Abstract
Background
Intracardiac echocardiography and 3D mapping systems allow catheter ablation for atrial fibrillation (AF) to be conducted without fluoroscopy; however, the safety and effectiveness of fluoroless AF ablation are not well defined.
Methods
We examined consecutive radiofrequency AF catheter ablations at a large academic teaching hospital from November 2017 to July 2019. Outcomes for fluoroscopy-guided (N = 176) and fluoroless (N = 147) ablations were compared. Cases were designated as fluoroless at the outset of the procedure.
Results
Mean age was 59.5 ± 10 years, 66.9% were male, 71.8% had paroxysmal AF, and the mean CHA2DS2-VASc score was 1.7 ± 1.4. There were no differences in patient baseline characteristics. In the fluoroless group, minimal fluoroscopy was used in 17 patients (median, 3 seconds; interquartile range, 1.2-4.8). Mean procedure time, fluoroscopy time, and radiation dose (± standard deviation) were greater in the fluoroscopy group compared with the fluoroless group (194 ± 56 vs 176 ± 46 minutes, P = 0.0021; 10.7 ± 6.6 vs 0.008 ± 0.03 minutes, P < 0.0001; 2759.2 ± 1911 vs 5.4 ± 24 μGy m2, P < 0.0001). In multivariable linear regression models, fluoroless AF ablation was independently associated with reduced procedure times (ß = −16.5 minutes, P = 0.01). Acute procedural success (95.5% vs 98.6%, P = 0.1), complication rates (4.5% vs 2.0%, P = 0.24), and 1-year AF recurrence rates (28.7% vs 27.1%, log-rank P = 0.69) were similar between fluoroscopy and fluoroless groups. Excluding the 17 patients receiving fluoroscopy in the fluoroless group did not impact our results (P = 0.013). After exclusion of redo cases, fluoroless AF ablation was no longer associated with reduced procedure times (ß = −11.4 minutes, P = 0.106).
Conclusions
Fluoroless radiofrequency AF ablation had similar effectiveness and safety compared with conventional fluoroscopy-guided AF ablation.
Résumé
Contexte
L'échocardiographie intracardiaque et les systèmes de cartographie 3D permettent l'ablation par cathéter de la fibrillation auriculaire (FA) sans fluoroscopie; l'innocuité et l'efficacité d'une telle approche ne sont toutefois pas bien connues.
Méthodologie
Nous avons examiné les résultats d'ablations par cathéter de la FA par radiofréquences menées de façon consécutive dans un hôpital universitaire d’envergure entre novembre 2017 et juillet 2019. Les résultats des ablations par fluoroscopie (n = 176) et des ablations sans fluoroscopie (n = 147) ont été comparés. Les cas étaient désignés comme n'ayant pas utilisé la fluoroscopie à la fin de l'intervention, le cas échéant.
Résultats
L'âge moyen était de 59,5 ± 10 ans, 66,9 % des patients étaient des hommes, 71,8 % étaient atteints de FA paroxystique, et le score CHA2DS2-VASc moyen était de 1,7 ± 1,4. Il n'y avait pas de différences entre les caractéristiques des patients au départ. Dans le groupe ayant subi une ablation sans fluoroscopie, une fluoroscopie minimale a été utilisée chez 17 patients (médiane : 3 secondes; intervalle interquartile : 1,2-4,8). La durée moyenne de l'intervention, la durée de la fluoroscopie, et la dose de rayonnements (± écart type) ont été plus élevées dans le groupe avec fluoroscopie que dans le groupe sans fluoroscopie (194 ± 56 vs 176 ± 46 minutes, p = 0,0021; 10,7 ± 6,6 vs 0,008 ± 0,03 minute, p < 0,0001; 2759,2 ± 1911 vs 5,4 ± 24 μGy m2, p < 0,0001). Dans des modèles de régression linéaire multivariables, l'ablation de la FA sans fluoroscopie a été associée de façon indépendante à des interventions de plus courte durée (ß = −16,5 minutes, p = 0,01). Le succès immédiat de l'intervention (95,5 % vs 98,6 %, p = 0,1), le taux de complications (4,5 % vs 2,0 %, p = 0,24), et le taux de récidive de la FA après 1 an (28,7 % vs 27,1 %, p (test du log-rank = 0,69) ont été comparables dans les groupes avec et sans fluoroscopie. L'exclusion des 17 patients chez qui la fluoroscopie avait été utilisée dans le groupe sans fluoroscopie n'a pas modifié ces résultats (p = 0,013). Après l'exclusion des cas où l'intervention était une reprise, l'ablation de la FA sans fluoroscopie n'était plus associée à une réduction des durées d'intervention (ß = −11,4 minutes, p = 0,106).
Conclusions
L'ablation de la FA par radiofréquences sans fluoroscopie est associée à une efficacité et à une innocuité comparables à celles de l'ablation de la FA classique guidée par fluoroscopie.
Radiofrequency (RF) catheter ablation is more effective than antiarrhythmic drugs at maintaining sinus rhythm in patients with atrial fibrillation (AF), and increasing evidence suggests that the procedure can be used as first-line therapy for most patients with AF.1, 2, 3 However, catheter ablation for AF is typically performed using fluoroscopy, which exposes patients and operators to radiation, increasing their cumulative risk of cancer.4, 5, 6 Medical staff must also wear heavy protective equipment, increasing their risk of musculoskeletal injury and disability.7
Recently, strategies for fluoroless catheter ablation for AF have been described;8, 9, 10, 11, 12 however, efficacy and safety data are lacking. Limited data suggest that fluoroless methods for AF ablation may have similar acute procedural success rates when compared with conventional fluoroscopy-based ablations.9,11,12 However, there are still concerns that fluoroless methods will lead to increased procedural duration times, procedure-related complication rates, and an increased risk of AF recurrence after ablation.
In the present analysis, we examined our local outcomes for fluoroless RF catheter ablation for AF to evaluate its safety and effectiveness in comparison with conventional fluoroscopy-guided RF catheter ablation for AF. We hypothesized that fluoroless AF ablation would have a similar safety profile, procedure duration, and effectiveness compared with conventional AF ablation.
Methods
Patients
The fluoroless AF catheter ablation program at Hamilton Health Sciences/McMaster University (Hamilton, Ontario, Canada) started on May 14, 2018, after which there was a gradual uptake of the procedure and the majority of cases have been performed without fluoroscopy. Consecutive patients who underwent an RF catheter ablation procedure for AF with or without atrial flutter (AFL) between November 2017 and July 2019 were included in this analysis (N = 323). To minimize bias, this cohort included all early fluoroless cases for each individual operator, and no patients were excluded. From clinical records, we retrospectively obtained patient baseline characteristics and procedural outcomes. Four operators (G.A., S.D., J.G.A., J.A.W.) conducted AF ablations on 3 different mapping systems (EnSite, n = 175; CARTO, n = 144; Rhythmia, n = 3). Use of fluoroscopy during a case was at the operator’s discretion; however, once the fluoroless program began, fluoroless AF ablation became the standard of care and the first option for all procedures. Cases were designated as fluoroless from the beginning of the procedure, and this designation did not change if fluoroscopy was subsequently used. The study was approved by the Hamilton Integrated Research Ethics Board.
Procedural methodology
Procedures were performed using either conscious sedation or general anaesthesia. Periprocedural anticoagulation was uninterrupted in patients receiving warfarin, whereas individuals on a direct oral anticoagulant held anticoagulation for 24 hours. Venous access was obtained using direct ultrasound visualization. An intracardiac echocardiography (ICE) catheter was advanced to the low right atrium to start in all procedures. In cases using CARTO, we additionally created a 3D shell of the left atrium (LA) using Soundstar software (Biosense Webster, Irvine, CA).
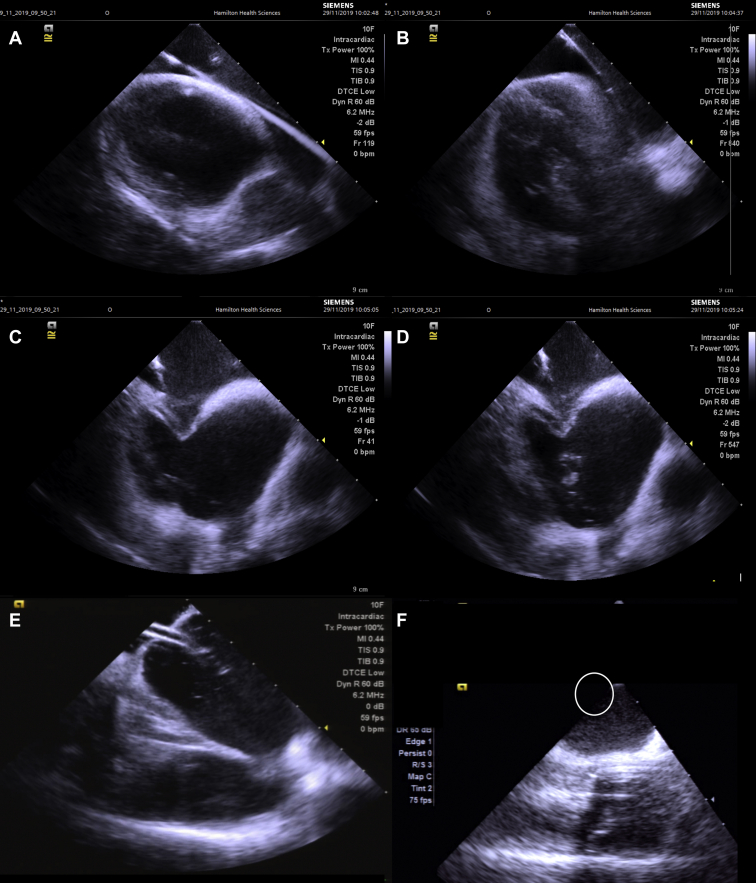
In all fluoroless cases, we started with creation of a 3D shell of the right atrium including the superior and inferior vena cava, coronary sinus ostium and body, with catheter manipulation. A deflectable multielectrode catheter was then placed in the distal coronary sinus and secured. Figure 1 summarizes transseptal access in a fluoroless case. A long, flexible guidewire was advanced to the superior vena cava (SVC) as confirmed on ICE, and then a transseptal sheath (SLO) was advanced to the SVC over the wire under ICE guidance. The guidewire was removed, and the sheath was meticulously flushed for air bubbles and a BRK-1 transseptal needle was advanced within the lumen of the SLO sheath. The sheath and needle were pulled down together under ICE guidance until there was observed displacement of the sheath onto the fossa ovalis, as evidenced by tenting of the fossa. The needle was advanced against the interatrial septum under ICE to monitor access into the LA. For individuals with cardiac implantable electronic devices (CIEDs), movement of the leads during sheath manipulation in the right atrium was monitored on ICE before transseptal puncture. If there was evidence of lead interaction, the transseptal sheath would be returned to the SVC under ICE using a guidewire, with the sheath pull-down repeated. After access into the LA was confirmed on ICE with heparinized saline injection, the sheath is advanced over the dilator into the LA under ICE guidance and connected to a pressurized, heparinized saline solution. A multielectrode catheter is advanced via the SLO sheath into the LA, and a 3D shell of the LA is created. Using an identical technique as above, a second transseptal puncture is performed to advance an SL1 sheath into the LA. After the sheath is flushed for air bubbles and connected to a pressurized saline solution, an irrigated ablation catheter is advanced into the LA. Heparin boluses were administrated during the case to maintain the activated clotting time between 300 and 350 seconds. Use of deflectable sheaths after transseptal puncture was left to operator discretion.
Figure 1.
Intracardiac echocardiography–guided transseptal puncture and placement of esophageal temperature probe. (A) View of the right atrium (RA), left atrium (LA), and superior vena cava (SVC) with guidewire in the SVC. (B) Transseptal sheath is advanced over the wire to the SVC. (C) Brockenbrough (BRK) needle is inserted in the lumen of the transseptal sheath, and both are dragged down together until there is displacement onto the fossa ovalis. (D) When in acceptable anteroposterior orientation, the BRK needle is advanced against the interatrial septum crossing into the LA. A microbubble injection confirms entry into the LA. (E) Dilator and sheath are advanced over the body of the BRK needle into the LA, and the dilator and needle are removed, leaving the sheath in the LA. (F) Placement of esophageal temperature probe tip in the posterior LA (circle).
Pulmonary vein isolation consisted of creating circumferential RF ablation lesions around the left and right set of pulmonary veins with the goal of entrance and exit conduction block. Additional ablation was performed at the discretion of the operator and could have included other linear ablation such as creation of LA roofline, posterior LA wall isolation, lateral isthmus line, cavotricuspid isthmus line, and isolation of the SVC. RF energy settings were operator dependent and included a range of 20-50 W. Contact force was used in all cases when available (n = 318), with a minimum contact of 8 g per lesion used when possible.
Positioning of an esophageal temperature probe was confirmed with ICE and demarcated using Soundstar when available. In the fluoroless group, minimal fluoroscopy (median, 3 seconds; interquartile range, 1.2-4.8) was used in 17 cases to confirm positioning of an esophageal temperature probe. This was generally a preplanned activity that took place at the beginning of each case, and required for only the operator to wear lead protection for a short period of time. For the majority of cases, operators and support staff did not wear lead protection at all. Procedure start was defined as the time of femoral sheath insertion, whereas the procedure was deemed completed at the time of sheath removal in procedures without general anaesthesia, or at the time of extubation in cases performed with general anaesthesia. In patients with CIEDs, device interrogation was performed after procedure to confirm that lead parameters were unchanged.
Patient follow-up
Patients were typically followed up in clinic at 3-6 months and 12 months after the ablation procedure. Subsequent visits took place depending on the operator’s preference. Procedural complications and AF recurrence data were collected. Follow-up electrocardiogram (ECG) monitoring consisted of a 48-hour Holter monitor at 3 months followed by 7- to 14-day Holter monitoring at 1 year. Additional ECG monitoring may have been performed if the patient exhibited symptoms. AF recurrence was defined as the occurrence of AF or AFL as seen on 12-lead ECG, or any AF/AFL episode that led to hospital presentation/admission or cardioversion, or an AF/AFL episode recorded on Holter or telemetry monitoring that was > 30 seconds in duration.
Statistical analyses
Continuous variables were reported as means ± standard deviations or medians (interquartile range) if not normally distributed. Categorical variables were reported as frequencies (%). Continuous variables were compared by Student’s t test, whereas categorical variables were compared using the χ2 test. Multivariable linear regression was used to adjust for confounders of procedure time, which included age, sex, body mass index (BMI), Congestive Heart Failure, Hypertension, Age (≥ 75 years), Diabetes, Stroke/Transient Ischemic Attack, Vascular Disease, Age (65-74 years), Sex (Female) (CHA2DS2-VASc) score, prior cardiac surgery, AF pattern, redo case, operator, presence of a trainee, use of linear ablation, mapping system used, use of general anaesthesia, and use of high-power ablation. Because the fluoroless program started later in time compared with the conventional AF ablation program, adjustment by temporal period was also performed. Temporal period was modeled as a dichotomous variable, reflecting the time period before and after the start of the fluoroless ablation program. One-year AF recurrence data are shown using Kaplan-Meier curves, with the log-rank test being used to demonstrate statistical significance. As sensitivity analyses, we excluded the 17 patients in the fluoroless group who received fluoroscopy and examined the effect on procedure duration, acute procedural success, and 1-year AF recurrence. In addition, we also performed a stratified analysis examining the above outcomes separately for patients undergoing first-time AF ablation and redo AF ablation. Statistical significance was defined as a 2-tailed P value of ≤ 0.05. Stata v.13.0 was used for all statistical analyses (College Station, TX).
Results
Patient baseline characteristics are summarized in Table 1. The mean age of participants was 59.5 ± 10 years, with 66.9% being male and the mean CHA2DS2-VASc score of 1.7 ± 1.4. A total of 71.8% of patients had paroxysmal AF. Individuals in the fluoroscopy group had borderline significantly lower BMI (29.5 ± 4.9 vs 30.7 ± 5.3 kg/m2, P = 0.043). Otherwise, there were no statistically significant differences in baseline characteristics found between groups, including key procedure-outcome deciding factors such as sex, age, history of hypertension, diabetes, sleep apnea, CHA2DS2-VASc scores, LA diameter, pattern of AF, or history of prior AF ablation.
Table 1.
Baseline characteristics of the study population
| Characteristic | Total (n = 323) | Fluoroscopy group (n = 176) | Fluoroless group (n = 147) | P value |
|---|---|---|---|---|
| Age (y) | 59.5 ± 10.0 | 59.7 ± 9.9 | 59.2 ± 10.1 | 0.63 |
| Age ≥ 75 y | 17 (5.3%) | 10 (5.7%) | 7 (4.8%) | 0.71 |
| Age 65-74 y | 88 (27.2%) | 53 (30.1%) | 35 (23.8%) | 0.21 |
| Male sex | 216 (66.9%) | 118 (67.1%) | 98 (66.7%) | 0.94 |
| BMI (kg/m2) | 30.0 ± 5.1 | 29.5 ± 4.9 | 30.7 ± 5.3 | 0.043 |
| Congestive heart failure | 49 (15.2%) | 27 (15.3%) | 22 (15.0%) | 0.93 |
| Hypertension | 176 (54.5%) | 95 (54.0%) | 81 (55.1%) | 0.84 |
| Diabetes | 23 (7.1%) | 11 (6.3%) | 12 (8.2%) | 0.51 |
| Prior stroke/TIA | 25 (7.7%) | 15 (8.5%) | 10 (6.8%) | 0.57 |
| Vascular disease | 42 (13.0%) | 24 (13.6%) | 18 (12.2%) | 0.71 |
| CHA2DS2-VASc score | 1.7 ± 1.4 | 1.8 ± 1.4 | 1.7 ± 1.4 | 0.82 |
| Prior cardiac surgery | 18 (5.6%) | 12 (6.8%) | 6 (4.1%) | 0.29 |
| Prior valve replacement | 9 (2.8%) | 6 (3.4%) | 3 (2.0%) | 0.46 |
| Congenital heart disease | 2 (0.6%) | 1 (0.6%) | 1 (0.7%) | 0.9 |
| Creatinine (mmol/L) | 86 ± 24 | 87 ± 28 | 85 ± 19 | 0.37 |
| Sleep apnea | 104 (4.3%) | 50 (28.4%) | 54 (36.7%) | 0.11 |
| LA diameter (mm) | 42 ± 8 | 41 ± 8 | 42 ± 7 | 0.17 |
| LVEF (%) | 55 ± 7 | 55 ± 7 | 56 ± 7 | 0.16 |
| Type of AF | ||||
| Paroxysmal AF | 232 (71.8%) | 128 (72.7%) | 104 (70.8%) | 0.69 |
| Persistent AF | 91 (28.2%) | 48 (27.3%) | 43 (29.2%) | |
| Pacemaker/ICD | 15 (4.6%) | 11 (6.3%) | 4 (2.7%) | 0.13 |
Continuous variables were reported as means ± standard deviation. Categorical variables were reported as n (%). Continuous variables were compared by Student’s t test, whereas categorical variables were compared using Pearson’s χ2 test.
AF, atrial fibrillation; BMI, body mass index; CHA2DS2-VASc, Congestive Heart Failure, Hypertension, Age (≥75 years), Diabetes, Stroke/Transient Ischemic Attack, Vascular Disease, Age (65-74 years), Sex (Female); ICD, implanted cardiac defibrillator; LA, left atrium; LVEF, left ventricular ejection fraction; TIA, transient ischemic attack.
Procedure-related details are summarized in Table 2. There was no significant difference in the proportion of redo AF cases between the conventional group and the fluoroless group (32.4% vs 40.1%, P = 0.15). Patients in the conventional group were more likely to undergo pulmonary vein isolation only compared with patients in the fluoroless group (61.9% vs 44.2%, P = 0.001) and more frequently underwent ablation using EnSite (65.9% vs 40.8%, P < 0.001) compared with the fluoroless group.
Table 2.
Procedure details of fluoroscopy-guided and fluoroless radiofrequency catheter ablations for AF
| Characteristic | Fluoroscopy group (n = 176) | Fluoroless group (n = 147) | P value |
|---|---|---|---|
| Redo AF case | 57 (32.4) | 59 (40.1) | 0.15 |
| PVI only | 109 (61.9) | 65 (44.2) | 0.001 |
| PVI + CTI | 35 (19.9) | 36 (24.5) | 0.32 |
| PVI + lines | 10 (5.7) | 17 (11.6) | 0.06 |
| PVI + CTI + lines | 22 (12.5) | 29 (19.7) | 0.08 |
| Mapping system | |||
| EnSite | 116 (65.9) | 60 (40.8) | < 0.001 |
| CARTO | 59 (33.5) | 85 (57.8) | |
| Rhythmia | 1 (0.6) | 2 (1.4) | |
| High-power short-duration ablation | 23 (13.1) | 36 (24.7) | 0.008 |
| Presence of a trainee | 42 (23.9) | 45 (30.6) | 0.17 |
Data presented as n (%). Statistical comparisons made using Pearson’s χ2 test.
Lines consisted of left atrial ablation lines and/or superior vena cava isolation.
AF, atrial fibrillation; CTI, cavotricuspid isthmus ablation; PVI, pulmonary vein isolation.
Procedural outcomes are summarized in Table 3. Conventional AF ablations were of longer duration compared with the fluoroless group (194 ± 56 vs 176 ± 46 minutes, P = 0.0021). Mean RF ablation time (34.5 ± 18 vs 27.4 ± 15 minutes, P = 0.0002), fluoroscopy time (10.7 ± 6.6 vs 0.008 ± 0.03 minutes, P < 0.0001), and dose area product (2759.2 ± 1911 vs 5.4 ± 24 μGy m2, P < 0.0001) were also greater in the fluoroscopy group compared with the fluoroless group. After multivariable adjustment with potential confounders of procedure duration including age, sex, BMI, CHA2DS2-VASc score, history of prior cardiac surgery, AF pattern, operator, presence of a trainee during the case, mapping system, prior AF ablation, use of additional linear RF ablation, use of general anaesthesia, high-power ablation, and temporal period, fluoroless ablation remained associated with reduced total procedure time compared with fluoroscopy-guided AF ablation (ß = −16.5 minutes, 95% confidence interval [CI]: −28.9 to −4.0, P = 0.01). Supplemental Table S1 summarizes the independent predictors of total procedure time. Exclusion of the 17 patients who received fluoroscopy from the fluoroless group did not meaningfully affect the result (ß = −16.7 minutes, 95% CI: −29.9 to −3.5, P = 0.013). On stratified analysis, when examining the patients who underwent first-time AF ablation and redo AF ablation separately, fluoroless ablation was associated with a trend towards shorter procedure times in both groups (first-time: ß = −11.4 minutes, 95% CI: −25.3 to +2.5, P = 0.106; redo: ß = −24.3 minutes, 95% CI: −51.3 to +2.8, P = 0.078). In multivariable adjusted models, fluoroless AF ablation was not associated with RF time (ß = −1.7 minutes, 95% CI: −5.3 to +1.9, P = 0.35).
Table 3.
Procedural outcomes of fluoroscopy-guided and fluoroless radiofrequency catheter ablations for atrial fibrillation
| Outcome | Fluoroscopy group (n = 176) | Fluoroless group (n = 147) | P value |
|---|---|---|---|
| Procedural time (min) | 194 ± 56 | 176 ± 46 | 0.0021 |
| Applied RF time (min) | 34.5 ± 18 | 27.4 ± 15 | 0.0002 |
| Fluoroscopy time (min) | 10.7 ± 6.6 | 0.008 ± 0.03 | < 0.0001 |
| Dose area product (μGy m2) | 2759.2 ± 1911 | 5.4 ± 24 | < 0.0001 |
| Acute procedural success | 168 (95.5%) | 145 (98.6%) | 0.1 |
Continuous variables were reported as means ± standard deviation. Categorical variables were reported as n (%). Continuous variables were compared by Student’s t test, and categorical variables were compared by Pearson’s χ2 test.
RF, radiofrequency.
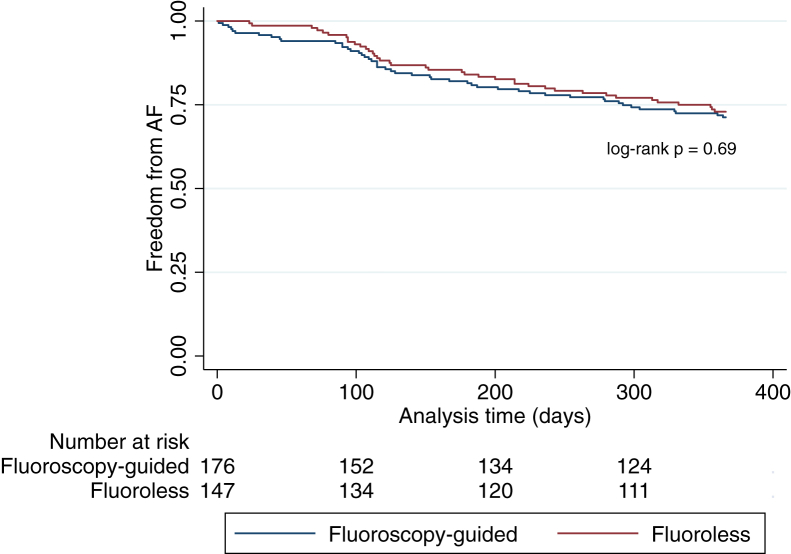
Acute procedural success, defined as the successful isolation of all 4 pulmonary veins and/or bidirectional block across ablation lines or isolation of peripheral arrhythmogenic sites, was found to be similar between the 2 groups, with 95.5% and 98.6% (P = 0.1) success for fluoroscopy and fluoroless groups, respectively. Procedural complications were not increased in the fluoroless group (Table 4; fluoroscopy: 4.5%, fluoroless: 2.0%, P = 0.24). There were no lead dislodgements among all CIED patients. Figure 2 depicts 1-year AF recurrence data for both fluoroscopy-guided and fluoroless AF ablation. At 1 year, 28.7% of patients in the fluoroscopy group had a recurrence of AF, whereas 27.1% of the patients in the fluoroless group recurred (log-rank P = 0.69). Exclusion of the 17 patients who received fluoroscopy from the fluoroless group did not significantly affect our results: acute procedural success (95.5% vs 98.5%, P = 0.14), procedural complications (4.5% vs 1.5%, P = 0.14), and 1-year recurrence rates (28.7% vs 26.8%, log-rank P = 0.66) for fluoroscopy-guided vs fluoroless, respectively. Similar results were also observed when we examined the patients who underwent first-time AF ablation only: acute procedural success (97.5% vs 98.9%, P = 0.47), procedural complications (3.4% vs 3.4%, P = 0.99), and 1-year recurrence rates (Supplemental Fig. S1; 32.7% vs 28.4%, log-rank P = 0.48).
Table 4.
Acute complications of fluoroscopy-guided and fluoroless RF catheter ablations for atrial fibrillation
| Complication type | Fluoroscopy group (n = 176) | Fluoroless group (n = 147) | P value |
|---|---|---|---|
| Pericardial effusion or tamponade | 3 (1.7) | 2 (1.4) | 0.79 |
| Stroke | 0 (0) | 0 (0) | N/A |
| Atrioesophageal fistula | 0 (0) | 1 (0.7) | 0.28 |
| Vascular access | 4 (2.3) | 0 (0) | 0.064 |
| Pulmonary vein stenosis | 1 (0.6) | 0 (0) | 0.36 |
| Total | 8 (4.5) | 3 (2.0) | 0.24 |
Data expressed as n (%). Categorical variables were compared by Pearson’s χ2 test.
RF, radiofrequency.
Figure 2.
Atrial fibrillation (AF) recurrence at 1 year by the type of AF ablation approach.
Supplemental Figure S2 depicts the procedure times associated with fluoroless AF ablation after the fluoroless program began. There was no appreciable learning curve when examining the duration of cases at the time of transition between fluoroscopy-guided cases and fluoroless cases.
Discussion
In this study of the outcomes and safety of fluoroless catheter AF ablation, we demonstrate that, compared with conventional fluoroscopy-guided AF ablation, fluoroless AF ablation was as efficacious with regard to procedure times, was associated with similar acute and long-term procedural success, and had a similar safety profile. The fluoroless technique also prevents radiation exposure to patients and staff, and obviates the need for wearing heavy protective equipment. Our study also demonstrates that it is feasible to implement a fluoroless approach to AF ablation for all operators in an electrophysiology laboratory and to rapidly achieve excellent results.
RF ablation is an important modality in the management of AF.1,3,13 Traditionally, RF ablation for AF has relied on the use of fluoroscopy to aid with navigation of catheters within the heart during the procedure. As the use of both ICE and 3D electroanatomic mapping systems has become commonplace during AF ablation, the tools that allow for the procedure to be performed completely free of fluoroscopy are thus now readily available.9,10 Despite the ubiquity of these tools, most operators continue to use fluoroscopy during AF ablation, perhaps due to habit, as most electrophysiologists have trained to perform the procedure in this fashion. However, operators may also be reluctant to make the transition to fluoroless AF ablation as there has been a general paucity of data regarding its safety and long-term effectiveness.
There have been few studies examining both the short-term and long-term outcomes of fluoroless AF ablation and how these contrast with a fluoroscopy-guided AF ablation approach. Only 1 larger study has reported on the long-term outcomes after fluoroless catheter ablation for AF. Lyan et al.14 reported similar recurrence rates between fluoroscopy-guided and fluoroless AF ablation among 481 patients. However, the authors may have potentially introduced selection bias by not reporting on consecutive patients, excluding 45 patients in the fluoroless group from the analysis, and not providing a reason for their exclusion.14 The only randomized trial of fluoroless AF ablation to date was a single-centre study of 80 patients, which did not identify a difference in procedure time between the 2 groups.15 Other studies of fluoroless catheter ablation have also had limitations, including small sample sizes and a primary focus on acute procedural success rather than important long-term outcomes such as AF recurrence,10,11,15, 16, 17, 18 whereas other studies have focused on a fluoro-reduction approach rather than a zero fluoroscopy one,12,19 or have not been specific to AF.8,9
Although minimal fluoroscopy was used in our study for the purposes of placement of an esophageal temperature probe among a few patients in the fluoroless group, this was typically a preplanned activity that took place near the start of each case and thus had little impact on workflow. Although we did not record lead protection use among staff in this study, operators and support staff did not wear lead protection at all for the majority of cases. Modifications to our fluoroless protocol, such as the tying of the esophageal temperature probe to a multielectrode catheter and use of an RF transseptal needle, allow for their respective visualization in 3D mapping systems and may further reduce the risk of requiring fluoroscopy during a case.
The benefits of a fluoroless approach to AF ablation are numerous. It has been described that use of fluoroscopy can significantly increase the risk of malignancy, both in patients and operators.4,6 Repeated exposure to fluoroscopy has been linked to an increased risk of breast cancer in females.5 In addition, AF ablation has been estimated to use an average of 16.6 mSV of radiation, or the equivalent of 830 chest radiographs.20 A cumulative dose >100 mSV has been associated with an increased 1% risk of malignancy.4 Furthermore, it has been estimated that electrophysiologists have a radiation exposure of 5 mSV per year due to fluoroscopy, leading to the development of cancer in 1 per 100 operators.6 There is no minimum acceptable or safe dose of radiation that has been described. Currently, the guiding principle in the use of radiation follows the concept of “As Low As Reasonably Achievable,”4 and fluoroless AF ablations make this goal attainable. Notwithstanding the risk of malignancy, the use of lead protective equipment in the procedure laboratory is associated with an increased risk of musculoskeletal injury among operators and support staff.7,21 In our study, support staff did not wear lead in all fluoroless cases, whereas operators only required minimal lead use in the few cases that used fluoroscopy. Given the typically long duration of AF ablation procedures, a fluoroless approach should significantly reduce the volume of orthopaedic injuries that are so common with the use of lead protection.22
There are several reasons why fluoroless AF ablation may lead to shorter procedure times compared with conventional AF ablation. First, operators may use significant fluoroscopy to confirm catheter placement despite the use 3D mapping systems, significantly slowing down the procedure. Fluoroless AF ablation may reduce procedure times by allowing operators to become more proficient in their use of ICE, which permits for real-time visual monitoring during the procedure and as a result may increase operator confidence in the accuracy of their electroanatomic maps. Second, operators, anaesthesiologists, and support personnel are less burdened as they no longer need to wear heavy, protective lead equipment during cases, which may have the effect of improving their overall efficiency. Finally, in contrast to fluoroscopy-guided protocols, which were heterogeneous among operators, the fluoroless workflow was shared among all operators and may have allowed for support staff to develop more anticipatory tendencies leading to greater procedural efficiencies.
There was no appreciable learning curve during the transition phase to fluoroless AF ablation. The reasons for this is likely multifactorial. First, before transitioning to fluoroless AF ablation, all operators were already comfortable with using ICE. Second, in the weeks before the transition, operators had the opportunity to familiarize themselves with the fluoroless workflow as well as the steps needed to perform a transseptal puncture with ICE alone, including practicing required ICE views and performing the transseptal sheath pull-down under ICE alone. Finally, an individual experienced with fluoroless AF ablation was present in the control room during the initial fluoroless cases. Confounding factors may have also affected procedure times around the time of transition including differences in the use of general anaesthesia, redo cases, and mapping systems used. Our data nonetheless suggest that it is possible to adapt to a fluoroless approach for AF ablation with minimal disruption to procedure times.
Although our study focuses on fluoroless RF catheter ablation for AF, a fluoroless approach may also be adapted to cryoballoon AF ablation with some modifications to our workflow. This has been described by Razminia et al.9,23 and would require exchange of the transseptal sheath with the steerable FlexCath under ICE, as well as use of a 3D-mapping system (EnSite) to aid with navigation of the Achieve multielectrode catheter in the LA. Occlusion of the pulmonary vein ostia with the cryoballoon can be visualized on ICE and confirmed with colour Doppler as well as the emergence of a wedge waveform on hemodynamic monitoring. Efficacy data on this technique are limited,9 with small studies reporting good acute success rates and low complications rates; however, its use may be limited in some centres due to cost.
Our study should be interpreted in light of some limitations. First, the study is retrospective in nature and is thus subject to the biases associated with this type of design. However, our study attempted to limit selection bias by including consecutive patients and by not excluding any patients from the analysis. Despite our best efforts to adjust our analysis for multiple potential confounders, we cannot exclude the possibility that uncontrolled confounding could have contributed to the findings we observed. Second, our study describes a single-centre experience on the implementation of a fluoroless AF ablation program, which may limit its generalizability. However, our study reports the experience of 4 operators, none of whom had significant exposure to fluoroless ablation before implementation of the program, and thus our experience likely reflects the “real-world” uptake of the technique. Third, time bias may have impacted our results because fluoroless AF ablations occurred later in time compared with fluoroscopy-guided ablation, and operators performing fluoroless cases could have become more experienced with time. However, after adjustment by time period in the multivariable models, fluoroless AF ablation remained independently associated with reduced procedure time. Finally, ascertainment of AF recurrence depended, in part, on patient reporting, and some AF episodes may have been asymptomatic; therefore, AF recurrence may have been underestimated. However, these factors were likely nondifferential and thus biased our results towards the null.
Conclusions
In the present study, we show our adaptation to a largely fluoroscopy-free approach at AF ablation and demonstrate no significant difference in procedural times, safety, acute procedural success, or 1-year AF recurrence compared with a fluoroscopy-guided approach. Fluoroless AF ablation is achievable with the required tools already being used for AF ablations in the majority of electrophysiology laboratories and only requires a mindset change by the operator. Fluoroless methods for RF catheter ablations for AF should be considered to become the standard-of-care due to increased patient and health care personnel safety, while maintaining similar effectiveness and procedural safety compared with conventional AF ablation using fluoroscopy.
Acknowledgement
The authors would like to thank Richard Matthew for his contributions to this manuscript.
Funding sources
J.A.W. holds a McMaster University Department of Medicine Early-Career Award. J.S.H. holds the Stuart Connolly Chair in Cardiology Research at the Population Health Research Institute, and the Salim Yusuf Chair at Hamilton Health Sciences.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: The study conforms to the declaration of Helsinki and the regional Ethics Review Board in Hamilton approved the study.
See page 310 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2020.11.002.
Supplementary Material
References
- 1.Amit G., Nyong J., Morillo C.A. Efficacy of catheter ablation for nonparoxysmal atrial fibrillation. JAMA Cardiol. 2017;2:812–813. doi: 10.1001/jamacardio.2017.0901. [DOI] [PubMed] [Google Scholar]
- 2.Morillo C.A., Verma A., Connolly S.J. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of paroxysmal atrial fibrillation (RAAFT-2): a randomized trial. JAMA. 2014;311:692–700. doi: 10.1001/jama.2014.467. [DOI] [PubMed] [Google Scholar]
- 3.Piccini J.P., Lopes R.D., Kong M.H. Pulmonary vein isolation for the maintenance of sinus rhythm in patients with atrial fibrillation: a meta-analysis of randomized, controlled trials. Circ Arrhythm Electrophysiol. 2009;2:626–633. doi: 10.1161/CIRCEP.109.856633. [DOI] [PubMed] [Google Scholar]
- 4.Heidbuchel H., Wittkampf F.H., Vano E. Practical ways to reduce radiation dose for patients and staff during device implantations and electrophysiological procedures. Europace. 2014;16:946–964. doi: 10.1093/europace/eut409. [DOI] [PubMed] [Google Scholar]
- 5.Linet M.S., Slovis T.L., Miller D.L. Cancer risks associated with external radiation from diagnostic imaging procedures. CA Cancer J Clin. 2012;62:75–100. doi: 10.3322/caac.21132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Picano E., Vano E., Rehani M.M. The appropriate and justified use of medical radiation in cardiovascular imaging: a position document of the ESC Associations of Cardiovascular Imaging, Percutaneous Cardiovascular Interventions and Electrophysiology. Eur Heart J. 2014;35:665–672. doi: 10.1093/eurheartj/eht394. [DOI] [PubMed] [Google Scholar]
- 7.Klein L.W., Miller D.L., Balter S. Occupational health hazards in the interventional laboratory: time for a safer environment. Catheter Cardiovasc Interv. 2018 doi: 10.1002/ccd.21772. [DOI] [PubMed] [Google Scholar]
- 8.Razminia M., Manankil M.F., Eryazici P.L. Nonfluoroscopic catheter ablation of cardiac arrhythmias in adults: feasibility, safety, and efficacy. J Cardiovasc Electrophysiol. 2012;23:1078–1086. doi: 10.1111/j.1540-8167.2012.02344.x. [DOI] [PubMed] [Google Scholar]
- 9.Razminia M., Willoughby M.C., Demo H. Fluoroless catheter ablation of cardiac arrhythmias: a 5-year experience. Pacing Clin Electrophysiol. 2017;40:425–433. doi: 10.1111/pace.13038. [DOI] [PubMed] [Google Scholar]
- 10.Reddy V.Y., Morales G., Ahmed H. Catheter ablation of atrial fibrillation without the use of fluoroscopy. Heart Rhythm. 2010;7:1644–1653. doi: 10.1016/j.hrthm.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Sadek M.M., Ramirez F.D., Nery P.B. Completely nonfluoroscopic catheter ablation of left atrial arrhythmias and ventricular tachycardia. J Cardiovasc Electrophysiol. 2019;30:78–88. doi: 10.1111/jce.13735. [DOI] [PubMed] [Google Scholar]
- 12.Sommer P., Rolf S., Piorkowski C. Nonfluoroscopic catheter visualization in atrial fibrillation ablation: experience from 375 consecutive procedures. Circ Arrhythm Electrophysiol. 2014;7:869–874. doi: 10.1161/CIRCEP.114.001542. [DOI] [PubMed] [Google Scholar]
- 13.Latchamsetty R., Morady F. Atrial fibrillation ablation. Annu Rev Med. 2018;69:53–63. doi: 10.1146/annurev-med-041316-090015. [DOI] [PubMed] [Google Scholar]
- 14.Lyan E., Tsyganov A., Abdrahmanov A. Nonfluoroscopic catheter ablation of paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 2018;41:611–619. doi: 10.1111/pace.13321. [DOI] [PubMed] [Google Scholar]
- 15.Bulava A., Hanis J., Eisenberger M. Catheter ablation of atrial fibrillation using zero-fluoroscopy technique: a randomized trial. Pacing Clin Electrophysiol. 2015;38:797–806. doi: 10.1111/pace.12634. [DOI] [PubMed] [Google Scholar]
- 16.Liu X., Palmer J. Outcomes of 200 consecutive, fluoroless atrial fibrillation ablations using a new technique. Pacing Clin Electrophysiol. 2018;41:1404–1411. doi: 10.1111/pace.13492. [DOI] [PubMed] [Google Scholar]
- 17.Rolf S., Schoene K., Kircher S. Catheter ablation of atrial fibrillation with nonfluoroscopic catheter visualization—a prospective randomized comparison. J Interv Card Electrophysiol. 2019;54:35–42. doi: 10.1007/s10840-018-0446-8. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez J.M., Yanics M.A., Wilson P. Fluoroless catheter ablation in adults: a single center experience. J Interv Card Electrophysiol. 2016;45:199–207. doi: 10.1007/s10840-015-0088-z. [DOI] [PubMed] [Google Scholar]
- 19.Sommer P., Bertagnolli L., Kircher S. Safety profile of near-zero fluoroscopy atrial fibrillation ablation with non-fluoroscopic catheter visualization: experience from 1000 consecutive procedures. Europace. 2018;20:1952–1958. doi: 10.1093/europace/eux378. [DOI] [PubMed] [Google Scholar]
- 20.Casella M., Dello Russo A., Russo E. X-ray exposure in cardiac electrophysiology: a retrospective analysis in 8150 patients over 7 years of activity in a modern, large-volume laboratory. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.008233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orme N.M., Rihal C.S., Gulati R. Occupational health hazards of working in the interventional laboratory: a multisite case control study of physicians and allied staff. J Am Coll Cardiol. 2015;65:820–826. doi: 10.1016/j.jacc.2014.11.056. [DOI] [PubMed] [Google Scholar]
- 22.Birnie D., Healey J.S., Krahn A.D. Prevalence and risk factors for cervical and lumbar spondylosis in interventional electrophysiologists. J Cardiovasc Electrophysiol. 2011;22:957–960. doi: 10.1111/j.1540-8167.2011.02041.x. [DOI] [PubMed] [Google Scholar]
- 23.Demo H., Willoughby C., Jazayeri M.A., Razminia M. Fluoroless catheter ablation of cardiac arrhythmias. Card Electrophysiol Clin. 2019;11:719–729. doi: 10.1016/j.ccep.2019.08.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.