Abstract
This report describes a case of a 35-year-old man who presented with acute coronary syndrome. An angiogram and intravascular ultrasound revealed atherosclerotic stenosis in the myocardial bridging segment of the mid-left anterior descending artery. The culprit lesion was treated using a drug-coated balloon, and no residual stenosis was observed, which was later confirmed by intravascular ultrasound and optical coherence tomography at a 1-year coronary angiographic follow-up. This case provides evidence that drug-coated balloon could be a potential treatment strategy for atherosclerosis located in the myocardial bridging segment and suggests advantages of the “leave nothing behind” strategy in such clinical scenarios.
Résumé
Nous décrivons le cas d’un homme de 35 ans présentant un syndrome coronarien aigu. L’angiographie et l’échographie intravasculaire ont révélé une sténose athéroscléreuse dans le segment du pont myocardique de l’artère interventriculaire antérieure moyenne. La lésion coupable a été traitée au moyen d’un ballonnet enduit de médicament; aucune sténose résiduelle n’a été observée, ce qui a par la suite été confirmé par échographie intravasculaire et tomographie par cohérence optique lors de la coronarographie de suivi à 1 an. Ce cas montre qu’il est possible de traiter l’athérosclérose du pont myocardique au moyen d’un ballonnet enduit de médicament et qu’il pourrait être avantageux d’adopter une stratégie ne laissant rien derrière (stratégie du « leave nothing behind ») dans une telle situation clinique.
Myocardial bridging (MB) is a term that describes an anatomic variant where the coronary artery runs underneath the myocardium for varying lengths before returning to the epicardium.1 MB could be found in any of the epicardial arteries but predominantly (over three-fourths) involves the left anterior descending (LAD) artery. From an anatomic perspective, MB has traditionally been considered a benign clinical condition.1 However, recent findings suggesting a relationship between MB and adverse cardiovascular outcomes have led to a reappraisal of the clinical relevance of MB.2 Considering that MB may physically compress the coronary artery during systole, treating a lesion in the MB-affected location during percutaneous coronary intervention remains clinically challenging. With the use of intravascular imaging, cardiologists can now detect the severity of MB and evaluate the spatial relationship between lesions and MB.1 Although atherosclerosis is usually present in an LAD segment proximal to the MB, intimal thickening occurs in tunnelled segment in a few patients. Here we report a case of MB-associated acute coronary syndrome (ACS) induced by plaque disruption at an LAD-MB segment.
Case
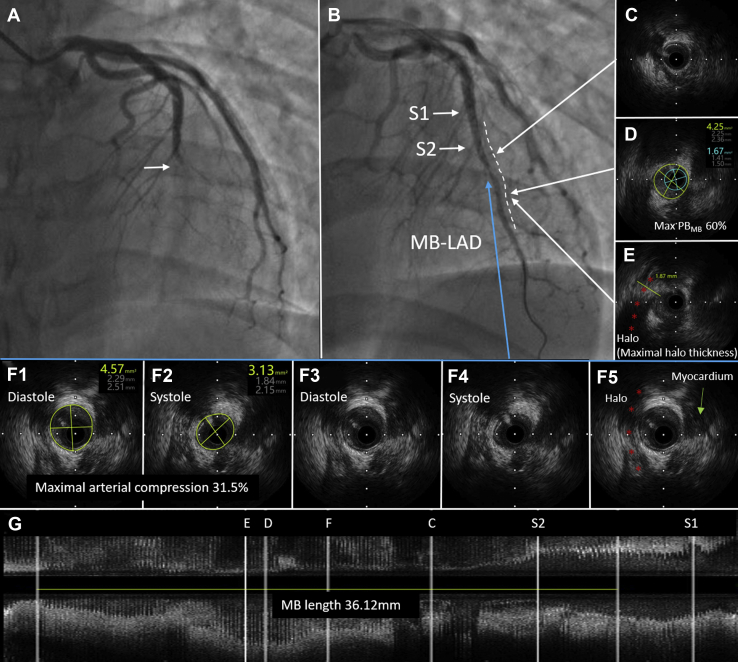
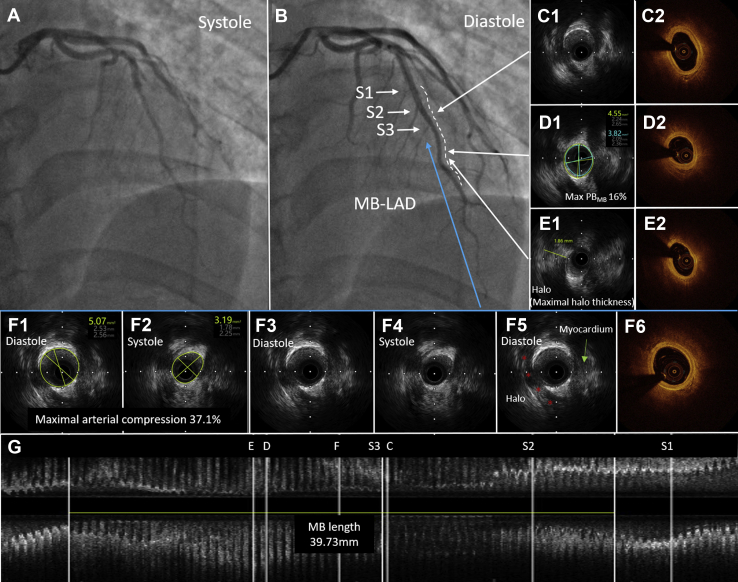
A 35-year-old man who was a smoker presented with paroxysmal retrosternal chest pain that had persisted for 2 weeks. This patient was diagnosed with ACS based on the abnormal findings of an electrocardiogram (Q waves in leads I and aVL and T-wave inversion in leads V1-V5, Supplemental Fig. S1) and elevated troponin I level (0.25 ng/mL). Transthoracic echocardiogram showed decreased contraction activity in septal and apical segments of the left ventricular anterior wall with a floating mural thrombus (10 × 4 mm) in the apical segment of the anterior wall of the left ventricle. Coronary angiography was performed, revealing a total occlusion at the middle segment of the LAD (Fig. 1A). We selected a 7F EBU 3.5 guiding catheter for the left coronary artery and a Sion Blue as the working wire. After passing the culprit lesion, we used a 2.0 × 15 mm Tazuna balloon for predilation at 8 atm. A repeated angiogram showed restored blood flow in the LAD with severe systolic compression (suggesting the presence of MB) at the middle segment (Fig. 1B). Intravascular ultrasound (IVUS) identified an MB with a length of 36.12 mm (Fig. 1G), maximal systolic arterial compression as 31.5% (Fig. 1F), and maximal halo thickness as 1.87 mm (Fig. 1E) in the middle segment of the LAD. Atherosclerosis in the MB segment was detected with a maximal plaque burden of 60% (Fig. 1D). Considering that the vessel diameter at the culprit lesion located in the MB segment was approximately 1.5-2.0 mm, we treated the culprit lesion with the use of a 2.0 × 20 mm Sequent Please drug-coated balloon (DCB) for dilation for 60 seconds at lesion at 10 atm. Thrombolysis in myocardial infarction flow grade 3 was observed after dilation. The patient was discharged with dual antiplatelet therapy (ie, aspirin plus clopidogrel) for 1 year and was instructed to take warfarin (INR range as 2-3) for 3 months along with atorvastatin, isosorbide mononitrate, and metoprolol. At the 1-year coronary angiographic follow-up, no residual stenosis was observed at the original location of the culprit lesion, which was confirmed by IVUS and optical coherence tomography (Fig. 2).
Figure 1.
Myocardial bridging (MB) and culprit lesions in acute coronary syndrome. (A) Angiographic appearance of culprit lesions in the mid-left anterior descending (LAD) artery (white arrowhead). (B) Angiographic appearance of opened coronary artery and MB in mid-LAD (white dashed line). (C) Intravascular ultrasound (IVUS) image of proximal MB. (D) Maximum plaque burden in the MB segment (Max PBMB) was 60%. (E) Site with maximal halo thickness. (F) Site with maximal arterial compression. (G) Longitudinal IVUS view. MB was identified by IVUS as an echolucent-band “halo” (red asterisk; E, F). S1, septal 1; S2, septal 2.
Figure 2.
One-year follow-up by intravascular ultrasound (IVUS) and optical coherence tomography (OCT) of myocardial bridging (MB). (A, B) Systolic and diastolic angiographic appearance of MB at the left anterior descending artery (LAD-MB) (white dashed line), respectively. (C) IVUS and OCT image of proximal MB. (D) Site of maximum plaque burden in the MB segment (Max PBMB). (E) Site with maximal halo thickness. (F) Site with maximal arterial compression. (G) Longitudinal IVUS view. MB was identified by IVUS as an echolucent-band “halo” (red asterisk; E, F). S1, septal 1; S2, septal 2; S3, septal 3.
Discussion
MB is a common coronary abnormality present in approximately a quarter of adults.1 Although cases of MB could be asymptomatic or benign, several reports have shown that it could cause severe mechanical compression of the LAD, and symptomatic patients with MB may present with myocardial ischemia, ACS, coronary spasm, or sudden death, even in those without significant clinical risk factors. The anatomic properties of MB relevant to clinical pathophysiology include depth (superficial: > 1-2 mm vs deep: > 2 mm) and length of the encasement.1 In this case, the patient was young with a low cardiovascular risk (ie, 10-year risk of fatal cardiovascular disease estimated as < 1% using the systematic coronary risk estimation system)3 but suffered ACS caused by a thick (1.87 mm) and long (36.12 mm) LAD-MB.
The primary treatment of symptomatic patients with MB is pharmacologic therapy.1 Intravascular imaging (eg, IVUS) during coronary angiography may provide additional assistance in the recognition of plaque location and its spatial relationship with MB.1 Stent implantation may rectify the abnormal hemodynamics in patients with flow-limiting plaque but with concerns on perforation during stent deployment, stent fracture,4 in-stent restenosis,5 and stent thrombosis.6 However, it may not be possible to avoid these interventional complications, especially when the target lesion is located at the MB segment. DCB could inhibit smooth muscle cell proliferation and thus eliminate stent thrombosis and reduce restenosis by directly contacting the antiproliferative drug with the vessel wall by a semicompliant balloon. Thus, this approach could be an alternative treatment option in de novo coronary lesions.7 With the “leave nothing behind” strategy afforded by DCB angioplasty, complications associated with stent implantation may be avoided in patients undergoing interventional treatment of the coronary lesion in the MB segment. Accordingly, in this case, we recognized MB using IVUS, treated the lesion with DCB only, and prescribed optimal medical therapy. No recurring angina had presented within 1 year of the procedure, and no residual stenosis was observed at the 1-year coronary angiographic follow-up, indicating that IVUS-guided DCB angioplasty could optimize the outcome in patients with MB-associated ACS. However, because MB exists at the original location, long-term MB-associated changes in wall shear stress and formation and progression of atherosclerosis may occur.8 A careful long-term management of the patients’ lifestyle and medical treatment should be provided and another interventional procedure or coronary artery bypass grafting may be required if MB patients are resistant to optimal medical treatment.
Conclusion
This case demonstrates the utility of an IVUS-guided interventional treatment of MB-induced ACS. DCB angioplasty may avoid coronary stent–associated complications when the lesion is located within the tunnelled segment of the MB.
Novel Teaching Points.
-
•
Given the physical compression of the coronary artery during systole in patients with myocardial bridging (MB), interventional treatment of the MB-associated lesion remains clinically challenging.
-
•
This case report presented the usefulness of intravascular imaging on detecting and assessing the severity of MB and the spatial relationship between the lesion and MB during percutaneous coronary intervention.
-
•
Drug-coated balloon angioplasty may avoid coronary stent–associated complications, especially when the lesion is located at the tunnelled segment of MB.
Funding Sources
This work was supported by Shanghai Science and Technology Committee (Grant/Award Number: 18411950400). K.X. was sponsored by Shanghai Sailing Program (Grant Number: 20YF1444200). W.Z. was sponsored by Shanghai Sailing Program (Grant Number: 19YF1444600).
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: The present research has adhered to the relevant ethical guidelines.
See page 375 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2020.10.013.
Supplementary Material
References
- 1.Tarantini G., Migliore F., Cademartiri F., Fraccaro C., Iliceto S. Left anterior descending artery myocardial bridging: a clinical approach. J Am Coll Cardiol. 2016;68:2887–2899. doi: 10.1016/j.jacc.2016.09.973. [DOI] [PubMed] [Google Scholar]
- 2.Ural E., Bildirici U., Celikyurt U. Long-term prognosis of non-interventionally followed patients with isolated myocardial bridge and severe systolic compression of the left anterior descending coronary artery. Clin Cardiol. 2009;32:454–457. doi: 10.1002/clc.20570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piepoli M.F., Hoes A.W., Agewall S. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Eur Heart J. 2016;37:2315–2381. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu H., Ge L., Ge J. Coronary aneurysm and stent fracture following stenting of a myocardial bridge. Catheter Cardiovasc Interv. 2016;87:E15–E18. doi: 10.1002/ccd.25815. [DOI] [PubMed] [Google Scholar]
- 5.Kunamneni P.B., Rajdev S., Krishnan P. Outcome of intracoronary stenting after failed maximal medical therapy in patients with symptomatic myocardial bridge. Catheter Cardiovasc Interv. 2008;71:185–190. doi: 10.1002/ccd.21358. [DOI] [PubMed] [Google Scholar]
- 6.Jiang Q., Liang C., Wu Z. Myocardial bridging is a potential risk factor of very late stent thrombosis of drug eluting stent. Med Sci Monit. 2012;18 doi: 10.12659/MSM.882717. HY9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yerasi C., Case B.C., Forrestal B.J. Drug-coated balloon for de novo coronary artery disease: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:1061–1073. doi: 10.1016/j.jacc.2019.12.046. [DOI] [PubMed] [Google Scholar]
- 8.Ishikawa Y., Akasaka Y., Suzuki K. Anatomic properties of myocardial bridge predisposing to myocardial infarction. Circulation. 2009;120:376–383. doi: 10.1161/CIRCULATIONAHA.108.820720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.