Abstract
Background
Fetuses of diabetic mothers develop left ventricular (LV) hypertrophy and are at increased long-term risk of cardiovascular disease. In our previous longitudinal study from midgestation to late infancy we showed persistence of LV hypertrophy and increased aortic stiffness compared with infants of healthy mothers, the latter of which correlated with third trimester maternal hemoglobin A1c. In the present study, we reexamined the same cohort in early childhood to determine if these cardiovascular abnormalities persisted.
Methods
Height, weight, and right arm blood pressure were recorded. A full functional and structural echocardiogram was performed with offline analysis of LV posterior wall and interventricular septal diastolic thickness (IVSd), systolic and diastolic function, and aortic pulse wave velocity. Vascular reactivity was assessed using digital thermal monitoring. Participants also completed a physical activity questionnaire.
Results
Twenty-five children of diabetic mothers (CDMs) and 20 children from healthy pregnancies (mean age, 5.6 ± 1.7 and 5.3 ± 1.3 years, respectively; P = not significant) were assessed. Compared with controls, IVSd z score was increased in CDMs (1.2 ± 0.6 vs 0.5 ± 0.3, respectively; P = 0.006), with one-fifth having a z score of more than +2.0. Aortic pulse wave velocity was increased in CDMs (3.2 ± 0.6 m/s vs 2.2 ± 0.4 m/s; P = 0.001), and correlated with IVSd z score (R2 = 0.81; P = 0.001) and third trimester maternal A1c (R2 = 0.65; P < 0.0001). Body surface area, height, weight, blood pressure, vascular reactivity, and physical activity scores did not differ between groups. Our longitudinal analysis showed that individuals with greater IVSd, and aortic stiffness in utero, early and late infancy also tended to have greater measures in early childhood (P < 0.001 and P < 0.0001, respectively).
Conclusions
CDMs show persistently increased interventricular septal thickness and aortic stiffness in early childhood.
Résumé
Introduction
Les fœtus des mères diabétiques présentent une hypertrophie du ventricule gauche (VG) et sont exposés à un risque accru à long terme de souffrir d’une maladie cardiovasculaire. Dans notre étude longitudinale précédente qui portait sur la période mi-gestationnelle à la fin de la première enfance, nous avons montré la persistance de l’hypertrophie du VG et l’augmentation de la rigidité aortique par rapport aux bébés des mères bien portantes, qui sont en corrélation avec le troisième trimestre de l’hémoglobine A1c maternelle. Dans la présente étude, nous avons réexaminé la même cohorte au début de la seconde enfance pour déterminer si ces anomalies cardiovasculaires persistaient.
Méthodes
Nous avons enregistré la taille, le poids et la pression artérielle au bras droit. Nous avons réalisé une échocardiographie complète pour évaluer l’état fonctionnel et structurel par analyse hors ligne de la paroi postérieure du VG et de l’épaisseur du septum interventriculaire en diastole (SIVd), la fonction systolique et diastolique, et la vitesse de l’onde de pouls aortique. Nous avons évalué la réactivité vasculaire à l’aide de la surveillance thermique numérique. Les participants ont également rempli un questionnaire sur l’activité physique.
Résultats
Vingt-cinq enfants issus de mères diabétiques (EMD) et 20 enfants de mères bien portantes (âge moyen, 5,6 ± 1,7 et 5,3 ± 1,3 ans, respectivement ; P = non significatif) ont fait l’objet d’une évaluation. Comparativement aux témoins, les EMD avaient un score z du SIVd plus élevé (1,2 ± 0,6 vs 0,5 ± 0,3, respectivement ; P = 0,006), et un cinquième de ces enfants avaient un score z de plus de +2,0. La vitesse de l’onde de pouls aortique était plus élevée chez les EMD (3,2 ± 0,6 m/s vs 2,2 ± 0,4 m/s ; P = 0,001), et était en corrélation avec le score z du SIVd (R2 = 0,81 ; P = 0,001) et le troisième trimestre de l’A1c maternelle (R2 = 0,65 ; P < 0,0001). Les scores de la surface corporelle, la taille, le poids, la pression artérielle, la réactivité vasculaire et l’activité physique ne différaient pas entre les groupes. Notre étude longitudinale a montré que les individus qui avaient une plus grande SIVd et une rigidité aortique in utero, au début et à la fin de la première enfance, avaient également tendance à avoir des mesures plus grandes au début de la seconde enfance (P < 0,001 et P < 0,0001, respectivement).
Conclusions
Les EMD montrent une persistance de l’augmentation de l’épaisseur du septum interventriculaire et de l’augmentation de la rigidité aortique au début de la seconde enfance.
Barker’s revolutionary hypothesis, “the developmental origins of health and disease,” which postulated that adult disease might have origins during fetal development, has spawned an important area of cardiovascular health research. It has been proposed that fetal adaptations to a suboptimal in utero environment result in permanent structural and functional differences in key homeostatic systems predisposing the individual to metabolic and cardiovascular disease (CVD).1 Other evidence from epidemiologic, clinical, and basic studies have supported this concept.2,3 Because > 30% of all global deaths can be attributed to CVD,4 investigations into how fetal programming might influence long-term cardiovascular health and contribute to CVD are increasingly relevant.
Pregestational diabetes mellitus (DM) is among the most common complications of pregnancy.5 Growing data suggest that maternal DM has transgenerational consequences, including higher incidences of metabolic syndrome and vascular abnormalities in older children and adult offspring of affected mothers.5, 6, 7, 8, 9 The pathogenesis including the contributing prenatal and postnatal insults experienced by the offspring of diabetic pregnancies remain ill defined.
It is well recognized that maternal DM has a significant fetal myocardial effect. In pregnancies complicated by maternal DM, the fetal and neonatal ventricular myocardium evolves hypertrophy, primarily affecting the septal wall. We and others have shown changes in fetal myocardial function in maternal DM possibly starting during the late first trimester.10, 11, 12, 13 In the first phase of this study, participants had 3 fetal echocardiograms. Our study also showed that fetuses from DM pregnancies showed significantly increased left ventricular (LV) posterior wall thickness (LVPWd) and interventricular septal thickness (IVSd) starting from the late second trimester.14 In this group of relatively well controlled diabetic mothers we also noted statistically significant differences in fetal mitral valve E wave Doppler echocardiography and LV Tei index in the third trimester.14 Although it had been assumed that myocardial abnormalities resolve by late infancy, we have recently shown in a small cohort that increased LV diastolic wall thickness (IVSd and LVPWd) persists in late infancy (6-12 months) but shows no direct relationship with maternal glycemic control.14 In the same prospective study, infants of diabetic mothers (IDMs) had increased aortic stiffness, which had a very strong correlation with maternal third trimester A1c and ventricular wall thickness in late gestation and late infancy.14 This suggested a potential causal relationship (ie, increased aortic stiffness contributes to increased LV mass) or at least a common pathogenic insult.
In the present continuation of our longitudinal study, we sought to explore whether increased LV mass and aortic stiffness in the IDMs persist into early childhood, whether there is altered myocardial function as a consequence, and whether there is evidence of altered vascular health including systemic hypertension and endothelial dysfunction.
Methods
This study represents the continuation of a prospective longitudinal investigation in which pregnant mothers with pregestational DM and healthy control mothers were recruited in the Fetal and Neonatal Cardiology Program at the University of Alberta between June 2009 and July 2014 to participate in serial fetal and postnatal cardiovascular assessments. The Research Ethics Board of the University of Alberta provided approval for this investigation. Mothers with a diagnosis of preconception DM who were receiving insulin and carrying a singleton pregnancy were recruited after informed consent. No DM-complicated pregnancies were included if there was a known or suspected major fetal abnormality other than fetal hypertrophic cardiomyopathy. Healthy control pregnancies included mothers with no fetal pathology or maternal illness that could affect fetal cardiovascular health. Pregnant mothers with abnormal glucose testing during pregnancy or gestational diabetes diagnosed in past pregnancies were excluded. Multiple pregnancies, those that delivered before 34 weeks, and those with fetal growth restriction (birth weight < 10th centile) were also excluded from both groups.
We recorded clinical and demographic data for the pregnant mothers. As a measure of glycemic control, we recorded hemoglobin A1c values in the first (closest to conception ≤ 12 weeks), second (> 12 to < 28 weeks), and third (≥ 28 weeks) trimesters.
The mothers underwent 3 fetal echocardiograms (approximately 20, 26, and 32 weeks gestation) and the offspring had an early (2-6 weeks of age) and late infancy (6-12 months of age) echocardiogram, the findings of which have been previously reported.14 The children of diabetic mothers (CDMs) and healthy controls who had originally participated in all 5 previous assessments were invited for follow-up assessment at 3-8 years.
During this phase the CDMs and their healthy control counterparts had weight, height, and right arm cuff blood pressure (average of 3 measures) recorded. We acquired a detailed interim clinical history and demographic data for the period since last assessment. The families completed a validated Habitual Activity Estimation Scale (HAES) physical activity in childhood survey.15
Echocardiography
CDMs and healthy control children underwent a complete anatomical and functional echocardiographic study using a General Electric Vivid e9 ultrasound system (General Electric, Boston, MA). Offline analysis was performed using GE Echopac software, version 7.1 (General Electric). Parameters measured included: LVPWd and IVSd, shortening fraction from m mode, LV longitudinal strain, and strain rate using speckle tracking, tissue Doppler-based mitral valve and septal annular velocities (e’, a’, s’), pulse wave velocity (PWV), e and a wave inflow Doppler, e/e’ ratios, e/a wave ratios, isovolumic relaxation and contraction, inflow, and ejection time. The Tei index was calculated using the formula: (isovolumic contraction time + isovolumic relaxation time)/ejection time.16 We converted raw measurements of LVPWd and IVSd to z scores on the basis of body surface area (BSA) using normative data from a large sample population of healthy infants and children.17 LV mass was calculated using the Devereaux method and converted to z score indexed to BSA using reference data of Foster et al.18
Aortic stiffness
Aortic stiffness was assessed using a previously validated echocardiographic Doppler method used to calculate aortic PWV.19 From the suprasternal notch view, the aortic arch length was measured summing serial measurements along the axis of the curved segment of aorta. Pulse-wave Doppler tracings were obtained sequentially in the ascending aorta (T1) and in the distal descending thoracic aorta (T2). All measurements were averaged over 3 cardiac cycles. We calculated transit time (T2-T1), measured from the onset of the R wave in the QRS waveform to the onset of flow, and together with the distance from T1 to T2 along the aortic arch, we calculated the PWV using the equation: PWV = distance/(T2-T1).
Peripheral arterial endothelial function testing
Peripheral arterial reactivity was assessed using the VENDYS I system (Endothelix Inc, Palo Alto, CA). This device uses a computer-based thermometry system with 2 temperature fingertip probes that are placed on the index finger of both hands. A sphygmomanometer cuff is placed around the right arm to facilitate the hyperemia protocol. The test is conducted with the patient at rest in a quiet room at standard room temperatures. A 15-minute protocol is used: 5 minutes of rest/calibration during which the participant lies still on a bed with the cuff and temperature probes, 5 minutes of cuff occlusion, followed by 5 minutes of data collection immediately after the blood pressure cuff deflates. The cuff is deflated rapidly after the 5 minutes of occlusion to invoke reactive hyperemia distally. Thermal tracings are measured continuously. The data are used to generate a vascular reactivity quotient, which represents a measure of vascular responsivity to flow. Although studies in children have been limited, this method has been validated in adult populations for endothelial function testing.20
Statistics
All values are reported as mean ± SD. Statistical analysis was completed using GraphPad Prism software version 7 (GraphPad Software, Inc, Palo Alto, CA) and IBM SPSS version 24 (IBM Corp, Armonk, NY). Graphical and linear analyses were performed using GraphPad Prism (GraphPad Software, Inc). Parameters between the 2 groups were compared using an unpaired t test. We performed longitudinal analysis to estimate means of cardiac outcomes over time (fetal stage, infancy 2-6 weeks, infancy 6-12 months, and 3-8 years) according to group (control and DM), and to estimate differences in means between the groups per each time period (post hoc contrast). We used a generalized estimation equations (GEE) approach to estimate the predictive means according to group for each time period. The GEE approach (also named marginal models or population average models) takes into account the correlation among the repeated responses per subject. The GEE approach differs from the traditional analysis of variance repeated measure, in that it does not require distributional assumptions for the observations either that subjects have the same number of measures or that they are measured at a common set of occasions.21 However, it provides consistent estimations of marginal effects on the basis of robust standards errors (sandwich estimator).21 Differences in the means of the outcomes between the groups for each time period were estimated using Bonferroni correction contrasts. We report graphs of the estimated marginal means with 95% confidence intervals and tables of results for the contrasts. Outputs from Stata (StataCorp, College Station, TX) for the GEE models, estimated marginals (with 95% confidence intervals) and contrast values (with P values adjusted for Bonferroni correction) were used in the analysis.
Results
All families that took part in all 5 of the previous study periods (3 fetal and 2 postnatal studies) were eligible to take part in this phase. We attempted to contact all 36 control and 36 diabetes families. Because it had been many years since most of these families had participated in a study with our group we were unable to contact a number of them. The main reasons for this were relocation and being unable to reach the families through our previously recorded contact information (eg, likely changed phone numbers). Twenty control children and 25 CDMs returned for repeat assessment at a mean age of 5.3 ± 1.3 years and 5.6 ± 1.7 years, respectively (not significant).
Table 1 shows a summary their demographic and general findings. Birth weight, length, and method of delivery were not different between groups. Weight, height, BSA, and systolic and diastolic blood pressures did not differ between the 2 groups. There were no significant differences in self-reported total minutes of activity per day or percentage of time spent inactive, somewhat inactive, somewhat active, and very active on either week days or weekend days between the 2 groups (Table 2).
Table 1.
Demographic and baseline clinical data for children of control and diabetic mothers
| Parameter | Control | Diabetes | P |
|---|---|---|---|
| n | 20 | 25 | NS |
| Age, years | 5.6 ± 1.7 | 5.3 ± 1.3 | 0.14 |
| Birth mode, vaginal/C-section | 17/3 | 21/4 | NS |
| Birth weight, kg | 3.7 ± 0.67 | 3.9 ± 1.3 | 0.21 |
| Female/male | 10/10 | 11/14 | NS |
| Body surface area, m2 | 0.84 ± 0.2 | 0.81 ± 0.18 | 0.12 |
| Height, m | 1.21 ± 0.12 | 1.19 ± 0.11 | 0.39 |
| Weight, kg | 22.2 ± 5.5 | 22.7 ± 6.1 | 0.46 |
| Systolic/diastolic BP, mm Hg | 99 ± 8/60 ± 6 | 101 ± 9/60 ± 5 | 0.2 |
| Family incomes > $100,000 | 16 | 20 | 0.32 |
BP, blood pressure; NS, nonsignificant used for all P values > 0.9.
Table 2.
Parent-reported physical activity of CDMs vs control children
| Activity level∗ | Weekday activity, min |
Weekend activity, min |
||
|---|---|---|---|---|
| Mean ± SD | P | Mean ± SD | P | |
| Inactive | 0.21 | 0.41 | ||
| Control children | 64 ± 7 | 72 ± 6 | ||
| CDMs | 71 ± 9 | 76 ± 10 | ||
| Somewhat Active | 0.31 | 0.13 | ||
| Control children | 211 ± 23 | 243 ± 43 | ||
| CDMs | 218 ± 13 | 254 ± 25 | ||
| Active | 0.43 | 0.23 | ||
| Control children | 278 ± 20 | 241 ± 31 | ||
| CDMs | 271 ± 24 | 238 ± 34 | ||
| Very Active | 0.21 | 0.56 | ||
| Control children | 198 ± 17 | 200 ± 27 | ||
| CDMs | 193 ± 32 | 195 ± 17 | ||
CDMs, children of diabetic mothers.
According to the Habitual Activity Estimation Scale Childhood Activity Survey.15
Myocardial findings
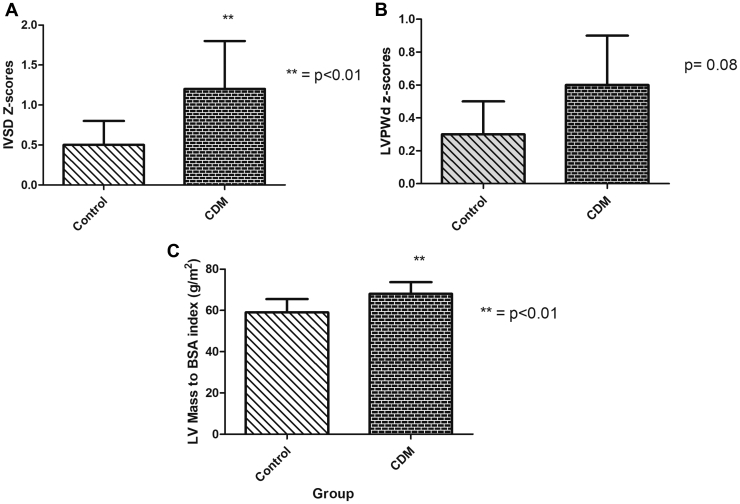
IVSd z score was significantly increased in CDMs compared with controls (P = 0.006; Fig. 1A). One-fifth of the CDMs had an IVSd z score of greater than +2, consistent with hypertrophy. LVPWd z scores tended to be increased in CDMs compared with control children, but no LVPWd z scores in the CDM group were greater than +2 (Fig. 1B). LV mass indexed to BSA was significantly increased in CDMs vs control children (Fig. 1C). LV end diastolic and end systolic dimensions did not differ between the 2 groups.
Figure 1.
Comparisons of interventricular septal and left ventricular (LV) wall thickness and mass between children of diabetic mothers (CDM) and control children: (A) intraventricular septal thickness in diastole (IVSd) z scores (mean ± SD values) for CDM and control children (P < 0.01); (B) left ventricular (LV) posterior wall thickness in diastole (LVPWd) z scores (mean ± SD values) for CDM vs control children (P = 0.08); (C) LV mass z scores (mean ± SD) for CDM vs control children (P < 0.01). BSA, body surface area.
Parameters of LV function including shortening and ejection fraction, strain and strain rate, tissue Doppler index annular velocities, PWV inflow Dopplers, e/e’ ratios, e/a and e’/a’ wave ratios, and isovolumic contraction, relaxation, inflow and ejection times did not differ between the 2 groups. The LV Tei index, which had been mildly increased in late infancy IDMs,14 was similar to that in control children in early childhood. No functional measures correlated with LVPWd or IVSd z scores or measures of LV mass indexed to BSA.
Vascular health assessments
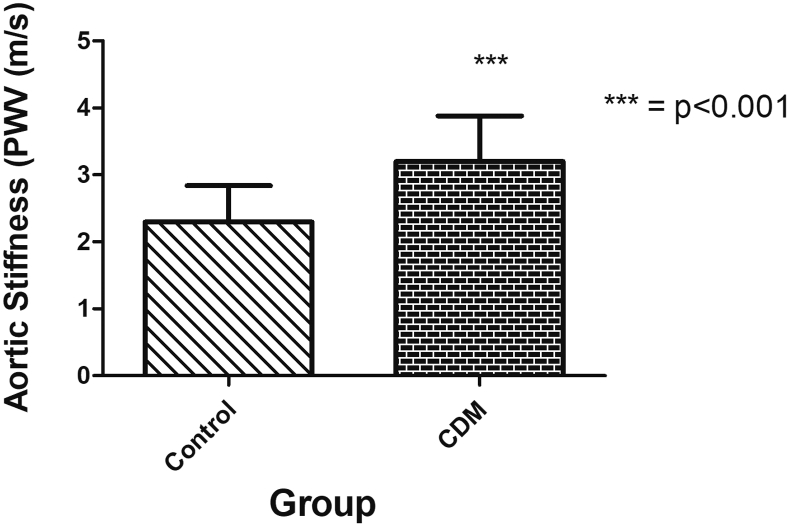
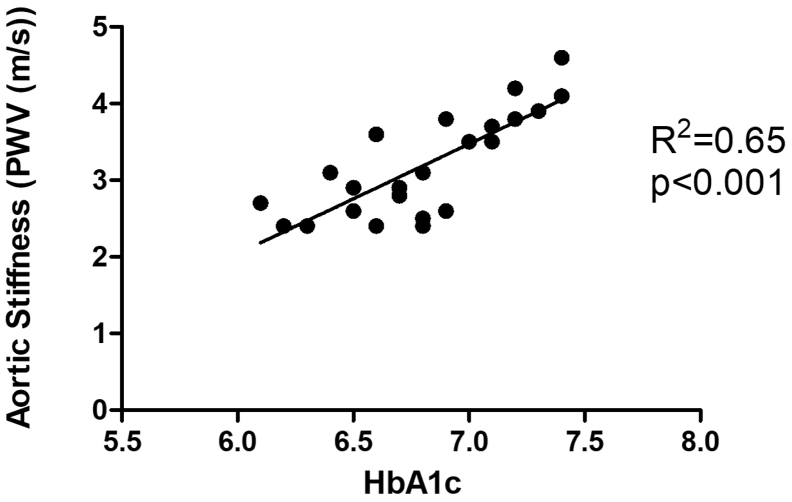
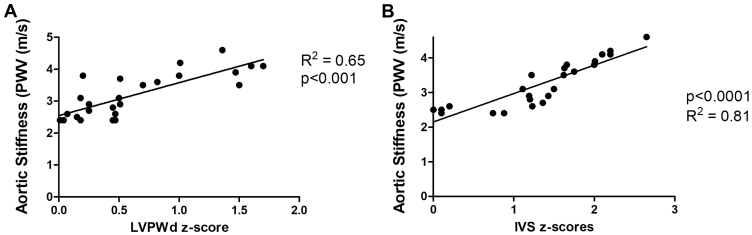
Aortic PWV was significantly increased in CDMs compared with control children (P < 0.001; Fig. 2) and showed a correlation with third-trimester maternal A1c (r2 = 0.65; P < 0.001; Fig. 3). There were strong correlations between aortic PWV and IVSd z scores measured in CDMs (R2 = 0.81; P < 0.001; Fig. 4A). Of particular note, although LVPWd z scores did not differ significantly between the CDMs and control children, there was still a significant positive relationship between LVPWd and aortic PWV in CDMs (R2 = 0.65; P < 0.001; Fig. 4B). There were no differences in the vascular reactivity index between the 2 groups (control 2.4 ± 0.4 vs CDM 2.6 ± 0.7; P = 0.12).
Figure 2.
Aortic stiffness in children of diabetic mothers (CDM) vs control children (mean ± SD values): aortic stiffness was significantly increased in CDMs (P < 0.001). PWV, pulse wave velocity.
Figure 3.
Maternal A1c in the third trimester and aortic stiffness in children of diabetic mothers. Despite a narrow window of relatively good maternal glycemic control, a strong correlation existed between maternal A1c in the third trimester and aortic pulse wave velocity (PWV). Hb, hemoglobin.
Figure 4.
Correlation between left ventricular wall thickness and mass and aortic pulse wave velocity (PWV) in children of diabetic mothers. There was a significant positive relationship between (A) left ventricular posterior wall thickness in diastole (LVPWd) and aortic PWV (P < 0.001) and (B) interventricular septal diastolic thickness (IVSd) and aortic PWV (P < 0.0001).
Longitudinal analysis
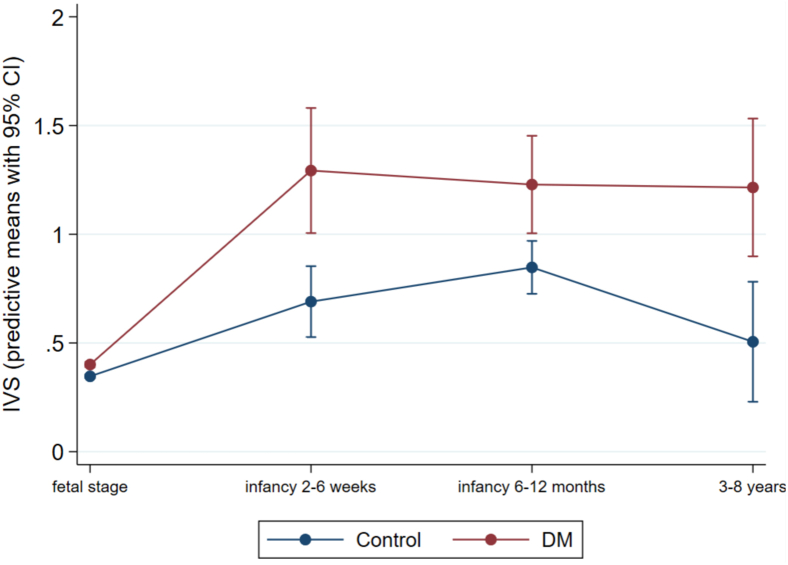
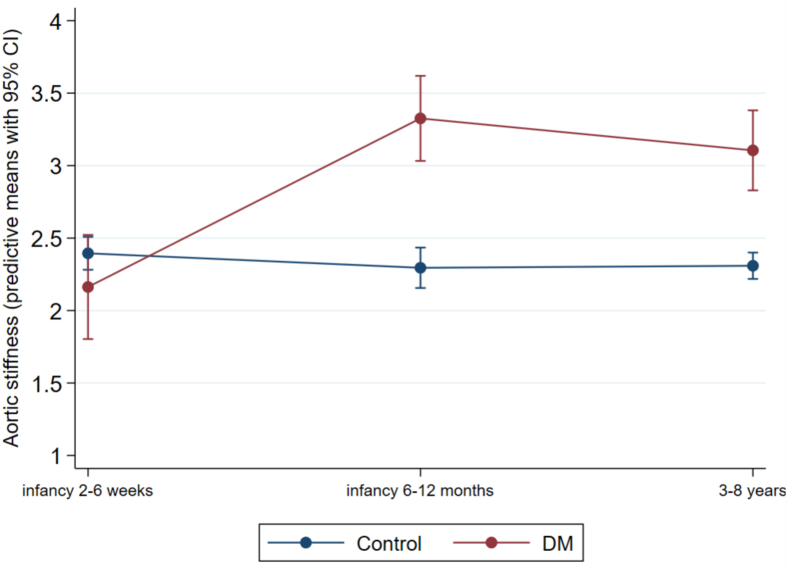
Our statistical methods were done to try to determine if CDMs with greater ventricular thickness had also been those with more hypertrophy in utero, and early and late infancy. Our longitudinal analysis showed that group means for IVSd (which were estimated on all the individuals and their individual changes) between the CDMs and control children were significantly different. IVSd analysis supported a trend that those that had increased thickness in utero, and early and late infancy were also the CDMs that were more likely to have greater ventricular thickness (P < 0.001). Figure 5 illustrates the longitudinal predictive means comparison between the control and CDM group for IVSd. In the diabetes group the individuals who had relatively higher aortic stiffness during early and late infancy are also the individuals who tended to have increased aortic stiffness in our follow-up (P < 0.0001). Figure 6 illustrates the longitudinal predictive means comparison between the control and CDM group for aortic stiffness.
Figure 5.
Longitudinal predictive means between in children of diabetic mothers (CDM) and control children for interventricular septal thickness (IVS) measures. The difference at each of our assessment points is significant. CI, confidence interval.
Figure 6.
Longitudinal predictive means between children of diabetic mothers (CDM) and control children for aortic stiffness measures. The difference at each of our assessment points is significant. CI, confidence interval.
Discussion
Although fetal and neonatal hypertrophic cardiomyopathy are well recognized features of maternal DM in pregnancy, it had previously been believed the myocardial pathology resolves within a few months of birth.11, 12, 13 Our group was the first to report that increased LV thickness in IDMs persists into late infancy.14 Our current continuation of this study provides evidence that the increased LV mass and wall thickness observed in fetuses and IDMs persist even into childhood. We found differences in LV mass not to be associated with significant abnormalities of systolic or diastolic ventricular function. Of particular interest, however, we showed persistence of increased aortic stiffness and a continued strong relationship between aortic stiffness and maternal third-trimester glycemic control. This is particularly interesting because the mothers in our study had relatively good glycemic control on the basis of A1c data. We also observed a persistent relationship between aortic stiffness (PWV) and ventricular septal and LV posterior wall thickness. Those with worse hypertrophy as fetuses were also those with increased ventricular thickness as infants and in childhood, which suggests persistence of the myocardial pathology from fetal stages. Although subclinical, we suspect these findings might be relevant to the increased long-term cardiovascular risks observed in adults of diabetic mothers. It should also be noted that although it might be expected that the offspring of diabetic mothers be macrosomic, our cohort had good glycemic control, which might account for this difference.
Increased LV wall thickness in CDMs
Although some studies have suggested a direct etiologic link between maternal hyperglycemia and fetal cardiac hypertrophy11, 12, 13 this has not been a consistent finding.14,22,23 Other theories as to the etiology of LV hypertrophy have centred on the role of upregulated growth factors including insulin-like growth factor (IGF)-1 and IGF-2 in the placenta, fetal liver, and heart.24, 25, 26 The role of IGF-1, a potent cardiac myocyte mitogen and promoter of myocardial growth,27,28 in particular, has been recently supported by a strong relationship shown between IGF-1 umbilical cord blood levels and myocardial abnormalities in newborn IDMs.27 The persistence of increased LV thickness in early childhood suggests that other factors beyond direct effects of upregulated growth factors before birth contribute to ongoing myocardial changes and potentially gives credence to the concept of longer-term “cardiovascular programming.”
The mechanisms of cardiovascular programming underlying persistent ventricular hypertrophy into childhood are currently unknown. In addition to hyperglycemia and upregulated growth factors leading to myocyte hypertrophy with subsequent failure of remodelling, adverse genetic reprogramming in the cardiac muscle itself, or a secondary response to vascular stiffness are potential mechanisms. The adverse hyperglycemic milieu also includes increased free fatty acids, excessive production of reactive oxygen species, stimulation of connective tissue growth factors, and formation of advanced glycation end-products among others.29 Rodent models have shown myocyte hypertrophy and changes in myocardial extracellular matrix including increased collagens I and III and matrix metalloproteinase activity in adult offspring exposed to a diabetic intrauterine environment, findings that might precede clinical functional changes.30 Because these findings are akin to observations in diabetic cardiomyopathy this could suggest the possibility of similar adverse reprogramming in CDMs.
The coexistence of increased aortic stiffness in CDMs could represent an additional postnatal insult that contributes to persistently increased LV mass, further supported by strong correlations between aortic PWV and LV wall thickness and mass in late infancy and now in early childhood. Peripheral arterial stiffness contributes to increased pulse pressure, greater LV work and remodelling, and is a well established risk factor for the evolution of ventricular hypertrophy independent of blood pressure in adults.31,32 Studies in adult patients have shown that increased arterial PWV contributes to altered LV global longitudinal strain and is associated with subclinical altered LV mechanics33 and predictive of LV mass.34 Although in our study we were not able to show cause and effect, these observations suggest a common insult in diabetic pregnancies that affects myocardial and vascular health that has been underexplored in this population.
Pathogenesis of aortic stiffness in CDMs
The correlation between third-trimester maternal A1c and aortic stiffness in late infancy and childhood provides insight into timing of the insult that might lead to longer-term arterial abnormalities. Poor glycemic control in adults contributes to increased deposition of advanced glycosylated end-products, inflammation, and oxidative stress, which further contributes to functional and structural vascular changes.35,36 More recent investigations have suggested that inflammation is a part of the intrauterine milieu of diabetic pregnancies. Increased umbilical cord plasma levels of C-reactive protein and intracellular adhesion molecule 1, for instance, have been shown in affected pregnancies at term.37 That vascular changes exist even before birth is suggested by the presence of upregulated genes responsible for vascular development in general, and integrity and function of the umbilical cord, and the finding of increased intima-medial wall thickness of the umbilical vein and artery in diabetic pregnancies.38 Interestingly, school-aged CDMs have been shown to have increased circulating markers associated with endothelial dysfunction in adults and often found in those with significant family histories of CVD.39,40 Most of these inflammatory markers have also been implicated in long-term development of metabolic syndrome.41,42 Such changes might be at least in part the consequence of epigenetic alterations.43 Moodley et al.44 interestingly noted that maternal arterial stiffness is increased in pregestational and gestational diabetic mothers and although this did not correlate with the altered fetal cardiovascular assessments, the authors hypothesized that these findings might show a shared response to the metabolic changes in diabetes. Our cohorts were similar in that the mothers had relatively tight glucose control for diabetes patients and their cohort did not include large for gestational age babies or have obvious evidence of placental insufficiency. Maternal DM might therefore contribute to a plethora of direct and indirect mediators of angiogenesis and adverse vascular remodelling in the fetus, which could contribute to aortic wall changes during critical periods of prenatal and postnatal development.
Longer-term, offspring in animal models of maternal DM show increased blood pressure, altered endothelium-dependent vascular dysfunction,45 decreased baroreceptor function, and impaired vascular responsiveness to vasoactive drugs.46 Taken together, this builds a case that a hyperglycemic intrauterine environment contributes to fetal vascular changes that persist and might contribute further to myocardial structural changes. Whether aortic stiffness observed in childhood contributes to the later evolution of vascular dysfunction and myocardial disease observed in adults of diabetic mothers is uncertain but should prompt future investigations into key mediators. Furthermore, how other postnatal lifestyle factors modify risk of longer-term CVD should be further studied.
We also considered how IVSd and aortic stiffness changed over time from early infancy to childhood. We were unable to include our fetal studies in this analysis because z scores in fetal echocardiography are done according to gestational age instead of BSA. Interestingly, our study showed that those with increased IVSd and aortic stiffness compared with the mean for the CDM group generally continued to have more elevated IVSd and further increased aortic stiffness at the childhood study. Factors that contribute to this, whether they are more indicative of the prenatal insult or a reflection of common postnatal changes and behaviours, need to be further studied.
Study limitations
The sample size for CDMs and healthy control children, which depended on a return years after initial participation, was small and therefore might have been underpowered to show more subtle functional differences. Longitudinal changes for individuals was difficult to explore from fetal through postnatal stages because of lack of normative fetal wall thickness data for z score generation and differences in cardiac structural normalization in fetal (according to gestational age) vs the pediatric population (according to BSA). Our vascular health assessments were limited to measurements of aortic stiffness and a gross measure of vascular reactivity. Most of our DM mothers had reasonable glycemic control, thus we were unable to explore the influence of worse glycemic control. Still, that there are findings of increased LV wall thickness that persist from fetal life, and increased aortic stiffness from late infancy that correlates with late gestation glycemic control, suggests even mild increases in blood sugars in combination with other factors of the diabetic intrauterine milieu influence postnatal cardiovascular health.
Conclusion
Young CDMs show persistent ventricular septal thickening, which is strongly related to aortic stiffness, a relationship that persists from late infancy. Increased aortic stiffness is present in CDMs from late infancy into childhood and positively correlates with late gestation maternal glycemic control. Our findings might have relevance to cardiovascular programming that could contribute to or increase the risk of longer-term adult CVD.
Funding Sources
This study was funded by the University of Alberta’s Women’s and Children’s Health Research Institute through donations from the Royal Alexandra Hospital Foundation, the Stollery Children’s Hospital Foundation and supporters of the Lois Hole Hospital for Women. Victor Do was supported by a Summer Student Grant through Alberta Innovates Health Solutions and a Women and Children's Health Research Institute Summer Studentship.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: This study adhered to the WMA Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects guidelines.
See page 351 for disclosure information.
References
- 1.Barker D.J. The fetal and infant origins of adult disease. BMJ. 1990;301:1111. doi: 10.1136/bmj.301.6761.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander B. Fetal programming of hypertension. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1–10. doi: 10.1152/ajpregu.00417.2005. [DOI] [PubMed] [Google Scholar]
- 3.Nijland M.J., Ford S.P., Nathanielsz P.W. Prenatal origins of adult disease. Curr Opin Obstet Gynecol. 2008;20:132–138. doi: 10.1097/GCO.0b013e3282f76753. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization Media Centre Fact Sheet 317, May 2017. July-August 2017. https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) Available at: Accessed February 4, 2021.
- 5.Nyut A.M., Alexander B.T. Developmental programming and hypertension. Curr Opin Neph Hyperten. 2009;18:144–152. doi: 10.1097/MNH.0b013e328326092c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gillman M.W., Rifas-Shiman S., Berkey C.S., Field A.E., Colditz G.A. Maternal gestational diabetes. Birth weight, and adolescent obesity. Pediatrics. 2003;111:e221–e226. doi: 10.1542/peds.111.3.e221. [DOI] [PubMed] [Google Scholar]
- 7.Akcakus M., Koklu E., Naykan A. Macrosomic newborns of diabetic mothers are associated with increased aortic intima-media thickness and lipid concentrations. Horm Res. 2007;67:277–283. doi: 10.1159/000098157. [DOI] [PubMed] [Google Scholar]
- 8.Clausen T.D., Mathiesen E.R., Hansen T. Overweight and the metabolic syndrome in adult offspring of women with diet treated gestational diabetes mellitus or type 1 diabetes. J Clin Endocrinol Metab. 2009;94:2464–2470. doi: 10.1210/jc.2009-0305. [DOI] [PubMed] [Google Scholar]
- 9.Simeoni U., Marker D.J. Offspring of diabetic pregnancy: long-term outcomes. Semin Fetal Neonatal Med. 2009;14:119–124. doi: 10.1016/j.siny.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Hornberger L.K. Maternal diabetes and the fetal heart. Heart. 2006;92:1019–1021. doi: 10.1136/hrt.2005.083840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turan S., Turan O.M., Miller J. Decreased fetal cardiac performance in the first trimester correlates with hyperglycemia in pregestational maternal diabetes. Ultrasound Obstet Gynecol. 2011;38:325–331. doi: 10.1002/uog.9035. [DOI] [PubMed] [Google Scholar]
- 12.Wong S.F., Chan F.Y., Cincotta R.B., McIntyre H.D., Oats J.J. Cardiac function in fetuses of poorly controlled pregestational diabetic pregnancies-a pilot study. Gynecol Obstet Invest. 2003;56:113–116. doi: 10.1159/000073191. [DOI] [PubMed] [Google Scholar]
- 13.Agoudemos M., Reinking B.E., Koppenhafer S.L., Segar J.L., Scholz T.D. Programming of adult cardiovascular disease following exposure to late-gestation hyperglycemia. Neonataolgy. 2011;100:198–205. doi: 10.1159/000324863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Do V., Al-Hashmi H., Ojala T. Cardiovascular health of offspring of diabetic mothers from the fetal through late infancy stages. JACC Cardiovasc Imaging. 2019;12:932–934. doi: 10.1016/j.jcmg.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 15.Hay J.A., Cairney J. Development of the habitual activity estimation scale for clinical research: a systematic approach. Pediatr Exerc Sci. 2006;18:193–202. [Google Scholar]
- 16.Tei C., Ling L.H., Hodge D.O. New index of combined systolic and diastolic myocardial performance: a simple and reproducible measure of cardiac function--a study in normals and dilated cardiomyopathy. J Cardiol. 1995;26:357–366. [PubMed] [Google Scholar]
- 17.Rumman R.K., Slorach C., Hui W. Cardiovascular structure and function in children with middle aortic syndrome and renal artery stenosis. Hypertension. 2017;70:1193–1200. doi: 10.1161/HYPERTENSIONAHA.117.10040. [DOI] [PubMed] [Google Scholar]
- 18.Foster B.J., Mackie A.S., Mitsnefes M. A novel method of expressing left ventricular mass relative to body size in children. Circulation. 2008;117:2769–2775. doi: 10.1161/CIRCULATIONAHA.107.741157. [DOI] [PubMed] [Google Scholar]
- 19.Sandor G.G.S., Hishitani T., Petty R.E. A novel echo Doppler method of measuring the biophysical properties of the aorta in pediatric patients. J Am Soc Echocardiog. 2003;16:745–750. doi: 10.1016/S0894-7317(03)00407-3. [DOI] [PubMed] [Google Scholar]
- 20.Ahmadi N., McQuilkin G.L., Akhtar M.W. Reproducibility and variability of digital thermal monitoring of vascular reactivity. Clin Physiol Funct Imaging. 2011;31:422–428. doi: 10.1111/j.1475-097X.2011.01037.x. [DOI] [PubMed] [Google Scholar]
- 21.Fitzmaurice G.M., Laird N.M., Ware J.H. john wiley & sons; NJ, USA: 2004. Applied Longitudinal Analysis. [Google Scholar]
- 22.Aman J., Hansson Y., Ostlund I., Wall K., Persson B. Increased fat mass and cardiac septal hypertrophy in newborn infants of mothers with well-controlled diabetes during pregnancy. Neonatology. 2011;100:147–154. doi: 10.1159/000323741. [DOI] [PubMed] [Google Scholar]
- 23.Sheehan P.Q., Rowland T.W., Shah B.L., McGravey V.J., Reiter E.O. Maternal diabetic control and hypertrophic cardiomyopathy in infants of diabetic mothers. Clin Pediatr (Phila) 1986;25:266–271. doi: 10.1177/000992288602500507. [DOI] [PubMed] [Google Scholar]
- 24.Han H.J., Kang W.K., Park S.H. Tissue-specific regulation of insulin-like growth factors and insulin-like growth factor binding proteins in male diabetic rates in vivo and in vitro. Clin Exp Pharmacol Physiol. 2006;33:1172–1179. doi: 10.1111/j.1440-1681.2006.04495.x. [DOI] [PubMed] [Google Scholar]
- 25.White V., Jawerbaum A., Mazzucco M.B. Diabetes-associated changes in the fetal insulin/insulin-like growth factor system are organ specific in rats. Pediatr Res. 2015;77:48–55. doi: 10.1038/pr.2014.139. [DOI] [PubMed] [Google Scholar]
- 26.El-Ganzoury M.M., El-Masry S.A., El-Farrash R.A., Anwar M., Ellatife R.Z.A. Infants of diabetic mothers: echocardiographic measurements and cord blood IGF-I and IGFBP-1. Pediatr Diabetes. 2012;13:189–196. doi: 10.1111/j.1399-5448.2011.00811.x. [DOI] [PubMed] [Google Scholar]
- 27.Hiden U., Glitzner E., Hartmann M., Desoye G. Insulin and the IGF system in the human placenta of normal and diabetic pregnancies. J Anat. 2009;215:60–68. doi: 10.1111/j.1469-7580.2008.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindsay R.S., Westgate J.A., Beattie J. Inverse changes in fetal insulin-like maternal diabetes. Clin Endocrinol (Oxf) 2007;66:322–328. doi: 10.1111/j.1365-2265.2006.02719.x. [DOI] [PubMed] [Google Scholar]
- 29.Jia G., Hill M.A., Sower J.R. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res. 2018;122:624–638. doi: 10.1161/CIRCRESAHA.117.311586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Musikant D., Sato H., Capobianco E. Altered FOXO1 activation in the programming of cardiovascular alterations by maternal diabetes. Mol Cell Endocrinol. 2019;479:78–86. doi: 10.1016/j.mce.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Theilade S., Lajer M., Persson F., Joergensen C., Rossing P. Arterial stiffness is associated with cardiovascular, renal, retinal, and autonomic disease in type 1 diabetes. Diabetes Care. 2013;36:715–721. doi: 10.2337/dc12-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watanabe K., Rajarajan A.T., Harima M. Role of differential signaling pathways and oxidative stress in diabetic cardiomyopathy. Curr Cardiol Rev. 2010;6:280–290. doi: 10.2174/157340310793566145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim D., Shim C.Y., Hong G.R., Park S. Differences in left ventricular functional adaptation to arterial stiffness and neurohormonal activation in patients with hypertension: a study with two-dimensional layer-specific speckle tracking echocardiography. Clin Hypertens. 2017;23:21. doi: 10.1186/s40885-017-0078-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J., Chowienczyk P.J., Spector T.D., Jiang B. Relation of arterial stiffness to left ventricular structure and function in healthy women. Cardiovasc Ultrasound. 2018;16:21. doi: 10.1186/s12947-018-0139-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X., Chen C. A new insight of mechanisms, diagnosis and treatment of diabetic cardiomyopathy. Endocrine. 2012;41:398–409. doi: 10.1007/s12020-012-9623-1. [DOI] [PubMed] [Google Scholar]
- 36.Holemans K., Gerber R.T., Meurrens K. Streptozotocin diabetes in the pregnant rat induces cardiovascular dysfunction in adult offspring. Diabetologia. 1999;42:81–89. doi: 10.1007/s001250051117. [DOI] [PubMed] [Google Scholar]
- 37.Koskinen A., Lehtoranta L., Laiho A. Maternal diabetes induces changes in the umbilical cord gene expression. Placenta. 2015;36:767–774. doi: 10.1016/j.placenta.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 38.Sarikabadayi U., Aydemir O., Kanmaz G. Umbilical artery intima-media and wall thickness in infants of diabetic mothers. Neonatology. 2012;102:157–162. doi: 10.1159/000339278. [DOI] [PubMed] [Google Scholar]
- 39.Nelson S.M., Sattar N., Freeman D.J., Walker J.D., Lindsay R.S. Inflammation and endothelial activation is evident at birth in offspring of mothers with type I diabetes. Diabetes. 2007;56:2697–2704. doi: 10.2337/db07-0662. [DOI] [PubMed] [Google Scholar]
- 40.Juonala M., Viikari J.S.A., Ronnemaa T. Childhood C-reactive protein in predicting CRP and carotid intima-media thickness in adulthood: the Cardiovascular Risk in Young Fins Study. Arterioscler Thromb Vasc Biol. 2006;26:1883–1888. doi: 10.1161/01.ATV.0000228818.11968.7a. [DOI] [PubMed] [Google Scholar]
- 41.Wojakowski W., Gminski J. Soluble ICAM-1, VCAM-1 and E-selectin in children from families with high risk of atherosclerosis. Int J Mol Med. 2001;7:181–185. doi: 10.3892/ijmm.7.2.181. [DOI] [PubMed] [Google Scholar]
- 42.Manderson J.G., Mullan B., Patterson C.C. Cardiovascular and metabolic abnormalities in the offspring of diabetic pregnancies. Diabetologia. 2002;45:991–996. doi: 10.1007/s00125-002-0865-y. [DOI] [PubMed] [Google Scholar]
- 43.Hjort L., Martino D., Grunnet L.G. Gestational diabetes and maternal obesity are associated with epigenome-wide methylation changes in children. JCI Insight. 2018;3 doi: 10.1172/jci.insight.122572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moodley S., Arunamata A., Stauffer K.J. Maternal artieral stiffness and fetal cardiovascular physiology in diabetic pregnancy. Ultrasound Obstet Gynecol. 2017 doi: 10.1002/uog.17528. [DOI] [PubMed] [Google Scholar]
- 45.Rueda-Clausen C.F., Morton J.S., Davidge S.T. Effects of hypoxia-induced intrauterine growth restriction on cardiopulmonary structure and function during adulthood. Cardiovasc Res. 2009;81 doi: 10.1093/cvr/cvn341. 713-2. [DOI] [PubMed] [Google Scholar]
- 46.El-Osta A., Brasacchio D., Yao D. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med. 2008;205:2683. doi: 10.1084/jem.20081188. [DOI] [PMC free article] [PubMed] [Google Scholar]