Abstract
Background
Elevated levels of cardiac troponin T as measured by a high-sensitivity test (hscTnT) are common in geriatric patients with a large spectrum of comorbidities but without acute coronary syndrome (ACS). However, the relative contribution of individual comorbidities has never been clearly addressed. This study aimed to determine the relationship between hscTnT elevation as a response variable and individual comorbidities, and to estimate the impact of individual comorbidities on hscTnT elevation in geriatric patients free of ACS.
Methods
A nonexperimental, retrospective, matched, longitudinal cohort study was designed to evaluate the files of 7062 geriatric patients (aged ≥ 65 years) without ACS. The hscTnT levels of the patients have already been measured in all evaluated medical records. The dataset was split into 2 groups (0 and 1) based on the individual comorbidity (0 and 1) and hscTnT levels (≤ 14 ng/L = 0 and > 14 ng/L = 1).
Results
Our results show that although age was positively and significantly correlated with hscTnT (r = 0.17, P < 0.0001), the likelihood of experiencing elevated hscTnT levels in older individuals after having excluded ACS was related to the presence of comorbidities independently of their number (P < 0.0001). The regression coefficients (β) associated with renal insufficiency (0.71), cardiomyopathy (0.63), chronic obstructive pulmonary disease (0.30), diabetes (0.25), and anemia (0.22) indicated that there exists a significant association between these comorbidities and the elevated hscTnT levels (P < 0.001). The receiver operating characteristic curve for predictive modeling was estimated at 71% (P < 0.0001).
Conclusions
Elevated hscTnT levels were mostly associated with renal insufficiency, cardiac myopathies, chronic obstructive pulmonary disease, diabetes, and anemia in geriatric patients without ACS. Developing guidelines to accurately evaluate hscTnT elevation in geriatric patients with comorbidities, without ACS, is clinically essential.
Résumé
Contexte
Il arrive fréquemment que les patients âgés présentant un grand éventail d’affections concomitantes, mais pas de syndrome coronarien aigu (SCA), aient un taux élevé de troponine T cardiaque mesuré par un test de haute sensibilité (TnTc-hs). La contribution relative des différentes affections concomitantes prises individuellement n’a toutefois jamais été clairement évaluée. Cette étude a tenté de déterminer la relation entre l’élévation du taux de TnTc-hs en tant que variable réponse et différentes affections concomitantes, et d’estimer l’effet individuel de ces affections concomitantes sur le taux de TnTc-hs chez les patients âgés exempts de SCA.
Méthodologie
Nous avons conçu une étude de cohorte longitudinale, non expérimentale, rétrospective et avec appariement afin d’évaluer les dossiers de 7 062 patients âgés (≥ 65 ans) ne présentant pas de SCA. Les taux de TnTc-hs des patients avaient déjà été mesurés et figuraient dans tous les dossiers médicaux examinés. Les données ont été séparées en 2 groupes (0 et 1) en fonction de la présence d’une affection concomitante particulière (0 et 1) et du taux de TnTc-hs (≤ 14 ng/l = 0 et > 14 ng/l = 1).
Résultats
Nos résultats indiquent que bien que l’âge soit corrélé de manière positive et significative avec le taux de TnTc-hs (r = 0,17, p < 0,0001), la probabilité qu’une personne âgée présente un taux de TnTc-hs élevé alors qu’un SCA a été exclu est liée à la présence d’affections concomitantes, indépendamment de leur nombre (p < 0,0001). Les coefficients de régression (β) associés à l’insuffisance rénale (0,71), à la cardiomyopathie (0,63), à la maladie pulmonaire obstructive chronique (0,30), au diabète (0,25) et à l’anémie (0,22) indiquent qu’il existe un lien significatif entre ces affections et un taux élevé de TnTc-hs (p < 0,001). La courbe caractéristique de la performance d’un test correspondant à la modélisation prédictive a été estimée à 71 % (p < 0,0001).
Conclusions
Chez les patients âgés exempts de SCA, les taux élevés de TnTc-hs étaient principalement associés à l’insuffisance rénale, à la cardiomyopathie, à la maladie pulmonaire obstructive chronique, au diabète et à l’anémie. Il est donc essentiel, sur le plan clinique, d’établir des lignes directrices permettant de mesurer avec précision l’élévation du taux de TnTc-hs chez les patients âgés présentant des affections concomitantes, mais exempts de SCA.
Although cardiac troponin T and I are the biomarkers of choice for detecting acute coronary syndrome (ACS) events,1 available data are scarce with respect to levels of cardiac troponin T from the high-sensitivity test (hscTnT) in older adult patients with comorbidities but without ACS.
Recent studies have shown that several noncoronary diseases cause elevated hscTnT levels in geriatric patients without ACS.2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 Although the significance of the effect of individual comorbidities on hscTnT elevation is a crucial and often-overlooked aspect of geriatric patient care, it has never been clearly addressed.
A recent study reported that elevated hscTnT levels in the absence of ACS are independently associated with age.17 However, the study did not verify the impact of other individual comorbidities on hscTnT elevation.
The significance of comorbid health conditions should be addressed because of their high prevalence in older adult populations18 and because of the high prevalence of ACS among the older adults.19,20 Indeed, elevated hscTnT levels are common in older adult patients within a large spectrum of clinical settings and comorbidities.3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 It is thus crucial for clinicians to have a systemic approach for accurately assessing the impact of hscTnT elevation.
Interpreting elevated hscTnT levels in terms of individual comorbidities in older adult patients is a major challenge. In addition, the presence of multiple chronic conditions in older adult patients may result in ''atypical'' or ''asymptomatic'' presentations.21
It should be emphasized that atypical presentation of ACS not only is more frequent in older adult patients but also results in higher mortality rates than it does in younger patients.22
The aims of the present study were, first, to determine the relationship between hscTnT levels (elevated or low) and comorbidities, after having excluded the presence of ACS, and second, to evaluate the predictive power of a model associating hscTnT levels with a comorbidity in order to predict, based on comorbidities, the hscTnT levels of geriatric patients presenting at an emergency room.
Methods
Study design and population
A nonexperimental, retrospective, matched, longitudinal multicenter cohort study of patient medical records between January 2012 and December 2016 was conducted. The study population (7062 patients) consisted of all older adult patients admitted to the emergency department or to the hospital of the Sherbrooke University Health Center, or elderly patients who were hospitalized for any reason, for ACS that was suspected but not confirmed in the final diagnosis. An Excel data entry form has been used to collect each medical record meeting the inclusion criteria from the standard format of the Sherbrooke University Health Center electronic database. The selection of patient files retained for this study was carried out in a separate manner by 2 physicians, and any disagreement was resolved by discussion and reevaluation by all authors.
Selection of participants
A medical record was included in the present study if the patient presented to the emergency room or evaluated at the hospital with suspected ACS. Only medical records of patients for whom hscTnT had been measured were selected, and only geriatric patients (aged ≥ 65 years) whose final diagnosis confirming the absence of ACS were retained for this study (7062 patients). The exclusion of ACS in the studied population was confirmed in their medical record by the attending physician. The latter also documented diagnoses of comorbidities in the medical records of the 7062 geriatric patients. In order to reduce the impact of any known ACS that could affect hscTnT elevation, the medical records of patients with confirmed coronary artery diseases were also excluded. Finally, duplicate medical records of patients and that of a patient who suffered out-of-hospital/in-hospital cardiac arrest were excluded, reducing the sample size of the study to 6822.
hscTnT assay
The level of hscTnT was measured using an electrochemiluminescence immunoassay and an Elecsys analyzer (Troponin T Stat; Roche Diagnostics, F. Hoffmann-La Roche Ltd., Basel, Switzerland) with a limit of detection of 3 ng/L, a 99th-percentile cutoff point of 14 ng/L, and a coefficient of variation of < 10 at 13 ng/L.23 Only the first documented measurement of hscTnT was retained. A high troponin value in the geriatric population was defined as > 14 ng/L.
Variables
The variables were separated into the response variable (the first hscTnT measurement, collected at time of admission to the hospital or emergency department) and the categorical variables. The categorical variables were 14 comorbidities: anemia, cerebrovascular accident (CVA), cancer, cardiomyopathy (CM), diabetes mellitus (DM), pulmonary embolism, subarachnoid hemorrhage (SAH), hypertension (HTN), pulmonary hypertension (PHTN), hypothyroidism, pneumonia, chronic obstructive pulmonary disease (COPD), renal insufficiency (RI), and arteriosclerotic vascular disease (ASVD). The considered comorbidities in the present study have been individually adopted from the Canadian Clinical Practice Guidelines.24
Smoking status was included as a confounding variable for patients who smoked ≥ 25 cigarettes a day as indicated in their medical record.
Statistical analysis
Continuous variables were expressed as means, standard deviations, and standard errors. Pearson's correlation test was used for the response variable. An independent 2-sample t test was conducted to compare hscTnT levels in the presence vs absence of an individual comorbidity. Yates’ χ2 test was used to evaluate differences in the prevalence of comorbidities based on hscTnT levels. Multiple logistic regressions were used to evaluate hscTnT outcomes; other categorical variables were used as well. Machine learning (ML) analytical methods were used to predict an appropriate statistical model directly from the database without any prior assumptions about a predefined equation. The statistical significance level was set at P < 0.01. All analyses were done in R 3.6.1.
Results
The mean age of our population was 78.39 ± 8.36 years, and the mean hscTnT value was 29.51 ± 20.7 ng/L (Supplemental Table S1). Two patients in our database had the same maximum age of 104 years: one man with an hscTnT value of 42 ng/L, and 4 comorbidities (anemia, SAH, HTN, and pneumonia); and one woman with an hscTnT value of 30 ng/L who suffered from Alzheimer’s disease and 5 other comorbidities (CVA, HTN, RI, hypothyroidism, and pneumonia). The assumption of normality was rejected by the Anderson-Darling test (P < 0.0001). Our result showed that the hscTnT levels were not normally distributed (Supplemental Fig. S1).
After changing the sex, hscTnT levels, and comorbidities to binary values, the patients were stratified into 2 subgroups based on hscTnT levels. Table 1 lists the characteristics of the patients with a comorbidity (including smoking) stratified by hscTnT level. The highest and lowest hscTnT levels were associated with CM and cancer, respectively. The ranges of hscTnT levels in the presence and absence of each categorical variable are displayed as boxplots (Supplemental Fig. S2).
Table 1.
Patient characteristics with respect to the presence of a comorbidity stratified by hscTnT concentration
| Variables | n (%) | Mean | SD | SE |
|---|---|---|---|---|
| hscTnT > 14ng/L | 5039 (73.9) | 37.05 | 19.95 | 0.281 |
| hscTnT ≤ 14ng/L | 1783 (26.1) | 9.74 | 2.84 | 0.067 |
| HTN | 5488 (80.5) | 22.02 | 21.94 | 0.296 |
| RI | 3326 (48.7) | 33.57 | 18.50 | 3.208 |
| Anemia | 2852 (41.8) | 19.40 | 18.50 | 0.346 |
| COPD | 2805 (30.5) | 28.35 | 18.09 | 0.342 |
| DM | 2354 (34.5) | 26.67 | 18.64 | 0.384 |
| Hypothyroidism | 2122 (31.1) | 21.74 | 18.73 | 0.407 |
| Pneumonia | 2030 (29.7) | 23.51 | 18.59 | 0.413 |
| Cancer | 1230 (33.7) | 18.78 | 22.71 | 0.648 |
| PHTN | 960 (14) | 24.13 | 12.44 | 0.401 |
| CVA | 844 (12.4) | 21.20 | 18.06 | 0.622 |
| ASVD | 607 (8.9) | 27.33 | 14.01 | 0.569 |
| SAH | 582 (8.5) | 23.66 | 13.10 | 0.543 |
| PE | 539 (7.9) | 29.63 | 12.44 | 0.536 |
| CM | 502 (7.4) | 36.01 | 16.79 | 0.749 |
| Smoking | 148 (2.2) | 12.03 | 8.13 | 0.668 |
ASVD, and arteriosclerotic vascular disease; CM, cardiomyopathy; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; DM, diabetes mellitus; hscTnT, cardiac troponin T from the high-sensitivity test; HTN, hypertension; PE, pulmonary embolism; PHTN, pulmonary hypertension; RI, renal insufficiency; SAH, subarachnoid hemorrhage; SD, standard deviation; SE, standard error.
As can be seen in Figure 1, age was positively and significantly correlated with hscTnT for the 2-tailed hypothesis (R2 = 0.17, n = 6820, P < 0.0001).
Figure 1.
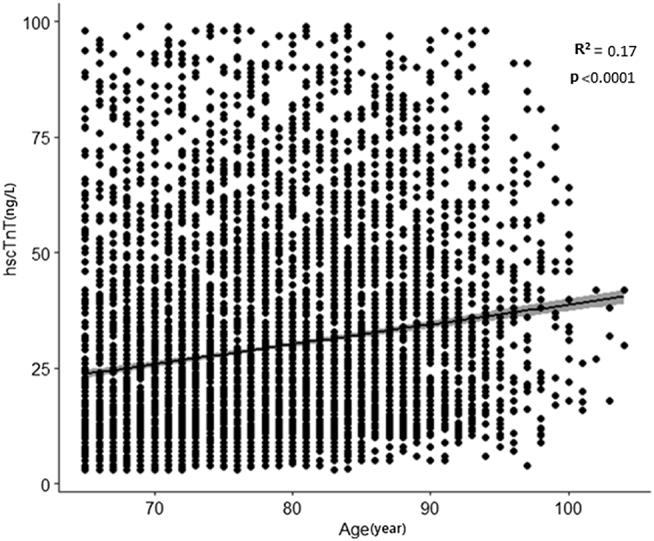
Scatterplot of age (year) vs concentration of cardiac troponin T as measured by a high-sensitivity test (hscTnT), and fitted regression line.
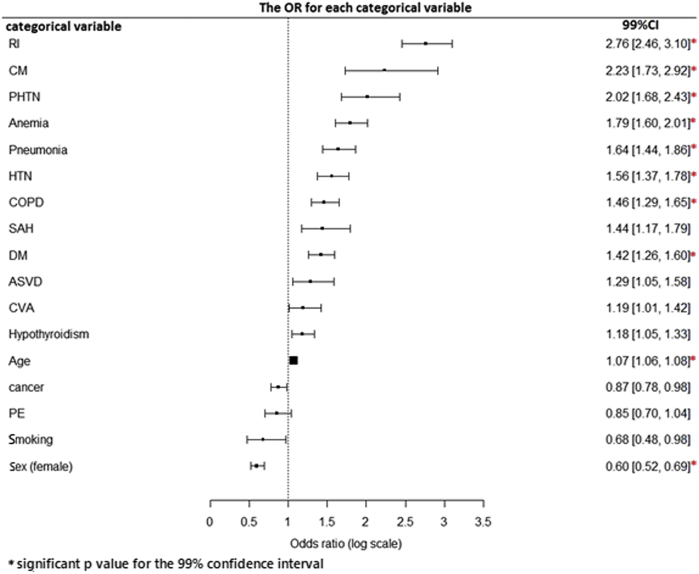
RI, CM, PHTN, hypothyroidism, anemia, pneumonia, HTN, COPD, SAH, DM, ASVD, CVA, and age were significantly associated with a greater odds ratio of having an elevated hscTnT level (Table 2). The forest plot in Figure 2 shows the estimated odds ratio of associating each comorbidity with an elevated hscTnT level.
Table 2.
OR estimation and CI for each categorical variable
| Explicative variable | OR | CI (99%) | P |
|---|---|---|---|
| RI | 2.759613 | 2.459986, 3.098012 | < 0.0001 |
| CM | 2.233875 | 1.730249, 2.917241 | < 0.0001 |
| PHTN | 2.016835 | 1.682592, 2.428753 | < 0.0001 |
| Hypothyroidism | 1.839311 | 1.051194, 1.334454 | < 0.01 |
| Anemia | 1.792583 | 1.598966, 2.010891 | < 0.0001 |
| Pneumonia | 1.638679 | 1.444997, 1.860625 | < 0.0001 |
| HTN | 1.560791 | 1.369257, 1.777863 | < 0.0001 |
| COPD | 1.461346 | 1.292729, 1.653674 | < 0.0001 |
| SAH | 1.442762 | 1.168971, 1.791256 | < 0.001 |
| DM | 1.419833 | 1.262365, 1.598257 | < 0.0001 |
| ASVD | 1.289541 | 1.054831, 1.584422 | 0.01 |
| CVA | 1.194432 | 1.007535, 1.420380 | 0.03819 |
| Age | 1.071711 | 1.061858, 1.081788 | < 0.0001 |
| Cancer | 0.872834 | 0.779055, 0.978249 | 0.01786 |
| PE | 0.852775 | 0.701055, 1.040778 | 0.1056 |
| Smoking | 0.680201 | 0.478510, 0.976310 | 0.0313 |
| Sex (female) | 0.601462 | 0.520871, 0.694027 | < 0.0001 |
ASVD, and arteriosclerotic vascular disease; CI, confidence interval; CM, cardiomyopathy; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; DM, diabetes mellitus; HTN, hypertension; OR, odds ratio; PE, pulmonary embolism; PHTN, pulmonary hypertension; RI, renal insufficiency; SAH, subarachnoid hemorrhage.
Figure 2.
Forest plot showing the odds ratio (OR) associated with the level of cardiac troponin T as measured by a high-sensitivity test (hscTnT) in the presence of each categorical variable. ASVD, arteriosclerotic vascular disease; CI, confidence interval; CM, cardiomyopathy; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident DM, diabetes mellitus; HTN, hypertension; PE, pulmonary embolism; PHTN, pulmonary hypertension RI, renal insufficiency; SAH, subarachnoid hemorrhage.
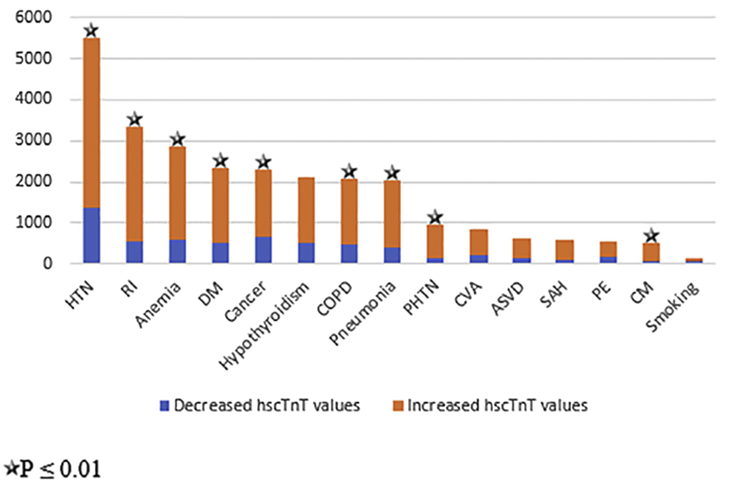
Yates's χ2 test and Fisher's exact test were used to evaluate differences in the prevalence of a comorbidity according to the hscTnT level (Supplemental Table S2). There was a significant relationship between anemia, cancer, CM, DM, HTN, PHTN, RI, COPD, and pneumonia, with elevated hscTnT levels (P < 0.0001; Supplemental Table S2).
Figure 3 shows the categorical variables (indicated with stars) that were significantly associated with elevated hscTnT levels. To make the variables more suitable for the multiple regression, the natural log of the odds was used. The primary regression equation is thus given as follows:
Figure 3.
Changes in level of cardiac troponin T as measured by a high-sensitivity test (hscTnT) for each categorical variable. ASVD, arteriosclerotic vascular disease; CI, confidence interval; CM, cardiomyopathy; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; DM, diabetes mellitus; HTN, hypertension; PE, pulmonary embolism; PHTN, pulmonary hypertension RI, renal insufficiency; SAH, subarachnoid hemorrhage.
Logit (probability of having an elevated hscTnT level) = Intercept + (a) age + (b) sex + (c) anemia + (d) CVA + (f) cancer + (g) CM + (h) DM + (i) PE + (j) SAH + (k) HTN + (l) PHTN + (m) hypothyroidism + (n) RI + (o) ASVD + (p) COPD + (q) pneumonia + (r) smoking. The letters in parentheses represent the suggested coefficient (β) for each category.
A binary multiple logistic regression was used to test predictors of the outcome variable and to create the risk-predictive model. The results of the association between all the categorical variables (the presence of a comorbidity) and elevated hscTnT levels are determined (Supplemental Table S3). The main results indicated that age, sex (male), and cancer had a positive association with elevated hscTnT levels (P ≤ 0.01), whereas anemia, CM, DM, PHTN, RI, and COPD together with intercept had a negative association with hscTnT levels.
Based on the first analytical outcome, we retained only the significant variables in our analysis. The primary regression equation for hscTnT resulted in the following equation:
Logit (probability of having elevated hscTnT levels) = Intercept + (a) age + (b) sex + (c) RI + (d) CM + (f) DM + (g) anemia + (h) COPD + (i) cancer. The letters in parentheses represent the coefficient (β) for each category.
After dividing our database randomly into train and test, the coefficients to estimate the values were determined. The associations between significant categorical variables and elevated hscTnT levels were then reassessed to develop an algorithm to determine the accuracy of our equation (Table 3).
Table 3.
Reduced multiple logistic regression coefficients of the categorical variables
| Coefficient | Estimation | SD | z | P |
|---|---|---|---|---|
| Intercept | –4.299439 | 0.352472 | –12.198 | < 0.0001 |
| Age | 0.644846 | 0.004619 | 14.039 | < 0.0001 |
| Sex (female) | 0.463643 | 0.071306 | –6.502 | < 0.0001 |
| Anemia | 0.219002 | 0.077597 | 2.822 | < 0.0001 |
| Cancer | –0.23736 | 0.073528 | –3.228 | 0.001 |
| CM | 0.62755 | 0.156162 | 4.109 | < 0.0001 |
| DM | 0.245569 | 0.076868 | 3.193 | 0.001 |
| RI | 0.712729 | 0.077121 | 9.242 | < 0.0001 |
| COPD | 0.30381 | 0.080064 | 3.794 | < 0.0001 |
CM, cardiomyopathy; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; RI, renal insufficiency; SD, standard deviation.
As can be seen in Table 3, of the 14 categorical variables, only anemia, cancer, CM, DM, RI, COPD, sex (female), and age had a significant impact on cTnT levels. The fitted estimated equation is given as:
Logit (probability of having elevated hscTnT levels in a geriatric patient without ACS) = –4.299439 + (0.644846) age – 0.463643 (if female) + (0.219002) anemia – 0.23736 (for any type of cancer) + (0.627550) CM + (0.245569) DM + (0.712729) RI + (0.303810) COPD.
To calculate the highest true-positive rate (sensitivity) and the lowest false-positive rate of the reduced model, a confusion matrix for hscTnT (kappa = 0.1856, McNamara’s test P value < 0.0001) was calculated.
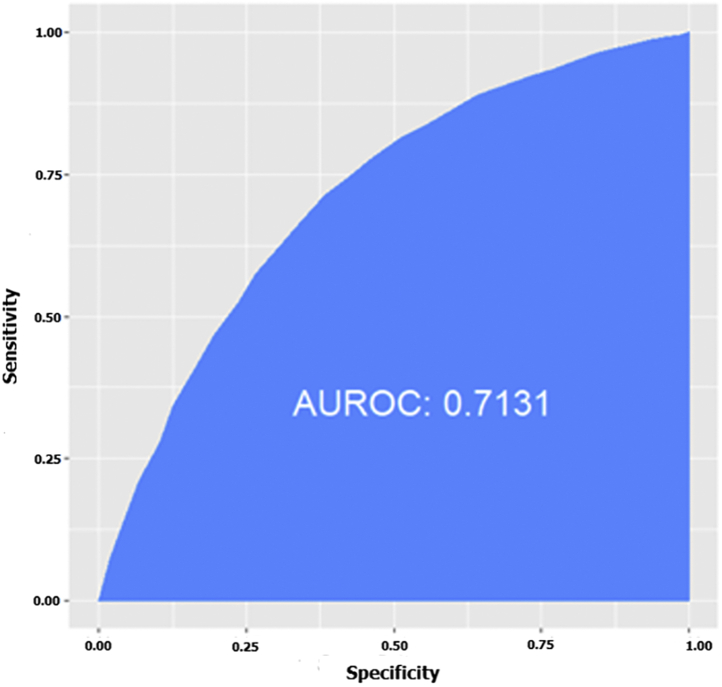
The Hosmer-Lemeshow test was used as a goodness-of-fit test for our risk-predictive model. The results of the Hosmer-Lemeshow test showed that our predictive model is a good fit for the data (χ2 = 25.30, df = 8, P = 0.001). The receiver operating characteristic curve of the data-driven predictive equation was then calculated in order to summarize the performance of the proposed model over all possible thresholds. Based on the curve predictive model, the usefulness of the reduced multiple logistic regression was estimated at about 71% (area under the receiver operating characteristic curve = 0.71, 99% confidence interval [0.70-0.89]; Fig. 4).
Figure 4.
Receiver operating characteristics and area under the receiver operating characteristics (AUROC) curve illustrating the performance of the predictive model in predicting elevation in level of cardiac troponin T as measured by a high-sensitivity test.
Given the heterogeneous nature of the comorbidities and the need to identify the impact of individual comorbidities on hscTnT levels more precisely, different ML methods were used to predict or explore and classify comorbidity subtypes. The following classifiers were used to pick the best predictive model: Adabooster, BernolliNB, Extra Trees, GaussianNB, Gaussian Process, K-(Nearest) Neighbors, Logistic Regression, MultinomialNB, Neural Network, RandomForest, Ridge, and Support Vector Classifier (SVC) (Supplemental Fig. S3). The multiple logistic regression used in the present study was the preferred ML model because its accuracy for the test and train datasets was approximately equal, and was greater than 75%. It was thus similar to or more robust and accurate than the other models.
Discussion
This study aimed, first, to determine the relationship between hscTnT as a response variable and individual comorbidities, and to estimate which comorbidity had the strongest impact on hscTnT in a geriatric population. The exact mechanism of comorbidity-induced hscTnT elevation is not well understood24 and remains to be proven; however, a wide spectrum of clinical conditions are known to cause hscTnT elevation.
Although hscTnT elevation has been studied with respect to CVA,3 CM, HTN,4,5 cancer,6,7 RI,8,9 COPD,10, 11, 12, 13 PHTN,14 septic shock,15 and epileptic seizures,16 the use of simultaneous combinations of different comorbidities to estimate the relative contribution of each has not been addressed. Given the higher prevalence of multimorbidities in geriatric populations,25 the present study was designed to predict the impact of individual comorbidities on hscTnT levels. To our knowledge, this study is the first to document 14 comorbidities in older adult patients with elevated hscTnT levels and to assess the relative impact of each comorbidity on HscTnT elevation.
The primary data analysis showed that 73.1% of the geriatric patients had elevated hscTnT levels without ACS, and had 14 multiple comorbidities compared to 7 in a previous study.26
The conventional cutoff value (99th percentile cutoff point of 14 ng/L) did not provide enough accuracy in our older adult patients with comorbidities. Given this, the 99th percentile cutoff point for hscTnT levels in the presence of a given comorbidity in a geriatric population without ACS should be reassessed. In other words, the diagnostic cutoff value for this population should take into consideration the impacts of age and comorbidities, given that elevated hscTnT levels are associated with the presence of pre-existing silent diseases that might not be detected when older adult patients present at the emergency room to have their hscTnT levels measured.
Unlike previous studies that considered age as the only factor with a significant impact on hscTnT elevation,26,27 the present study showed that various comorbidities can have a much more significant intrinsic impact on hscTnT levels than age. Given this, the importance of age as a cause of elevated hscTnT levels should be reconsidered in the presence of a comorbid condition. The present study, which used several statistical analysis methods, showed that age had the least impact on hscTnT elevation (odds ratio = 1.07, 99% confidence interval [1.06185, 1.081788], P < 0.0001), in agreement with a previous study.17
Like previous studies24,28 the present study showed that base hscTnT levels are higher in men than in women, indicating that being female may protect against hscTnT elevation to a certain degree. However, a recent study reported that variable thresholds for abnormal troponin levels related to sex indicated that as many as 5 times more women than the number identified using current thresholds may have elevated hscTnT levels.29
Elevated cTnT levels associated with breast cancer,7 epidermoid carcinoma, rhabdomyosarcoma, and metastatic forms of cancers6 have been reported. The present study showed that although hscTnT levels were elevated in cancer patients, they were lower than those in the other subgroups (β = –0.23736, P = 0.001). This result may be related to the effectiveness of the cardiotoxic effect of the chemotherapy used (eg, doxorubicin) and may explain the lack of correlation between hscTnT levels and other silent diseases. Elevated hscTnT levels may thus indicate the presence of a residual cancer or may be a response to a cancer treatment.
The elevated hscTnT levels observed in RI8,9 may be used as a parameter for determining the severity of RI or predicting RI mortality.28 In our suggestive logistic model, RI had the strongest impact on hscTnT levels (β = 0.712729, P < 0.0001), whereas cancer had the lowest impact. It is thus crucial to clarify the role of hscTnT levels in RI,30 regardless of whether they are elevated or not, and to determine how elevated hscTnT levels influence the long-term outcomes of RI. Given the relatively high prevalence of elevated hscTnT levels associated with RI, these issues should be the topic of future research.
Based on the data-driven predictive equation and the comorbidity-caused elevation of hscTnT levels observed in the present study, RI (β = 0.712729, P < 0.0001), CM (β = 0.62755, P < 0.0001), COPD (β = 0.30381, P < 0.0001), DM (β = 0.245569, P < 0.0001), and anemia (β = 0.219002, P < 0.0001) contribute the most to the elevated hscTnT levels in geriatric patients without ACS.
Although age has been associated with hscTnT elevation in geriatric patients without ACS, the present study showed that RI plays the most prominent role in hscTnT elevation among all the categorical variables studied (β = 0.712729, P < 0.0001 for RI vs β = 0.644846, P < 0.0001 for age).
Conclusions
Elevated hscTnT levels in geriatric patients without ACS should be attributed to the presence of silent comorbidities and should prompt a thorough evaluation to identify these asymptomatic diseases. Based on the present study, it is vital to emphasize that hscTnT could be released as a result of extracardiac diseases in older adults.
Geriatric patients without ACS but with known or unknown comorbidities and elevated hscTnT levels represent a significant portion of patient referrals to emergency rooms. This patient volume warrants the development of accurate guidelines for assessing hscTnT elevation. The management of geriatric patients with multimorbidities and elevated hscTnT levels requires further investigations.
Study strengths
Our cohort includes a sufficient number of geriatric patients, with 14 prevalent comorbidities overall, to address the research objectives adequately. In addition, the data-driven results can be generalized to and across the same population.
The present study is unparalleled with respect to the number of comorbidities evaluated. To our knowledge, it assesses the impact of the largest number of comorbidities ever evaluated in a single predictive model.
An important aspect of the present study is that it includes a strong predictive model that increases the probability that hscTnT elevation is not due to ACS, which has profound clinical implications. It contradicts existing literature with strong supporting arguments with respect to the relationship between elevated hscTnT levels and age. It refutes any significant impact of age on hscTnT level and suggests that the hscTnT elevation observed in geriatric patients is most likely due to the increased prevalence of various comorbidities in that population.
The elderly population investigated in the present study was very diversified in terms of age (65-104 years old), which increases the generalizability of the results.
Study limitations
This retrospective study was based on the collection of data from the electronic medical records of geriatric patients. We adjusted for smoking with multiple analyses, but likely could not account for all confounding variables, such as severity of each comorbidity. Indeed, the severity of the underlying comorbidity is likely to be relevant to understanding its impact on cardiac stress, and in turn, hscTnT elevations. Although we cannot be absolutely certain that all patients with ACS were excluded, to the best of our capacity, we have excluded patients with primary and secondary diagnoses of ACS, based on the medical records.
Future directions
A comprehensive interpretation of hscTnT elevation may provide a better understanding of the physio-pathologic mechanism of comorbidity-induced hscTnT elevation in older adults. In addition, future studies should be designed to determine the cutoff for hscTnT levels in the presence of multimorbidities, and to determine the relationship between mortality and elevated hscTnT levels in older adult patients, specifically with respect to comorbidities.
Funding Sources
This project was supported by a grant from the Canadian Institutes of Health Research (PJT-162366).
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: The present study complied with the ethical principles of the Declaration of Helsinki and was approved by the local ethics committee of CIUSSS de l'Estrie-CHUS in June 2019 (F9 - 25363).
See page 254 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2020.07.017.
Supplementary Material
References
- 1.Thygesen K., Alpert J.S., Jaffe A.S. Fourth universal definition of myocardial infarction. Glob Heart. 2018;13:305–338. doi: 10.1016/j.gheart.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Vestergaard K.R., Jespersen C.B., Arnadottir A. Prevalence and significance of troponin elevations in patients without acute coronary disease. Int J Cardiol. 2016;222:819–825. doi: 10.1016/j.ijcard.2016.07.166. [DOI] [PubMed] [Google Scholar]
- 3.VanHouten J., Fricker G., Collins B. Circulating troponin I level in patients with acute ischemic stroke. Curr Neurol Neurosci Rep. 2018;18:32. doi: 10.1007/s11910-018-0842-6. [DOI] [PubMed] [Google Scholar]
- 4.Kubo T., Kitaoka H., Yamanaka S. Significance of high-sensitivity cardiac troponin T in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2013;62:1252–1259. doi: 10.1016/j.jacc.2013.03.055. [DOI] [PubMed] [Google Scholar]
- 5.Kaypakli O., Gur M., Gozukara M.Y. Association between high-sensitivity troponin T, left ventricular hypertrophy, and myocardial performance index. Herz. 2015;40:1004–1010. doi: 10.1007/s00059-015-4322-3. [DOI] [PubMed] [Google Scholar]
- 6.Johnston J.R., Chase P.B., Pinto J.R. Troponin through the looking-glass: emerging roles beyond regulation of striated muscle contraction. Oncotarget. 2017;9:1461–1482. doi: 10.18632/oncotarget.22879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simoes R., Silva L.M., Cruz A. Troponin as a cardiotoxicity marker in breast cancer patients receiving anthracycline-based chemotherapy: a narrative review. Biomed Pharmacother. 2018;107:989–996. doi: 10.1016/j.biopha.2018.08.035. [DOI] [PubMed] [Google Scholar]
- 8.McGill D., Talaulikar G., Potter J.M., Koerbin G., Hickman P.E. Over time, high-sensitivity TnT replaces NT-proBNP as the most powerful predictor of death in patients with dialysis-dependent chronic renal failure. Clin Chim Acta. 2010;411:936–939. doi: 10.1016/j.cca.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 9.De Zoysa J.R. Cardiac troponins and renal disease. Nephrology (Carlton) 2004;9:83–88. doi: 10.1111/j.1440-1797.2003.00235.x. [DOI] [PubMed] [Google Scholar]
- 10.Neukamm A.M., Hoiseth A.D., Hagve T.A., Soyseth V., Omland T. High-sensitivity cardiac troponin T levels are increased in stable COPD. Heart. 2013;99:382–387. doi: 10.1136/heartjnl-2012-303429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harvey M.G., Hancox R.J. Elevation of cardiac troponins in exacerbation of chronic obstructive pulmonary disease. Emerg Med Australas. 2004;16:212–215. doi: 10.1111/j.1742-6723.2004.00589.x. [DOI] [PubMed] [Google Scholar]
- 12.Manikkan A.T. Elevated troponin I levels in diabetic ketoacidosis without obstructive coronary artery disease. J Endocr Soc. 2018;19:1020–1023. doi: 10.1210/js.2018-00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lankeit M., Friesen D., Aschoff J. Highly sensitive troponin T assay in normotensive patients with acute pulmonary embolism. Eur Heart J. 2010;31:1836–1844. doi: 10.1093/eurheartj/ehq234. [DOI] [PubMed] [Google Scholar]
- 14.Filusch A., Giannitsis E., Katus H.A., Meyer F.J. High-sensitive troponin T: a novel biomarker for prognosis and disease severity in patients with pulmonary arterial hypertension. Clin Sci (Lond) 2010;119:207–213. doi: 10.1042/CS20100014. [DOI] [PubMed] [Google Scholar]
- 15.Rosjo H., Varpula M., Hagve T.A. Circulating high sensitivity troponin T in severe sepsis and septic shock: distribution, associated factors, and relation to outcome. Intensive Care Med. 2011;37:77–85. doi: 10.1007/s00134-010-2051-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sieweke N., Allendorfer J., Franzen W. Cardiac troponin I elevation after epileptic seizure. BMC Neurol. 2012;12:58. doi: 10.1186/1471-2377-12-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sedighi S.M., Prud'Homme P., Ghachem A. Increased level of high-sensitivity cardiac troponin T in a geriatric population is determined by comorbidities compared to age. Int J Cardiol Heart Vasc. 2019;22:187–191. doi: 10.1016/j.ijcha.2019.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van den Akker M., Buntinx F., Metsemakers J.F., Roos S., Knottnerus J.A. Multimorbidity in general practice: prevalence, incidence, and determinants of co-occurring chronic and recurrent diseases. J Clin Epidemiol. 1998;51:367–375. doi: 10.1016/s0895-4356(97)00306-5. [DOI] [PubMed] [Google Scholar]
- 19.Alexander K.P., Newby L.K., Cannon C.P. Acute coronary care in the elderly, part I: Non-ST-segment-elevation acute coronary syndromes: a scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology: in collaboration with the Society of Geriatric Cardiology. Circulation. 2007;115:2549–2569. doi: 10.1161/CIRCULATIONAHA.107.182615. [DOI] [PubMed] [Google Scholar]
- 20.Saunderson C.E., Brogan R.A., Simms A.D. Acute coronary syndrome management in older adults: guidelines, temporal changes and challenges. Age Ageing. 2014;43:450–455. doi: 10.1093/ageing/afu034. [DOI] [PubMed] [Google Scholar]
- 21.Rittger H., Rieber J., Breithardt O.A. Influence of age on pain perception in acute myocardial ischemia: a possible cause for delayed treatment in elderly patients. Int J Cardiol. 2011;149:63–67. doi: 10.1016/j.ijcard.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 22.Elbarouni B., Goodman S.G., Yan R.T. Validation of the Global Registry of Acute Coronary Event (GRACE) risk score for in-hospital mortality in patients with acute coronary syndrome in Canada. Am Heart J. 2009;158:392–399. doi: 10.1016/j.ahj.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 23.Giannitsis E., Kurz K., Hallermayer K. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem. 2010;56:254–261. doi: 10.1373/clinchem.2009.132654. [DOI] [PubMed] [Google Scholar]
- 24.Mancini G.B.J., Gosselin G., Chow B. Canadian cardiovascular society guidelines for the diagnosis and management of stable ischemic heart disease. Can J Cardiol. 2014;30 doi: 10.1016/j.cjca.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Jeremias A., Gibson C.M. Narrative review: alternative causes for elevated cardiac troponin levels when acute coronary syndromes are excluded. Ann Intern Med. 2005;142:786–791. doi: 10.7326/0003-4819-142-9-200505030-00015. [DOI] [PubMed] [Google Scholar]
- 26.Olivieri F., Galeazzi R., Giavarina D. Aged-related increase of high sensitive troponin T and its implication in acute myocardial infarction diagnosis of elderly patients. Mech Ageing Dev. 2012;133:300–305. doi: 10.1016/j.mad.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Noeller T.P., Meldon S.W., Peacock W.F. Troponin T in elders with suspected acute coronary syndromes. Am J Emerg Med. 2003;21:293–297. doi: 10.1016/s0735-6757(03)00081-0. [DOI] [PubMed] [Google Scholar]
- 28.Dubin R.F., Li Y., He J. Predictors of high sensitivity cardiac troponin T in chronic kidney disease patients: a cross-sectional study in the chronic renal insufficiency cohort (CRIC) BMC Nephrol. 2013;14:229. doi: 10.1186/1471-2369-14-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferry A.V., Anand A., Strachan F.E. Presenting symptoms in men and women diagnosed with myocardial infarction using sex-specific criteria. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.012307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma L., Zhao S. Risk factors for mortality in patients undergoing hemodialysis: a systematic review and meta-analysis. Int J Cardiol. 2017;238:151–158. doi: 10.1016/j.ijcard.2017.02.095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.