess the mechanical properties of a single-layered cell sheet, the cell sheet would have to be probed while suspended. However, this is extremely challenging, given the fragile nature of the untreated cell sheets. Such a procedure would have to include edge fixation, which would unavoidably create trapped air pockets. Instead, confluent cells plated on untreated glass bottom Petri dishes were used as our samples. In addition, we noted that HDFa cells can be easily lifted in intact sheets from these dishes if exposed to room temperature for an hour, which reassured us that the samples serve as a relatively close approximation to recovered cell sheets. An alternative is to fix the cell sheets before AFM examination. However, this fixation can significantly and artificially increase sample stiffness,27 which would affect our results and potentially interfere with stiffness changes induced by collagen I treatment. For future investigation, this methodology of rapid augmentation of cell sheet mechanical properties can be confirmed on other cell types, such as smooth muscle cells and endothelial cells. Our laboratory has experimented with cell sheets composed of both smooth muscle cells and endothelial cells using Upcell plates.6 Since endothelial cells' natural tendency is to form a single-layer structure, our methodology of rapid cell sheet mechanical property augmentation can have an impact on the translational and future clinical application of the cell sheet technology.28 Other abundant ECM proteins can also be evaluated to assess for their impacts on cell sheet mechanics. Finally, a contact mode could be attempted with a conical probe to assess for cell sheet shear force thresholds before failure.
Conclusion
In conclusion, our study demonstrated that a 24-h collagen I treatment resulted in increased collagen I incorporation within the HDFa cell sheet without affecting cell morphology, cell type, or cell sheet quality. Increased co-localization of integrin and elastin with collagen I signals was also observed with high concentration of collagen I treatment. After the treatment with 100 μg/mL of collagen I, the resulting Young's modulus of the treated cell sheet was significantly higher compared to that of the control cell sheets. This methodology of rapid mechanical property augmentation can impact the modern cell sheet technology by improving the ease of construct manipulation, which may further facilitate cell sheet tissue engineering for translational applications.
Acknowledgment
We would also like to thank Dr. Marcin Walkiewicz for his tremendous technical support for this research effort.
Disclaimer
The article contents are solely the responsibility of the authors and do not necessarily represent the official views of the funders.
Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by the National Institutes of Health (NIH R01 HL089315-01, R01 HL 152155, Y.J.W.), the Thoracic Surgery Foundation Resident Research Fellowship (Y.Z.), the National Science Foundation Graduate Research Fellowship Program (A.M.I.), and, in part, the National Center for Research Resources (1S10OD021514-01).
References
- 1. Miyagawa, S., Sawa, Y., Sakakida, S., et al. . Tissue cardiomyoplasty using bioengineered contractile cardiomyocyte sheets to repair damaged myocardium: their integration with recipient myocardium. Transplantation 80, 1586, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Shudo, Y., Cohen, J.E., MacArthur, J.W., et al. . A tissue-engineered chondrocyte cell sheet induces extracellular matrix modification to enhance ventricular biomechanics and attenuate myocardial stiffness in ischemic cardiomyopathy. Tissue Eng Part A 21, 2515, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Miyagawa, S., Roth, M., Saito, A., Sawa, Y., and Kostin, S.. Tissue-engineered cardiac constructs for cardiac repair. Ann Thorac Surg 91, 320, 2011 [DOI] [PubMed] [Google Scholar]
- 4. L'Heureux, N., Dusserre, N., Konig, G., et al. . Human tissue engineered blood vessel for adult arterial revascularization. Nat Med 12, 361, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. von Bornstädt, D., Wang, H., Paulsen, M.J., et al. . Rapid self-assembly of bioengineered cardiovascular bypass grafts from scaffold-stabilized, tubular bilevel cell sheets. Circulation 138, 2130, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shudo, Y., Cohen, J.E., Macarthur, J.W., et al. . Spatially oriented, temporally sequential smooth muscle cell-endothelial progenitor cell Bi-level cell sheet neovascularizes ischemic myocardium. Circulation 128, S59, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shudo, Y., Miyagawa, S., Ohkura, H., et al. . Addition of mesenchymal stem cells enhances the therapeutic effects of skeletal myoblast cell-sheet transplantation in a rat ischemic cardiomyopathy model. Tissue Eng Part A 20, 728, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Celikkin, N., Rinoldi, C., Costantini, M., et al. . Naturally derived proteins and glycosaminoglycan scaffolds for tissue engineering applications. Mater Sci Eng C Mater Biol Appl 78, 1277, 2017 [DOI] [PubMed] [Google Scholar]
- 9. Kanta, J. Collagen matrix as a tool in studying fibroblastic cell behavior. Cell Adhes Migr 9, 308, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Artym, V.V., and Matsumoto, K.. Imaging cells in three-dimensional collagen matrix. Curr Protoc Cell Biol 10, 1, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Plant, A.L., Bhadriraju, K., Spurlin, T.A., and Elliott, J.T.. Cell response to matrix mechanics: focus on collagen. Biochim Biophys Acta Mol Cell Res 1793, 893, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Pirentis, A.P., Polydorou, C., Papageorgis, P., Voutouri, C., Mpekris, F., and Stylianopoulos, T.. Remodeling of extracellular matrix due to solid stress accumulation during tumor growth. Connect Tissue Res 56, 345, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Horimizu, M., Kawasea, T., Tanakac, T., et al. . Biomechanical evaluation by AFM of cultured human cell-multilayered periosteal sheets. Micron 48, 1, 2013 [DOI] [PubMed] [Google Scholar]
- 14. Allison, D.P., Mortensen, N.P., Sullivan, C.J., and Doktycz, M.J.. Atomic force microscopy of biological samples. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2, 618, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Basoli, F., Giannitelli, S.M., Gori, M., et al. . Biomechanical characterization at the cell scale: present and prospects. Front Physiol 9, 1449, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krieg, M., Fläschner, G., Alsteens, D., et al. . Atomic force microscopy-based mechanobiology. Nat Rev Phys 1, 41, 2019 [Google Scholar]
- 17. Briscoe, B.J., Sebastian, K.S., and Adams, M.J.. The effect of indenter geometry on the elastic response to indentation. J Phys D Appl Phys 27, 1156, 1994 [Google Scholar]
- 18. Altomare, L., Cochis, A., Carletta, A., Rimondini, L., and Farè, S.. Thermo-responsive methylcellulose hydrogels as temporary substrate for cell sheet biofabrication. J Mater Sci Mater Med 27, 95, 2016 [DOI] [PubMed] [Google Scholar]
- 19. Ko, I.K., Kato, K., and Iwata, H.. A thin carboxymethyl cellulose culture substrate for the cellulase-induced harvesting of an endothelial cell sheet. J Biomater Sci Polym Ed 16, 1277, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Aldana, A.A., and Abraham, G.A.. Current advances in electrospun gelatin-based scaffolds for tissue engineering applications Current advances in electrospun gelatin-based scaffolds for tissue engineering applications. Int J Pharm 523, 441, 2017 [DOI] [PubMed] [Google Scholar]
- 21. Shimizu, T., Yamato, M., Kikuchi, A., and Okano, T.. Two-dimensional manipulation of cardiac myocyte sheets utilizing temperature-responsive culture dishes augments the pulsatile amplitude. Tissue Eng 7, 141, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Sato, Y., Mera, H., Takahashi, D., et al. . Synergistic effect of ascorbic acid and collagen addition on the increase in type 2 collagen accumulation in cartilage-like MSC sheet. Cytotechnology 69, 405, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sanchez, A., Blanco, M., Correa, B., Perez-Martin, R.I., and Sotelo, C.G.. Effect of fish collagen hydrolysates on type I collagen mRNA levels of human dermal fibroblast culture. Mar Drugs 16, 144, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu, X., Long, X., Liu, W., et al. . Type I collagen induces mesenchymal cell differentiation into myofibroblasts through YAP-induced TGF-β1 activation. Biochimie 150, 110, 2018 [DOI] [PubMed] [Google Scholar]
- 25. Fisher, G.J., Shao, Y., He, T., et al. . Reduction of fibroblast size/mechanical force down-regulates TGF-β type II receptor: implications for human skin aging. Aging Cell 15, 67, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thomas, G., Burnham, N.A., Camesano, T.A., and Wen, Q.. Measuring the mechanical properties of living cells using atomic force microscopy. J Vis Exp 76, 50497, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hecht, F.M., Rheinlaender, J., Schierbaum, N., Goldmann, W.H., Fabry, B., and Schäffer, T.E.. Imaging viscoelastic properties of live cells by AFM: power-law rheology on the nanoscale. Soft Matter 11, 4584, 2015 [DOI] [PubMed] [Google Scholar]
- 28. Koizumi, N., Sakamoto, Y., Okumura, N., et al. . Cultivated corneal endothelial cell sheet transplantation in a primate model. Invest Ophthalmol Vis Sci 48, 4519, 2007 [DOI] [PubMed] [Google Scholar]