Abstract
Sustained attention and working memory were improved in young adults after they engaged in a recently developed, closed-loop, digital meditation practice. Whether this type of meditation also has a sustained effect on dominant resting-state networks is currently unknown. In this study, we examined the resting brain states before and after a period of breath-focused, digital meditation training versus placebo using an electroencephalography (EEG) microstate approach. We found topographical changes in postmeditation rest, compared with baseline rest, selectively for participants who were actively involved in the meditation training and not in participants who engaged with an active, expectancy-match, placebo control paradigm. Our results suggest a reorganization of brain network connectivity after 6 weeks of intensive meditation training in brain areas, mainly including the right insula, the superior temporal gyrus, the superior parietal lobule, and the superior frontal gyrus bilaterally. These findings provide an opening for the development of a novel noninvasive treatment of neuropathological states by low-cost, breath-focused, digital meditation practice, which can be monitored by the EEG microstate approach.
Impact statement
Breath-focused, digital meditation training leads to sustained changes of resting-state, brain network functional connectivity, as assessed with electroencephalography microstates.
Keywords: attention, bodily self-consciousness, EEG microstates, EEG source localization, meditation training
Introduction
Maintaining focused and sustained attention to a cognitive task, such as reading a book or listening to a talk, becomes increasingly difficult due to the constant external and internal distractions that prevent us from focusing on one thing at a time (Gazzaley and Rosen, 2016). Often, we are doing several things simultaneously, such as reading emails on the cell phone while watching TV or running to catch a bus. Particularly, the younger generation is increasingly involved in multitasking activities (Rideout et al., 2010), which leads to the question of whether this habit has positive or negative effects on focused attention (Moisala et al., 2016).
While some studies reported positive consequences of split attention (Yap and Wee Hun Lim, 2013) and task-switching skills (Alzahabi and Becker, 2013), others demonstrated increased distractibility (Loh and Kanai, 2016), increased impulsivity (Minear et al., 2013), and diminished attention (Moisala et al., 2016; Ophir et al., 2009), which has also been associated with a decrease in gray matter volume in the anterior cingulate cortex (Loh and Kanai, 2016). A healthy adult can remain focused on a task for some amount of time; however, someone suffering from a mental health issue may struggle with sustaining attention to a specific goal due to continuous distraction of the wandering mind and external environment (Smallwood and Andrews-Hanna, 2013).
Ultimately, the inability to sustain attention can be associated with pathological conditions such as attention-deficit/hyperactivity disorder, anxiety disorder, or depression. These neuropathological states might be possible to self-regulate by practicing internally focused meditation and engaging self-awareness on one's breath and body sensations while letting go of externally oriented thoughts and other distractions. The positive therapeutic effects of meditation have been previously observed in improvement of self-awareness (Farb et al., 2007; Vago and Silbersweig, 2012), emotion regulation (Holzel et al., 2008), and metacognition (Kerr et al., 2013). Recently, Ziegler and colleagues (2019) showed that using digital, closed-loop meditation training improved focused attention and working memory in healthy young adults (18–35 years old) and this improvement increased activity in posterior temporoparietal areas and the medial prefrontal cortex (MPFC). Mishra and colleagues (2020) confirmed previous results of the study by Ziegler and associates (2019) and further demonstrated that the closed-loop, digital meditation practice in adolescence with childhood neglect (10–18 years old) was associated with significantly enhanced resting-state functional connectivity between the dorsal anterior cingulate cortex and cingulo-opercular network (CON), including the insula, thalamus, and anterior prefrontal cortex, and that this modulation led to enhanced attention, reduced hyperactivity, and improvements in school performance 1 year after the treatment.
Overall, the studies by Ziegler and colleagues (2019) and Mishra and colleagues (2020) demonstrated that internally focused meditation seems to have an important effect on externally oriented behavior, cognition, and underlying brain networks related to the self. Cognitive neuroscience has been examining consciousness associated with the subject (i.e., the self of the conscious experience) and its related multisensory processing of bodily signals (Blanke, 2012; Blanke et al., 2015). Notably, recent research highlights two forms of the self (i.e., the bodily self and the autobiographical self), which are based on an integrated neural system that combines and interacts with both the internal and external multisensory processing (Bréchet et al., 2018; Grivaz et al., 2017; Park and Blanke, 2019). Not only does the bodily self fundamentally influence the autobiographical self (Bréchet et al., 2019, 2020b) but it also seems possible to manipulate and capture the self-related nature of brief conscious thoughts, whether they are internally or externally oriented (Bréchet et al., 2019). It is known that during rest, the brain dynamically organizes its activity in large-scale neuronal networks (Bassett and Sporns, 2017).
In functional magnetic resonance imaging (fMRI), these resting-state networks are characterized by the correlated blood oxygen level dependent activity of distributed brain areas (Biswal et al., 1995). In electroencephalography (EEG), such networks can be captured by microstates, representing short-lasting states of nonlagged synchronized activity of large-scale brain networks (Koenig and Valdes-Sosa, 2018; Michel and Koenig, 2018). Both, fMRI and EEG have reliably identified a few distinct network patterns and have related them to different cognitive functions, such as internal mentation, external attention, memory, and sensory processing (Britz et al., 2010; Panda et al., 2016).
Based on results of studies by Ziegler and associates (2019) and Mishra and associates (2020) and previous studies on EEG microstate changes in meditators (Faber et al., 2017; Panda et al., 2016), in this study, we first hypothesized that EEG resting-state networks that are related to the self can be modulated by intense meditation training. Second, we hypothesized that this effect of focused attention meditation on EEG resting-state networks would include brain areas such as the medial prefrontal, insular, and posterior parietal cortex, similar to previous studies (Bréchet et al., 2019; Panda et al., 2016). To examine the effects of internally self-focused meditation, we analyzed the resting-state 64-channel EEG of the participants reported by Ziegler and associates (2019). We used the EEG microstate approach to capture resting-state network dynamics before and after the meditation training and compared them with changes before and after placebo sessions.
EEG microstates, first described by Lehmann and colleagues (1987), are characterized by scalp potential maps that remain stable for short subsecond periods. Only a few prototypical map configurations dominate resting-state activity (Koenig et al., 2002). They can be obtained through k-means cluster analysis or other pattern recognition approaches (Pascual-Marqui et al., 1995). The optimal number of microstates may depend on the given dataset; however, four dominant maps (labeled A, B, C, and D, presently) are usually observed in resting-state EEG data (Michel and Koenig, 2018).
Numerous studies have shown that these most dominant EEG microstate maps are highly reproducible within and between participants (da Cruz et al., 2020; Khanna et al., 2014; Koenig et al., 2002; Liu et al., 2020; Tomescu et al., 2018; Zanesco et al., 2020) and are essentially independent of the functional state of the brain (Bréchet et al., 2020a; Brodbeck et al., 2012; Stefan et al., 2018). However, the temporal dynamics of these microstates and their occurrence, duration, appearance, and sequence are highly sensitive to the trait and the momentary state of the brain. For example, altered states of consciousness (e.g., sleep, disorders of consciousness, anesthesia, hypnosis, and meditation) influence the temporal dynamics of microstates (Bréchet et al., 2020a; Brodbeck et al., 2012; Faber et al., 2017; Katayama et al., 2007; Panda et al., 2016; Shi et al., 2020; Stefan et al., 2018). Notably, it has also been demonstrated that instructed mentation influences the temporal dynamics of specific EEG microstates (Bréchet et al., 2019; Croce et al., 2018b; Milz et al., 2016; Seitzman et al., 2017).
Methods
Participants
The study included 23 participants (18–35 years old) who performed the meditation condition and 20 participants (19–32 years old) who were assigned to the placebo condition. Details of the participant recruitment and inclusion criteria are given by Ziegler and colleagues (2019). All participants gave informed consent to participate in the study according to procedures approved by the Committee for Human Research at the University of California San Francisco (IRB 16-18680).
Training programs
The MediTrain program requested the participants to attend to the sensations of their breath in a quiet location with headphones on and eyes closed. Initially, they received detailed instructions about how to engage in the treatment and use the iPad, and participants listened to a short lesson about mindful breathing practices. While focusing on their breath, they were asked to monitor the quality of their attention and to acknowledge any distracting thoughts. The length of the initial treatment trial was set at 20 sec, after which participants were asked to report whether their attention remained on their breathing throughout the trial or whether they experienced moments of mind wandering. Depending on this report, the duration of the next trial was either increased or decreased, thereby individually adapting the training to each participant's ability to self-regulate internal attention. The participants gradually increased the amount of time each day that they successfully sustained their attention to their breath without distractions, averaging 20 sec on the first day and progressing to an average of 6 min after 25 days of training. More details about the MediTrain program can be found in the study by Ziegler and associates (2019). The placebo condition consisted of a set of three commercially available apps (i.e., foreign language learning, logic games, and Thai Chi apps) that were selected a priori based on matching the expectation of improvement with that of the MediTrain program [see Experiment 1 in the study by Ziegler et al. (2019)]. All participants were instructed to spend ∼10 min with each placebo app each training day (5 days/week for 6 weeks).
EEG recordings and analysis
EEG was recorded with an active two-head cap (Cortech Solutions) with a BioSemiActiveTwo 64-channel EEG acquisition system. Signals were amplified and digitized at 1024 Hz with a 16-bit resolution. Antialiasing filters were used and data were band-pass filtered between 0.01 and 100 Hz during data acquisition.
EEG preprocessing
All EEG analyses were performed using the freely available academic software, Cartool (Brunet et al., 2011). Continuous segments of 3-min eyes-closed EEG were analyzed. The data were first downsampled to 256 Hz using a cascaded integrator–comb filter and band-pass filtered between 1 and 40 Hz (second-order Butterworth, 12 db/octave roll-off). Data were then carefully visually inspected and bad electrodes and artifactual segments (eye blinks and muscle artifacts, etc.) were marked. Bad electrodes were interpolated using a spherical spline interpolation method.
k-Means cluster analysis
Using an automatic detection algorithm, all global field power (GFP) peaks of artifact-free periods were marked. Data at these time points were concatenated and subjected to an adapted k-means cluster analysis (Pascual-Marqui et al., 1995) for each participant and each recording. The polarity of the maps was ignored. To determine the optimal number of clusters for each participant, six criteria were used to independently evaluate the quality of each clustering. They were then merged to derive a single synthetic metacriterion (Bréchet et al., 2019). The optimal number of clusters ranged between 4 and 10. These cluster maps of each participant were then exposed to a second k-means cluster analysis across participants. For this group clustering, we deliberately fixed the number of clusters to the four most dominant maps in terms of global explained variance (GEV) to compare the microstates between the different conditions in our cohort and with the microstate maps of previous studies. The GEV is the sum of the explained variances weighted by the GFP at each moment in time.
Spatial correlation analysis
To compare the microstate maps between conditions, the following analysis was performed: the four microstate maps of each condition were back-fitted to the original EEG of each participant by calculating the spatial correlation between the microstate maps and the GFP-normalized map at each data point (ignoring polarity). Each time point was then labeled with the microstate map with the highest correlation. All time points with the same maps were then averaged by ignoring polarity, resulting in four mean maps per participant representing the four microstates. These mean maps of one condition were then correlated with the mean maps of all participants of another condition and the correlation coefficients were averaged after Fisher z-transformation.
Source localization
The approach described by Bréchet and associates (2019) was used for determining the sources underlying each microstate. A distributed linear inverse solution (LAURA) (Grave de Peralta Menendez et al., 2004) was calculated using the average brain of the Montreal Neurological Institute as the head model and restricting the solution space to the gray matter using the LSMAC approach with 5016 solution points (Birot et al., 2014; Brunet et al., 2011). A standardization across time was applied for each solution point to eliminate activation biases (Michel and Brunet, 2019). The estimated current densities of each participant were then averaged across all time points that were attributed to a given microstate in each condition. A randomization test was used to statistically evaluate whether the sources of the microstates differed between conditions. Similar to the conjunction analysis suggested for fMRI data (Friston et al., 1999), we first thresholded each source map to the solution points that were above the 90th percentile of activation in the mean map across all participants (Bréchet et al., 2020a).
Results
Based on previous studies that examined EEG microstates (Khanna et al., 2014; Koenig et al., 2002; Liu et al., 2020; Tomescu et al., 2018; Zanesco et al., 2020), we hypothesized that the meditation training would not alter the dominant microstates in terms of their spatial distribution over the scalp, but would rather influence their temporal dynamics. We thus first evaluated whether similar microstates were present in all four conditions (i.e., before and after the placebo as well as before and after the meditation training). To do so, we applied the k-means spatial clustering separately for the four conditions and compared the resulting microstate maps.
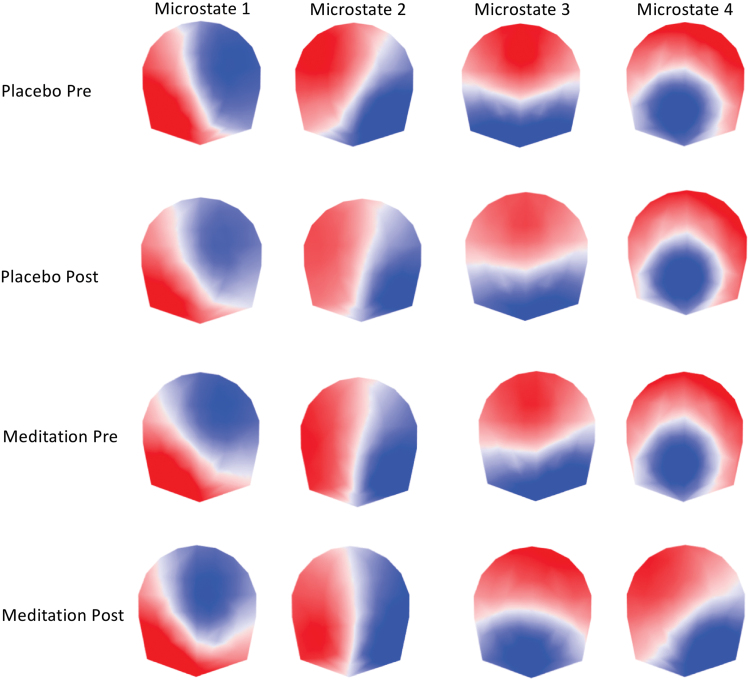
Figure 1 illustrates the four microstate maps for each condition and shows that three of the four conditions (preplacebo, postplacebo, and premeditation) were characterized by highly similar maps. Comparing them with the maps described in the literature reveals that the first three maps replicate the canonical microstates usually labeled as A, B, and C. The fourth microstate map is less consistent in the literature. The maps observed in the preplacebo, postplacebo, and premeditation conditions not only resemble microstate D in studies by Brodbeck and associates (2012), Gschwind and associates (2016), and Seitzman and associates (2017) but were also similar to maps previously described in studies that used more than four cluster maps (Bréchet et al., 2019; Custo et al., 2017; D'Croz-Baron et al., 2019; Zanesco et al., 2020).
FIG. 1.
The four dominant microstate topographies resulting from the k-means clustering of each condition (pre- and postplacebo, pre- and postmeditation). The microstates are ordered according to their topographic similarity. Note similar topographies in the pre- and postplacebo and the premeditation conditions and the difference of maps (particularly maps 3 and 4) in the postmeditation condition. Color images are available online.
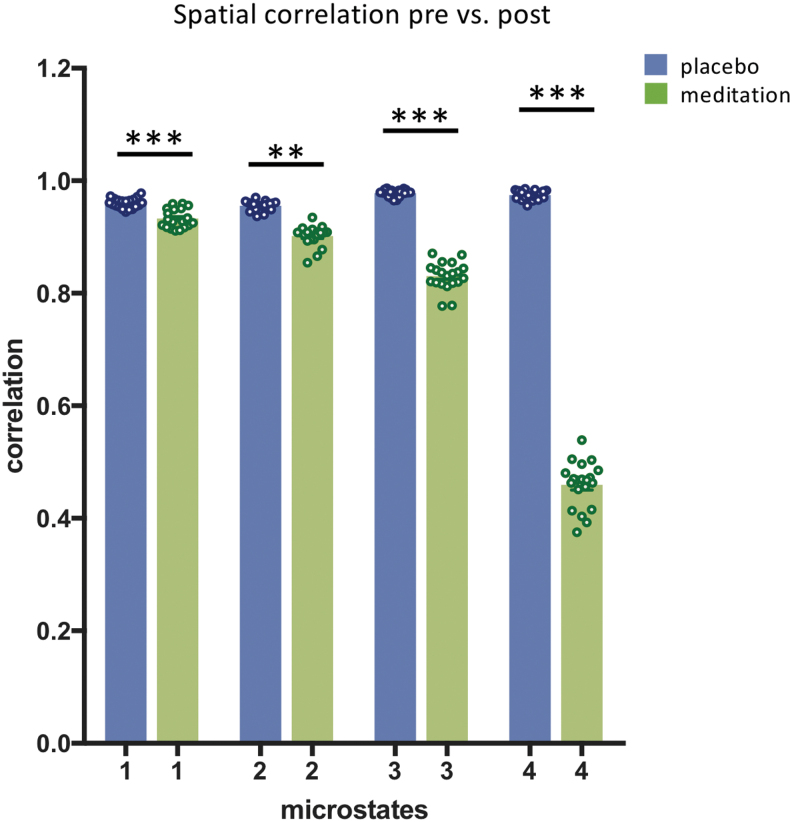
The crucial observation from the cluster analysis was that maps 3 and 4 showed a distinct topography in postmeditation compared with all other conditions. To verify this observation, we calculated the spatial correlation of the strength-normalized maps of each microstate between pre- and postplacebo and between pre- and postmeditation. Remarkably, we found that the differences in correlations between premeditation and postmeditation were significantly lower than differences between the preplacebo and postplacebo conditions [microstate 1: t(38) = 6.59, <0.0001, 95% CI = −0.03 to −0.01, η2 = 0.53; microstate 2: t(38) = 12.09, <0.0001, 95% CI = −0.06 to −0.04, η2 = 0.79; microstate 3: t(38) = 25.89, <0.0001, 95% CI = −0.15 to −0.13, η2 = 0.94; and microstate 4: t(38) = 54.92, <0.0001, 95% CI = −0.53 to −0.49, η2 = 0.98] (Fig. 2).
FIG. 2.
Mean spatial correlation of the four microstate maps between pre- and postplacebo and pre- and postmeditation. The means and standard errors were obtained by correlating the mean maps of each participant in one condition with the mean maps of all participants in the other condition and averaging the correlation values after z-transformation. **p < 0.001; ***p < 0.0001. Color images are available online.
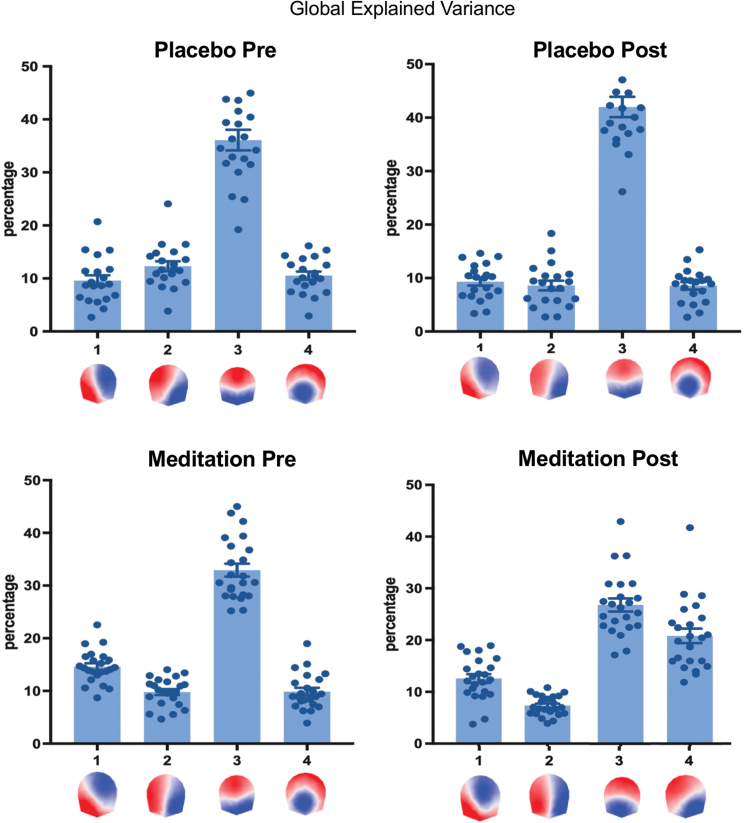
Given this result, we rejected our a priori hypothesis that the topography of the four canonical microstate maps is similar across conditions, as commonly observed in the literature (Michel and Koenig, 2018). While the EEG maps were highly similar before and after placebo, they were different after the meditation training. This result prevented us from comparing the temporal dynamics of the microstates between conditions since this would require that the microstates have the same topography (Grieder et al., 2016). However, we evaluated how much of the data these four microstates explained in each condition. For this, we calculated the GEV for each microstate and each participant and condition (Fig. 3). The result shows that in the three nonmeditation conditions (i.e., preplacebo, postplacebo, and premeditation), the GEV was very similar and the four microstates together explained around 70% of the data in all conditions (preplacebo: 68.5%, postplacebo: 68.5%, and premeditation: 67.2%). In addition, microstate 3 dominated in all three conditions, explaining alone more than 30% of the data (preplacebo: 36.1%, postplacebo: 42.0%, and premeditation: 32.9%). This result confirms many previous studies showing the dominance of microstate C in the eyes-closed EEG (Michel and Koenig, 2018). However, this dominance was not observed in the postmeditation condition. Interestingly, in the postmeditation group, variance of data is equally well explained by both microstates 3 and 4 (microstate 3: 26.8%, microstate 4: 20.8%, and GEV of all microstates: 67.6%).
FIG. 3.
GEV of each of the four EEG microstate maps in the four experimental conditions (pre- and postplacebo and pre- and postmeditation). The four microstates together explained around 70% of the data in each of the four conditions (preplacebo: 68.5%, postplacebo: 68.5%, premeditation: 67.2%, and postmeditation: 67.6%). EEG, electroencephalography; GEV, global explained variance. Color images are available online.
We then wanted to determine the sources generating these EEG microstate maps. For this, we converted the data to the source space (Michel et al., 2004) by calculating a distributed linear inverse solution [LAURA; Grave de Peralta Menendez et al. (2004)] using a gray matter restricted solution space of 5016 solution points in the Montreal Neurological Institute brain template (Michel and Brunet, 2019). Each data point of the original EEG of each participant was localized, normalized over time, and then averaged over all data points that were labeled with a given microstate map (Bréchet et al., 2019). Supplementary Figures S1 to S4 illustrate the result of this analysis, displaying only voxels that were above the 0.9 percentile of the mean across all solution points. The figures show that the source maps of each microstate were very similar across the three nonmeditation conditions, supporting the finding of similar topographies on the scalp level.
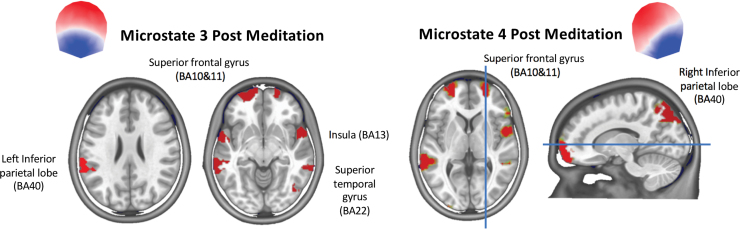
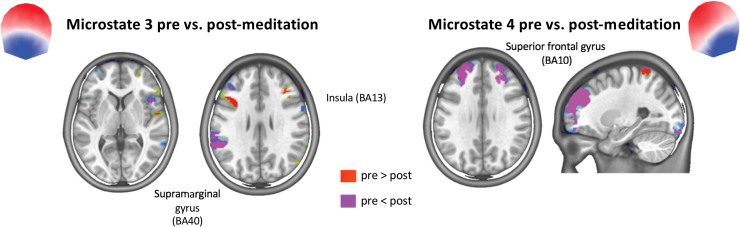
In Figure 4, we depict the main brain sources of microstates 3 and 4 that showed a distinct topography in the postmeditation condition compared with all other conditions. Microstate 3 in the postmeditation condition was dominated by activity in the superior frontal gyrus (Brodmann areas, BA10 and BA11), the superior temporal gyrus (BA22), the insula (BA13), and the left inferior parietal lobule (BA40). The sources of microstate 4 in the postmeditation condition were mainly found in the superior frontal gyrus (BA10 and BA11), the superior temporal gyrus (BA22), the insula (BA13), and the right superior parietal lobule, particularly in the precuneus (BA7).
FIG. 4.
Source localization of microstates 3 and 4 derived by averaging all normalized source maps that were labeled with a given microstate across participants in the postmeditation conditions. Only sources above the 0.9 percentile of the mean across all solution points are shown. For detailed source maps of each condition, see Supplementary Figures S1, S2, S3, S4. Color images are available online.
To statistically test for differences in the source configuration of the microstates between conditions, we performed paired randomization tests for all voxels that were above the 0.9 percentile of the mean maps across all participants. Figure 5 shows the comparison of the sources of the microstates 3 and 4 between pre- and postmeditation. For microstate 3, postmeditation showed increased activity bilaterally in the insula and the left supramarginal gyrus (BA40). For microstate 4, increased activity was found in the superior frontal gyrus (BA10) bilaterally. As expected, given the similarity of the scalp maps, these tests did reveal only very few significant voxels for any of the microstates between the conditions preplacebo and postplacebo. In addition, the spatial correlation (in the inverse space) was very high (above 0.98 in all comparisons). See Supplementary Figures S5 to S8 for a detailed analysis of all conditions.
FIG. 5.
Result of paired randomization tests (p < 0.01 Bonferroni corrected) between the sources of the microstates in the pre- and postmeditation conditions (red areas indicate significantly greater activation in the precondition and blue areas indicate significantly greater activation in the postcondition). Color images are available online.
Discussion
EEG microstates may be sensitive to changes of spontaneous mentation
In this study, we evaluated whether EEG microstates can be modulated by intense meditation training. We indeed show that the digital meditation training causally led to topographical changes, specifically in two microstates, and these new postmeditation EEG microstates together explained nearly 50% of the variance of the data. Interestingly, these two microstate topographies were associated with activity in the insula, the supramarginal gyrus, and the superior frontal gyrus, which are all known to be involved in self-related, multisensory conscious experiences. Several previous studies related EEG microstates to the ongoing mental activity and showed that the temporal dynamics of EEG microstates may be sensitive to instruction and changes in the content of spontaneous mentation. For example, Seitzman and colleagues (2017) altered the temporal features of the four canonical microstates by instructing their participants to either mentally subtract numbers or to spontaneously mind-wander. They found a significant decrease in the occurrence and duration of microstate C and an increase in microstate D during a mental calculation, supporting the hypothesis that this state is related to the externally oriented attentional system. The study by Milz and colleagues (2016) showed an increase in microstate A while visualizing and microstate B while verbalizing. A recent meta-analysis on EEG microstates (Rieger, 2016) showed that the default mode network (DMN)-related microstate C increased in occurrence in patients with schizophrenia, who suffer from an exaggerated sense of self. Contrary to schizophrenia, a reduced occurrence of EEG microstate C has been observed in dementia patients (Nishida et al., 2013). Similarly, findings from fMRI studies show that DMN connectivity increases in schizophrenic patients (Whitfield-Gabrieli and Ford, 2012) and decreases in vegetative and coma patients (Vanhaudenhuyse et al., 2010). Unlike in previous studies, our result prevented us from comparing the temporal dynamics of microstates between conditions, which would be only possible if the microstates would have had the same topography (Grieder et al., 2016).
Postmeditation EEG microstates show different topographies
Based on the previous literature, we here hypothesized that the training will not change the topography of the prototypical microstate maps, but that the temporal dynamics of some of the microstates, as particularly shown for participants exercising transcendental meditation (Faber et al., 2017), and the temporal dynamics of the DMN may characterize meditation-induced changes in consciousness, as shown by Panda and associates (2016). Surprisingly, our analysis did not confirm this hypothesis. Compared with a placebo condition and compared with the premeditation resting EEG data, the microstates in the postmeditation EEG data showed different topographies. In addition, the usually observed dominance of microstate C was not present after meditation, and notably, two microstate maps dominated the EEG resting data. Changes in microstate topographies between sessions or between groups have rarely been observed. On the contrary, recent studies with repeated measures of the same participants showed high interindividual stability and reliability (Khanna et al., 2014; Liu et al., 2020). This observation was also made in the present study between the pre- and postplacebo sessions, but not between the premeditation and postmeditation sessions.
Modulation-specific topographical changes in the resting-state EEG
Only a handful of EEG studies on microstates showed topographical changes after a specific modulation. Recently, a study by Croce and associates (2018a) tested the effect of repeated transcranial magnetic stimulation (rTMS) on EEG microstates and showed that stimulation over the intraparietal sulcus (IPS), that is, the key node of the dorsal attention network (DAN), and the angular gyrus, that is, the key node of the DMN, changed the topography of EEG microstate C, while stimulation over the temporal parietal junction (TPJ) as well as sham stimulation showed no effect. The authors interpreted this result of local inhibition through rTMS as a global reorganization of brain activity of different brain areas. Furthermore, the authors speculate that since the topography of the microstate C, which has been previously associated with the activity in the CON, has been modified after inactivation of the essential nodes of DAN and DMN, the results provide indirect evidence that DMN–DAN interactions are mediated by CON, the higher-order control system (Sestieri et al., 2014).
Interestingly, a permanent brain lesion after stroke also led to changes in EEG microstates (Zappasodi et al., 2017). In this study, patients with a left hemisphere lesion inducing a language impairment showed modulation of microstate C topography, while patients with a right hemisphere lesion inducing neglect symptoms showed a change in the topography of microstate D. Another study by Grieder and associates (2016) showed topographic alteration of microstate B, and particularly microstate C, in patients with semantic dementia, which might be due to network reorganization caused by brain atrophy and cognitive deficits. A global reorganization of brain activity might also explain the current findings: after 6 weeks of meditation training, the brain areas that generated the new EEG microstates 3 and 4 were mainly localized in the right insula, the superior temporal gyrus, the superior parietal lobule, and the superior frontal gyrus bilaterally. When statistically comparing the sources of these microstates in postmeditation conditions with the premeditation condition, increased activity in the superior frontal gyrus bilaterally, the right insula, and the left supramarginal gyrus was found after meditation.
Brain areas related to bodily self and autobiographical self and their relationship with self-focused meditation
In line with the study by Ziegler and colleagues (2019), we found activity in the superior and middle frontal gyri, which are commonly associated with the DMN and DAN (Posner and Petersen, 1990). Similar to our results, the study by Ziegler and colleagues showed increased ERP activity in a widely distributed temporoparietal network, including the precuneus, which is also considered a hub within the DMN. Interestingly, a previous fMRI study by Ionta and associates (2011) demonstrated that experimental manipulation using multisensory stimuli induced changes in clearly defined aspects of bodily self, such as self-identification or self-location, and showed increased activity at the left and right temporal parietal junction (TPJ) and included the posterior part of the superior temporal gyrus, the parietal operculum, the posterior insula, and superior portion of the supramarginal gyrus. Furthermore, recent evidence (Bréchet et al., 2018) showed that both aspects of autobiographical self and bodily self recruit the lateral parietal cortex and thus demonstrated a fundamental link between both forms of the self and multimodal, subjective conscious experiences.
A large number of studies revealed that the insular cortex (INS) is recruited by mental processing such as interoceptive awareness (Critchley et al., 2004), emotional feeling (Johnstone et al., 2006), self-recognition (Devue et al., 2007), self-reflection (Modinos et al., 2009), and personal relevance (Enzi et al., 2009). Interestingly, Mishra and colleagues (2020) showed strong functional connectivity between anterior cingulate cortex (ACC) and the insula/frontal opercular region during adolescent development. Based on earlier work, it has been suggested that self-referential processing in the brain involves integration of information from multiple cortical networks, including the INS, MPFC, and TPJ (Blanke et al., 2015; Bréchet et al., 2018; Grivaz et al., 2017). Particularly, the INS and medial regions of the cortex, that is, the cortical midline structures, including the ventromedial prefrontal cortex, the ACC, and the posterior cingulate cortex, play a primary role in interoceptive signals related to self-consciousness. The two subnetworks of the proposed cortical system seem to overlap in the inferior parietal sulcus area (Park and Blanke, 2019).
Experience-dependent changes in the resting-state EEG
Murphy and associates (2018) aimed to identify characteristics of EEG microstates as potential markers of offline memory processing. The authors hypothesized that a specific topography related to navigation in a virtual maze would also reappear during periods of post-task rest and sleep. The results confirm their hypothesis of recurrence of task-related topography and the source localization specified that this microstate was produced by activity in temporal and parietal regions, with maximal cortical activity in the superior parietal cortex. This study is the first one to use scalp EEG to demonstrate task-related activity patterns during post-training rest. Interestingly, a recent study by Bréchet and associates (2020a) found that two microstates dominated during non-rapid eye movement (NREM) sleep compared with the resting wake state. Using the source localization of these microstates, this study showed that short-lasting synchronous activity of specific brain networks in frontal and parietal regions changes systematically whether we dream or not in the course of NREM sleep. Poskanzer and colleagues (2020) studied the postencoding quiet rest after performing a word-pairs memory task and found an increase in microstate D, while microstate C decreased significantly. The authors argue that offline periods are critical for memory consolidation and that their study provides important insights into offline brain processes using the EEG microstate approach.
Short- and long-term effects of meditation on key brain networks captured with microstates
It could be argued that if EEG microstates are perceptive of the level of consciousness, then they should be modulated by changes in the state of mind. The study by Panda and colleagues (2016) recorded simultaneous EEG-fMRI while experienced participants meditated. This study showed that at rest, the meditators exhibited increased duration and occurrence of DMN-related microstate C, which further increased during meditation. Another study tested two phases of transcendental meditation—transcending (i.e., self-awareness becomes primary) and undirected (i.e., the mind becomes engaged in an undirected stream of thoughts) mentation—that were compared using EEG microstates. Compared with the transcending mentation, undirected mentation was marked by significantly higher coverage and occurrence of microstate C, while transcending meditation was characterized by higher coverage and occurrence of microstate D. Interestingly, light hypnosis was characterized by a decrease in microstate C, while deep hypnosis was associated with a decrease in microstate D (Katayama et al., 2007).
Conclusion
In conclusion, this is the first study to show that breath-focused, digital meditation practice leads to significant topographical changes in EEG microstates, which are visible during a quiet rest period postmeditation training. These results suggest the beneficial outcomes of 6 weeks of self-regulating meditation training on topographical reorganization of functional connectivity between brain structures in the fronto-insular-parietal networks, which are related to the self and attention. These results are valuable in opening new avenues for development of novel noninvasive treatment of neuropathological states such as anxiety, hyperactivity, or depression by low-cost, widely applicable, breath-focused, digital meditation practice, which can be captured and monitored by the EEG microstate approach.
Supplementary Material
Authors' Contributions
All authors contributed significantly to the work. D.A.Z. and A.J.S. collected the data and preprocessed the EEG. D.A.Z. and A.G. designed and supervised the experiment and the data collection. L.B., D.B., and C.M.M. analyzed the data. L.B. and C.M.M. wrote the manuscript. D.A.Z. and A.G. corrected the manuscript.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
The NIH, grants R21 AG041071 and R01 AG049424, provided financial support for the collection of data reported in this study. The data analysis was supported by the Swiss National Science Foundation, Grant No. 320030_184677 (to C.M.M.).
Supplementary Material
References
- Alzahabi R, Becker MW. 2013. The association between media multitasking, task-switching, and dual-task performance. J Exp Psychol Hum Percept Perform 39:1485–1495 [DOI] [PubMed] [Google Scholar]
- Bassett DS, Sporns O. 2017. Network neuroscience. Nat Neurosci 20:353–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birot G, Spinelli L, Vulliemoz S, Megevand P, Brunet D, Seeck M, Michel CM. 2014. Head model and electrical source imaging: a study of 38 epileptic patients. Neuroimage Clin 5:77–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. 1995. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34:537–541 [DOI] [PubMed] [Google Scholar]
- Blanke O. 2012. Multisensory brain mechanisms of bodily self-consciousness. Nat Rev Neurosci 13:556–571 [DOI] [PubMed] [Google Scholar]
- Blanke O, Slater M, Serino A. 2015. Behavioral, neural, and computational principles of bodily self-consciousness. Neuron 88:145–166 [DOI] [PubMed] [Google Scholar]
- Bréchet L, Brunet D, Birot G, Gruetter R, Michel CM, Jorge J. 2019. Capturing the spatiotemporal dynamics of self-generated, task-initiated thoughts with EEG and fMRI. Neuroimage 194:82–92 [DOI] [PubMed] [Google Scholar]
- Bréchet L, Brunet D, Perogamvros L, Tononi G, Michel CM. 2020a. EEG microstates of dreams. Sci Rep 10:17069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bréchet L, Grivaz P, Gauthier B, Blanke O. 2018. Common recruitment of angular gyrus in episodic autobiographical memory and bodily self-consciousness. Front Behav Neurosci 12:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bréchet L, Hausmann SB, Mange R, Herbelin B, Blanke O, Serino A. 2020b. Subjective feeling of re-experiencing past events using immersive virtual reality prevents a loss of episodic memory. Brain Behav 10:e01571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brechet L, Mange R, Herbelin B, Theillaud Q, Gauthier B, Serino A, Blanke O. 2019. First-person view of one's body in immersive virtual reality: influence on episodic memory. PLoS One 14:e0197763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britz J, Van De Ville D, Michel CM. 2010. BOLD correlates of EEG topography reveal rapid resting-state network dynamics. Neuroimage 52:1162–1170 [DOI] [PubMed] [Google Scholar]
- Brodbeck V, Kuhn A, von Wegner F, Morzelewski A, Tagliazucchi E, Borisov S, et al. 2012. EEG microstates of wakefulness and NREM sleep. Neuroimage 62:2129–2139 [DOI] [PubMed] [Google Scholar]
- Brunet D, Murray MM, Michel CM. 2011. Spatiotemporal analysis of multichannel EEG: CARTOOL. Comput Intell Neurosci 2011:813870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. 2004. Neural systems supporting interoceptive awareness. Nat Neurosci 7:189–195 [DOI] [PubMed] [Google Scholar]
- Croce P, Zappasodi F, Capotosto P. 2018a. Offline stimulation of human parietal cortex differently affects resting EEG microstates. Sci Rep 8:1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce P, Zappasodi F, Spadone S, Capotosto P. 2018b. Magnetic stimulation selectively affects pre-stimulus EEG microstates. Neuroimage 176:239–245 [DOI] [PubMed] [Google Scholar]
- Custo A, Van De Ville D, Wells WM, Tomescu MI, Brunet D, Michel CM. 2017. Electroencephalographic resting-state networks: source localization of microstates. Brain Connect 7:671–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Croz-Baron DF, Baker M, Michel CM, Karp T. 2019. EEG microstates analysis in young adults with autism spectrum disorder during resting-state. Front Hum Neurosci 13:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Cruz JR, Favrod O, Roinishvili M, Chkonia E, Brand A, Mohr C, et al. 2020. EEG microstates are a candidate endophenotype for schizophrenia. Nat Commun 11:3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devue C, Collette F, Balteau E, Degueldre C, Luxen A, Maquet P, Brédart S. 2007. Here I am: the cortical correlates of visual self-recognition. Brain Res 1143:169–182 [DOI] [PubMed] [Google Scholar]
- Enzi B, de Greck M, Prosch U, Tempelmann C, Northoff G. 2009. Is our self nothing but reward? Neuronal overlap and distinction between reward and personal relevance and its relation to human personality. PLoS One 4:e8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber PL, Travis F, Milz P, Parim N. 2017. EEG microstates during different phases of Transcendental Meditation practice. Cogn Process 18:307–314 [DOI] [PubMed] [Google Scholar]
- Farb NA, Segal ZV, Mayberg H, Bean J, McKeon D, Fatima Z, Anderson AK. 2007. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Soc Cogn Affect Neurosci 2:313–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Price CJ, Buchel C, Worsley KJ. 1999. Multisubject fMRI studies and conjunction analyses. Neuroimage 10:385–396 [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Rosen LD. 2016. The Distracted Mind: Ancient Brains in a High-Tech World. Cambridge, MA, USA: The MIT Press [Google Scholar]
- Grave de Peralta Menendez R, Murray MM, Michel CM, Martuzzi R, Gonzalez Andino SL. 2004. Electrical neuroimaging based on biophysical constraints. Neuroimage 21:527–539 [DOI] [PubMed] [Google Scholar]
- Grieder M, Koenig T, Kinoshita T, Utsunomiya K, Wahlund LO, Dierks T, Nishida K. 2016. Discovering EEG resting state alterations of semantic dementia. Clin Neurophysiol 127:2175–2181 [DOI] [PubMed] [Google Scholar]
- Grivaz P, Blanke O, Serino A. 2017. Common and distinct brain regions processing multisensory bodily signals for peripersonal space and body ownership. Neuroimage 147:602–618 [DOI] [PubMed] [Google Scholar]
- Gschwind M, Hardmeier M, Van De Ville D, Tomescu MI, Penner IK, Naegelin Y, et al. 2016. Fluctuations of spontaneous EEG topographies predict disease state in relapsing-remitting multiple sclerosis. Neuroimage Clin 12:466–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzel BK, Ott U, Gard T, Hempel H, Weygandt M, Morgen K, Vaitl D. 2008. Investigation of mindfulness meditation practitioners with voxel-based morphometry. Soc Cogn Affect Neurosci 3:55–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionta S, Heydrich L, Lenggenhager B, Mouthon M, Fornari E, Chapuis D, et al. 2011. Multisensory mechanisms in temporo-parietal cortex support self-location and first-person perspective. Neuron 70:363–374 [DOI] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Oakes TR, Davidson RJ. 2006. The voice of emotion: an FMRI study of neural responses to angry and happy vocal expressions. Soc Cogn Affect Neurosci 1:242–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama H, Gianotti LR, Isotani T, Faber PL, Sasada K, Kinoshita T, Lehmann D. 2007. Classes of multichannel EEG microstates in light and deep hypnotic conditions. Brain Topogr 20:7–14 [DOI] [PubMed] [Google Scholar]
- Kerr CE, Sacchet MD, Lazar SW, Moore CI, Jones SR. 2013. Mindfulness starts with the body: somatosensory attention and top-down modulation of cortical alpha rhythms in mindfulness meditation. Front Hum Neurosci 7:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna A, Pascual-Leone A, Farzan F. 2014. Reliability of resting-state microstate features in electroencephalography. PLoS One 9:e114163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig T, Prichep L, Lehmann D, Sosa PV, Braeker E, Kleinlogel H, et al. 2002. Millisecond by millisecond, year by year: normative EEG microstates and developmental stages. Neuroimage 16:41–48 [DOI] [PubMed] [Google Scholar]
- Koenig T, Valdes-Sosa PA. 2018. Results obtained by combining different estimators of EEG connectivity become uninterpretable if the underlying models are incompatible. Brain Connect 8:57–59 [DOI] [PubMed] [Google Scholar]
- Lehmann D, Ozaki H, Pal I. 1987. EEG alpha map series: brain micro-states by space-oriented adaptive segmentation. Electroencephalogr Clin Neurophysiol 67:271–288 [DOI] [PubMed] [Google Scholar]
- Liu J, Xu J, Zou G, He Y, Zou QH, Gao J-H. 2020. Reliability and individual specificity of EEG microstate characteristics. Brain Topogr 30:438–449 [DOI] [PubMed] [Google Scholar]
- Loh KK, Kanai R. 2016. How has the Internet reshaped human cognition? Neuroscientist 22:506–520 [DOI] [PubMed] [Google Scholar]
- Michel CM, Brunet D. 2019. EEG source imaging: a practical review of the analysis steps. Front Neurol 10:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel CM, Koenig T. 2018. EEG microstates as a tool for studying the temporal dynamics of whole-brain neuronal networks: a review. Neuroimage 180:577–593 [DOI] [PubMed] [Google Scholar]
- Michel CM, Murray MM, Lantz G, Gonzalez S, Spinelli L, Grave de Peralta R. 2004. EEG source imaging. Clin Neurophysiol 115:2195–2222 [DOI] [PubMed] [Google Scholar]
- Milz P, Faber PL, Lehmann D, Koenig T, Kochi K, Pascual-Marqui RD. 2016. The functional significance of EEG microstates—associations with modalities of thinking. Neuroimage 125:643–656 [DOI] [PubMed] [Google Scholar]
- Minear M, Brasher F, McCurdy M, Lewis J, Younggren A. 2013. Working memory, fluid intelligence, and impulsiveness in heavy media multitaskers. Psychon Bull Rev 20:1274–1281 [DOI] [PubMed] [Google Scholar]
- Mishra J, Sagar R, Parveen S, Kumaran S, Modi K, Maric V, et al. 2020. Closed-loop digital meditation for neurocognitive and behavioral development in adolescents with childhood neglect. Transl Psychiatry 10:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modinos G, Ormel J, Aleman A. 2009. Activation of anterior insula during self-reflection. PLoS One 4:e4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisala M, Salmela V, Hietajarvi L, Salo E, Carlson S, Salonen O, et al. 2016. Media multitasking is associated with distractibility and increased prefrontal activity in adolescents and young adults. Neuroimage 134:113–121 [DOI] [PubMed] [Google Scholar]
- Murphy M, Stickgold R, Parr ME, Callahan C, Wamsley EJ. 2018. Recurrence of task-related electroencephalographic activity during post-training quiet rest and sleep. Sci Rep 8:5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida K, Morishima Y, Yoshimura M, Isotani T, Irisawa S, Jann K, et al. 2013. EEG microstates associated with salience and frontoparietal networks in frontotemporal dementia, schizophrenia and Alzheimer's disease. Clin Neurophysiol 124:1106–1114 [DOI] [PubMed] [Google Scholar]
- Ophir E, Nass C, Wagner AD. 2009. Cognitive control in media multitaskers. Proc Natl Acad Sci U S A 106:15583–15587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda R, Bharath RD, Upadhyay N, Mangalore S, Chennu S, Rao SL. 2016. Temporal dynamics of the default mode network characterize meditation-induced alterations in consciousness. Front Hum Neurosci 10:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HD, Blanke O. 2019. Coupling inner and outer body for self-consciousness. Trends Cogn Sci 23:377–388 [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui RD, Michel CM, Lehmann D. 1995. Segmentation of brain electrical activity into microstates: model estimation and validation. IEEE Trans Biomed Eng 42:658–665 [DOI] [PubMed] [Google Scholar]
- Poskanzer C, Denis D, Herrick A, Stickgold R. 2020. Using EEG microstates to examine post-encoding quiest rest and subsequent word-pair memory. bioRxiv (preprint). DOI: 10.1101/2020.05.08.085027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. 1990. The attention system of the human brain. Annu Rev Neurosci 13:25–42 [DOI] [PubMed] [Google Scholar]
- Rideout VJ, Foehr UG, Roberts DF. 2010. Generation M2: Media in the Lives of 8- to 18-Year-Olds. Menlo Park, CA: The Henry J. Kaiser Family Foundation [Google Scholar]
- Rieger K, Diaz Hernandez L, Baenninger A, Koenig T. 2016. 15 years of microstate research in Schizophrenia - where are we? A meta-analysis. Front Psychiatry 7:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitzman BA, Abell M, Bartley SC, Erickson MA, Bolbecker AR, Hetrick WP. 2017. Cognitive manipulation of brain electric microstates. Neuroimage 146:533–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestieri C, Tosoni A, Mignogna V, McAvoy MP, Shulman GL, Corbetta M, Luca Romani G. 2014. Memory accumulation mechanisms in human cortex are independent of motor intentions. J Neurosci 34:6993–7006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W, Li Y, Liu Z, Li J, Wang Q, Yan X, Wang G. 2020. Non-canonical microstate becomes salient in high density EEG during propofol-induced altered states of consciousness. Int J Neural Syst 30:2050005. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Andrews-Hanna J. 2013. Not all minds that wander are lost: the importance of a balanced perspective on the mind-wandering state. Front Psychol 4:441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan S, Schorr B, Lopez-Rolon A, Kolassa IT, Shock JP, Rosenfelder M, et al. 2018. Consciousness indexing and outcome prediction with resting-state EEG in severe disorders of consciousness. Brain Topogr 31:848–862 [DOI] [PubMed] [Google Scholar]
- Tomescu MI, Rihs TA, Rochas V, Hardmeier M, Britz J, Allali G, et al. 2018. From swing to cane: sex differences of EEG resting-state temporal patterns during maturation and aging. Dev Cogn Neurosci 31:58–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vago DR, Silbersweig DA. 2012. Self-awareness, self-regulation, and self-transcendence (S-ART): a framework for understanding the neurobiological mechanisms of mindfulness. Front Hum Neurosci 6:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaudenhuyse A, Noirhomme Q, Tshibanda LJ, Bruno MA, Boveroux P, Schnakers C, et al. 2010. Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients. Brain 133:161–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Ford JM. 2012. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol 8:49–76 [DOI] [PubMed] [Google Scholar]
- Yap JY, Wee Hun Lim S. 2013. Media multitasking predicts unitary versus splitting visual focal attention. J Cogn Psychol 25:889–902 [Google Scholar]
- Zanesco AP, King BG, Skwara AC, Saron CD. 2020. Within and between-person correlates of the temporal dynamics of resting EEG microstates. Neuroimage 211:116631. [DOI] [PubMed] [Google Scholar]
- Zappasodi F, Croce P, Giordani A, Assenza G, Giannantoni NM, Profice P, et al. 2017. Prognostic value of EEG microstates in acute stroke. Brain Topogr 30:698–710 [DOI] [PubMed] [Google Scholar]
- Ziegler DA, Simon AJ, Gallen CL, Skinner S, Janowich JR, Volponi JJ, et al. 2019. Closed-loop digital meditation improves sustained attention in young adults. Nat Hum Behav 3:746–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.