Abstract
The Working Group on Severe Asthma of the Korean Academy of Allergy and Clinical Immunology recently published an expert opinion paper on the management of severe asthma in Korea. When developing a consensus, the working group encountered several diagnostic and treatment issues and decided to perform a questionnaire survey of Korean specialists with regard to severe asthma. An e-mail with a uniform resource locator link to the questionnaire was sent to 121 asthma specialists, of whom 44.6% responded. The most commonly accepted definitions of severe asthma were a history of fatal exacerbation or an asthma-triggered need for mechanical ventilation, 3–4 oral corticosteroid (OCS) bursts/year, and maintenance of OCS therapy for 3–6 months per year. Before diagnosing severe asthma, most physicians contemplate chest computed tomography, seek to control chronic rhinosinusitis, and consider poor inhaler compliance. For patients with uncontrolled severe asthma accompanied by type 2 (T2)-high inflammation, most biologics available in Korea were considered appropriate, but gaps were apparent in terms of T2-low asthma treatments. These findings about specialist perception of diagnosis and treatment of severe asthma will inform the use of emerging new drugs and facilitate personalized therapy.
Keywords: Asthma, diagnosis, therapeutics, disease management, surveys and questionnaires, biological products, standards, consensus
INTRODUCTION
More than 50% of the total medical costs of asthma are attributed to severe asthma, which affects only 3%–5% of all asthmatics.1,2,3,4 The Global Initiative for Asthma and the European Respiratory Society/American Thoracic Society have proposed definitions of severe asthma.5,6 A key shared feature is uncontrolled asthma even after the highest level of treatment─high-dose inhaled corticosteroid (ICS)/long-acting beta-2 agonist (LABA) combined with second controllers. However, it is well known that a gap exists between the guidelines and real clinical practice especially in terms of diagnosis and treatment.4,7,8,9,10 The Korean Academy of Allergy and Clinical Immunology (KAAACI) organized a Working Group on Severe Asthma and recently published an expert opinion paper on the management of severe asthma in Korea.11 When developing the consensus, the working group encountered several diagnostic and treatment issues.
A concept of severe asthma was once investigated in the previous survey by asking a key feature of severe asthma, such as asthma aggravation by stepping down treatment, frequent exacerbation, uncontrolled asthma despite higher treatment steps, and serious exacerbation.10 In that survey, asthma aggravation by stepping down treatment was the most common feature of severe asthma. Nevertheless, we still meet several questions during our daily clinical practice. For example, how should the examinations prior to a differential diagnosis be scheduled? Is it appropriate to consider risk factors or comorbidities? What level of OCS use, or number of oral corticosteroid (OCS) bursts, define severe asthma? Clinician preferences also differ widely in terms of biologics for patients with severe asthma (sometimes used off-label). No uniform treatment flow has appeared. It is also not clear whether inflammatory biomarkers can be used to select biologics. To clarify these issues, the working group performed a questionnaire survey exploring the diagnostic and treatment choices of Korean specialists in cases of severe asthma. This brief report summarizes the results.
MATERIALS AND METHODS
We performed a questionnaire survey of specialists in adult asthma recruited from 2 representative societies: the Working Group on Severe Asthma of the KAAACI and the steering committee of the Asthma and Chronic Obstructive Respiratory Disease (COPD) study group of the Korean Academy of Tuberculosis and Respiratory Diseases. Additional participants were recruited during participation in the “Severe Asthma Symposium” held on September 21, 2019, where an “Expert opinion paper about the diagnosis and treatment of severe asthma” was discussed in depth. An e-mail was sent to 121 specialists; it explained why the questionnaire was being circulated (the need to learn more about the diagnosis and treatment of severe asthma) and provided a uniform resource locator link to the questionnaire. In total, 54 specialists completed the questionnaire (response rate 44.6%). Respondents also provided demographic data (age, sex, and area of practice [allergy or pulmonology]).
The questionnaire was developed on the basis of the results of semi-structured interviews with the members of the Working Group of Severe Asthma of the KAAACI who participated in the preparation of an expert opinion paper entitled “Evaluation and management of difficult-to-treat and severe asthma.” A draft questionnaire (5 questions) was prepared in May 2019 after brainstorming the most ambiguous issues. A pilot survey was completed by 29 physicians from May 25 to June 1, 2019, and the results and additional feedback were reviewed by the working group. The results revealed that more detailed questions were required to improve clarity, especially in areas in which knowledge seemed to be lacking and to explore issues to which the physicians did not respond. Participants also suggested 21 additional questions, of which 15 were chosen by the working group. The revised questionnaire included 4 parts (as the expert opinion paper did), and explored asthma definitions (3 items), diagnosis (3 items), treatment (8 items), and asthma-COPD overlap (ACO, 1 item). Questions explored how clinicians defined severe asthma, the roles played by comorbidities and modifiable risk factors, and treatment options including add-on therapies. The answers were of 2 types: 1) ticking a box indicating the extent of agreement with a statement (from 1 [disagree] to 5 [strongly agree]); and 2) selecting from a multiple-choice list. Agreement scores were assessed based on means ± standard deviations of scores awarded and the numbers (with percentages) of answers to multiple-choice questions.
RESULTS
In total, 54 physicians answered the questionnaire. The age distribution was: 30–39 years (42.6%); 40–49 years (35.2%); over 50 years (20.4%); and no age given (1.9%). Of the respondents, 51.9% were males, 55.6% had an allergy subspecialty, and 44.4% had a pulmonology subspecialty.
Definition of severe asthma
As shown in Table 1, the most agreed-upon criterion was a history of fatal exacerbation or an asthma-triggered need for mechanical ventilation in addition to uncontrolled asthma symptoms and decreased lung function (Supplementary Fig. S1). Most specialists considered that 3–4 OCS bursts/year was an appropriate criterion. Additionally, most physicians agreed that asthma patients who required OCS for 3–6 months/year had severe asthma.
Table 1. Defining severe asthma.
| Questions | Value | |
|---|---|---|
| Q1. Do you agree that the criteria listed below appropriately define severe asthma? | ||
| A fatal exacerbation or at least one episode of mechanical ventilation required because of asthma during the last year | 4.69 ± 0.58 | |
| At least 2 hospitalizations or ER visits/year | 4.31 ± 0.82 | |
| Uncontrolled asthma symptoms (at least 3 of daytime symptoms, night awakening, activity limitations, and/or reliever use) | 4.30 ± 0.72 | |
| At least 2 OCS bursts/year | 4.06 ± 0.76 | |
| Decreased lung function (FEV1 < 80%) | 3.56 ± 0.90 | |
| Q2. How many OCS bursts/year appropriately define severe asthma? | ||
| 3–4 | 36 (66.7) | |
| 1–2 | 8 (14.8) | |
| 5–6 | 6 (11.1) | |
| Not a useful criterion | 4 (7.5) | |
| More than 7 | 0 (0) | |
| Q3. What duration of OCS maintenance/year appropriately defines severe asthma? | ||
| 6 mon | 21 (38.9) | |
| 3 mon | 16 (29.7) | |
| Not a useful criterion | 11 (20.4) | |
| 9 mon | 4 (7.4) | |
| 12 mon | 1 (1.9) | |
| No answer | 1 (1.9) | |
Each score is a mean ± standard deviation determined by considering all answers from 1 (disagree) to 5 (strongly agree); †Each multiple-choice question was scored as a number (%).The answers are given in decreasing order.
ER, emergency room; OCS, oral corticosteroid; FEV1, forced expiratory volume in 1 second.
Diagnosis of severe asthma
As shown in Table 2 and Supplementary Fig. S2, most physicians agreed that chest computed tomography (CT) and chronic rhinosinusitis (CRS) control should be considered prior to diagnosis of severe asthma. The most agreed-upon risk factor that should be modified before diagnosis was poor compliance with or cessation of inhaler use.
Table 2. Considerations prior to diagnosis of severe asthma.
| Questions | Value | |
|---|---|---|
| Q4. Do you agree that the tests listed below are appropriate during differential diagnosis of severe asthma? | ||
| Chest CT | 4.32 ± 1.07 | |
| Anti-Aspergillus IgE level | 3.18 ± 0.96 | |
| Serum ANCA | 3.06 ± 1.17 | |
| Laryngoscopy | 2.98 ± 1.26 | |
| Bronchoscopy | 2.43 ± 1.06 | |
| Q5. Do you agree that the risk factors listed below should be modified prior to diagnosis of severe asthma? | ||
| Chronic rhinosinusitis | 4.31 ± 0.87 | |
| Obesity | 3.91 ± 1.01 | |
| Depression/anxiety disorder | 3.74 ± 0.89 | |
| Gastro-esophageal reflux disease | 3.64 ± 1.02 | |
| Obstructive sleep apnea | 3.47 ± 0.99 | |
| Q6. Do you agree that the risk factors listed below should be modified prior to diagnosis of severe asthma? | ||
| Poor inhaler compliance | 4.82 ± 0.51 | |
| Lack of inhaler skill | 4.64 ± 0.68 | |
| Stoppage of asthma medication because of side-effects | 4.61 ± 0.68 | |
| Smoking | 4.31 ± 0.93 | |
| Exposure to sensitized allergens or nonspecific stimuli that worsen the respiratory symptoms | 4.08 ± 0.96 | |
Each score is a mean ± standard deviation determined by considering all answers from 1 (disagree) to 5 (strongly agree). The answers are given in decreasing order.
CT, computed tomography; IgE, immunoglobulin E; ANCA, anti-neutrophil cytoplasmic antibodies.
Treatment of severe asthma
As shown in Table 3, the most agreed-upon indications for biologic commencement were a combination of high-dose ICS-LABA with tiotropium or a need for OCS maintenance. However, most physicians did not start biologics when asthma was well-controlled with high-dose ICS-LABA without other add-on treatments such as tiotropium or a leukotriene receptor antagonist. They considered commencing biologics when asthmatic patients required OCS for 3–6 months/year and stopped biologics when no asthma exacerbation was noted for 1 year. Notably, no clinician answered “yes” to: “I do not consider biologics if asthma is well-controlled via the maintenance of OCS.” Thus, all agreed that OCS maintenance should be avoided if at all possible.
Table 3. Treatment of severe asthma.
| Questions | Value | ||
|---|---|---|---|
| Q7. Do you agree that the prior treatments listed below would be appropriate when considering whether to start biologics in patients with severe asthma? | |||
| High dose ICS-LABA + tiotropium | 4.42 ± 0.83 | ||
| Maintenance of OCS | 4.35 ± 1.07 | ||
| High dose ICS-LABA + tiotropium + LTRA | 4.30 + 0.84 | ||
| High dose ICS-LABA | 4.17 ± 0.80 | ||
| Medium dose ICS-LABA + tiotropium + LTRA | 3.79 + 1.15 | ||
| Q8. What duration of prior high-dose ICS-LABA treatment is appropriate when considering whether to start biologics in patients with severe asthma? | |||
| I do not consider biologics if the asthma is well controlled by high-dose ICS-LABA | 22 (40.7) | ||
| 6 mon | 12 (22.3) | ||
| More than 12 mon | 8 (14.9) | ||
| 3 mon | 7 (13.0) | ||
| 9 mon | 5 (9.3) | ||
| Q9. How much prior OCS maintenance is appropriate when considering whether to start biologics in patients with severe asthma? | |||
| 6 mon | 21 (38.9) | ||
| 3 mon | 16 (29.7) | ||
| I prefer biologics to OCS maintenance if the biologics are available and affordable | 10 (18.5) | ||
| 9 mon | 5 (9.3) | ||
| More than 12 mon | 2 (3.8) | ||
| I do not consider biologics if the asthma is well controlled by OCS | 0 (0) | ||
| Q10. Do you agree that it would be appropriate to consider stopping biologics in patients with severe asthma under the following conditions? | |||
| No asthma exacerbation for 1 yr | 3.98 + 0.77 | ||
| I do not consider stopping biologics if they are available and affordable | 3.50 + 0.96 | ||
| A target amount has been delivered | 3.35 + 1.23 | ||
| Improved lung function (FEV1) | 3.33 + 1.03 | ||
| A decreased blood eosinophil or FeNO level | 3.08 + 1.05 | ||
| Q11. How much improvement in lung function (FEV1) is appropriate before considering whether to stop biologics in severe asthma patients? | |||
| I do not consider stop biologics according to lung function improvement | 27 (50.0) | ||
| 20%–30% | 10 (18.5) | ||
| 10%–20% | 5 (9.3) | ||
| 30%–40% | 3 (5.6) | ||
| ≥ 50% | 3 (5.6) | ||
| No answer | 6 (11.1) | ||
| Q12 & Q13. Do you agree that the add-on treatments listed below are appropriate for patients with uncontrolled T2-high or T2-low severe asthma in addition to high-dose ICS-LABA with tiotropium? | |||
| T2-high | |||
| Reslizumab | 4.67 ± 0.48 | ||
| Benralizumab | 4.59 ± 0.53 | ||
| Mepolizumab | 4.59 ± 0.53 | ||
| Dupilumab | 4.51 ± 0.67 | ||
| Omalizumab | 3.72 ± 0.95 | ||
| T2-low | |||
| Macrolide | 3.75 ± 0.92 | ||
| Tezepelumab | 3.63 ± 1.09 | ||
| Roflumilast | 3.44 ± 1.00 | ||
| Bronchial thermoplasty | 2.59 ± 1.00 | ||
| Imatinib | 2.52 ± 0.91 | ||
| Q14. Do you agree that the biomarkers mentioned below are of assistance when considering anti-IL5/5R therapy for patients with severe asthma? | |||
| Blood eosinophil count | 4.74 ± 0.45 | ||
| FeNO level | 4.17 ± 0.86 | ||
| Atopic status | 3.40 ± 1.17 | ||
| Total IgE level | 3.17 ± 1.11 | ||
| Periostin level | 3.15 ± 1.05 | ||
Each score is a mean ± standard deviation determined by considering all answers from 1 (disagree) to 5 (strongly agree); Each multiple-choice question was scored as a number (%). The answers are given in decreasing order.
ICS, inhaled corticosteroid; LABA, long-acting beta-2 agonist; OCS, oral corticosteroid; LTRA, leukotriene receptor antagonist; FEV1, forced expiratory volume in 1 second; FeNO, fractional exhaled nitric oxide; T2, type 2; IL5, interleukin 5; IL5R, interleukin 5 receptor; IgE, immunoglobulin E.
Most physicians did not consider stopping biologics according to lung function improvement. For patients with uncontrolled severe asthma and high-type 2 (T2) airway inflammation, all biologics available in Korea were considered appropriate, but add-on treatments for T2-low asthma were considered variously, with a wide gap. The blood eosinophil level was the most agreed-upon biomarker for the choice of anti-interleukin 5 (IL5)/interleukin 5 receptor (5R) therapy.
ACO in severe asthma
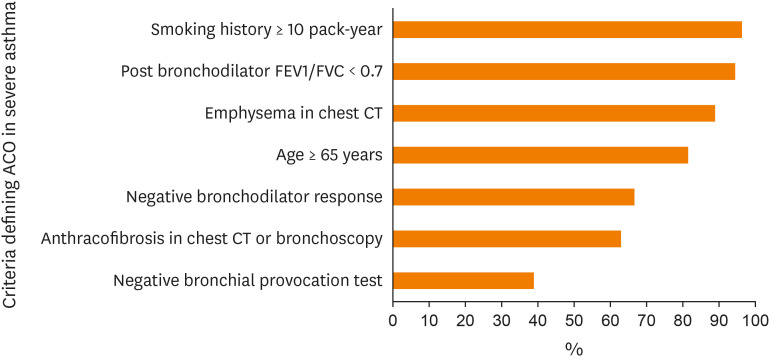
Respondents were asked about which criteria are appropriate when defining ACO in patients with severe asthma. As shown in Fig. 1, the most agreed-upon criteria were a smoking history of more than 10 packs-year (96.3%) and a post-bronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio < 0.7 (94.4%).
Fig. 1. Proportions of agreement (mean scores of over 3) in terms of defining ACO in patients with severe asthma.
ACO, asthma-Chronic Obstructive Respiratory Disease overlap; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; CT, computed tomography.
DISCUSSION
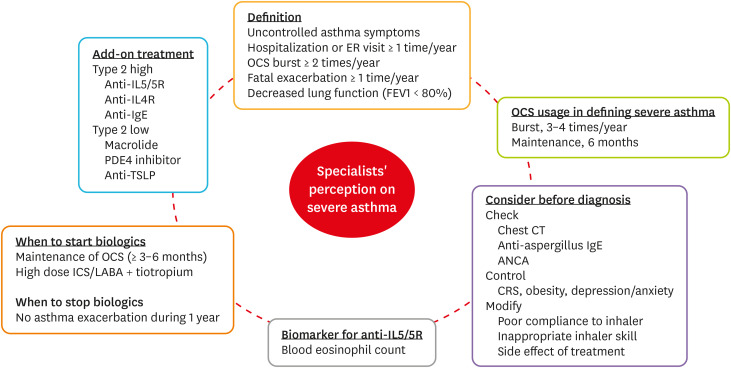
Fig. 2 presents the results of our questionnaire survey of specialist perception of severe asthma in Korea. First, we identified the need to use a lung function test when seeking to define severe asthma; most physicians agreed because the test is widely available in primary clinics and the National Health Insurance Service emphasizes the importance of lung function tests when assessing the quality of asthma management.12 Secondly, we found that chest CT, CRS improvement, and inhaler adherence were usually scheduled/checked prior to the diagnosis of severe asthma. Thirdly, we clarified opinions about add-on treatments, including when to start or stop biologics, useful biomarkers, and medications. Generally, physicians consider add-on biologics when OCS maintenance is required for more than 3–6 months/year or when high-dose ICS-LABA with tiotropium is consistently needed for asthma control. The blood eosinophil level was the most agreed-upon biomarker in terms of when to start IL5/5R therapy. For patients with T2-high, severe uncontrolled asthma, most biologics available in Korea were considered useful, but differences were evident in terms of add-on treatment choices for patients with T2-low severe asthma. Drugs that are effective in such patients require further research. Physicians consider ACO status when patients with severe asthma have smoking histories of more than 10 packs-year or fixed airflow limitations (post-bronchodilator FEV1/FVC ratio < 0.7).
Fig. 2. A graphical summary of specialists' perceptions of severe asthma.
ER, emergency room; OCS, oral corticosteroid; FEV1, forced expiratory volume in 1 second; CT, computed tomography; IgE, immunoglobulin E; ANCA, anti-neutrophil cytoplasmic antibodies; CRS, chronic rhinosinusitis; ICS, inhaled corticosteroid; LABA, long-acting beta-2 agonist; IL5, interleukin 5; IL5R, interleukin 5 receptor; IL4R, interleukin 4 receptor; PDE4, phosphodiesterase 4; TSLP, thymic stromal lymphopoietin.
Most guidelines suggest that severe asthma be diagnosed after chronological observation of the patient and the success (or not) of asthma control, and the exacerbation risk. Many physicians find this confusing; a gap is evident between the guidelines and real clinical practice.6,7,8,9 Our survey results reflect expert opinions about the chronological definition of severe asthma formed by personal clinical experience. We explored issues such as what duration of OCS maintenance is required before starting biologics, what examinations should be scheduled, and what comorbidities and risk factors should be considered prior to a diagnosis of severe asthma. Our results are informative, but may be appropriate only in Korea; medical circumstances differ worldwide. Also, the response rate to questionnaire survey was under 50%, which could be increased if diverse forms of survey would be applied. In conclusion, the findings of our survey on specialist perception of severe asthma diagnosis and treatment will inform the use of emerging new drugs and help facilitate personalized therapy.
ACKNOWLEDGMENTS
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HC19C0318).
Footnotes
Disclosure: There are no financial or other issues that might lead to a conflict of interest.
SUPPLEMENTARY MATERIALS
The extents of agreement (mean scores of over 3) in terms of defining severe asthma.
The extents of agreement (mean scores of over 3) in terms of items considered before diagnosing severe asthma.
References
- 1.Kang SY, Song WJ, Cho SH, Chang YS. Time trends of the prevalence of allergic diseases in Korea: a systematic literature review. Asia Pac Allergy. 2018;8:e8. doi: 10.5415/apallergy.2018.8.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen S, Golam S, Myers J, Bly C, Smolen H, Xu X. Systematic literature review of the clinical, humanistic, and economic burden associated with asthma uncontrolled by GINA Steps 4 or 5 treatment. Curr Med Res Opin. 2018;34:2075–2088. doi: 10.1080/03007995.2018.1505352. [DOI] [PubMed] [Google Scholar]
- 3.Ivanova JI, Bergman R, Birnbaum HG, Colice GL, Silverman RA, McLaurin K. Effect of asthma exacerbations on health care costs among asthmatic patients with moderate and severe persistent asthma. J Allergy Clin Immunol. 2012;129:1229–1235. doi: 10.1016/j.jaci.2012.01.039. [DOI] [PubMed] [Google Scholar]
- 4.Kim S, Kim J, Kim K, Kim Y, Park Y, Baek S, et al. Healthcare use and prescription patterns associated with adult asthma in Korea: analysis of the NHI claims database. Allergy. 2013;68:1435–1442. doi: 10.1111/all.12256. [DOI] [PubMed] [Google Scholar]
- 5.Global Initiative for Asthma. Global strategy for asthma management and prevention, 2020. Fontana: GINA; 2020. [cited 2020 Sep 15]. Available from: www.ginasthma.org. [Google Scholar]
- 6.Holguin F, Cardet JC, Chung KF, Diver S, Ferreira DS, Fitzpatrick A, et al. Management of severe asthma: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2020;55:1900588. doi: 10.1183/13993003.00588-2019. [DOI] [PubMed] [Google Scholar]
- 7.Mincheva R, Ekerljung L, Bossios A, Lundbäck B, Lötvall J. High prevalence of severe asthma in a large random population study. J Allergy Clin Immunol. 2018;141:2256–2264.e2. doi: 10.1016/j.jaci.2017.07.047. [DOI] [PubMed] [Google Scholar]
- 8.Backman H, Jansson SA, Stridsman C, Eriksson B, Hedman L, Eklund BM, et al. Severe asthma—A population study perspective. Clin Exp Allergy. 2019;49:819–828. doi: 10.1111/cea.13378. [DOI] [PubMed] [Google Scholar]
- 9.Ilmarinen P, Tuomisto LE, Niemelä O, Kankaanranta H. Prevalence of patients eligible for anti-IL-5 treatment in a cohort of adult-onset asthma. J Allergy Clin Immunol Pract. 2019;7:165–174.e4. doi: 10.1016/j.jaip.2018.05.032. [DOI] [PubMed] [Google Scholar]
- 10.Kim SH, Moon JY, Lee JH, Ban GY, Kim S, Kim MA, et al. Perceptions of severe asthma and asthma-COPD overlap syndrome among specialists: a questionnaire survey. Allergy Asthma Immunol Res. 2018;10:225–235. doi: 10.4168/aair.2018.10.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim BK, Park SY, Ban GY, Kim MA, Lee JH, An J, et al. Evaluation and management of difficult-to-treat and severe asthma: an expert opinion from the Korean Academy of Asthma, Allergy and Clinical Immunology, the Working Group on Severe Asthma. Allergy Asthma Immunol Res. 2020;12:910–933. doi: 10.4168/aair.2020.12.6.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Health Insurance Review and Assessment (KR) Comprehensive report on quality assessment of national health insurance 2016. Wonju: HIRA; 2018. [cited 2020 Sep 15]. Available from: https://www.hira.or.kr/bbsDummy.do?pgmid=HIRAJ030000001000&brdScnBltNo=4&brdBltNo=46914. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The extents of agreement (mean scores of over 3) in terms of defining severe asthma.
The extents of agreement (mean scores of over 3) in terms of items considered before diagnosing severe asthma.