Abstract
Objective
To investigate if routine audiometry in Bell’s palsy patients has prognostic value.
Methods
Retrospective case review was conducted on all Bell’s palsy patients (n=191) seen at the tertiary otolaryngology specialist outpatient clinic from 2015 to 2017. Correlation of ipsilesional audiometric thresholds with patients’ time-to-recovery and initial clinical severity (measured by House-Brackmann (HB) scoring) were used for the prognostic outcome measure. Audiometry results were analyzed using three contiguous frequency pure-tone average (1kHz, 2kHz, 4kHz). Statistical analysis was done via Stata (v13.1), significance tests were 2-sided at 5% significance level.
Results
There was no significant difference between audiometric thresholds between the ipsilesional ear and the contralateral ear (p=0.87). Time-to-recovery was significantly longer for patients with severe initial presentation as compared to mild and moderate severity (p<0.01). There was no correlation found between the audiometry results and HB score at presentation (p=0.39). There was no correlation found between ipsilesional audiometric thresholds and time-to-recovery (p=0.58).
Conclusion
Our study suggests that routine audiometry has limited prognostic value in Bell’s palsy patients.
Keywords: Audiometry, Bell’s palsy, Audit, Facial nerve, Audiology, Prognostic, Outcomes
1. Introduction
Bell’s palsy, a condition described first in 1821 by Sir Charles Bell, is an acute peripheral lower motor neuron facial nerve palsy of idiopathic etiology characterized by the partial or complete paralysis of facial muscles on the affected side. Bell’s palsy is a common, often self-limiting, condition that is often encountered in the Otolaryngology specialist outpatient clinic, with its incidence described between 20 and 30 cases per 100,000 (Marson and Salinas, 2000; Peitersen, 2002, Yanagihara, 1988). In Singapore, routine audiometry is often done for patients with Bell’s palsy to establish any concomitant subclinical hearing loss. However, the clinical utility and the value of performing routine audiometry in Bell’s palsy patients have yet to be evaluated in literature to the authors’ best knowledge.
2. Methods
2.1. Study population
A retrospective review was conducted on all patients with Bell’s palsy (n = 191) seen at our tertiary otolaryngology specialist outpatient clinics from 2015 to 2017. Audiological results and clinical outcomes were extracted from electronic health records using a standardized data collection form. All patients initially diagnosed with Bell’s palsy were identified via ICD-10 and were included in our study.
This study aimed to evaluate if audiometry has prognostic value in patients with Bell’s palsy, by evaluating if audiometric thresholds correlated with time-to-recovery and severity of initial clinical presentation in Bell’s palsy patients.
Audiometry results were analyzed using a three contiguous frequency pure-tone average (PTA) (1 kHz, 2 kHz, 4 kHz). Severity of clinical presentation was based on House-Brackmann (HB) scoring (Baugh et al., 2013). HB score of 1 and 2 were defined as “mild”, 3 and 4 were defined as “moderate”, and 5 and 6 were defined as “severe”. Time-to-recovery was defined as clinically recovering to a “mild” severity as based on HB scoring.
This study was approved by the Institutional Review Board of National Healthcare Group Singapore. Waiver of informed consent was granted for retrospective record review.
2.2. Statistical analysis
Statistical analysis was performed using Stata (v13.1, College Station, TX: StataCorp LP). Significance tests were 2-sided at the 5% significance level.
Comparisons were made using t-test or ANOVA for normally distributed data and Mann-Whitney U test or Kruskal Wallis test for skewed data. Chi-square or Fisher’s exact test were used for categorical data as appropriate. Results are reported as count (n) and percentage by category for categorical data and mean, standard deviation (SD) or median, interquartile range (IQR) for continuous data. Spearman’s correlation was used to assess the relationship between continuous variables and the spearman’s correlation co-efficient is reported.
3. Results
The mean age of the series was 50.6 (SD 17) years. There was approximately equal gender distribution, with 52.4% being males. Commonest co-morbidities were hypertension (36.1%), hyperlipidemia (30.4%), and diabetes mellitus (21.5%).
The left (51.3%) and right side (48.7%) were equally involved with no cases of bilateral Bell’s palsy or herpes zoster oticus in the study. Other than facial paresis, majority of patients did not have any other symptoms (88.5%). Hyperacusis (8.9%) was reported in a small number of patients, followed by subjective hearing loss (2.6%). In terms of clinical severity, most Bell’s palsy patients presented with moderate severity (64.9%), followed by severe (25.6%), and mild (9.42%) severities. Audiometry was performed in a majority of the patients (91.1%) in our series. The median (IQR) time-to-recovery was 64 (102) days.
There was no significant difference between audiometric thresholds between the ipsilesional ear and the contralateral ear (p = 0.87). The mean (SD) PTA for ipsilesional-side was 28.13 (16.5) dB and for the contralateral-side was 27.85 (16.1) dB.
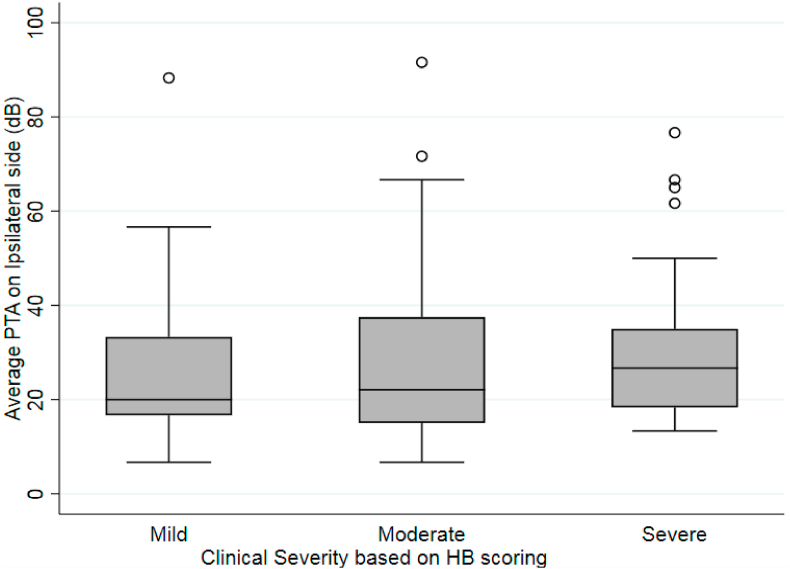
There was no significant association between ipsilesional audiometric thresholds and the severity of initial clinical presentation on House-Brackmann score (Fig. 1). The median (IQR) PTA did not differ significantly when comparing mild (20.0 dB; IQR 16.6 dB), moderate (22.1 dB; IQR 22.5 dB), and severe (26.7 dB; IQR 16.7 dB) groups (p = 0.393).
Fig. 1.
Comparing ipsilesional pure-tone average (PTA) and severity of initial clinical presentation on House-Brackmann Score (p = 0.393).
Time-to-recovery was found to be significantly longer for severe group as compared to mild and moderate severity groups based on House-Brackmann scoring (p = 0.001) (Table 1).
Table 1.
Correlation between initial clinical severity and time-to-recovery (p = 0.001).
| Severity of Clinical Presentation |
|||
|---|---|---|---|
| Mild (n = 13) | Moderate (n = 71) | Severe (n = 35) | |
| Time-to-recovery (days), median (IQR) | 42 (67) | 40 (99) | 110 (186) |
Kruskal-Wallis test, p = 0.001.
There was no significant correlation between time-to-recovery and ipsilesional audiometric thresholds (Spearman’s correlation co-efficient = 0.056) (p = 0.584) (Fig. 2). Of the Bell’s palsy patients with continued follow-up (n = 119), 97 patients (81.5%) were eventually able to recover to mild severity with a median (IQR) time-to-recovery of 64 (102) days.
Fig. 2.
Correlation of ipsilesional audiometric thresholds to time-to-recovery.
4. Discussion
This study attempts to examine the value of audiometry in patients clinically diagnosed with Bell’s palsy, by evaluating if audiometric results in Bell’s palsy patients correlated with time-to-recovery or the severity of initial Bell’s palsy presentation.
Our study suggests audiometry to have limited prognostic value in Bell’s palsy patients. There was no significant correlation between audiometric results and time-to-recovery. This is supported by Chung et al. (2015), who found no association between ipsilateral pure-tone audiometry thresholds and the rate of recovery from Bell’s palsy (Chung et al., 2015). Our study also found that there was no association between audiometry results and initial clinical severity of Bell’s palsy based on House-Brackmann scoring, which is a known prognostic factor in Bell’s palsy patients (Chung et al., 2015; Hydean and Sandstedt, 1982). In our study, time-to-recovery was found to be significantly longer for Bell’s palsy patients with severe initial clinical presentation as compared to mild and moderate severities based on House-Brackmann scoring, which supports the prognostic value of initial severity of Bell’s palsy based on House-Brackmann scoring.
Bell’s palsy is an idiopathic dysfunction of the facial nerve (cranial nerve VII), which manifests with acute lower motor neuron facial nerve palsy. Hearing loss is not expected in Bell’s palsy patients, who have a peripheral facial nerve palsy. Our study supports that hearing is not significantly affected in Bell’s patients, with no difference in audiometric thresholds found between the ipsilesional ear and the contralateral ear. This finding is corroborated by Kopala and Kukwa (2017), who similarly found no difference between the affected and healthy sides in terms of the average hearing threshold for peripheral facial nerve palsy patients (Kopala and Kukwa, 2017).
Routine audiometry for Bell’s palsy patients is sometimes practiced in various centers to detect any subclinical hearing loss. Although the intention is to detect any retrocochlear pathology affecting both facial and vestibulocochlear nerves, such lesions, if present, are unlikely to only have lower motor neuron facial nerve palsy as its sole presentation. For example, in vestibular schwannoma, the most common presentation is unilateral sensorineural hearing loss and tinnitus (Wexler et al., 1990; Wiegand et al., 1996; Neely and Neblett, 1983), which can often be found on clinical examination. Other possible pathologies for example intra-canalicular lesions, such as facial nerve schwannoma (Jacob et al., 2012; Park et al., 2007), meningiomas (Hilton et al., 2002), and arachnoid cysts (Nedzelski and Tator, 1982) have been reported in literature as exceedingly rare entities to cause lesions of both the vestibulocochlear nerve and facial nerve. Almost all of these conditions presented with other symptoms such as hearing loss and tinnitus in addition to facial paresis (Asaoka et al., 2002). The authors would not suggest audiometry as a routine investigation for Bell’s palsy patients, although further studies are required to evaluate if routine audiometry led to detection of retrocochlear pathologies by way of subclinical sensorineural hearing loss on audiometry. However, audiometry should still be performed in Bell’s palsy patients complaining of subjective hearing loss.
Though this study is limited by its retrospective design, the strength of this study is that this is the first study to the authors’ knowledge that critically examines the value of audiometry in the Bell’s palsy patient.
5. Conclusion
Our study suggests that routine audiometry has limited prognostic value in Bell’s palsy patients.
Declaration of competing interest
No funding or conflicts of interests to declare.
Footnotes
Peer review under responsibility of PLA General Hospital Department of Otolaryngology Head and Neck Surgery.
References
- Asaoka K., Barrs D.M., Sampson J.H. Intracanalicular meningioma mimicking vestibular schwannoma. AJNR Am J Neuroradiol. 2002;23:1493–1496. [PMC free article] [PubMed] [Google Scholar]
- Baugh R., Basura G., Ishii L. Clinical practice guideline. Otolaryngol. Head Neck Surg. (Tokyo) 2013;149(Suppl. l):S1–S27. doi: 10.1177/0194599813505967. [DOI] [PubMed] [Google Scholar]
- Chung J., Lee S., Kim S., Yeo S., Park M., Byun J. Neurotological parameters and prognosis of Bell’s palsy patients. Audiol. Neurotol. 2015;20(2):117–121. doi: 10.1159/000369609. [DOI] [PubMed] [Google Scholar]
- Hilton M.P., Kaplan D.M., Ang L. Facial nerve paralysis and meningioma of the internal auditory canal. J. Laryngol. Otol. 2002;116:132–134. doi: 10.1258/0022215021909863. [DOI] [PubMed] [Google Scholar]
- Hydean D., Sandstedt P. Prognosis in Bell’s palsy based on symptoms, signs and laboratory data. Acta Otolaryngol. 1982;93(1–6):407–414. doi: 10.3109/00016488209130898. [DOI] [PubMed] [Google Scholar]
- Jacob J.T., Driscoll C.L., Link M.J. Facial nerve schwannomas of the cerebellopontine angle: the Mayo Clinic experience. J Neurol Surg B Skull Base. 2012;73:230–235. doi: 10.1055/s-0032-1312718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopala W, Kukwa A. Hearing organ assessment using pure-tone audiometry in children and adolescents with peripheral paralysis of the facial nerve. Res. J. Ear Nose Throat. 2017;1:1–4. [Google Scholar]
- Marson A.G., Salinas R. Bell’s palsy. West. J. Med. 2000;173(4):266–268. doi: 10.1136/ewjm.173.4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedzelski J., Tator C. Other cerebellopontine angle (nonacoustic neuroma) tumors. J. Otolaryngol. 1982;11:248–252. [PubMed] [Google Scholar]
- Neely J.G., Neblett C.R. Differential facial nerve function in tumors of the internal auditory meatus. Ann. Otol. Rhinol. Laryngol. 1983;92:39–41. doi: 10.1177/000348948309200109. [DOI] [PubMed] [Google Scholar]
- Park H.Y., Kim S.H., Son E.J. Intracanalicular facial nerve schwannoma. Otol. Neurotol. 2007;28:376–380. doi: 10.1097/01.mao.0000265191.24131.ed. [DOI] [PubMed] [Google Scholar]
- Peitersen E. Bell’s palsy: the spontaneous course of 2,500 peripheral facial nerve palsies of different etiologies. Acta Otolaryngol. 2002;122(6):4–30. doi: 10.1080/000164802320401694. [DOI] [PubMed] [Google Scholar]
- Wexler D., Fetter T., Gantz B. Vestibular schwannoma presenting with sudden facial paralysis. Arch. Otolaryngol. Head Neck Surg. 1990;116(4):483–485. doi: 10.1001/archotol.1990.01870040105024. [DOI] [PubMed] [Google Scholar]
- Wiegand D., Ojemann R., Fickel V. Surgical treatment of acoustic neuroma (vestibular schwannoma) in the United States. Laryngoscope. 1996;106(1):58–66. doi: 10.1097/00005537-199601000-00012. [DOI] [PubMed] [Google Scholar]
- Yanagihara N. Incidence of Bell’s palsy. Ann. Otol. Rhinol. Laryngol. Suppl. 1988;137:3–4. doi: 10.1177/00034894880976s301. [DOI] [PubMed] [Google Scholar]