Abstract
Introduction
Meniere’s disease (MD) is characterized by episodic symptoms, including vertigo, hearing loss, and tinnitus.
Objectives
in this study, cases of unilateral tinnitus were investigated for MD.
Method
Twenty-two patients who presented with chronic unilateral tinnitus on puretone audiograms showing an average threshold better than 25 dB HL and thresholds in the lower frequencies worse than those in the higher frequencies by more than 10 dB were suspected to have early-stage MD and underwent electrocochleography (ECochG). Patients showing ECochG findings conclusive for MD were compared to a control group of patients presenting with chronic unilateral tinnitus on pure-tone audiograms showing an average threshold better than 25 dB and thresholds in the higher frequencies worse than those in the lower frequencies by more than 10 dB.
Results
Eighteen of the 22 patients included in this study showed elevated summating potential amplitude to action potential amplitude ratios in ECochG (suggestive of endolymphatic hydrops due to MD) and were followed up for 2 months; 14 of them experienced at least two attacks of vertigo or unsteadiness. In contrast, only one patient in the control group reported two or more attacks of vertigo or unsteadiness in the 2 month observation period. The incidence of this finding in the two groups was significantly different.
Conclusion
Patients with early-stage MD can present with only unilateral tinnitus. Thus, the addition of “pure-tone audiograms showing lower-frequency thresholds worse than higher-frequency thresholds” to the probable MD category in the globally.
agreed diagnostic criteria for MD, may be useful.
Keywords: Meniere’s disease, Unilateral tinnitus, Vertigo, Electrocochleography, Dizziness
1. Introduction
Meniere’s disease (MD) is an idiopathic inner ear disease characterized by recurrent spontaneous vertigo accompanied by tinnitus,fluctuating or progressive sensorineural hearing loss, and aural fullness in the affected ear (Van RompaeyWatkinson and Clarke, 2018). Among the numerous diagnostic criteria that have been proposed in the past decades, the most widely accepted guidelines were issued in 2015 by the Barany Society, Japan Society for Equilibrium Research, European Academy of Otology and Neurotology, the American Academy of Otolaryngology–Head and Neck Surgery, and Korean Balance Society (Lopez-Escamez et al., 2015), as shown in Table 1.
Table 1.
“Diagnostic criteria for Meniere’s disease” (Van RompaeyWatkinson and Clarke, 2018).
| “Definite Meniere’s Disease” | “≥ Two definitive spontaneous episodes of vertigo lasting 20 min to 12 h + Audiometrically documented low- to medium-frequency sensorineural hearing loss in the affected ear on at least one occasion before, during, or after one of the episodes of vertigo + Fluctuating aural symptoms (hearing, tinnitus, or fullness) in the affected ear” |
| “Probable Meniere’s Disease” | “≥ Two episodes of vertigo or dizziness, each lasting 20 min to 24 h + Fluctuating aural symptoms (hearing, tinnitus, or fullness) in the reported ear” |
Many authors have reported that in MD, the cochlear symptoms often present before the vertigo and tend to be ignored (Zhang et al., 2016), while the vertigo episodes are the most incapacitating feature of the disease and the patients’ major complaint (Cunha et al., 2005); hearing loss is usually used to identify the stage of the disease (Van RompaeyWatkinson and Clarke, 2018). Although the diagnosis of MD is primarily based on clinical findings according to the criteria in Table 1, some tests can confirm the presence of endolymphatic hydrops, and the most important of these tests is electrocochleography (ECochG). The typical expected result in an ear positive for MD is an increase in the summating potential amplitude relative to the action potential amplitude, i.e., the SP/AP ratio (Jacobson and Neil, 2016). The presence of hydrops is believed to affect the resting position of the basilar membrane, displacing it toward the scala tympani and leading to changes in the electro-anatomy of the hair cells, increasing the value of the summating potential (Jacobson and Neil, 2016).
As shown in Table 1, the presence of at least two definitive spontaneous episodes of vertigo or dizziness is an essential for the diagnosis of both definite and probable MD, and the vertigo appears after cochlear symptoms in most of the cases (Zhang et al., 2016). Accordingly, this study investigated the incidence of MD in cases presenting with unilateral tinnitus accompanied by pure-tone audiometric pattern showing average low-frequency thresholds worse than the average high-frequency thresholds by more than 10 dB, since these cases may represent early stages of MD before the occurrence of the vestibular symptoms.
2. Material and methods
Twenty-two patients who attended the specialized otolaryngology and audio-vestibular medical center between March 1, 2019 and January 1, 2020 complaining of unilateral tinnitus were included in this study. The protocol of this study was accepted by (medical ethics council committee no 23/2020).
Patients presenting with any of the following findings were excluded from the study:
-
1.
Bilateral tinnitus
-
2.
History of unilateral or bilateral hearing loss
-
3.
History of attacks of vertigo, dizziness, or imbalance
-
4.
Tinnitus of less than 6 weeks duration
-
5.
Otorhinolaryngologic examination showing abnormalities in the tympanic membrane or the middle ear
-
6.
Abnormal impedance curve
-
7.
Pure-tone audiometry showing down sloping toward the higher frequencies
-
8.
Pure-tone audiometry showing air–bone gap
-
9.
Moderate, severe, or profound hearing loss
-
10.
Hearing threshold average equal or worse than 25 dB HL
-
11.
Age below 10 years
-
12.
Age above 60 years
-
13.
Any signs or symptoms of acute or chronic systemic disease
-
14.
History of migraine or chronic headache
Thus, patients included in this study typically had.
-
1.
Chronic unilateral tinnitus without noticeable hearing loss
-
2.
Threshold of hearing better than 25 dB HL in the involved ear
-
3.
Uprising pure-tone audiogram showing lower-frequency thresholds worse than the higher-frequency thresholds by more than 10 dB.
For all patients included in the study, the possibility of MD was discussed with the patients and informed consent form for study participation and ECochG examinations was obtained. Accordingly, ECochG was performed in the ears with tinnitus using gold foil-insert phones via the extratympanic method with click stimulus at 80 dB nHL intensity and 80 Hz stimulus rate; Sentiero AdvancedR: Path Medical GmbH, Germering, Germany was used to test all patients included in this study. Patients refused to perform ECochG were excluded from the study, borderline results for ECochG (SP/AP amplitudes ratio between 0.4 and 0.5) were excluded from the study too. For cases with suspicions findings in ECochG (SP/AP amplitude ratio greater than 0.5) the author discussed the diagnostic criteria of MD with the patient and also explained that treatment would not be started unless the patient’s findings meet the internationally agreed criteria for a diagnosis of MD. Patients were followed up by weekly phone calls for 2 months to monitor any new attacks of vertigo or dizziness, as these are important to include the patient to probable MD according to the agreed criteria, other symptoms like fullness of the ear and worsening tinnitus was also asked for during the phone calls. In addition, 15 age-matched patients who attended the same otolaryngology and audio-vestibular medical center complaining of unilateral chronic tinnitus for more than 6 weeks duration, showed pure-tone audiograms with normal thresholds at speech frequencies with high-frequency drops, with no air bone gap and normal impedance curve and had no history of vertigo, dizziness, migraine, chronic headache or other systemic disease were selected as the control group and followed up by weekly phone calls for 2 months to monitor any new attacks of vertigo or dizziness and other associated symptoms.
This study received was not supported financially by any public or commercial agency, and there was no any kind of support from not-for-profit sectors.
2.1. Statistical analysis
Chi square used in the in this study to identify ant statistical deference between the study and the control groups of patients.
3. Results
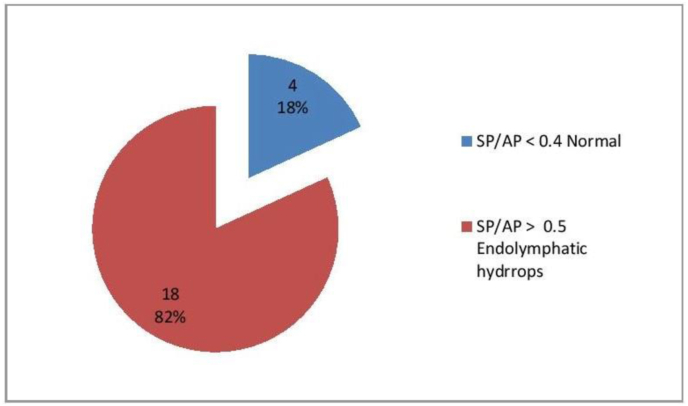
A total of 22 patients (19 female and 3 male; male to female ratio 1:6.33) aging from 22 to 43 years (median age 33 years) were included and agreed to undergo the ECochG assessments. The results of ECochG examinations are shown in Fig. 1. In the fellow up period; in the group of eighteen patients who showed ECochG findings confirming the presence of endolymphatic hydrops; 14 (77.7%) experienced at least two attacks of vertigo or dizziness during the 2-month observation period and categorized as probable MD according to the agreed international criteria. In contrast, only one of the 15 patients in the control group (6.6%) experienced two attacks or more of vertigo or dizziness during the 2-month observation period. The intergroup difference for this finding was statistically highly significant (p = 0.000044). No one from the four patients who showed normal ECochG finding developed attack of vertigo or dizziness in the 2-month observation period.
Fig. 1.
Number and percentage of patients with normal and suspicious electrocochleography findings.
The calculated positive predictive value (PPV) of patients with unilateral tinnitus and low frequencies drop to progress to probable MD was 0.933 and negative predictive value (NPV) was 0.636.
4. Discussion
Most patients included in the study showed ECochG results confirming the presence of endolymphatic hydrops suggestive of the early stage of MD. Patients with ECochG suggesting MD showed significant differences compared to the control group in the development of at least two attacks of vertigo or dizziness in the subsequent 2 months; accordingly, such patients with unilateral low-frequency drops and tinnitus can be categorized as having probable MD.
The exclusion criteria for this study addressed multiple conditions that did not meet the diagnostic criteria for definite or probable MD and also aimed to exclude other possible causes. Cases with bilateral tinnitus and any signs and symptoms of acute or chronic systemic disease were excluded, since the tinnitus in these patients may indicate more systemic or generalized problems rather than MD (Crummer and Hassan, 2004). Similarly, cases presenting with vertigo; dizziness; imbalance; moderate, severe, or profound hearing loss on audiograms; mild hearing loss with pure-tone average worse than 25 dB; and any noticeable hearing loss by the patient were excluded since these findings are cardinal symptoms of MD (Table 1). Cases showing tinnitus of less than 6 weeks’ duration were also excluded, since tinnitus is one of the chronic and recurrent symptoms of MD (Committee on Hearing and Equilibrium, 1995), and this study was designed to investigate the possibility of MD in patients with chronic unilateral tinnitus. Noguchi et al. (2004) showed that the pathogenesis of acute low-tone sensorineural hearing loss arises from an endolymphatic hydrops with little or no impairment of hair cells; accordingly, only cases showing chronic unilateral tinnitus of more than 6 weeks’ duration were included to exclude cases of slight acute low-tone sensorineural hearing loss with pure-tone average better than 25 dB HL.
Patients presenting with abnormal middle-ear function, including abnormal impedance curves, abnormalities of the tympanic membrane detected by clinical examination, and the presence of air–bone gap detected by pure-tone audiometry, were also excluded to exclude cases of tinnitus induced by middle-ear problems (Crummer and Hassan, 2004). Similarly, patients showing downward sloping toward the higher frequencies in pure-tone audiometry were excluded, because these patients have pure cochlear deficit rather than MD and are candidates for tinnitus retraining therapy (Alsarhan, 2012). Kotimäki et al. (1999) showed that only 1%–7% of MD cases are seen in the pediatric population, and Ballester et al. (2002) showed that only 9% of MD patients experience the onset of MD at the age of 65 years or more. Thus, MD onset usually occurs after puberty and is rarely observed in the elderly; consequently, the age range for the study population was fixed to above 10 and below 60 years. In addition, Radtke et al. (2002) suggested that there is a patho-physiologic relation between MD and migraine, while Weinreich et al. (Weinreich and Carey, 2016) reported that tinnitus can be observed in the context of migraine. Therefore, all cases with history of migraine were excluded to exclude possible cases of tinnitus due to migraine. Most of the patients identified and included were young women in child-bearing age (M:F ratio was 1:6.33 and median age 33). Andrews and Honrubia (2010) reported that hormonal stress can act on the volatile inner ear with MD to induce dysfunction, which supports the findings of this study.
Tinnitus due to primary cochlear deficit caused by aging or cochlear deficit secondary to ototoxicity, acoustic traumas or instant noise exposure usually presented by high frequencies drop by pure tone audiogram (Alsarhan, 2012), while most of the patients with MD especially in early stage represented with low frequencies drop (Zhang et al., 2016); for that reason no ECochG was not recommended for patients in control group.
Diagnostic guidelines for MD have been updated over the last century. In 1995, the American Academy of Otolaryngology – Head and Neck foundation reported guidelines for diagnosis and classified MD as possible, probable, definite, and certain disease (Committee on Hearing and Equilibrium, 1995). In 2015, multiple international committees agreed upon the updated guidelines for diagnosis and classified MD into definite and probable categories (Lopez-Escamez et al., 2015). Although clear diagnosis of definite MD can be easily obtained on the basis of the criteria in Table 1, confirmation of the diagnosis of probable MD may require additional diagnostic assessments such as ECochG. Devaiah et al. (2003) reported that early diagnosis of probable MD is important to initiate dietary restrictions and/or treatment protocols and prevent further progression and destruction of neural function of the inner ear, since early intervention can preserve inner ear function. Won-Ho Chung et al. (2004) stated that the sensitivity and specificity of ECochG in the diagnosis of MD were 71% and 96%, respectively, and that extratympanic ECochG may play an important role, especially in patients with less definite symptoms, supporting the use of extratympanic ECochG in this study; Fulvio Mammarella et al. (2017) reported sensitivity of ECochG in MD is greater than 80%. Quatre R. et al. (Quatre et al., 2019) reported that ECochG can be performed regardless of hearing loss and can enhance the chances to confirm the diagnosis of MD with a better confidence; supporting the finding of this study. Devaiah et al. (2003) also showed that the SP/AP area curve ratio yielded significantly better diagnostic sensitivity for ECochG in possible MD in comparison to the standard SP/AP ratio; accordingly, the cut-off diagnostic criterion for endolymphatic hydrops in this study showed normal findings in four patients (18.2%) that could have been lowered if the SP/AP area curve ratio had been used.
Among the patients who showed ECochG findings conclusive of MD, 77.7% experienced at least two attacks of vertigo or dizziness compared to only 6.6% in the control group; this difference was statistically highly significant. Thus, the null hypothesis was rejected, and the author’s idea of diagnosing these cases as early-stage or probable MD depending on internationally agreed criteria was valid. Calculating PPV for patient with unilateral tinnitus and low-frequencies drop to develop MD of 0.933 was highly encouraging to the author’s idea that this category of patients may be potentially MD.
Some additional hints can be added to the diagnostic criteria for probable MD. Noguchi et al. (2004) concluded that low-tone sensorineural hearing loss resembles early-stage MD, supporting the results of this study; additionally, Hiroaki et al. (Fushiki et al., 2009) showed that idiopathic sudden low-tone sensorineural hearing loss without vertigo has a high recurrence rate when associated with elevated SP/AP ratios. These findings are also consistent with the findings of this study.
Vestibular evoked myogenic potentials (VEMPs) have been recently used as a diagnostic parameter in cases of MD. Maxwell et al. (2017) studied 42 patients with certain unilateral MD and 21 healthy controls and reported that multi-frequency VEMPs analysis offers a simple and cost-effective method to address the diagnostic difficulties presented by MD. Noij et al. (2019) showed that the cervical VEMPs metrics most useful in distinguishing definite MD patients from healthy controls are VEMPs threshold, VEMPs -normalized peak-to-peak amplitude, and VEMPs inhibition depth. In addition, Singh and Barman (2016) reported that the sensitivity and specificity of the frequency amplitude ratio at 1000/500 Hz and 750/500 Hz in ocular VEMPs for differentiating definite MD from healthy controls was 90% and 100%, respectively. All of these studies concluded that VEMPs were useful in the diagnosis of definite MD. Therefore, future studies should attempt VEMP analysis for patients with probable MD and for a population with similar characteristics to the patients included in this study.
Imaging studies are used also in MD; to confirm the diagnosis and to exclude other conditions like vestibular schwanoma and other cerebellopontine angle tumors; Venkatasamy A. et al. (Venkatasamy et al., 2017) concluded in their study that T2-weighted sequence MRI is an easy method to diagnose MD; in spite of that MRI was not recommended to the patients included in this study as Quatre R. et al. (Quatre et al., 2019) concluded that inner ear MRI usually showing hydrops when hearing loss is higher than 35 dB HL; and vestibular schwanoma was not expected in the group of patients in this study as this tumor usually yields high frequencies drop in pure tone audiograms. (Kim et al., 2016)., (Gimsing, 2010)
Treatment of patients with MD includes dietary restrictions and medications like diuretics, betahistine and intra-tympanic injection of dexamethasone or rarely gentamycin (Van RompaeyWatkinson and Clarke, 2018). Additional researches are needed to identify the best treatment option for early-stage MD and probable MD; and whether dietary salt and caffeine restriction is needed alone or with specific pharmacological agents; on the other hand, in this research studied a limited number of patients; further expanded and multicenter future researches should be conducted to confirm the results of this research.
5. Conclusion
Early-stage MD can present with cochlear symptoms alone like chronic unilateral tinnitus. Furthermore, if the outcome of larger sample future similar researches confirm the findings of this research; it is recommended to supplement the diagnostic criteria for probable MD as shown in Table 2.
Table 2.
Suggested addition to the diagnostic criteria for probable Meniere’s disease.
| Probable Meniere’s disease | “≥ Two episodes of vertigo or dizziness, each lasting 20 min to 24 h + Fluctuating aural symptoms (hearing, tinnitus, or fullness) in the reported ear |
| OR | |
| Fluctuating aural symptoms (hearing, tinnitus, or fullness) in the reported ear + Audiometric demonstration of Unilateral low-frequency thresholds worse than higher-frequency thresholds by more than 10 dB (low-frequency sensorineural drop) in the reported ear’’ |
Declaration of conflicting interests
None.
Acknowledgment
I really appreciate the efforts of (www.editage.com) for English language review of this article.
Footnotes
Peer review under responsibility of PLA General Hospital Department of Otolaryngology Head and Neck Surgery.
References
- Alsarhan H. Treatment of tinnitus with tinnitus retraining therapy in Iraq. Iraqi J Commun Med. 2012;25:179–185. [Google Scholar]
- Andrews J.C., Honrubia V. Premenstrual exacerbation of Meniere’s disease revisited. Otolaryngol. Clin. 2010;43:1029–1040. doi: 10.1016/j.otc.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Ballester M., Liard P., Vibert D., Häusler R. Meniere’s disease in the elderly. Otol. Neurotol. 2002;23:73–78. doi: 10.1097/00129492-200201000-00017. [DOI] [PubMed] [Google Scholar]
- Chung W.H., Cho D.Y., Choi J.-Y., Hong S.H. Clinical usefulness of extratympanic electrocochleography in the diagnosis of Ménière’s disease. Otol. Neurotol. 2004;25:144–149. doi: 10.1097/00129492-200403000-00011. [DOI] [PubMed] [Google Scholar]
- Committee on Hearing and Equilibrium Guidelines for the diagnosis and evaluation of therapy in Meniere’s disease. Otolaryngol. Head Neck Surg. 1995;113:181–185. doi: 10.1016/S0194-5998(95)70102-8. [DOI] [PubMed] [Google Scholar]
- Crummer R.W., Hassan G.A. Diagnostic approach to tinnitus. Am. Fam. Physician. 2004;69:120–126. [PubMed] [Google Scholar]
- Cunha F., Settanni F.A., Ganança F.F. What is the effect of dizziness on the quality of life for patients with Meniere’s disease? Rev. Laryngol. Otol. Rhinol. 2005;126:155–158. [PubMed] [Google Scholar]
- Devaiah A.K., Dawson K.L., Ferraro J.A., Ator G.A. Utility of area curve ratio electrocochleography in early Meniere disease. Arch. Otolaryngol. Head Neck Surg. 2003;129:547–551. doi: 10.1001/archotol.129.5.547. [DOI] [PubMed] [Google Scholar]
- Fushiki H., Junicho M., Aso S., Watanabe Y. Recurrence rate of idiopathic sudden low-tone sensorineural Hearing loss without vertigo: a long-term follow-up study. Otol. Neurotol. 2009;30:295–298. doi: 10.1097/mao.0b013e31819d3496. [DOI] [PubMed] [Google Scholar]
- Gimsing S. Vestibular schwanoma : when to look for it? J. Laryngol. Otol. 2010;124(3):258–264. doi: 10.1017/S0022215109991423. [DOI] [PubMed] [Google Scholar]
- Jacobson G.P., Neil T.S. second ed. Plural Publishing, Inc; 2016. Balance Function Assessment and Management; p. 604. [Google Scholar]
- Kim S.H., Lee S.H., Choi S.K., Lim Y.J., Na S.Y., Yeo S.G. Audiometric evaluation of vestibular schwanoma and other cerebellopontine ange tumors. Acta Otolaryngol. 2016;135(2):149–153. doi: 10.3109/00016489.2015.1100326. [DOI] [PubMed] [Google Scholar]
- Kotimäki J., Sorri M., Aantaa E., Nuutinen J. Prevalence of Meniere disease in Finland. Laryngoscope. 1999;109:748–753. doi: 10.1097/00005537-199905000-00013. [DOI] [PubMed] [Google Scholar]
- Lopez-Escamez J.A., Carey J., Chung W.H., Goebel J.A., Magnusson M., Mandala M. Diagnostic criteria for Meniere’s disease. J. Vestib. Res. 2015;25:1–7. doi: 10.3233/VES-150549. [DOI] [PubMed] [Google Scholar]
- Mammarella F., Zelli M., Varakliotis T., Eibenstein A., Pianura C.M., Bellocchi G. Is electrocochleography still helpful in early diagnosis of Meniere disease? J Audiol Otol. 2017;21(2):72–76. doi: 10.7874/jao.2017.21.2.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell R., Jerin C., Gürkov R. Utilisation of multi-frequency VEMPs improves diagnostic accuracy for Meniere’s disease. Eur. Arch. Oto-Rhino-Laryngol. 2017;274:85–93. doi: 10.1007/s00405-016-4206-z. [DOI] [PubMed] [Google Scholar]
- Noguchi Y., Nishida H., Tokano H., Kawashima Y., Kitamura K. Comparison of acute low-tone sensorineural hearing loss versus Meniere’s disease by electrocochleography. Ann. Otol. Rhinol. Laryngol. 2004;113:194–199. doi: 10.1177/000348940411300304. [DOI] [PubMed] [Google Scholar]
- Noij K.S., Herrmann B.S., Guinan J.J., Jr., Rauch S.D. Cervical vestibular evoked myogenic potentials in Menière’s disease: a comparison of response metrics. Otol. Neurotol. 2019;40:e215–e224. doi: 10.1097/MAO.0000000000002092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quatre R., Attye A., Karkas A., Job A., Dumas G., Schmerber S. Relationship between Audio-vestibular Tests and inner ear MRI in Meniere’s disease. Ear Hear. 2019;1:168–176. doi: 10.1097/AUD.0000000000000584. [DOI] [PubMed] [Google Scholar]
- Radtke A., Lempert T., Gresty M.A., Brookes G.B., Bronstein A.M., Neuhauser H. Migraine and Ménière’s disease: is there a link? Neurology. 2002;59:1700–1704. doi: 10.1212/01.wnl.0000036903.22461.39. [DOI] [PubMed] [Google Scholar]
- Singh N.K., Barman A. Frequency-amplitude ratio of ocular vestibular-evoked myogenic potentials for detecting Meniere’s disease: a preliminary investigation. Ear Hear. 2016;37:365–373. doi: 10.1097/AUD.0000000000000263. [DOI] [PubMed] [Google Scholar]
- Van Rompaey Vincent WFM. Meniere’s disease. In: Watkinson J.C., Clarke R.W., editors. eighth ed. vol. 2. CRC Press, Taylor and Francis Group; 2018. pp. 817–827. (Scott Brown’s Otolaryngology Head & Neck Surgery). [Google Scholar]
- Venkatasamy A., Veillon F., Fleury A., Eliezer M., Abu Eid M., Romain B. Imaging of the saccule for diagnosis of Endolymphatic hydrops in Meniere disease, using athreee-simensional T2-weighted steady state free precession sequence: accurate, fast and with out contract material intravenous injection. Eur. Radiol. Exp. 2017;1:14. doi: 10.1186/s41747-017-0020-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreich H.M., Carey J.P. Prevalence of pulsatile tinnitus among patients with migraine. Otol. Neurotol. 2016;37:244–247. doi: 10.1097/MAO.0000000000000968. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Liu B., Wang R., Jia R., Gu X. Characteristics of the cochlear symptoms and functions in Meniere’s disease. Chin Med J (Engl) 2016;129:2445–2450. doi: 10.4103/0366-6999.191767. [DOI] [PMC free article] [PubMed] [Google Scholar]