Abstract
Spasticity causes an array of disabilities, which in turn may lead to the need for surgical intervention. Spasticity itself may also negatively affect surgical outcomes. This report reviews the potential benefit of perioperative (before, during, or after surgery) botulinum toxin (BoNT) injections for 3 patients with spasticity due to spinal cord injury, stroke, or multiple sclerosis. We discuss perioperative BoNT in 3 time periods: preoperatively, intraoperatively, and postoperatively. The cases demonstrate the use of perioperative BoNT in decreasing pain, improving wound healing, and improving surgical outcomes. We conclude by discussing the potential use of perioperative BoNT for surgical interventions in patients with spasticity and the need for further high-quality research in this field.
Keywords: Botulinum toxin, Perioperative, Rehabilitation, Spasticity
List of abbreviations: BoNT, botulinum toxin; CP, cerebral palsy; MS, multiple sclerosis; RCT, randomized controlled trial; SCI, spinal cord injury; UMN, upper motor neuron
Spasticity is a disorder of sensorimotor control resulting from upper motor neuron (UMN) pathologies, characterized by intermittent or sustained involuntary activation of muscles.1 Spasticity can have a negative effect on quality of life, function, and self-esteem and can also increase caregiver burden, cause pain, and lead to joint deformities and contractures.2
Spasticity management can involve nonpharmacologic, oral pharmacologic, chemodenervation, and surgical interventions.3 Surgical techniques include tendon lengthening, tendon releases, tendon transfers, and neurotomies.4 Patients with spasticity may also require operations that are not directly related to their underlying neurologic condition, such as hip replacement or wound flap surgery.5, 6, 7 Spasticity may interfere with the success of surgical interventions.8
Previous animal studies have demonstrated positive results from preoperative treatment with botulinum toxin (BoNT), such as decreased passive tension in the muscle-tendon unit, which may lead to increased ease of intraoperative manipulation, lowered risk of rupture of tendon repairs, and facilitation of rehabilitation.9, 10, 11
Human studies have suggested that perioperative BoNT administration (the perioperative period includes the following 3 time frames: preoperative, intraoperative, or postoperative)12 may reduce pain, spasticity, and analgesic use in patients with preexisting spasticity.5,6,13, 14, 15 Similarly, patients undergoing orthopedic procedures unrelated to their spasticity, such as hip replacement for hip dysplasia, wound flap surgery for pressure ulcers, or neck stabilization for cervical myelopathy, may also benefit from perioperative BoNT injections to help with a successful surgical outcome.5, 6, 7,16, 17, 18
A recent systematic review demonstrated that perioperative BoNT injection might theoretically improve the outcomes of operations performed on spastic limb(s) in children with cerebral palsy (CP), especially regarding controlling pain and alleviating spasticity.8
Two randomized controlled trials (RCTs)5,13 and 1 retrospective case series14 on the use of perioperative BoNT for children with CP undergoing orthopedic procedures to their spastic limb as well as a case report on the use of perioperative BoNT in adults with CP6 were identified in this systematic review.8 The systematic review, however, identified no studies regarding individuals with spasticity from a spinal cord injury (SCI), stroke, or multiple sclerosis (MS). While the mechanism of action of BoNT is similar in all etiologies, each patient’s diagnosis creates unique operative challenges because of differences in spasticity patterns. The focus on CP in these studies limits the generalizability of the results to other patient populations. Further, all studies analyzed in this perioperative systematic review were from either the preoperative or intraoperative period, and no studies were found from the postoperative period.
We, therefore, to our knowledge present the first case series of 3 adult patients with different UMN etiologies of spasticity (SCI, stroke, MS), undergoing perioperative BoNT injections in 3 different perioperative time periods: (1) preoperative, (2) intraoperative, and (3) postoperative. These patients were seen in our interdisciplinary spasticity management clinic in a tertiary center in Canada. Because this report is composed of individual case reports, Institutional Review Board approval was not required. Patient consent for publication has been obtained by the authors.
Case 1
Our first patient is a 64-year-old woman with a C6 American Spinal Injury Association Impairment Scale D incomplete hemicord SCI sustained after a motor vehicle collision 43 years ago. She was managed conservatively for her SCI. She adapted to her left-sided upper and lower limb weakness, reduced right-sided sensation, and hip adductor spasticity and was independent with all her activities of daily living.
She then developed worsening right hip joint pain secondary to osteoarthritis 5 years ago associated with an increase in her adductor spasticity affecting her gait.
She was subsequently referred to our spasticity clinic, and our orthopedic surgeon recommended a right-sided total hip replacement. We were concerned that surgery would increase her spasticity, thereby impairing her ability to maintain hip precautions such that she would be at an increased risk of hip dislocation and that this could impede her participation in postoperative rehabilitation.
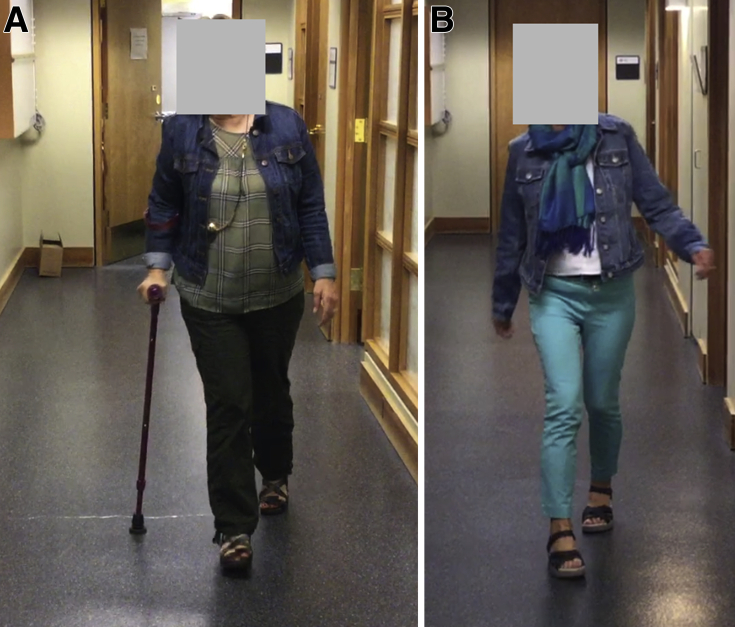
We collaborated with her orthopedic surgeon to plan for BoNT injections 2 weeks before her scheduled surgical date for her right total hip replacement. We injected incobotulinumtoxinA (Xeomina) reconstituted in a 2:1 dilution with normal saline, using the following protocol: 75 U into the hip adductors bilaterally and 50 U into the right tensor fasciae latae muscle (200 U total) using electrical stimulation (E-Stimb) guidance. The primary goal was to prevent postoperative complications such as dislocation. Additional goals included minimizing a postoperative increase in her spasticity and enabling early rehabilitation. All her goals were met, and she was able to participate in rehabilitation without delay. Figure 1 shows the patient in the double limb support phase of gait pre- and post operation. In the preoperative phase, she ambulated with a gait aid because of pain. Postoperatively, she no longer required a gait aid. Partly because of decreased pain with walking, she increased her physical activity and therefore lost weight.
Fig 1.
Pre- and postoperative double limb support phase of gait. The success of the total hip replacement allowed the patient to walk without a gait aid. Additionally, partly because of increased ambulatory endurance, she was able to lose weight.
Case 2
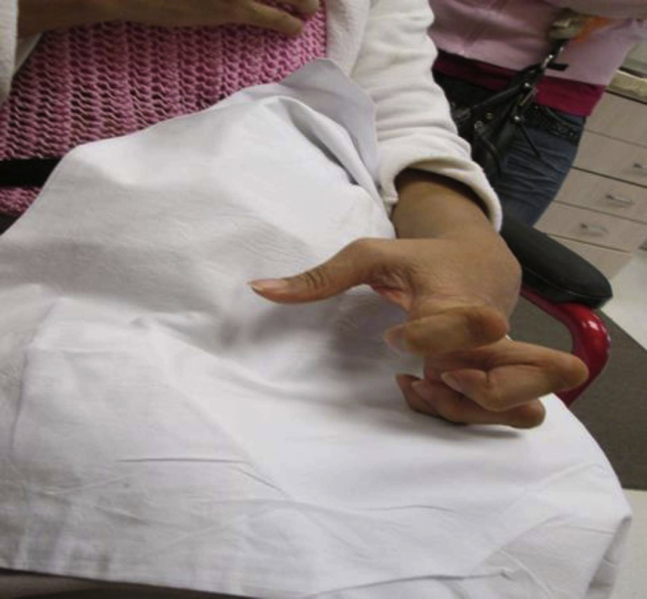
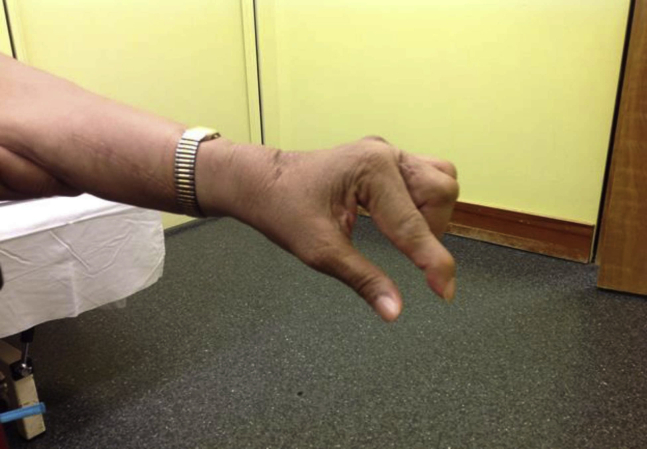
Our second patient is a 63-year-old right-hand-dominant woman with a history of tuberculosis meningitis and subsequent stroke, which occurred in 2006. The stroke caused dystonic posturing and spasticity in the left hand. When assessed in our spasticity clinic, she was noted to have poor proprioceptive control of her left hand in addition to dystonic posturing. Her posturing was characterized by wrist extension, metacarpophalangeal joint extension, interphalangeal joint flexion, and thumb extension. Figure 2 shows her hand before BoNT administration with onabotulinumtoxinA (Botoxc). She found BoNT reduced her pain from 8 of 10 to 6 of 10 on the visual analog scale and reduced her dystonic posturing by 50%. Despite the improved pain and decreased dystonic posturing, she was only able to use the left hand as a helper. We reviewed her case in March of 2014 with an upper limb orthopedic surgeon, and we collaboratively devised a plan for tendon transfer surgery. The patient underwent surgery involving the transfer of her left extensor carpi radialis brevis into the lateral bands of digits 2, 3, 4, and 5 with the hope of enabling finger extension at the proximal and distal interphalangeal joints. Unfortunately, after discharge home, severe wrist extension and finger flexion spasticity led to the destruction of her postoperative splint and failure of her tendon graft. The orthopedic surgeon assessed her and felt that muscle overactivity of her extensor digitorum communis and flexor digitorum superficialis led to the rupture of the tendon transfer. Thus, we made plans to perform BoNT injection at the time of her salvage surgery. She had emergency surgery performed for repair of the tendon ruptures. During this operation, 50 U of onabotulinumtoxinA were injected directly into her extensor digitorum communis, and 50 U were injected directly into flexor digitorum superficialis, for a total dose of 100 U. A 2:1 dilution was used for reconstitution. Postoperatively, she did well. She was able to wear the postoperative splint, she had only mild dystonia and spasticity, and the tendons healed well. There was a change in the appearance of the hand. She went from a claw hand to a near-neutral hand, a change she embraced. Figure 3 shows her hand postoperatively.
Fig 2.
Case 2 preoperative hand position.
Fig 3.
Case 2 postoperative hand positioning.
She gained function in the left hand after surgery and rehabilitation. She achieved the ability to carry out a pinch and key precision and cylindrical power grip. She continued to work with occupational therapy to improve her function in the left hand, and she used finger splints to manage the residual flexion deformity in digits 3-5. Further BoNT injections were deemed unnecessary, and no further surgical intervention was required.
Case 3
Our third patient is a 47-year-old woman diagnosed as having MS in 1998 and now secondary progressive MS with an Expanded Disability Status Scale score of 9. She developed spasticity in her hip adductors, which impaired pericare. She had repeated BoNT injections into her adductor brevis, adductor longus, semimembranosus, and semitendinosus muscles.
She had a chronic sacral wound with underlying osteomyelitis that worsened in early 2020. The wound required surgical closure by plastic surgery. She had a V-Y flap surgery that completely covered the open sacral wound. We were asked to see her on day 12 postoperatively to help manage her increasing adductor spasticity so as to help prevent dehiscence of the recent flap surgery. On examination, she had no volitional movement in her lower limbs and Modified Ashworth Scale grade 3 spasticity in her bilateral hip flexors, knee flexors, and hip adductors. Modified Tardieu Scale measurements are shown in table 1.
Table 1.
Case 1: Modified Tardieu spasticity measurements
| Muscle | Pre |
|||||
|---|---|---|---|---|---|---|
| Right |
Left |
|||||
| V0 | V1 | V3 | V0 | V1 | V3 | |
| Hamstrings | 90 | 110 | 95 | 90 | 100 | 95 |
| Rectus femoris | 110 | 135 | 120 | 110 | 10 | 120 |
| Adductors | Neutral | 25 | 20 | Neutral | 30 | 30 |
| Muscle | Post |
|||||
|---|---|---|---|---|---|---|
| Right |
Left |
|||||
| V0 | V1 | V3 | V0 | V1 | V3 | |
| Hamstrings | 100 | 125 | 120 | 90 | 140 | 140 |
| Rectus femoris | 110 | 135 | 120 | 110 | 150 | 140 |
| Adductors | Neutral | 40 | 20 | Neutral | 40 | 30 |
Our primary goal of treatment was to reduce the risk of skin shearing and dehiscence and promote wound healing of the flap surgery by decreasing her hip flexor, adductor, and hamstring spasticity using BoNT. Our secondary goals were to facilitate hygiene, facilitate dressing, facilitate catheterization, and ease positioning in her wheelchair. Twelve days after her surgery, we injected onabotulinumtoxinA using E-Stim and a 2:1 dilution into the following muscles: (right/left) rectus femoris (50U/50U), adductor longus (nil/50U), gracilis (50U/nil), and biceps femoris (50U/50U).
The combination of the surgery and postoperative BoNT was successful. Postoperatively her wound completely healed, and the underlying osteomyelitis resolved. Prior to the operation, she had been struggling with her sacral wound and associated osteomyelitis for approximately 2 years. By providing her with perioperative BoNT, we helped guide her toward a positive endpoint for her V-Y flap. This in turn resulted in improvement in her spasticity and a complete resolution of her sacral wound and associated osteomyelitis.
Discussion
To our knowledge, this is the first publication of 3 cases describing the use of perioperative BoNT in patients with spasticity from SCI, stroke, and MS. This is also the first case series describing the use of perioperative BoNT in 3 time periods including preoperative (2wk prior to surgery), intraoperative, and postoperative (12d post surgery). Case 1 demonstrates preoperative BoNT to minimize postoperative pain, reduce risk of postoperative hip dislocation, and maximize rehabilitation in a patient with spasticity secondary to a SCI. Case 2 demonstrates intraoperative BoNT to reduce the risk of tendon ruptures in a patient with spasticity secondary to a stroke after prior operative failure directly attributable to spasticity. Case 3 demonstrates postoperative BoNT to enable surgical wound healing in a patient with spasticity secondary to MS. All 3 cases demonstrated successful outcomes.
The optimal timing for perioperative BoNT is unknown, but different studies have described preoperatively up to 5 weeks presurgery,6,13,14 intraoperatively during the surgery,5,15 and postoperatively as early as 1 week post surgery.19 Our cases demonstrate an important role for perioperative BoNT in patients with spasticity undergoing surgical interventions where their spasticity could affect the surgical outcome. Our cases revealed that perioperative BoNT can potentially help decrease the risk of hip arthroplasty dislocation, increase postoperative rehabilitation participation, reduce postoperative pain, protect tendon repairs, decrease wound dehiscence, and improve wound healing post wound flap surgery. The heterogeneity of our 3 cases highlights the potential benefits of perioperative BoNT for different patient populations. This contributes to the current literature in which the majority of the case studies, retrospective case series, and RCTs have focused on the use of perioperative BoNT in patients, predominantly children, with CP. We recommend that perioperative BoNT be considered during operative planning for patients undergoing surgical interventions such as joint replacement, tendon repairs, and wound flap operations where postoperative spasticity could lead to complications or failures of these surgical procedures.
Our study is limited by the fact that it is a case series rather than an RCT or cohort study. Thus, we cannot draw firm conclusions about the efficacy of BoNT injections in the perioperative period. Rather, our aim is to raise awareness of the potential utility of perioperative BoNT for a wide variety of patient groups with spasticity and to stimulate further research. We have presented 3 spasticity cases from differing UMN etiologies (SCI, stroke, MS), each of these diagnoses may necessitate a unique treatment plan for perioperative toxin because of differences in their spasticity patterns.
Future directions
Despite the suggestion of utility in our case reports, there is a lack of high-quality studies on the perioperative use of BoNT in adult patients with spasticity. The timing of perioperative BoNT injections varied in our 3 cases. This is reflected in the lack of literature regarding optimal timing for the administration of perioperative BoNT in adults with spasticity. This timing requires standardization in future prospective studies. More data and studies are therefore required regarding the optimal timing, dosage, and possible reinjection intervals for perioperative BoNT.
The outcome measures identified from our cases included time of perioperative BoNT injection, pre- and postoperative BoNT range of motion, Modified Ashworth Scale, Modified Tardieu Scale, video/photograph pre- and post-BoNT injection, and visual analog scale pain scale. We suggest that future prospective and RCT studies include at the minimum these outcome measures as well as the Goal Attainment Scale20 when designing future research of perioperative BoNT to enhance outcomes in patients with spasticity.
Suppliers
-
a.
Xeomin; Merz Pharmaceuticals GmbH.
-
b.
E-Stim, Natus.
-
c.
Botox; Allergan.
Footnotes
Disclosures: Dr Finlayson reports personal fees from Allergan Inc and Ipsen Inc outside the submitted work. Dr Reebye reports personal fees from Allergan Inc, Ipsen Inc, and Merz Inc outside the submitted work. The other authors have nothing to disclose.
References
- 1.Pandyan A.D., Gregoric M., Barnes M.P. Spasticity: clinical perceptions, neurological realities and meaningful measurement. Disabil Rehabil. 2005;27:2–6. doi: 10.1080/09638280400014576. [DOI] [PubMed] [Google Scholar]
- 2.Fheodoroff K., Rekand T., Medeiros L. Quality of life in subjects with upper-and lower-limb spasticity treated with incobotulinumtoxinA. Health Qual Life Outcomes. 2020;18:1–10. doi: 10.1186/s12955-020-01304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rekand T. Clinical assessment and management of spasticity: a review. Acta Neurol Scand. 2010;122:62–66. doi: 10.1111/j.1600-0404.2010.01378.x. [DOI] [PubMed] [Google Scholar]
- 4.Genêt F., Denormandie P., Keenan M.A. Orthopaedic surgery for patients with central nervous system lesions: concepts and techniques. Ann Phys Rehabil Med. 2019;62:225–233. doi: 10.1016/j.rehab.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Will E., Magill N., Arnold R. Preoperative botulinum neurotoxin A for children with bilateral cerebral palsy undergoing major hip surgery: a randomized double-blind placebo-controlled trial. Dev Med Child Neurol. 2019;61:1074–1079. doi: 10.1111/dmcn.14145. [DOI] [PubMed] [Google Scholar]
- 6.Eibach S., Krug H., Lobsien E., Hoffmann K.T., Kupsch A. Preoperative treatment with botulinum toxin A before total hip arthroplasty in a patient with tetraspasticity: case report and review of literature. NeuroRehabilitation. 2011;28:81–83. doi: 10.3233/NRE-2011-0635. [DOI] [PubMed] [Google Scholar]
- 7.Negosanti L., Sanguinetti G., Gaiani L. Spinal cord injury patients with spasticity and pressure sores: preliminary report on reconstruction with botulinum toxin treated muscle flaps. Integr Mol Med. 2019;6:1–3. [Google Scholar]
- 8.Saeidiborojeni S., Mills P.B., Reebye R., Finlayson H. Perioperative botulinum neurotoxin injection to improve outcomes of surgeries on spastic limbs: a systematic review. Toxicon. 2020;188:48–54. doi: 10.1016/j.toxicon.2020.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Mannava S., Callahan M.F., Trach S.M. Chemical denervation with botulinum neurotoxin a improves the surgical manipulation of the muscle-tendon unit: an experimental study in an animal model. J Hand Surg Am. 2011;36:222–231. doi: 10.1016/j.jhsa.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Ma J., Shen J., Smith B.P., Ritting A., Smith T.L., Koman L.A. Bioprotection of tendon repair: adjunctive use of botulinum toxin A in Achilles tendon repair in the rat. J Bone Joint Surg Am. 2007;89:2241–2249. doi: 10.2106/JBJS.D.03054. [DOI] [PubMed] [Google Scholar]
- 11.Tuzuner S., Özkan Ö., Erin N., Özkaynak S., Cinpolat A., Özkan Ö. Effects of botulinum toxin A injection on healing and tensile strength of ruptured rabbit Achilles tendons. Ann Plast Surg. 2015;74:496–500. doi: 10.1097/SAP.0b013e31829aa2e1. [DOI] [PubMed] [Google Scholar]
- 12.McQueen K., Coonan T., Ottaway A. Anesthesia and perioperative care. In: Debas H.T., Donkor P., Gawande A., Jamison D.T., Kruk M.E., Mock C.N., editors. Essential surgery: disease control priorities. The World Bank; Washington (DC): 2015. p. 265. [Google Scholar]
- 13.Barwood S., Baillieu C., Boyd R. Analgesic effects of botulinum toxin A: a randomized, placebo-controlled clinical trial. Dev Med Child Neurol. 2000;42:116–121. doi: 10.1017/s0012162200000220. [DOI] [PubMed] [Google Scholar]
- 14.Dohin B., Garin C., Vanhems P., Kohler R. [Botulinum toxin for postoperative care after limb surgery in cerebral palsy children] [French] Rev Chir Orthop Reparatrice Appar Mot. 2007;93:674–681. doi: 10.1016/s0035-1040(07)73252-x. [DOI] [PubMed] [Google Scholar]
- 15.Kubat W.D., Rekant M. Botulinum toxin use as an adjunctive modality in a patient with multiple flexor tendon ruptures. Hand. 2008;3:237–239. doi: 10.1007/s11552-008-9091-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adler C.H., Zimmerman R.S., Lyons M.K., Simeone F., Brin M.F. Perioperative use of botulinum toxin for movement disorder-induced cervical spine disease. Mov Disord. 1996;11:79–81. doi: 10.1002/mds.870110114. [DOI] [PubMed] [Google Scholar]
- 17.Traynelis V.C., Ryken T., Rodnitzky R.L., Menezes A.H. Botulinum toxin enhancement of postoperative immobilization in patients with cervical dystonia. Technical note. J Neurosurg. 1992;77:808–809. doi: 10.3171/jns.1992.77.5.0808. [DOI] [PubMed] [Google Scholar]
- 18.Jameson R., Rech C., De Loubresse C.G. Cervical myelopathy in athetoid and dystonic cerebral palsy: Retrospective study and literature review. Eur Spine J. 2010;19:706–712. doi: 10.1007/s00586-009-1271-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang R.L., Ho C.K., Tremp M., Xie Y., Li Q., Zan T. Early postoperative application of botulinum toxin type A prevents hypertrophic scarring after epicanthoplasty: a split-face, double-blind, randomized trial. Plast Reconstr Surg. 2019;144:835–844. doi: 10.1097/PRS.0000000000006069. [DOI] [PubMed] [Google Scholar]
- 20.Turner-Stokes L. Goal attainment scaling (GAS) in rehabilitation: a practical guide. Clin Rehabil. 2009;23:362–370. doi: 10.1177/0269215508101742. [DOI] [PubMed] [Google Scholar]