Abstract
Background
The etiology of sudden cardiac arrest (SCA) in individuals without known cardiovascular heart disease remains elusive in nearly half of all patients after systematic testing. We investigated the relationship between stressful life events and SCA risk in cases of explained and unexplained SCA (USCA) events.
Methods
Individuals who previously experienced SCA were enrolled prospectively and divided into a USCA or explained SCA (ESCA) subgroup dependent on whether a diagnosis was ascribed after SCA. Participants completed either the 1997 Recent Life Changes Questionnaire, Student Stress Scale, or Social Re-adjustment Rating Scale for Non-Adults recalling events during the year preceding their SCA, depending on age at SCA presentation; all measure stress in life change units (LCUs). SCA group scores were compared with an age- and sex-matched control group.
Results
We compared 36 SCA group participants (22 USCA, 14 ESCA, age 47 ± 15 years, age at SCA 40 ± 14 years, 50% male) with 36 control participants (age 47 ± 15 years, 50% male). There was no significant difference in LCU score between the control group and the SCA group (248 ± 181 LCU vs 252 ± 227 LCU; P > .05). The ESCA subgroup had significantly lower mean LCU scores than the USCA subgroup (163 ± 183 LCU vs 308 ± 237 LCU; P = .030).
Conclusions
Stressful life events, especially those producing chronic stress, might predispose otherwise healthy individuals to lethal arrhythmias. Further investigation into the role of stress in SCA precipitation is warranted.
Résumé
Contexte
La cause de l’arrêt cardiaque subit (ACS) chez les personnes n’ayant pas de maladie cardiovasculaire connue demeure nébuleuse dans près de la moitié des cas, même après des examens systématiques. Nous avons étudié la relation entre les événements stressants de la vie et le risque d’ACS chez des patients présentant un ACS expliqué (ACSe) ou inexpliqué (ACSi).
Méthodologie
Des sujets ayant déjà subi un ACS ont été recrutés de manière prospective et répartis en deux sous-groupes (ACSe et ACSi), selon qu’un diagnostic a pu ou non être posé après l’ACS. On a demandé aux participants de répondre au questionnaire RLCQ (Recent Life Changes Questionnaire, questionnaire sur les changements de vie récents, version de 1997), au questionnaire SSS (Student Stress Scale, échelle d’évaluation du stress vécu par les étudiants) ou au questionnaire SRRS (Social Readjustment Rating Scale, échelle d’évaluation du réajustement social) pour les non-adultes en repensant aux événements survenus dans l’année précédant l’ACS, selon leur âge au moment de l’ACS; tous ces questionnaires mesurent le stress en unités de changement de vie (UCV). Les scores des patients ayant subi un ACS ont été comparés à ceux de sujets témoins appariés selon l’âge et le sexe.
Résultats
Nous avons comparé 36 sujets ayant subi un ACS (22 ACSi et 14 ACSe; âge : 47 ± 15 ans; âge au moment de l’ACS : 40 ± 14 ans; proportion d’hommes : 50 %) à 36 sujets témoins (âge : 47 ± 15 ans; proportion d’hommes : 50 %). Il n’y avait pas de différence significative quant au score UCV entre le groupe témoin et le groupe ACS (248 ± 181 UCV vs 252 ± 227 UCV; p > 0,05). Les sujets du sous-groupe ACSe avaient un score UCV moyen significativement plus faible que ceux du sous-groupe ACSi (163 ± 183 UCV vs 308 ± 237 UCV; p = 0,030).
Conclusions
Les événements stressants, plus particulièrement ceux qui entraînent un stress chronique, peuvent prédisposer des personnes autrement en bonne santé aux arythmies mortelles. Une étude plus poussée du rôle du stress dans la survenue précipitée d’un ACS s’impose.
Most sudden cardiac arrest (SCA) cases occur in older adults with underlying coronary heart disease (CHD) or structural heart disease.1,2 In relatively younger adults, inherited heart rhythm disorders have been identified as important risk factors for SCA.2,3 However, despite extensive evaluations, the etiology of SCA or sudden cardiac death (SCD) in those without known coronary or structural heart disease remains elusive in nearly half of cases.2,4,5
The autonomic nervous system (ANS) is known to be a critical modulator of arrhythmogenesis in structurally normal and abnormal hearts.6,7 Sympathetic activation of the ANS has been shown to modulate substrates of ventricular arrhythmias in either normal or diseased myocardium8 as well as play a role in cardiovascular disease progression and risk of SCD.9 Stress—a mental, physical, or emotional tension in response to various unexpected or uncertain factors and/or circumstances—induces physiological changes by shifting tone between the sympathetic and parasympathetic systems of the ANS.10
Psychosocial factors such as distress, anxiety, depression, and life dissatisfaction have been connected with increased risk for SCD and stroke.9,11,12 Lane et al. investigated the role of psychological stress in idiopathic ventricular fibrillation (IVF) and reported that IVF survivors experienced moderate or severe psychological stress before their cardiac event.13
The Cardiac Arrest Survivors With Preserved Ejection Fraction Registry (CASPER) is a large national, multicentre registry for cases of unexplained SCA (USCA), which promotes systematic evaluation post SCA.5 Within CASPER, the cause of approximately 50% of SCA cases remain elusive after rigourous examination.5 The role of stressful life events (SLEs) as antecedents to SCA in individuals who had no etiology after rigourous testing has not been investigated.
The purpose of this exploratory study was to investigate whether life stress contributed significantly to SCA risk in a population of USCA victims vs those with an explained SCA (ESCA). The results of this study are intended to generate hypotheses to be tested in a larger sample of cardiac arrest survivors.
Methods
Recruitment
Institutional approval for this study was obtained from the UBC Children’s and Women's Health Centre of British Columbia Research Ethics Board, as well as from the research ethics board at each participating site. This exploratory study was conducted within the context of a larger program of research involving cardiac arrest survivors—CASPER. Participants were identified from 3 centres already part of the CASPER network.
Children and adults older than the age of 11 years who presented with SCA were recruited for this study. Thorough systematic evaluation of SCA in participants included but was not limited to cardiac magnetic resonance imaging, signal-averaged electrocardiogram, exercise testing, drug provocation testing, electrophysiological study, and genetic testing.5 SCA survivors who had no identifiable cardiac abnormalities on clinical testing and no pathogenic or likely pathogenic mutations, according to criteria by the American College of Medical Genetics and Genomics, identified on genetic evaluation were included in the USCA group. SCA survivors found to have cardiac abnormalities upon testing and/or one or more pathogenic mutations identified were included in the ESCA group. Participants who were unable to provide consent or complete the forms and questionnaires were excluded.
Recruited control group members were age- and sex-matched with those in the SCA survivors group. Control group participants were recruited through advertisement via flyers posted throughout BC Children’s Hospital, the BC Children’s Hospital Research Institute intranet mailing list, the Hearts in Rhythm Organization Web site, and Facebook. Those who were unable to provide consent, complete the questionnaires, had a history of SCA, or clinically relevant diagnoses were excluded.
Evaluation of life stress
Study consent was obtained and questionnaires were administered using REDCap (Vanderbilt University, Nashville, TN) housed at the BC Children’s Hospital Research Institute. We measured life stress in study participants who first experienced an SCA between ages 11 and 18 years, between ages 19 and 22 years, and at age 23 years or older, using the Social Readjustment Rating Scale (SRSS) for nonadults, Student Stress Scale (SSS), and the validated 1997 Recent Life Changes Questionnaire (RLCQ), respectively.14, 15, 16 These questionnaires list a series of potentially SLEs each with an assigned, differentially weighted numerical value measured in life change units (LCUs). Evaluations were administered to age- and sex-matched control participants.
SCA survivors were asked to mark which SLEs occurred for them in the 12 months before their SCA. Similarly, control group participants answered for the same period as their sex- and age-matched participant in the SCA group. To illustrate this, if an SCA group participant was recruited in 2018 but had their first SCA event in May of 2014, they were asked to complete the questionnaires for the year period from May 2013 to May 2014. The control group participant matched to that SCA group participant would also complete the questionnaires for the year period from May 2013 to May 2014. Aggregate scores > 500 LCUs in a 12 month period denote high recent life stress, and greater risk of illness.14
Statistical analysis
Elevated LCU scores reported on any of the questionnaires indicated elevated life stress and readjustment. Normalcy of our data was analyzed using a Kolmogorov-Smirnov test, and mean +/- SDs calculated from each of the groups and compared using a t test. A P value < 0.05 was interpreted as significant. All analyses were conducted using SPSS for Windows Version 23 (IBM Corp, Armonk, NY).
Results
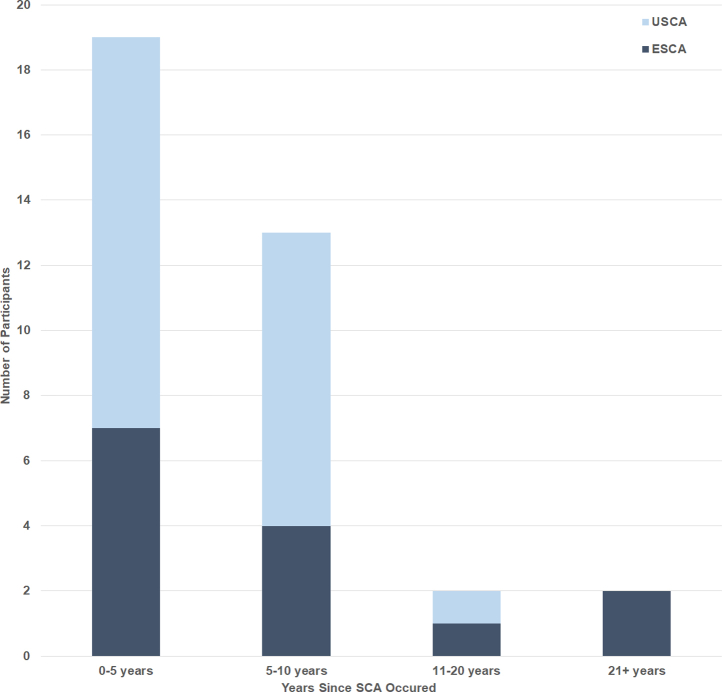
Thirty-six survivors of SCA (50% male) and 36 control participants (50% male) were recruited into the study. At time of participation, the mean age of the SCA group participants was 47 ± 15 years and control group participants was 47 ± 15 years. The mean age at the time of the SCA event in the SCA group was 40 ± 14 years. Most patients presented with SCA within the previous 5 years (19/36; 53%; Fig. 1). The SCA participants (n = 36) were categorized into USCA (22/36; 61%) and ESCA (14/36; 39%).
Figure 1.
Number of years between first SCA event and participation in current study for SCA group members. ESCA, explained sudden cardiac arrest subgroup; SCA, sudden cardiac arrest; USCA, unexplained sudden cardiac arrest subgroup.
Details of circumstances of SCA
Full details and circumstantial information related to the SCA events are outlined in Table 1. The SCA events were witnessed in 33/36 (92%) of cases, and most frequently occurred in the mornings (14/36; 39%). SCA events commonly occurred at home (14/36; 39%) or in public places (14/36; 39%). At the time of, or immediately before, the SCA event, individuals were commonly at rest (12/36; 33%) or engaged in exercise (7/36; 19%). Of the 14 patients who were exercising, moderately active or vigorously active, the most common activity was jogging or running (10; 71%).
Table 1.
Demographic descriptors and circumstance of SCA group participants
| Characteristic | n | % |
|---|---|---|
| Unexplained SCA subgroup | 22 | 61 |
| Explained SCA subgroup | 14 | 39 |
| Sex | ||
| Male | 18 | 50 |
| Female | 18 | 50 |
| Age at cardiac arrest, years | ||
| 11-18 | 2 | 6 |
| 19-22 | 2 | 6 |
| ≥ 23 | 32 | 89 |
| Time of day of SCA | ||
| Morning | 14 | 39 |
| Afternoon | 8 | 22 |
| Evening | 6 | 17 |
| Night | 2 | 6 |
| Unknown | 6 | 17 |
| Setting of SCA event | ||
| Home | 14 | 39 |
| Public place | 14 | 39 |
| School | 2 | 6 |
| Other | 5 | 14 |
| Unknown | 1 | 3 |
| Level of activity | ||
| Sleep | 2 | 6 |
| Rest | 12 | 33 |
| Mildly active | 6 | 17 |
| Moderately active | 4 | 11 |
| Vigorously active | 3 | 8 |
| Exercise | 7 | 19 |
| Unknown | 2 | 6 |
| Neurological damage from SCA | ||
| No damage | 18 | 50 |
| Yes, but it went away | 6 | 17 |
| Yes, and it hasn’t affected my life much | 6 | 17 |
| Yes, and it has changed my life a lot | 5 | 14 |
| Unknown | 1 | 3 |
| SCA witnessed by bystanders | ||
| Yes | 33 | 92 |
| No | 2 | 6 |
| Unknown | 1 | 3 |
| CPR performed by bystander | ||
| Yes | 29 | 81 |
| No | 3 | 8 |
| Unknown | 4 | 11 |
| AED used by bystander | ||
| Yes | 9 | 25 |
| No | 21 | 58 |
| Unknown | 6 | 17 |
AED, automated external defibrillator; CPR, cardiopulmonary resuscitation; SCA, sudden cardiac arrest.
Most of the SCA events that occurred were witnessed by bystanders (33/36; 92%). Of the 36 survivors, bystanders performed onsite cardiopulmonary resuscitation in 29 (81%) cases and deployed an automated external defibrillator in 9 (25%) cases. More than half of the patients (16/29; 55%) who received bystander cardiopulmonary resuscitation showed no evidence of neurological impairment. Of the SCA survivors, 6 (17%) had transient neurological impairment, 6 (17%) had mild neurological impairment, and 5 (15%) had severe neurological impairment.
SLEs
The most common SLEs are outlined in Table 2. For the SCA survivor group, 14 (39%) experienced changes in work responsibilities, 12 (33%) experienced changes in work hours, and 11 faced a major decision about the future (31%). The control group had many overlapping SLEs. The control group respondents listed vacation most often (20; 56%), followed by change in work responsibilities (12; 33%), and change in work hours (11; 30%) whereas some reported a relationship gain (8; 22%) as stressful.
Table 2.
Commonly reported stressful life events
| SCA group | n | % |
|---|---|---|
| Change in work responsibilities | 14 | 39 |
| Change in work hours | 12 | 33 |
| Major decision about future | 11 | 31 |
| Vacation | 11 | 31 |
| Control group | ||
| Vacation | 20 | 56 |
| Change in work responsibilities | 12 | 33 |
| Change in work hours | 11 | 31 |
| Gain relationship | 8 | 22 |
SCA, sudden cardiac arrest.
The mean aggregate LCU score of the SCA survivor group (252 ± 227) was not significantly different from the mean aggregate LCU score of the age- and sex-matched control group (248 ± 181; P > 0.05; Fig. 2). Within the SCA group, the time that had passed since their cardiac arrest did not differ significantly between the 2 subgroups (USCA group: 4.5 ± 3.1 years; ESCA group: 10.1 ± 14.1 years; P > 0.05). When we compared responses of participants within the SCA survivors group, the ESCA group had lower LCU scores compared with the USCA group (163 ± 183 LCUs vs 308 ± 237 LCUs; P = 0.03; Fig. 3). When we compared each of the SCA subgroups with their respective age- and sex-matched control participants, LCU scores were not significantly different (USCA subgroup vs controls: 308 ± 237 LCUs vs 265 ± 205 LCUs; P > 0.05; ESCA subgroup vs controls: 163 ±183 LCUs vs 218 ± 134 LCUs; P > 0.05). Similarly, there was no correlation between LCU scores and overall time from cardiac arrest (P > 0.05) nor between LCU score vs time from cardiac arrest for each of the 2 subgroups (USCA time from SCA vs LCU score, P > 0.05; ESCA time from SCA vs LCU score, P > 0.05).
Figure 2.
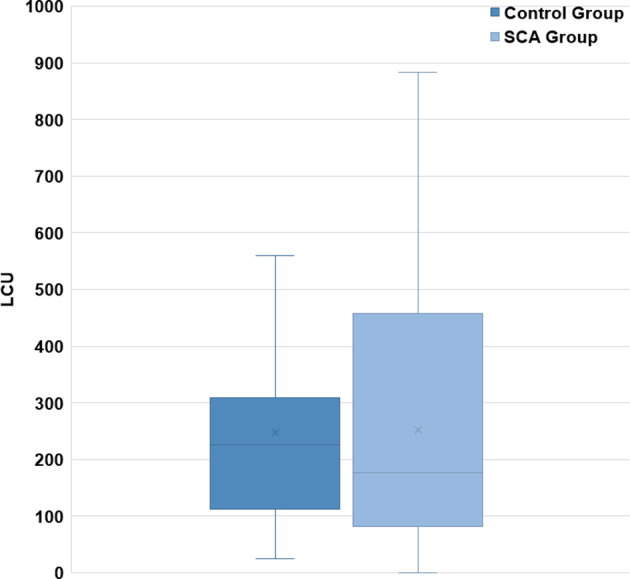
Comparison of LCU scores of the SCA and control group participants on stress inventories. The difference between the 2 groups was nonsignificant (P > 0.05). LCU, life change units; SCA, sudden cardiac arrest.
Figure 3.
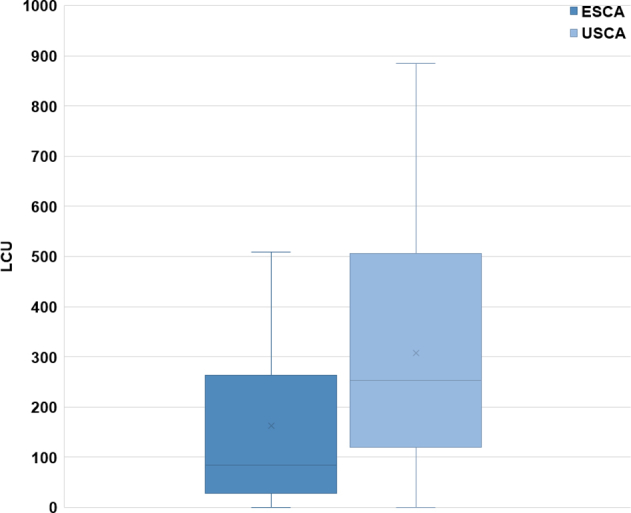
Comparison of LCU scores of ESCA and USCA subgroup participants on stress inventories. The difference between the 2 groups was significant (P = 0.03). ESCA, explained sudden cardiac arrest subgroup; LCU, life change units; USCA, unexplained sudden cardiac arrest subgroup.
Discussion
The main purpose of this exploratory study was to investigate the potential role of life stress in SCA events. Half of SCA and SCD among younger individuals remain unexplained despite extensive investigation.2,4,5 Stress has been shown previously to precede cardiac events13 and psychosocial factors including distress, anxiety, depression, and life dissatisfaction have been connected with increased risk for SCD and stroke.9,11,12 We investigated whether SCA survivors had elevated life stress in the year before SCA. There was no significant difference between SLEs measured as LCUs between the SCA group and their age- and sex-matched control participants, which was unexpected. We expected SCA survivors to have experienced more SLEs leading up to their cardiac arrest compared with age- and sex-matched control participants because previous reports indicate that stress influences cardiac health. The result was surprising and could be because of the limitations of this study as outlined in the Limitations. The critical relationship between life stress and the heart is perhaps most effectively illustrated by Takotsubo syndrome, which is classically triggered by acute emotional stressors, such as the loss of a loved one.17 A significant increase in myocardial infarction incidence is established around potentially stressful times of the year, especially Mondays, Christmas and New Year’s, and spring daylight savings.18, 19, 20, 21 Temporal relationships between SCD and acutely stressful events, such as extreme threats to personal safety or hearing of a loved one’s death, have also been reported.22 Phobic anxiety has been linked to CHD and SCD in men and women, and additional psychosocial factors such as distress, anxiety, depression, and life dissatisfaction have been connected with increased risk for SCD and stroke.9,11,12
The lower rates of life stress in individuals from the ESCA group compared with the USCA subgroup complimented the findings from the study by Lane et al. In their study, a significantly greater number of the IVF survivors experienced moderate or severe psychological stress before their cardiac event compared with the CHD patients.13 They compared IVF survivors and CHD patients who had survived acute myocardial infarction or angina. The study by Lane et al. provided evidence for psychological stress playing a role in USCA, but reliance on participants’ perceptions of the stressful events left room for memory error and bias especially without using a proper control group.
In the current study, we observed that mornings were the most common time individuals experienced SCA. This finding aligns with the robust body of literature that identify a circadian pattern of SCA incidence. A morning peak in SCA occurrence has been reported in cohort studies and prospective studies,23, 24, 25, 26 although the exact mechanisms for this circadian variation is still not well understood. However, some recent studies have noted a loss of morning peak, possibly because of the shifting sleep patterns.27 Alternatively, the higher LCU scores in the USCA group could be attributed to the unexplained nature of their SCA and merits further investigation.
The connection between stress and cardiac events likely lies with the ANS. Ventricular fibrillation is a common cause of SCD, and cardiac autonomic function might be an important factor in setting the arrhythmic threshold.28,29 Psychosocial factors like stress might affect arrhythmic risk by altering the sympathetic-parasympathetic balance.30 This imbalance could create an unstable cardiac state with increased risk for deadly arrhythmias, even in ostensibly healthy individuals. Although what exactly disposes individuals without an identified etiology to deadly arrhythmias is likely multifactorial; our findings suggest a role for environmental factors including chronic life stress.
Limitations
Reliability of participants’ responses on SLE checklists decreases as the recall period increases.31 Although the recall period between the USCA and ESCA groups did not differ, we are limited by the accuracy of individuals’ retrieval. Additionally, an individual's current state affects how they interpret past events and can distort memories, even if the memory prompt asked is considered objective.31,32 Our study design attempted to limit this bias by using an objective list and scoring system for SLEs, but as already noted, even objective measures are susceptible to participants’ biases. It is possible that those who lack a proper explanation for their sudden cardiac event are open to alternative explanations, such as stress, might recall stressful events more easily, might report greater stress than they were actually experiencing, or believe stressful events are worth reporting. Also, our control group might not be a true representation of the general population and perhaps were more cognizant of stress in their lives and sought participation after exposure to our advertising materials. Furthermore, because of the small sample size, we were unable to determine possible associations between other confounders such as lifestyle (exposure to illicit substances, alcohol, stimulant drinks, participation in vigorous sports, etc) and onset of SCA in this cohort.
Conclusions
Although there were no significant differences in SLEs between the SCA group and age- and sex-matched control group, the unexplained nature of the SCA might give rise to an elevated life stress score or alternatively SLEs might predispose certain individuals to SCA, particularly those in whom a cardiac etiology is not identified. The ANS is a likely system that mediates the interaction between stress and SCA. Further, larger scale, prospective studies using more robust techniques are warranted to determine the association between potential confounders such as lifestyle for those whose SCA remains unexplained. The relationship between stress, the ANS, and other confounders such as lifestyle and SCA merits further investigation.
Acknowledgements
The authors thank the participants for their cooperation and contribution to this study.
Footnotes
Ethics Statement: The study was approved by the UBC Children's and Women's Health Centre of British Columbia Research Ethics Board as well as the research ethics board at each participating site. Informed consent was obtained from each study participant.
See page 290 for disclosure information.
Funding Sources
This study was supported by the Heart in Rhythm Organization (Dr Krahn, Principal Investigator), which receives support from the Canadian Institutes of Health Research (RN380020-406814).
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Chugh S.S., Reinier K., Teodorescu C. Epidemiology of sudden cardiac death: clinical and research implications. Prog Cardiovasc Dis. 2008;51:213–228. doi: 10.1016/j.pcad.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagnall R.D., Weintraub R.G., Ingles J. A prospective study of sudden cardiac death among children and young adults. N Engl J Med. 2016;374:2441–2452. doi: 10.1056/NEJMoa1510687. [DOI] [PubMed] [Google Scholar]
- 3.Risgaard B. Sudden cardiac death: a nationwide cohort study among the young. Dan Med J. 2016;63:B5321. [PubMed] [Google Scholar]
- 4.Lahrouchi N., Raju H., Lodder E.M. Utility of post-mortem genetic testing in cases of sudden arrhythmic death syndrome. J Am Coll Cardiol. 2017;69:2134–2145. doi: 10.1016/j.jacc.2017.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krahn A.D., Healey J.S., Chauhan V. Systematic assessment of patients with unexplained cardiac arrest: Cardiac Arrest Survivors With Preserved Ejection Fraction Registry (CASPER) Circulation. 2009;120:278–285. doi: 10.1161/CIRCULATIONAHA.109.853143. [DOI] [PubMed] [Google Scholar]
- 6.Franciosi S., Perry F.K.G., Roston T.M. The role of the autonomic nervous system in arrhythmias and sudden cardiac death. Auton Neurosci. 2017;205:1–11. doi: 10.1016/j.autneu.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Shen M.J., Zipes D.P. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ Res. 2014;114:1004–1021. doi: 10.1161/CIRCRESAHA.113.302549. [DOI] [PubMed] [Google Scholar]
- 8.Gilmour R.F. Life out of balance: the sympathetic nervous system and cardiac arrhythmias. Cardiovasc Res. 2001;51:625–626. doi: 10.1016/s0008-6363(01)00402-3. [DOI] [PubMed] [Google Scholar]
- 9.Hering D., Lachowska K., Schlaich M. Role of the sympathetic nervous system in stress-mediated cardiovascular disease. Curr Hypertens Rep. 2015;17:80. doi: 10.1007/s11906-015-0594-5. [DOI] [PubMed] [Google Scholar]
- 10.McEwen B.S. Neurobiological and systemic effects of chronic stress. Chronic Stress (Thousand Oaks) 2017;1 doi: 10.1177/2470547017692328. 2470547017692328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albert C.M., Chae C.U., Rexrode K.M. Phobic anxiety and risk of coronary heart disease and sudden cardiac death among women. Circulation. 2005;111:480–487. doi: 10.1161/01.CIR.0000153813.64165.5D. [DOI] [PubMed] [Google Scholar]
- 12.Kawachi I., Colditz G.A., Ascherio A. Prospective study of phobic anxiety and risk of coronary heart disease in men. Circulation. 1994;89:1992–1997. doi: 10.1161/01.cir.89.5.1992. [DOI] [PubMed] [Google Scholar]
- 13.Lane R.D., Laukes C., Marcus F.I. Psychological stress preceding idiopathic ventricular fibrillation. Psychosom Med. 2005;67:359–365. doi: 10.1097/01.psy.0000160476.67536.41. [DOI] [PubMed] [Google Scholar]
- 14.Miller M.A., Rahe R.H. Life changes scaling for the 1990s. J Psychosom Res. 1997;43:279–292. doi: 10.1016/s0022-3999(97)00118-9. [DOI] [PubMed] [Google Scholar]
- 15.Holmes T.H., Rahe R.H. The Social Readjustment Rating Scale. J Psychosom Res. 1967;11:213–218. doi: 10.1016/0022-3999(67)90010-4. [DOI] [PubMed] [Google Scholar]
- 16.Insel P.R.W. Mayfield Publishing Company; Palo Alto, CA: 1985. Core Concepts in Health. 4th Ed. [Google Scholar]
- 17.Y-Hassan S., Tornvall P. Epidemiology, pathogenesis, and management of takotsubo syndrome. Clin Auton Res. 2018;28:53–65. doi: 10.1007/s10286-017-0465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wallert J., Held C., Madison G. Temporal changes in myocardial infarction incidence rates are associated with periods of perceived psychosocial stress: a SWEDEHEART national registry study. Am Heart J. 2017;191:12–20. doi: 10.1016/j.ahj.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 19.Phillips D.P., Jarvinen J.R., Abramson I.S. Cardiac mortality is higher around Christmas and New Year’s than at any other time: the holidays as a risk factor for death. Circulation. 2004;110:3781–3788. doi: 10.1161/01.CIR.0000151424.02045.F7. [DOI] [PubMed] [Google Scholar]
- 20.Collart P., Coppieters Y., Godin I. Day-of-the-week variations in myocardial infarction onset over a 27-year period: the importance of age and other risk factors. Am J Emerg Med. 2014;32:558–562. doi: 10.1016/j.ajem.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 21.Jiddou M.R., Pica M., Boura J. Incidence of myocardial infarction with shifts to and from daylight savings time. Am J Cardiol. 2013;111:631–635. doi: 10.1016/j.amjcard.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 22.Radnic B., Radojevic N., Vucinic J. The association between pro-arrhythmic agents and aortic stenosis in young adults: is it sufficient to clarify the sudden unexpected deaths? Cardiol Young. 2017;27:929–935. doi: 10.1017/S1047951116001864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willich S.N., Levy D., Rocco M.B. Circadian variation in the incidence of sudden cardiac death in the Framingham Heart Study population. Am J Cardiol. 1987;60:801–806. doi: 10.1016/0002-9149(87)91027-7. [DOI] [PubMed] [Google Scholar]
- 24.Levine R.L., Pepe P.E., Fromm R.E., Jr. Prospective evidence of a circadian rhythm for out-of-hospital cardiac arrests. JAMA. 1992;267:2935–2937. [PubMed] [Google Scholar]
- 25.Bagai A., McNally B.F., Al-Khatib S.M. Temporal differences in out-of-hospital cardiac arrest incidence and survival. Circulation. 2013;128:2595–2602. doi: 10.1161/CIRCULATIONAHA.113.004164. [DOI] [PubMed] [Google Scholar]
- 26.Peters R.W., McQuillan S., Gold M.R. Interaction of septadian and circadian rhythms in life-threatening ventricular arrhythmias in patients with implantable cardioverter-defibrillators. Am J Cardiol. 1999;84:555–557. doi: 10.1016/s0002-9149(99)00376-8. [DOI] [PubMed] [Google Scholar]
- 27.Ni Y.M., Rusinaru C., Reinier K. Unexpected shift in circadian and septadian variation of sudden cardiac arrest: the Oregon Sudden Unexpected Death Study. Heart Rhythm. 2019;16:411–415. doi: 10.1016/j.hrthm.2018.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meredith I.T., Broughton A., Jennings G.L. Evidence of a selective increase in cardiac sympathetic activity in patients with sustained ventricular arrhythmias. N Engl J Med. 1991;325:618–624. doi: 10.1056/NEJM199108293250905. [DOI] [PubMed] [Google Scholar]
- 29.Barron H.V., Lesh M.D. Autonomic nervous system and sudden cardiac death. J Am Coll Cardiol. 1996;27:1053–1060. doi: 10.1016/0735-1097(95)00615-X. [DOI] [PubMed] [Google Scholar]
- 30.Sgoifo A., De Boer S.F., Buwalda B. Vulnerability to arrhythmias during social stress in rats with different sympathovagal balance. Am J Physiol. 1998;275:H460–H466. doi: 10.1152/ajpheart.1998.275.2.H460. [DOI] [PubMed] [Google Scholar]
- 31.Dohrenwend B.P. Inventorying stressful life events as risk factors for psychopathology: toward resolution of the problem of intracategory variability. Psychol Bull. 2006;132:477–495. doi: 10.1037/0033-2909.132.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Southwick S.M., Morgan C.A., 3rd, Nicolaou A.L. Consistency of memory for combat-related traumatic events in veterans of Operation Desert Storm. Am J Psychiatry. 1997;154:173–177. doi: 10.1176/ajp.154.2.173. [DOI] [PubMed] [Google Scholar]