Abstract
Background
Von Willebrand factor (VWF) elevation correlates with the left atrial blood stasis in nonvalvular atrial fibrillation (NVAF). However, the long-term impact of elevated VWF in patients with NVAF is not well established.
Methods
To assess the impact of VWF and a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13) in conjunction with echocardiographic measures of left atrium blood stasis on clinical outcomes, 414 NVAF prospectively recruited (October 4, 2007, to April 27, 2009) patients were followed for 3 years. VWF antigen, VWF activity, ADAMTS13 activity, and echocardiographic findings were assessed at baseline. Thromboembolism (TE) (stroke/transient ischemic attack (TIA)), myocardial infarction, or TE of other locations), major bleeding, clinically relevant nonmajor bleeding, and all-cause mortality were assessed by clinical follow-up, questionnaire, or telephone communication.
Results
Among 374 patients (mean age, 63.4 ± 12.7 years; 25% females) who had complete follow-up data, there were 33 TE in 32 patients (8.6%), 18 deaths (5.1%), and 33 bleeding events (21 major bleeding and 12 clinically relevant nonmajor bleeding) in 25 patients (6.7%). VWF antigen was predictive of TE in the univariate examination (hazard ratio [HR]: 1.007, 95% confidence interval [CI]: 1.002, 1.013, P = 0.011) but not in multivariate analysis. VWF was an independent predictor of all-cause mortality (HR: 1.011, 95% CI: 1.003, 1.020, P = 0.011) and a composite of TE and all-cause mortality (HR: 1.006, 95% CI: 1.001, 1.012, P = 0.039) in multivariate analysis. ADAMTS13 was not predictive of clinical outcomes in multivariate analysis.
Conclusions
Among patients with NVAF, VWF is an independent predictor of poor outcomes including death and a composite of death and TE. As such, VWF measure may help identify high-risk patients and provide further stratification beyond CHA2DS2-VASc assessment.
Résumé
Contexte
Une élévation du facteur de Von Willebrand (FVW) concorde avec une stase sanguine dans l'oreillette gauche dans la fibrillation auriculaire non valvulaire (FANV). Les répercussions à long terme d'un taux élevé du FVW chez les patients présentant une FANV ne sont toutefois pas bien établies.
Méthodologie
Pour évaluer les répercussions sur les résultats cliniques du FVW et d'une désintégrine et métalloprotéinase de motif type 1 (ADAMTS13) conjointement avec les mesures échocardiographiques de la stase sanguine dans l'oreillette gauche, 414 patients atteints de FANV ont été inscrits de façon prospective (du 4 octobre 2007 au 27 avril 2009) pour faire l'objet d'un suivi de 3 ans. L'antigène du FVW, l'activité du FVW, l'activité d'ADAMTS13, et les résultats de l'échocardiographie ont été évalués au départ. La thromboembolie (TE) (accident vasculaire cérébral/accident ischémique transitoire, infarctus du myocarde, ou TE survenant ailleurs), l'hémorragie majeure, l'hémorragie non majeure pertinente sur le plan clinique et la mortalité toutes causes ont été évaluées au suivi clinique, par questionnaire, ou lors d'un appel téléphonique.
Résultats
Parmi les 374 patients (âge moyen : 63,4 ± 12,7 ans; 25 % de femmes) ayant participé au suivi jusqu'à sa fin, on a relevé 33 TE chez 32 patients (8,6 %), 18 décès (5,1 %) et 33 événements hémorragiques (21 hémorragies majeures et 12 hémorragies non majeures pertinentes sur le plan clinique) chez 25 patients (6,7 %). L'antigène du FW était prédictif d'une TE selon l'analyse univariée (risque relatif [RR] : 1,007; intervalle de confiance [IC] à 95 % : de 1,002 à 1,013; p = 0,011), mais non selon l'analyse multivariée. Le FVW était un facteur prédictif indépendant de la mortalité toutes causes (RR : 1,011; IC à 95 % : de 1,003 à 1,020; p = 0,011) et des événements regroupés de TE et de mortalité toutes causes (RR : 1,006; IC à 95 % : de 1,001 à 1,012; p = 0,039) dans l'analyse multivariée. La protéase ADAMTS13 ne constituait pas un facteur prédictif des résultats cliniques dans l'analyse multivariée.
Conclusions
Parmi les patients présentant une FANV, le FVW était un facteur prédictif indépendant de résultats défavorables, notamment de décès et des événements regroupant les décès et la TE. La mesure du FVW pourrait donc aider à cibler les patients à risque élevé, et permettre une stratification au-delà de l'évaluation du score CHA2DS2-VASc.
Ischemic stroke from nonvalvular atrial fibrillation (NVAF) occurs when a left atrial appendage thrombus (LAAT) embolizes into the cerebral circulation.1, 2, 3 The risk for LAAT development increases with worsening of left atrium appendage mechanical function as measured by reduction in left atrial appendage emptying velocity (LAAEV), development of spontaneous echocardiographic contrast (SEC), and left atrial distension assessed by left atrium volume index (LAVI).4, 5, 6, 7 However, blood stagnation alone is not sufficient to explain thrombotic events in these patients8, 9, 10 and additional prothrombotic variables are likely involved.11, 12, 13, 14, 15, 16, 17
Von Willebrand factor (VWF) is a large multimeric plasma glycoprotein that mediates platelet adhesion and aggregation and chaperones factor VIII.18 The thrombogenic potential of this protein is directly proportional to VWF activity determined by both plasma protein size and concentration. Protein size is highly regulated by the VWF-specific cleaving protease a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13).19 We have previously demonstrated a direct relationship between antigen concentration and activity of VWF and both left atrial blood flow stasis and the presence of LAAT in NVAF.20
The long-term consequences of increased VWF antigen concentration have been evaluated,21, 22, 23, 24, 25, 26, 27, 28, 29 but the assessment of both VWF concentration and activity with ADAMTS13 in patients with NVAF on adverse cardiovascular events in relation to echocardiographic measures, to our knowledge, has never been reported. To determine whether measures of VWF concentration and activity together with ADAMTS13 predict adverse events in NVAF, the 3-year outcomes of patients enrolled between October 4, 2007, and April 27, 2009, were analyzed.20
Methods
Study population
All patients with NVAF who had a transesophageal echocardiogram (TEE) and blood testing for VWF antigen concentration, VWF latex immunoassay activity, and VWF-specific protease ADAMTS13 activity between October 4, 2007, and April 27, 2009, were followed up for 3 years from the date of enrollment. Methods of patient identification, recruitment, exclusion criteria, and data acquisition were previously described.20 Briefly, all patients referred for TEE were approached for study participation unless they had: (1) acute illness, stroke, myocardial infarction, or surgery within 30 days; (2) more than moderate valvular heart disease; (3) artificial heart valves; (4) prior unprovoked venous or arterial thrombosis; (5) prior major bleeding unrelated to anticoagulation therapy; (6) liver disease; (7) active malignancy; or (8) hormonal stimulation (estrogen/progesterone therapy or pregnancy). The details related to atrial fibrillation (AF) onset, timing, chronicity, underlying etiologies, cardiac anatomy and physiology, relevant comorbidities, and medical and interventional treatment of AF, particularly anticoagulation, were collected prospectively at baseline and entered into a SAS-based system for managing clinical data.
Patient clinical course including exploration of stroke/transient ischemic attack (TIA), myocardial infarction, arterial thromboembolism (TE) events of a different location, major bleeding, clinically relevant nonmajor bleeding (CRNMB), and death was assessed during patient clinical follow-up, or by mailed written questionnaire or scripted phone interview.
Transesophageal echocardiogram
TEE was performed as previously described using commercially available ultrasound instruments and a multiplane probe.30,31 LAAT was defined as an echogenic mass in the appendage or body of the atrium, distinct from the underlying endocardium and pectinate muscles, and detected in more than one imaging plane.30,31 SEC was defined as a pattern of dynamic “smoke-like,” slowly swirling, intracavitary echodensities imaged with gain settings adjusted to eliminate background noise. SEC was graded as “absent,” “mild,” “moderate,” or “severe” according to the published, echocardiographic criteria.32 The LAAEV profiles were measured over 5 consecutive cardiac cycles using pulsed-wave Doppler interrogation with the sample volume positioned 1 cm within the orifice of the LAA.33 The left ventricular ejection fraction was visually estimated. Aortic atherosclerosis severity was defined as “simple” when atheroma thickness was <4 mm and immobile. Severe atheroma exceeded 4 mm or contained mobile components.30, 31, 32, 33 Given the known difficulties in measuring left atrial volume by TEE, LAVI was assessed by transthoracic echocardiography performed within 1 month of the TEE study and calculated by the biplane area-length method.33 All echocardiographic images were analyzed by the study cardiologist (NMA) who was blinded to clinical and laboratory data.
Original TEE studies at the time of enrollment were requested to exclude intracardiac thrombi before electrophysiological procedures (69%), cardioversion (17%), or for cardioembolic risk assessment (14%).
Sample collection
For each patient, 20 mL citrated blood was collected at the time of recruitment to the study by antecubital venipuncture using a 19-gauge thin-wall “butterfly” needle with a short plastic tube extension. For patients with AF scheduled for electric cardioversion or radiofrequency ablation, the blood sample was uniformly collected before this procedure.
Assays of plasma VWF antigen, VWF activity, and ADAMTS13 activity
VWF antigen concentration in plasma was measured using HemosIL von Willebrand Factor Antigen latex immunoassay kits (Instrumentation Laboratory, Lexington, MA) with 2 ACL TOP coagulation system analyzers (Beckman Coulter, Brea, CA), following the manufacturer’s instructions.
VWF activity in plasma was measured using HemosIL von Willebrand Factor Activity latex immunoassay kits (Instrumentation Laboratory) on 2 ACL TOP coagulation analyzers, following the manufacturer’s instructions.
The lower limit of normality was set up at 55 IU/dL and the higher limit of normal was 200 IU/dL for both concentration and activity of VWF. The standard relationship between activity and antigen was greater than 0.7. The validated normal range of the VWF antigen concentration to VWF activity (n = 452 normal donors) was 0.7-1.4 with a mean at 1.0.20
ADAMTS13 protease activity (expressed as a percentage of normal) was measured by a fluorescence resonance energy transfer–based assay using a VWF 73-amino-acid peptide substrate (commercial kit; GTI DIAGNOSTICS, Waukesha, WI).
Study definitions and event adjudication
The Congestive Heart Failure, Hypertension, Age (≥75 years), Diabetes, Stroke/Transient Ischemic Attack, Vascular Disease, Age (65-74 years), Sex (Female) (CHA2DS2-VASc) score was calculated for each patient.34 The presence of AF was confirmed by either electrocardiogram or Holter monitoring. Congestive heart failure was defined as the presence of clinical symptoms and signs of heart failure within the last 3 months, with or without evidence of LV systolic dysfunction by echocardiography.34 Diabetes mellitus was diagnosed based on the criteria recommended by the American Diabetes Association.35 Stroke and TIA were defined by criteria proposed by the American Heart Association.36 AF was classified as “paroxysmal,” “persistent,” or “permanent” per contemporary guidelines.37
Major bleeding was defined as overt bleeding plus a haemoglobin decrease of ≥2 g/dL after the incident, transfusion of ≥2 units of packed red blood cells, or intracranial, intraspinal, intraocular, pericardial, retroperitoneal, or fatal bleeding.38 CRNMB was defined as overt bleeding not meeting the criteria for major bleeding but associated with medical intervention, unscheduled contact with a member of the health care team, or temporary cessation of anticoagulation therapy. The third safety outcome was a composite of major and CRNMB.
To include the variable of anticoagulation into the univariate and multivariate analysis, patients who were treated with anticoagulation for more than 2 years over 3 years of follow-up were classified as “on anticoagulation for >2 years.”
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Mayo Clinic Institutional Review Board in Rochester, MN (IRB#: 18-011482).
Statistical analysis
Demographic, clinical, and echocardiographic patient characteristics are presented as mean ± standard deviation for continuous variables and as frequency (percentage) for discrete variables. Long-term follow-up event estimates were computed using the Kaplan-Meier method. Univariate and multivariate modelling of long-term outcomes was assessed using Cox proportional hazards models, modelling the time to the first event. Because of the limited number of events, multivariate models were developed by adding the most clinically relevant predictors that were statistically significant in the univariate analyses. The proportional hazards assumption was tested using the Supremum test. Results are presented with 95% confidence intervals and P values. Statistical analyses were completed with SAS 9.4 (SAS Institute Inc, Cary, NC).
Results
Of the original 414 patients recruited, complete 3-year follow-up data was available for 374 (90%) patients (mean age, 63.4 ± 12.7 years; 25% women). Demographic, clinical, and echocardiographic characteristics are reported in Table 1. Congestive heart failure was diagnosed in 25% and 15% had prior stroke/TIA. The mean CHA2DS2-VASc score was 2.4 ± 2.0, and 58% of patients had scores ≥ 2. SEC was identified in 54% of patients and 19 (5%) had LAAT confirmed by TEE. One-fourth of the patients had severely decreased left atrium appendage contractility (LAAEV ≤ 25 cm/s) and 14% had markedly enlarged left atria (LAVI > 60 cm/m2). Significant left ventricle systolic dysfunction (left ventricular ejection fraction < 40%) was present in 11%, and severe aortic atheromas were detected in 8% of the study group (Table 1). During 3 years of follow-up, 164 (43.8%) patients continued anticoagulation uninterrupted, 19 (5%) were anticoagulated for more than 2 years, 52 (14%) patients received anticoagulation for less than 2 years but more than 3 months, 108 (29%) for up to 3 months, and 31 (8%) were not treated with anticoagulation.
Table 1.
Demographic, clinical, and echocardiographic characteristics
| Variables | Patients (n = 374) |
|---|---|
| Age (y), mean ± SD | 63.4 ± 12.7 |
| Female, n (%) | 93 (25) |
| Type of atrial fibrillation, n (%) | |
| Paroxysmal | 180 (48) |
| Persistent | 105 (28) |
| Permanent | 57 (15) |
| Unspecified | 32 (9) |
| CHF, n (%) | 93 (25) |
| Hypertension, n (%) | 222 (59) |
| Age ≥ 75, n (%) | 70 (19) |
| Diabetes mellitus, n (%) | 51 (14) |
| Prior stroke/TIA, n (%) | 55 (15) |
| Vascular disease, n (%) | 97 (27) |
| Age 65-74, n (%) | 100 (27) |
| CHA2DS2-VASc score, mean ± SD | 2.4 ± 2.0 |
| Score 0, n (%) | 57 (16) |
| Score 1, n (%) | 96 (26) |
| Score 2-6, n (%) | 196 (54) |
| Score > 6, n (%) | 14 (4) |
| Spontaneous echocardiographic contrast (SEC), n (%) | 200 (54) |
| Mild | 96 (26) |
| Moderate | 70 (19) |
| Severe | 34 (9) |
| Left atrium appendage thrombus (LAAT), n (%) | 19 (5) |
| Left atrium appendage emptying velocity (cm/s), mean ± SD | 46.9 ± 25.2 |
| ≥ 75, n (%) | 52 (17) |
| 50-74, n (%) | 81 (26) |
| 26-49, n (%) | 96 (31) |
| ≤ 25, n (%) | 78 (25) |
| Left atrium volume index (cm/m2), mean ± SD | 43.3 ± 16.6 |
| > 60, n (%) | 44 (14) |
| Left ventricular ejection fraction, mean ± SD | 54.8 ± 11.6 |
| < 40%, n (%) | 40 (11) |
| Any atheromas of aorta, n (%) | 237 (65) |
| Simple | 209 (57) |
| Severe | 28 (8) |
| VWF activity (IU/dL) | |
| Mean (SD) | 145.9 (51.5) |
| Median (min, max) | 136.8 (49.2, 375.2) |
| VWF antigen (IU/dL) | |
| Mean (SD) | 159.6 (53.4) |
| Median (min, max) | 155.0 (49.0, 399.0) |
| VWF activity/antigen concentration ratio | |
| Mean (SD) | 0.9 (0.1) |
| Median (min, max) | 0.9 (0.6, 1.5) |
| ADAMTS13 activity | |
| Mean (SD) | 115.8 (46.0) |
| Median (min, max) | 109.3 (30.7, 289.3) |
ADAMTS13, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; CHA2DS2-VASc, Congestive Heart Failure, Hypertension, Age (≥75 years), Diabetes, Stroke/Transient Ischemic Attack, Vascular Disease, Age (65-74 years), Sex (Female); CHF, congestive heart failure; SD, standard deviation; TIA, transient ischemic attack; VWF, Von Willebrand factor.
The mean VWF antigen concentration in the group of NVAF with complete follow-up was 159.6 ± 53.4 IU/dL and VWF activity 145.9 ± 51.5 IU/dL, with VWF activity proportional to antigen concentration (mean ratio of 0.9 ± 0.1), consistent with the results from the whole cohort of the original study.20
Clinical outcomes
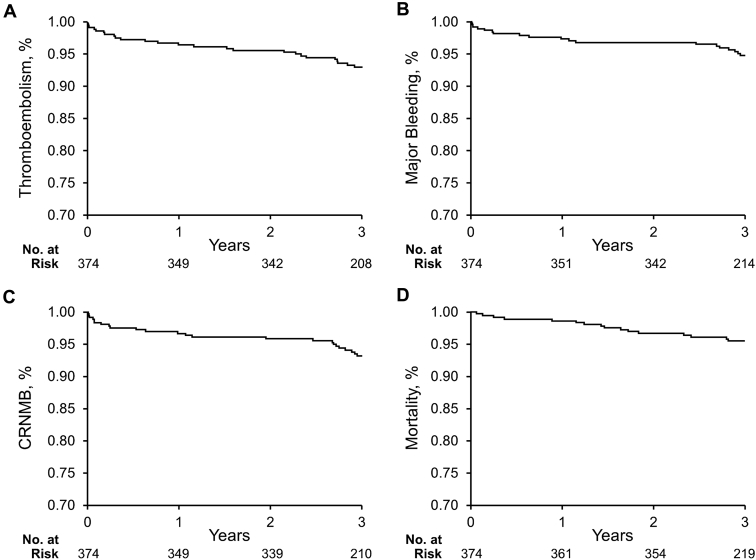
During 3 years of follow-up, there were 33 TE events in 32 patients (8.6%): 12 myocardial infarctions, 14 strokes/TIA (1 patient had TIA and then stroke), and 7 TE events of other locations (Table 2). There were also 33 bleeding events in 25 (6.7%) patients: 21 major and 12 CRNMB (13 patients had major bleeding only, 8 patients had major bleeding and CRNMB, and 4 patients had only CRNMB). Eighteen patients (5.1%) died during 3 years of follow-up. Cumulative incidence curves for the time to TE, major bleeding, CRNMB, and death are shown in Figure 1.
Table 2.
Thromboembolic and bleeding events during 3 years of follow-up
| Adverse event | Total | Anticoagulation therapy‡, n | Antiplatelet therapy‡, n | No antiplatelet or anticoagulant therapy, n |
|---|---|---|---|---|
| Thromboembolism, n (%) | 33 (8.8) | 14 | 16 | 3 |
| Myocardial infarction | 12 (3.2) | 8 | 4 | 0 |
| Stroke/TIA | 14 (3.7)∗ | 4 | 8 | 2 |
| Other thromboembolism | 7 (1.9) | 2 | 4 | 1 |
| Bleeding, n (%) | 33 (8.8)† | 32 | 14 | 0 |
| Major | 21 (5.6) | 21 | 8 | 0 |
| CRNMB | 12 (3.2) | 11 | 6 | 0 |
CRNMB, clinically relevant nonmajor bleeding; TIA, transient ischemic attack.
13 patients had 14 events.
25 patients had 33 events.
Some patients were both on anticoagulation and antiplatelet therapy.
Figure 1.
Kaplan-Meier cumulative event rates. Kaplan-Meier curves display the first event of (A) thromboembolism, (B) major bleeding, (C) composite of major and clinically relevant nonmajor bleeding (CRNMB), and (D) death event in patients with nonvalvular atrial fibrillation.
Patients who suffered TE during follow-up had significantly higher VWF activity (P = 0.07) and VWF antigen concentration (P = 0.02) compared with those without this event (Table 3, panel A). VWF activity and concentration were not different in patients with major bleeding or composite of major and CRNMB compared with those without bleeding complications (Table 3, panels B and C). ADAMTS 13 activity was not different in patients with TE or with major bleeding compared with those without these events (Table 3, panels A and B). However, within the group of patients who had either major bleeding or CRNMB, ADAMTS13 activity was lower compared with those without bleeding (P = 0.03).
Table 3.
Von Willebrand factor (VWF) activity, VWF antigen concentration, and ADAMTS13 activity in patients with nonvalvular atrial fibrillation who during follow-up had a thromboembolic event (panel A), major bleeding (panel B), or the composite of major bleeding and clinically relevant nonmajor bleeding (CRNMB, panel C), compared with those without these events
| A. Thromboembolic event | |||
|---|---|---|---|
| Variable | Thromboembolic event (n = 32) | No thromboembolic event (n = 342) | P value |
| VWF activity | 0.07 | ||
| Mean (SD) | 158.8 (46.2) | 144.7 (51.8) | |
| Median (min, max) | 153.5 (96.4, 271.7) | 135.0 (49.2, 375.2) | |
| VWF antigen | 0.017 | ||
| Mean (SD) | 180.5 (53.0) | 157.7 (53.1) | |
| Median (min, max) | 184.0 (98.0, 298.0) | 152.5 (49.0, 399.0) | |
| ADAMTS13 activity | 0.28 | ||
| Mean (SD) | 120.8 (35.2) | 115.4 (46.9) | |
| Median (min, max) | 125.2 (53.7, 187.8) | 106.1 (30.7, 289.3) | |
| B. Major bleeding | |||
|---|---|---|---|
| Variable | Major bleeding (n = 21) | No major bleeding (n = 353) | P value |
| VWF activity | 0.13 | ||
| Mean (SD) | 148.3 (39.6) | 145.8 (52.1) | |
| Median (min, max) | 139.0 (85.2, 218.6) | 136.1 (49.2, 375.2) | |
| VWF antigen | 0.65 | ||
| Mean (SD) | 162.5 (43.0) | 159.5 (54.0) | |
| Median (min, max) | 158.0 (77.0, 259.0) | 152.5 (49.0, 399.0) | |
| ADAMTS13 activity | 0.11 | ||
| Mean (SD) | 104.1 (50.5) | 116.5 (45.7) | |
| Median (min, max) | 86.8 (44.7, 258.0) | 110.7 (30.7, 289.3) | |
| C. CRNMB | |||
|---|---|---|---|
| Variable | CRNMB (n = 25) | No CRNMB (n = 349) | P value |
| VWF activity | 0.13 | ||
| Mean (SD) | 156.1 (41.4) | 145.2 (52.1) | |
| Median (min, max) | 164.5 (85.2, 229.8) | 135.6 (49.2, 375.2) | |
| VWF antigen | 0.19 | ||
| Mean (SD) | 169.3 (44.0) | 158.9 (54.0) | |
| Median (min, max) | 162.0 (77.0, 259.0) | 154.0 (49.0, 399.0) | |
| ADAMTS13 activity | 0.026 | ||
| Mean (SD) | 99.7 (47.7) | 117.0 (45.7) | |
| Median (min, max) | 86.1 (44.7, 258.0) | 111.0 (30.7, 289.3) | |
ADAMTS13, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; SD, standard deviation.
Among other clinical and echocardiographic variables, VWF antigen concentration was identified as a predictor of TE, all-cause mortality, composite of TE and all-cause mortality, and composite of any bleeding and all-cause mortality by univariate analysis (Supplemental Table S1). In addition, VWF activity was a predictor of all-cause mortality, composite of TE and all-cause mortality, and a composite of any bleeding and all-cause mortality, whereas ADAMTS13 activity was a predictor of all-cause mortality by univariate analysis.
By multivariate Cox proportional hazards analysis, both VWF antigen concentration and CHA2DS2-VASc scores were identified as independent predictors of all-cause mortality (Table 4). Furthermore, both VWF antigen and CHA2DS2-VASc were independent predictors of a composite of TE and death. Also, left atrium distension as assessed by volume index independently predicted a composite of TE and all-cause mortality. CHA2DS2-VASc score was the only predictor of TE and the composite of any bleeding and death.
Table 4.
Multivariate analysis of covariates modeled as a time to the first event, associated with the risk of thromboembolic events (subset A), major bleeding (subset B), composite of major and clinically relevant nonmajor bleeding (subset C), all-cause mortality (subset D), composite of thromboembolism and all-cause mortality (subset E), and the composite of major, clinically relevant nonmajor, and all-cause mortality (subset F)
| Variable | Hazard ratio | 95% CI | P value |
|---|---|---|---|
| A. Thromboembolic events (n = 32) | |||
| CHA2DS2-VASc score | 1.349 | (1.110, 1.639) | 0.003 |
| LAAT | 2.291 | (0.756, 6.940) | 0.143 |
| VWF antigen | 1.005 | (0.998, 1.011) | 0.170 |
| On anticoagulation for > 2 y | 0.777 | (0.303, 1.990) | 0.599 |
| B. Major bleeding (n = 21) | |||
| CHA2DS2-VASc score | 1.571 | (1.162, 2.123) | 0.003∗ |
| LAA emptying velocity ≤ 25 | 3.046 | (0.947, 9.798) | 0.062 |
| On anticoagulation for > 2 y | 2.695 | (0.297, 24.460) | 0.378 |
| C. Major or clinically relevant nonmajor bleeding (n = 32) | |||
| CHA2DS2-VASc score | 1.650 | (1.250, 2.177) | < 0.001 |
| On anticoagulation for > 2 y | 3.284 | (0.375, 28.776) | 0.283 |
| LAA emptying velocity ≤ 25 | 1.882 | (0.656, 5.402) | 0.240 |
| D. All-cause mortality (n = 18) | |||
| CHA2DS2-VASc score | 1.717 | (1.273, 2.315) | <0.001 |
| VWF antigen | 1.011 | (1.003, 1.020) | 0.011 |
| On anticoagulation for >2 y | 1.830 | (0.216, 15.465) | 0.579 |
| E. Thromboembolism and all-cause mortality (n = 44) | |||
| CHA2DS2-VASc score | 1.391 | (1.171, 1.651) | < 0.001 |
| VWF antigen | 1.006 | (1.000, 1.012) | 0.039 |
| Moderate/severe SEC | 0.907 | (0.413, 1.991) | 0.808 |
| LA volume index | 1.023 | (1.005, 1.041) | 0.011 |
| On anticoagulation for > 2 y | 0.885 | (0.366, 2.144) | 0.787 |
| F. Major or clinically relevant nonmajor bleeding and death (n = 41) | |||
| CHA2DS2-VASc score | 1.752 | (1.382, 2.222) | < 0.001 |
| LAAT | 1.155 | (0.293, 4.549) | 0.837 |
| LAA emptying velocity ≤ 25 | 1.830 | (0.745, 4.493) | 0.187 |
| VWF antigen | 1.004 | (0.997, 1.010) | 0.279 |
| On anticoagulation for > 2 y | 1.942 | (0.398, 9.478) | 0.412 |
CHA2DS2-VASc, Congestive Heart Failure, Hypertension, Age (≥75 years), Diabetes, Stroke/Transient Ischemic Attack, Vascular Disease, Age (65-74 years), Sex (Female); CI, confidence interval; LAAT, left atrium appendage thrombus; SEC, spontaneous echocardiographic contrast; VWF, Von Willebrand factor.
For this one evaluation, the proportional hazards P value, calculated using the Supremum test, has not proved the assumption of proportionality (P = 0.044 with the Supremum test). All other evaluations proved the assumption (P > 0.05).
To assess the possible violations of the proportional hazards assumption in Cox regression the Supremum test was used. It showed that the only hazard ratio of multivariate analysis that showed a violation of proportionality was the CHA2DS2-VASc score prediction of major bleeding (hazard ratio, 1.571; 95% confidence interval, 1.162, 2.123, but the proportional hazard P value by the Supremum test was 0.044); all other hazard ratio analyses showed adequate proportionality by this methodology (P > 0.05).
Discussion
The principal finding of the study is that VWF antigen concentration is an independent predictor of death and a composite of death and TE in patients with NVAF at 3 years of follow-up after controlling for both clinical and echocardiographic variables. Patients who developed TE had significantly higher VWF activity and concentration compared with those without a TE event. This finding underscores the general hypothesis that blood hypercoagulability adds to the risk calculation for all-cause mortality in these patients. VWF activity analysis has not revealed any better predictive power compared with VWF antigen concentration. The importance of clinical risk factors in the prediction of adverse clinical outcomes, as is captured by the CHA2DS2-VASc score tool, is also confirmed.
AF has been generally viewed as an acquired hypercoagulable condition.11, 12, 13, 14, 15, 16, 17 Out of a myriad of blood variables analyzed, VWF antigen concentration has been the only consistently elevated protein identified in this dysrhythmia.11,20, 21, 22, 23 AF is known to promote left atrial enlargement and decreased left atrial appendage contractility, which leads to blood stagnation. In fact, the LAVI was an independent predictor of the composite outcome of TE and all-cause mortality in the current analysis. Our previous studies have shown a direct correlation between measures of blood stasis in the fibrillating left atrium and increased measures of VWF antigen concentration and activity.20 Beyond blood stasis, these measures have been associated with left atrial appendage thrombi.11,20 Endocardial expression of VWF was significantly correlated with both platelet adhesion and thrombus formation in the left atrial appendage in patients with NVAF.39 Increased VWF levels were independently associated with prior cerebral ischemia in a cohort of 1531 patients with AF from the Stroke Prevention in Atrial Fibrillation (SPAF) III trial.21 However, in a follow-up study of 994 SPAF III participants receiving aspirin, there was no relation between VWF and subsequent stroke after adjusting for other clinical predictors.23 In the group of 829 patients with permanent AF on chronic anticoagulation during a median of 828 days (range, 18-1085 days) of follow-up, the high plasma VWF level (221 IU/dL) was an independent predictor of future adverse cardiovascular events, mortality, and major bleeding.24 In the study of 423 patients with NVAF followed for 19 (9-31) months, more cardiovascular adverse events (ischemic stroke, acute myocardial infarction, or all-cause mortality) occurred in the upper tertile of VWF compared with the lowest or middle tertiles.25 In another prospective, longitudinal single-centre study including 269 patients with AF followed for a median duration of 1933 (1517-2277) days, a high ratio of VWF/ADAMTS13 independently predicted major adverse cardiovascular events.26 In a recent meta-analysis, elevated circulating VWF was independently associated with a higher risk of cardiovascular complications and all-cause mortality.29 However, most of the studies of this meta-analysis investigated the VWF effect on clinical outcomes using either high tertile or certain cutoff points of VWF concentration (VWF >1434.92 mU/mL or 221 IU/dL), not VWF as a continuous variable. Moreover, none of those studies were using echocardiographic measures as confounding variables representing a high risk of adverse cardiovascular events and mortality. All prior studies had an older population of patients with AF. Our study cohort represents a more current clinical profile of the AF population with a significant proportion of patients treated with pulmonary vein isolation. Moreover, most of the previous studies had a wide range of follow-up periods such as from 18 to 2277 days, and the current study assessed clinical outcomes during a uniformed time frame of 3 years.
In the current study, although VWF was predictive of TE in the univariate examination, it lost significance after adjusting for other variables in the multivariate model. This finding may be accounted for by the introduction of antithrombotic therapy in those high-risk patients. Moreover, a substantial percentage (69%) of our population underwent pulmonary vein isolation that undoubtedly altered the natural history of this disease. The ideal longitudinal study would be to monitor outcomes in these patients without altering the dysrhythmia or prothrombotic status, which would be neither practical nor ethical. Furthermore, the discrepancy between a clear and strong association of elevated VWF and the presence of LAAT and the lack of predictive power for future TE events could reflect the heterogeneous etiology of stroke in NVAF. It is estimated that only half of strokes in NVAF are related to cardioembolism.40
In contrast to measures of VWF, ADAMTS13 activity was not an independent predictor of TE, bleeding, or death in multivariate analysis. ADAMTS13 is regulated in vivo by the unfolding of the substrate—the VWF multimer. Brisk laminar blood flow unfolds ultra-large VWF molecules, which allows rapid proteolysis by ADAMTS13.41 Under static conditions, proteolysis is retarded 1000-fold. Therefore, measuring ADAMTS13 activity in vitro does not reflect flow-mediated conditions in vivo and, not surprisingly, does not correlate with clinical outcomes.
Several limitations of this study deserve mention. First, we cannot exclude referral bias as only patients who had a TEE requested by their care provider were recruited. Moreover, 40 patients of the original study cohort were not seen in our institution within the 3 years after enrollment, have not submitted the mailed questionnaire, and we were not able to contact them by phone (lost to follow-up). However, the demographic characteristics of the current study cohort are not different from the original study group.20 Second, the blood type was not measured. Blood type “O” is associated with lower than normal values of circulating VWF and has been shown to impact outcomes.42 Third, there were no predetermined follow-up visits scheduled for the study. Clinical course and study outcome exploration were performed based on the analysis of clinical reports from visits scheduled by primary service, and from the information obtained from mailed questionnaires or a scripted phone interview. Because of this method of gathering the data, we were not always able to precisely establish the date of anticoagulation initiation or discontinuation. Fourth, the VWF level represents acute phase reactivity that raises concerns of reproducibility. A large study, however, found that VWF reproducibility is similar to blood pressure and serum cholesterol.43 Lastly, patients were treated with different medications that may have an impact on VWF and ADAMTS13. However, measures of VWF concentration, activity, and ADAMTS13 activity that we checked at baseline in our cohort were not affected by sex, or therapy with warfarin, aspirin, or statin.20
Conclusions
Among patients with NVAF, those with TE complications have higher VWF antigen concentration and activity, and VWF is an independent predictor of poor outcomes including death and a composite of death and TE after controlling for both clinical and echocardiographic variables. As such, this measure may help identify high-risk patients and provide further stratification beyond CHA2DS2-VASc assessment.
Funding Sources
This study was funded, in part, by Nr.17896 CR 20 grant from the Department of Internal Medicine, Mayo Clinic, and statistical support was provided by an internal grant from the Mayo Clinic Department of Cardiology.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Mayo Clinic Institutional Review Board in Rochester, MN (IRB#: 18-011482).
See page 324 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2020.10.018.
Supplementary Material
References
- 1.Aberg H. Atrial fibrillation: I. A study of atrial thrombosis and systemic embolism in a necropsy material. Acta Med Scand. 1969;185:373–379. [PubMed] [Google Scholar]
- 2.Manning W.J., Silverman D.I., Waksmonski C.A., Oettgen P., Douglas P.S. Prevalence of residual left atrial thrombi among patients with acute thromboembolism and newly recognized atrial fibrillation. Arch Intern Med. 1995;155:2193–2198. [PubMed] [Google Scholar]
- 3.Klein A.L., Grimm R.A., Murray R.D. Assessment of cardioversion using transesophageal echocardiography investigators. Use of transesophageal echocardiography to guide cardioversion in patients with atrial fibrillation. N Engl J Med. 2001;344:1411–1420. doi: 10.1056/NEJM200105103441901. [DOI] [PubMed] [Google Scholar]
- 4.Handke M., Harloff A., Hetzel A. Left atrial appendage flow velocity as a quantitative surrogate parameter for thromboembolic risk: determinants and relationship to spontaneous echocontrast and thrombus formation—a transesophageal echocardiographic study in 500 patients with cerebral ischemia. J Am Soc Echocardiogr. 2005;18:1366–1372. doi: 10.1016/j.echo.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Goldman M.E., Pearce L.A., Hart R.G. Pathophysiologic correlates of thromboembolism in nonvalvular atrial fibrillation: I. Reduced flow velocity in the left atrial appendage (The Stroke Prevention in Atrial Fibrillation [SPAF-III] Study) J Am Soc Echocardiogr. 1999;12:1080–1087. doi: 10.1016/s0894-7317(99)70105-7. [DOI] [PubMed] [Google Scholar]
- 6.Bernhardt P., Schmidt H., Hammerstingl C., Lüderitz B., Omran H. Patients with atrial fibrillation and dense spontaneous echo contrast at high risk a prospective and serial follow-up over 12 months with transesophageal echocardiography and cerebral magnetic resonance imaging. J Am Coll Cardiol. 2005;45:1813–1814. doi: 10.1016/j.jacc.2004.11.071. [DOI] [PubMed] [Google Scholar]
- 7.Zabalgoitia M., Halperin J.L., Pearce L.A. Transesophageal echocardiographic correlates of clinical risk of thromboembolism in nonvalvular atrial fibrillation. Stroke Prevention in Atrial Fibrillation III Investigators. J Am Coll Cardiol. 1998;31:1622–1626. doi: 10.1016/s0735-1097(98)00146-6. [DOI] [PubMed] [Google Scholar]
- 8.Thomas D.P. Venous thrombosis and the “Wessler” test. Thromb Haemost. 1996;76:1–4. [PubMed] [Google Scholar]
- 9.Schaub R.G., Simmons C.A., Koets M.H., Romano P.J., Stewart G.J. Early events in the formation of a venous thrombus following local trauma and stasis. Lab Invest. 1984;51:218–224. [PubMed] [Google Scholar]
- 10.Kumar A., Lindner V. Remodeling with neointima formation in the mouse carotid artery after cessation of blood flow. Arterioscler Thromb Vasc Biol. 1997;17:2238–2244. doi: 10.1161/01.atv.17.10.2238. [DOI] [PubMed] [Google Scholar]
- 11.Heppell R.M., Berkin K.E., McLenachan J.M., Davies J.A. Haemostatic and haemodynamic abnormalities associated with left atrial thrombosis in non-rheumatic atrial fibrillation. Heart. 1997;77:407–411. doi: 10.1136/hrt.77.5.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lip G.Y.H. Hypercoagulability and haemodynamic abnormalities in atrial fibrillation. Heart. 1997;77:395–399. doi: 10.1136/hrt.77.5.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sohara H., Amitani S., Kurose M., Miyahara K. Atrial fibrillation activates platelets and coagulation in a time-dependent manner: a study in patients with paroxysmal atrial fibrillation. J Am Coll Cardiol. 1997;29:106–111. doi: 10.1016/s0735-1097(96)00427-5. [DOI] [PubMed] [Google Scholar]
- 14.Minamino T., Kitakaze M., Sanada S. Increased expression of P-selectin on platelets is a risk factor for silent cerebral infarction in patients with atrial fibrillation: role of nitric oxide. Circulation. 1998;98:1721–1726. doi: 10.1161/01.cir.98.17.1721. [DOI] [PubMed] [Google Scholar]
- 15.Mondillo S., Sabatini L., Agricola E. Correlation between left atrial size, prothrombotic state and markers of endothelial dysfunction in patients with lone chronic nonrheumatic atrial fibrillation. Int J Cardiol. 2000;75:227–232. doi: 10.1016/s0167-5273(00)00336-3. [DOI] [PubMed] [Google Scholar]
- 16.Mitusch R., Siemens H.J., Gaber M. Detection of hypercoagulable state in nonvalvular atrial fibrillation and the effect of anticoagulant therapy. Thromb Haemost. 1996;75:219–223. [PubMed] [Google Scholar]
- 17.Feng D., D’Agostino R.B., Silbershatz H. Hemostatic state and atrial fibrillation (the Framingham Offspring Study) Am J Cardiol. 2001;87:168–171. doi: 10.1016/s0002-9149(00)01310-2. [DOI] [PubMed] [Google Scholar]
- 18.Ruggeri Z.M., Savage B. Biological functions of von Willebrand factor. In: Ruggeri Z.M., editor. Von Willebrand Factor and the Mechanisms of Platelet Function. Springer; Berlin: 1998. pp. 79–109. [Google Scholar]
- 19.Zheng X., Chung D., Takayama T.K. Structure of von Willebrand factor-cleaving protease (ADAMTS13), a metalloprotease involved in thrombotic thrombocytopenic purpura. J Biol Chem. 2001;276:41059–41063. doi: 10.1074/jbc.C100515200. [DOI] [PubMed] [Google Scholar]
- 20.Ammash N., Konik E.A., McBane R.D. Left atrial blood stasis and Von Willebrand Factor-ADAMTS13 homeostasis in atrial fibrillation. Arterioscler Thromb Vasc Biol. 2011;31:2760–2766. doi: 10.1161/ATVBAHA.111.232991. [DOI] [PubMed] [Google Scholar]
- 21.Feinberg W.M., Pearce L.A., Hart R.G. Markers of thrombin and platelet activity in patients with atrial fibrillation: correlation with stroke among 1531 participants in the stroke prevention in atrial fibrillation III study. Stroke. 1999;30:2547–2553. doi: 10.1161/01.str.30.12.2547. [DOI] [PubMed] [Google Scholar]
- 22.Conway D.S.G., Pearce L.A., Chin B.S., Hart R.G., Lip G.Y. Plasma von Willebrand factor and soluble P-selectin as indices of endothelial damage and platelet activation in 1321 patients with non-valvular atrial fibrillation: relationship to stroke risk factors. Circulation. 2002;106:1962–1967. doi: 10.1161/01.cir.0000033220.97592.9a. [DOI] [PubMed] [Google Scholar]
- 23.Conway D.S., Pearce L.A., Chin B.S., Hart R.G., Lip G.Y. Prognostic value of plasma von Willebrand factor and soluble P-selectin as indices of endothelial damage and platelet activation in 994 patients with nonvalvular atrial fibrillation. Circulation. 2003;107:3141–3145. doi: 10.1161/01.CIR.0000077912.12202.FC. [DOI] [PubMed] [Google Scholar]
- 24.Roldán V., Marín F., Muiña B. Plasma von Willebrand factor levels are an independent risk factor for adverse events including mortality and major bleeding in anticoagulated atrial fibrillation patients. J Am Coll Cardiol. 2011;57:2496–2504. doi: 10.1016/j.jacc.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 25.Krishnamoorthy S., Khoo C.W., Lim H.S. Prognostic role of plasma von Willebrand factor and soluble E-selectin levels for future cardiovascular events in a 'real-world' community cohort of patients with atrial fibrillation. Eur J Clin Invest. 2013;43:1032–1038. doi: 10.1111/eci.12140. [DOI] [PubMed] [Google Scholar]
- 26.Freynhofer M.K., Gruber S.C., Bruno V. Prognostic value of plasma von Willebrand factor and its cleaving protease ADAMTS13 in patients with atrial fibrillation. Int J Cardiol. 2013;168:317–325. doi: 10.1016/j.ijcard.2012.09.056. [DOI] [PubMed] [Google Scholar]
- 27.Joachim R., Ehrlich J.R., Kaluzny M. Biomarkers of structural remodelling and endothelial dysfunction for prediction of cardiovascular events or death in patients with atrial fibrillation. Clin Res Cardiol. 2011;100:1029–1036. doi: 10.1007/s00392-011-0337-9. [DOI] [PubMed] [Google Scholar]
- 28.Zhong C., Xin M., He L., Sun G., Shen F. Prognostic value of von Willebrand factor in patients with atrial fibrillation: a meta-analysis. Medicine (Baltimore) 2018;97:e11269. doi: 10.1097/MD.0000000000011269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ye Y.Z., Chang Y.F., Wang B.Z., Ma Y.T., Ma X. Prognostic value of von Willebrand factor for patients with atrial fibrillation: a meta-analysis of prospective cohort studies. Postgrad Med J. 2020;96:267–276. doi: 10.1136/postgradmedj-2019-136842. [DOI] [PubMed] [Google Scholar]
- 30.Melduni R.M., Chandrasekaran K., Friedman P.A. Does left atrial appendage peak emptying flow velocity predict the electrical energy required to achieve successful direct-current cardioversion in patients with persistent atrial fibrillation? J Am Soc Echocardiogr. 2007;20:1004–1008. doi: 10.1016/j.echo.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 31.Wysokinski W.E., Ammash N., Sobande F. Predicting left atrial thrombi in atrial fibrillation. Am Heart J. 2010;159:665–671. doi: 10.1016/j.ahj.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 32.Fatkin D., Kelly R.P., Feneley M.P. Relations between left atrial appendage blood flow velocity, spontaneous echocardiographic contrast and thromboembolic risk in vivo. J Am Coll Cardiol. 1994;23:961–969. doi: 10.1016/0735-1097(94)90644-0. [DOI] [PubMed] [Google Scholar]
- 33.Oh J.K., Seward J.B., Tajik A.J. The Echo Manual. Third Edition. Lippincott Williams and Wilkins; Philadelphia: 2006. The Echo Manual. 3rd ed; pp. 109–119. [Google Scholar]
- 34.Lip G.Y., Nieuwlaat R., Pisters R., Lane D.A., Crijns H.J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 35.Genuth S., Alberti K.G., Bennett P. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 36.Gillum R.F., Fortmann S.P., Prineas R.J., Kottke T.E. International diagnostic criteria for acute myocardial infarction and acute stroke. Am Heart J. 1984;108:150–158. doi: 10.1016/0002-8703(84)90558-1. [DOI] [PubMed] [Google Scholar]
- 37.Fuster V., Rydén L.E., Cannom D.S. American College of Cardiology/American Heart Association Task Force on Practice Guidelines; European Society of Cardiology Committee for Practice Guidelines; European Heart Rhythm Association; Heart Rhythm Society. ACC/AHA/ESC 2006 Guidelines for the Management of Patients With Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines. Circulation. 2006;114:e257–e354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- 38.Schulman S., Kearon C. Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 39.Fukuchi M., Watanabe J., Kumagai K. Increased von Willebrand factor in the endocardium as a local predisposing factor for thrombogenesis in overloaded human atrial appendage. J Am Coll Cardiol. 2001;37:1436–1442. doi: 10.1016/s0735-1097(01)01125-1. [DOI] [PubMed] [Google Scholar]
- 40.Hart R.G., Pearce L.A., Miller V.T. Cardioembolic vs. noncardioembolic strokes in atrial fibrillation: frequency and effect of antithrombotic agents in the stroke prevention in atrial fibrillation studies. Cerebrovasc Dis. 2000;10:39–43. doi: 10.1159/000016023. [DOI] [PubMed] [Google Scholar]
- 41.Tsai H.M. Physiologic cleavage of von Willebrand factor by a plasma protease is dependent on its conformation and requires calcium ion. Blood. 1996;87:4235–4244. [PubMed] [Google Scholar]
- 42.Blustin J.M., McBane R.D., Mazur M. The association between thromboembolic complications and blood group in patients with atrial fibrillation. Mayo Clin Proc. 2015;90:216–223. doi: 10.1016/j.mayocp.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 43.Danesh J., Wheeler J.G., Hirschfield G.M. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.