Abstract
Immune checkpoint inhibitor therapy has been shown to improve outcomes across many types of malignancies. However, immune checkpoint inhibitor has been associated with several immune-related adverse events including myocarditis. We describe the case of a 69-year-old man with fulminant myocarditis likely due to pembrolizumab therapy, complicated by biventricular failure with cardiogenic shock. Because of deterioration in hemodynamic status refractory to conventional immunosuppression, therapeutic plasma exchange was performed, resulting in a rapid reduction of serum pembrolizumab levels, and marked clinical, radiological, and biochemical improvement. To our knowledge, this is the first reported case on the successful use of plasma exchange for pembrolizumab-associated fulminant myocarditis.
Résumé
Il a été montré que le traitement par un inhibiteur du point de contrôle immunitaire améliore les résultats dans de nombreux types de cancer. Un inhibiteur du point de contrôle immunitaire a toutefois été associé à plusieurs effets indésirables d'origine immunologique, y compris la myocardite. Nous vous présentons le cas d'un homme de 69 ans ayant présenté une myocardite fulminante, probablement causée par un traitement par le pembrolizumab, compliquée par une insuffisance biventriculaire accompagnée d'un choc cardiogénique. En raison de la détérioration de l'état hémodynamique réfractaire à une immunosuppression classique, un échange plasmatique thérapeutique a été effectué, lequel a entraîné une réduction rapide des taux sériques de pembrolizumab, et une amélioration marquée sur les plans clinique, radiologique et biochimique. À notre connaissance, il s'agit du premier cas signalé dans lequel un échange plasmatique a été utilisé avec succès pour traiter une myocardite fulminante associée au pembrolizumab.
Immune checkpoint inhibitor (ICI) therapy with monoclonal antibodies directed against programmed cell death 1 (PD-1) or programmed cell death ligand (PD-L1) significantly improves outcomes across many malignancies including melanoma, lung cancer, Hodgkin’s lymphoma, urothelial carcinoma, gastric cancer, colorectal cancer, hepatocellular carcinoma, and renal cell carcinoma.1 Nivolumab and pembrolizumab are 2 ICIs used as therapeutic antibodies that block cancer cells’ ability to express PD-L1 and therefore its corresponding receptor, PD-1, which normally inactivates T-lymphocytes impeding their ability to target and destroy cancer cells. However, this unrestricted activation of T-lymphocytes can cause immune-related adverse events. Adverse events involving cardiac, respiratory, renal, hepatic, gastrointestinal, endocrine, neurologic, musculoskeletal, dermatologic, and ocular systems have been described.2 Cardiovascular adverse events include myocarditis, arrhythmia, pericardial disease, and Takotsubo cardiomyopathy.1 We describe a case of pembrolizumab-associated myocarditis complicated by cardiogenic shock.
Case Report
A 69-year-old man with metastatic castration-resistant prostate cancer with bone metastases, type 2 diabetes mellitus, hypertension, prior transient ischemic attack, and prehospital Eastern Cooperative Oncology Group performance status 0 was admitted to the cardiac intensive care unit for suspected myocarditis after 1 month of fatigue and dyspnea. Notably, there was no previous history of angina, presyncope, palpitations, or cardiac disease. He recently received 2 cycles of pembrolizumab (200 mg per cycle, intravenously) 10 and 4 weeks before admission, combined with abiraterone, a selective CYP17 inhibitor (which was dose reduced from standard 1000 mg daily to 750 mg daily for the second cycle due to elevated liver enzymes) and prednisone 5 mg twice daily. On admission, his blood pressure was 114/53 mm Hg, heart rate was 96 beats per minute, and oxygen saturation was 99%; physical examination revealed jugular venous distention and peripheral oedema.
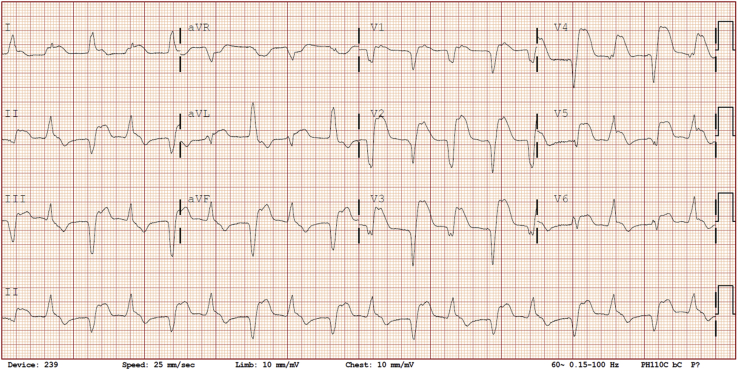
Investigations revealed markedly elevated troponin I of 17.7 μg/L (normal level, < 0.15 μg/L), creatinine kinase of 2437 U/L (normal level, < 250 U/L), and lactate of 2.8 mmol/L, along with alanine aminotransferase (ALT) of 257 U/L, aspartate transaminase (AST) of 353 U/L, alkaline phosphatase of 512 U/L, and a sodium level of 120 mmol/L. Creatinine was normal (68 μmol/L) as was complete blood count. Electrocardiogram (ECG) at presentation demonstrated bidirectional accelerated idioventricular rhythm (Fig. 1), which was a new finding (Supplemental Fig. S1). There was no evidence of noncardiac immune-related adverse effects apart from hepatitis and asymptomatic myositis; specifically, there were no ocular abnormalities, fatigable weakness, thyroid dysfunction, or colitis.
Figure 1.
Admission electrocardiogram demonstrating bidirectional accelerated idioventricular rhythm with the alternating QRS axis. Retrograde p-waves are best visualized in lead V1.
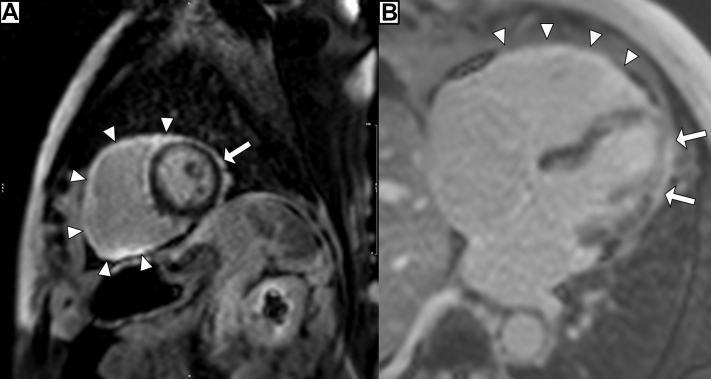
Coronary angiography demonstrated no significant coronary artery disease. Transthoracic echocardiogram demonstrated severe global biventricular systolic dysfunction with left ventricular ejection fraction (LVEF) of 30%. Cardiac magnetic resonance (CMR) imaging revealed severe biventricular dysfunction with the LVEF of 17% and the right ventricular (RV) ejection fraction of 24%, along with patchy, subepicardial enhancement of the LV and global transmural RV involvement with late-gadolinium enhancement (LGE) imaging consistent with myocarditis (Fig. 2, Videos 1-3
 , view videos online). A diagnosis of pembrolizumab-associated myocarditis causing cardiogenic shock and bidirectional accelerated idioventricular rhythm was made.
, view videos online). A diagnosis of pembrolizumab-associated myocarditis causing cardiogenic shock and bidirectional accelerated idioventricular rhythm was made.
Figure 2.
Cardiac magnetic resonance mid-ventricular short-axis (A) and 4-chamber (B) views with late-gadolinium enhancement imaging demonstrating subepicardial contrast uptake of the left ventricle (arrows) and diffuse transmural uptake of the right ventricle (arrowheads). Spatial resolution is poor due to decreased ability of the patient to follow commands.
The patient was in stage C (Classic) cardiogenic shock based on the Society for Cardiovascular Angiography and Interventions (SCAI) classification. He was started on oral mycophenolate 1 g twice daily and intravenous methylprednisolone 125 mg daily, which was subsequently escalated to a pulse dose of 1 g daily for 3 days due to worsening clinical status. Despite immunosuppression, his clinical status worsened to SCAI stage D (Deteriorating) with a Glasgow Coma Scale of 11 and he developed monomorphic ventricular tachycardia that required intravenous amiodarone. His ALT peaked at 1314 U/L and AST at 801 U/L. Lactate and creatinine rose to 3.7 mmol/L and 356 μmol/L, respectively. The patient was not a candidate for mechanical circulatory support due to his metastatic cancer.
Given pembrolizumab’s half-life of 22 days and the patient’s lack of response to immunosuppression, plasma exchange was proposed to remove the drug. Moreover, although pembrolizumab, an IgG4 isotope antibody, does not bind to plasma proteins, it has a small volume of distribution of approximately 6 L with limited extravascular redistribution, making it an ideal candidate for plasma exchange.
Two sessions of 2 body volumes worth of plasma exchange were performed 2 days apart on days 11 and 13. The plasma pembrolizumab drug level immediately before the first session was 10,200 ng/mL, and then decreased to 3340 ng/mL after the first session and 1220 ng/mL after the second session.
There was dramatic clinical improvement to SCAI stage A (At Risk) and Glasgow Coma Scale from 11 to 14. The ECG returned to normal sinus rhythm with right bundle branch block with no recurrence of accelerated idioventricular rhythm (Supplemental Fig. S2). Transthoracic echocardiography after plasma exchange revealed normal LVEF but persistent RV systolic dysfunction (Videos 4-6
 , view videos online). Troponin I, ALT, and AST normalized to 0.47 μg/L, 67 U/L, and 29 U/L, respectively. The patient was eventually transferred to the ward for rehabilitation.
, view videos online). Troponin I, ALT, and AST normalized to 0.47 μg/L, 67 U/L, and 29 U/L, respectively. The patient was eventually transferred to the ward for rehabilitation.
On day 28 of admission, he developed anaemia due to gastrointestinal bleeding. Upper endoscopy showed diffuse esophagitis/gastritis, large gastric and duodenal ulcers likely secondary to mycophenolate and methylprednisolone. Esophageal, gastric and duodenal biopsies were suggestive of focal erosions and healing ulceration. Cytomegalovirus immunohistochemistry was positive in the gastric and duodenal biopsies. On day 40 of admission, he developed increased somnolence with hypercapnic respiratory failure and had cytomegalovirus viremia. He required continuous noninvasive bilevel ventilation with vasopressor support. Given his poor oncologic prognosis, the family decided to withdraw active therapy.
Discussion
Immune checkpoint inhibitor myocarditis
Immune checkpoint therapy with PD-1 inhibitors, such as pembrolizumab, has led to marked improvements in survival in multiple cancers. These therapeutic antibodies prevent PD-L1 expressed on cancer cells from interacting with PD-1, thus enhancing T-lymphocyte antitumour activity.3 However, these therapies are associated with immune-related adverse events, affecting numerous systems, with incidence rising due to increased use and better recognition of adverse events.4 Unfortunately, randomized clinical trial data have inconsistently reported adverse cardiovascular events.1 These adverse events include arrhythmias such as supraventricular tachycardia and ventricular tachycardia, conduction disease (eg, complete heart block), pericardial disease (eg, pericarditis and pericardial effusion), myocardial disease (eg, myocarditis, cardiogenic shock, and takotsubo cardiomyopathy), and vascular disease (eg, temporal arteritis and coronary artery disease).1
Myocarditis is the most common cardiovascular toxicity of immune checkpoint inhibition, occurring in approximately 1% of patients and accounting for 45% of cardiovascular-related adverse events.1,4 Importantly, fulminant myocarditis, representing 15% of myocarditis, is associated with 46% mortality.4 Myocarditis from ICI therapy occurs early, with a median onset of 17-34 days, and appears to be consistent across cancer types.1,4
In the present case, myocarditis was diagnosed using the European Society of Cardiology Working Group criteria, including clinical presentation, ECG features, myocardial cytolysis markers, normal coronary angiography, and tissue characterization by CMR, obviating need for endomyocardial biopsy.5 Myositis, hepatitis, and colitis are the most common noncardiac immune-related adverse events noted in patients with myocarditis.2 On presentation, our patient had biochemical evidence of hepatitis and myositis, but otherwise no other apparent ICI-related adverse events involving other organs. Although the patient’s subsequent deterioration may be ascribed to other ICI-related adverse events, this is unlikely given the absence of symptoms or significant biochemical derangement on presentation. In addition, the temporal delay for clinical manifestations to arise well after the last dose of ICI therapy, especially given interim immunosuppression and plasma exchange, would be very atypical. Furthermore, the discovery of cytomegalovirus viremia makes this alternative diagnosis much more likely.
The prognostic role of cardiac MRI
Tissue characterization with LGE on CMR suggests significant fibrosis and provides prognostic information in patients with myocarditis. In our case, CMR demonstrated transmural RV involvement on LGE, whereas only subepicardial enhancement was observed in the LV. After resolution of the myocarditis, a follow-up echocardiogram demonstrated normalization of LV function but minimal RV recovery. This finding highlights the ability of LGE imaging on CMR to predict segmental recovery in patients with myocardial injury including fulminant myocarditis.
Management of immune checkpoint inhibitor myocarditis
High-dose corticosteroids are recommended for the management of myocarditis from ICI therapy.1,6 The addition of other immunosuppressive drugs, such as tacrolimus, infliximab, mycophenolate mofetil, or antithymocyte globulin, have been suggested for refractory cases.6 Alemtuzumab, a monoclonal antibody to CD52, has been successfully used to induce cytolytic immunosuppression for treatment of myocarditis caused by ICI refractory to conventional immunosuppression.7 However, alemtuzumab is not widely available and causes further immunosuppression. Given that pembrolizumab is a humanized IgG4 monoclonal antibody and therapeutic plasma exchange has been used for other diseases to rapidly deplete levels of deleterious immunoglobulins, we postulated that plasma exchange may be beneficial in the management of refractory myocarditis in our patient. In addition, removal of circulating systemic inflammatory cytokines may aid in recovery of cardiac function. The precedent of plasma exchange for immune-related adverse events has been established in patients with pembrolizumab-induced myasthenia gravis.8 We observed remarkable biochemical and clinical improvement, as well as recovery of LV function. Plasma exchange may represent an intriguing first-line therapy for ICI myocarditis.
Myocarditis is becoming an increasingly recognized adverse event of ICI therapy. Plasma exchange should be considered for the management of fulminant myocarditis. Further research is needed to determine whether plasma exchange for ICI myocarditis may allow for significant therapeutic benefit without exposing patients to risks associated with high levels of immunosuppression.
Novel Teaching Points.
-
•
Myocarditis has become recognized as an adverse event of immune checkpoint inhibitor.
-
•
Plasma exchange should be considered as part of the management of fulminant myocarditis.
Funding Sources
The authors have no funding sources to declare.
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
The authors would like to recognize the nurses, physicians, and other allied health care workers who were responsible for the care of this patient.
Footnotes
Ethics Statement: The above reported research adhered to all relevant ethical guidelines.
See page 382 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2020.11.004.
Supplementary Material
Four-chamber cardiac magnetic resonance cine demonstrating biventricular systolic dysfunction. Spatial and temporal resolution is poor due to decreased ability of the patient to follow commands.
Two-chamber cardiac magnetic resonance cine demonstrating biventricular systolic dysfunction. Spatial and temporal resolution is poor due to decreased ability of the patient to follow commands.
Short-axis cardiac magnetic resonance cine at the mid-ventricular level demonstrating biventricular systolic dysfunction. Spatial and temporal resolution is poor due to decreased ability of the patient to follow commands.
Four-chamber view of transthoracic echocardiogram demonstrating recovery of left ventricular systolic function with impaired right ventricular systolic function.
Two-chamber view of transthoracic echocardiogram demonstrating recovery of left ventricular systolic function.
Parasternal short-axis view transthoracic echocardiogram at the mid-ventricular level demonstrating recovery of left ventricular systolic function.
References
- 1.Ball S., Ghosh R.K., Wongsaengsak S. Cardiovascular toxicities of immune checkpoint inhibitors: JACC review topic of the week. J Am Coll Cardiol. 2019;74:1714–1727. doi: 10.1016/j.jacc.2019.07.079. [DOI] [PubMed] [Google Scholar]
- 2.Zimmer L., Goldinger S.M., Hofmann L. Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:210–225. doi: 10.1016/j.ejca.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 3.Buchbinder E.I., Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol. 2016;39:98. doi: 10.1097/COC.0000000000000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moslehi J.J., Salem J.E., Sosman J.A., Lebrun-Vignes B., Johnson D.B. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. 2018;391:933. doi: 10.1016/S0140-6736(18)30533-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caforio A.L., Pankuweit S., Arbustini E. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648. doi: 10.1093/eurheartj/eht210. [DOI] [PubMed] [Google Scholar]
- 6.Haanen J., Carbonnel F., Robert C. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv119–iv142. doi: 10.1093/annonc/mdx225. [DOI] [PubMed] [Google Scholar]
- 7.Esfahani K., Buhlaiga N., Thebault P. Alemtuzumab for immune-related myocarditis due to PD-1 therapy. N Engl J Med. 2019;380:2375–2376. doi: 10.1056/NEJMc1903064. [DOI] [PubMed] [Google Scholar]
- 8.Becquart O., Lacotte J., Malissart P. Myasthenia gravis induced by immune checkpoint inhibitors. J Immunother. 2019;42:309–312. doi: 10.1097/CJI.0000000000000278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Four-chamber cardiac magnetic resonance cine demonstrating biventricular systolic dysfunction. Spatial and temporal resolution is poor due to decreased ability of the patient to follow commands.
Two-chamber cardiac magnetic resonance cine demonstrating biventricular systolic dysfunction. Spatial and temporal resolution is poor due to decreased ability of the patient to follow commands.
Short-axis cardiac magnetic resonance cine at the mid-ventricular level demonstrating biventricular systolic dysfunction. Spatial and temporal resolution is poor due to decreased ability of the patient to follow commands.
Four-chamber view of transthoracic echocardiogram demonstrating recovery of left ventricular systolic function with impaired right ventricular systolic function.
Two-chamber view of transthoracic echocardiogram demonstrating recovery of left ventricular systolic function.
Parasternal short-axis view transthoracic echocardiogram at the mid-ventricular level demonstrating recovery of left ventricular systolic function.