Abstract
Background
Fulminant viral myocarditis (FVM) is a rare cause of cardiogenic shock associated with high morbidity and mortality rates. An inappropriately activated immune system results in severe myocardial inflammation. Acute immunosuppressive therapy for FVM therefore gained in popularity and was described in numerous retrospective studies.
Methods
We conducted an extensive review of the literature and compared it with our single-centre retrospective review of all cases of FVM from 2009-2019 to evaluate the possible effect of acute immunosuppression with intravenous immunoglobulins and/or high dose corticosteroids in patients with FVM.
Results
We report on 17 patients with a mean age of 46 ± 15 years with a mean left ventricular ejection fraction (LVEF) of 15 ± 9% at admission. Fourteen (82%) of our patients had acute LVEF recovery to ≥ 45% after a mean time from immunosuppression of 74 ± 49 hours (3.1 days). Extracorporeal membrane oxygenation (ECMO) was required in 35% (6/17) of our patients for an average support of 126 ± 37 hours. Overall mortality was 12% (2/17). No patient needed a long-term left ventricular assist device or heart transplant. All surviving patients achieved complete long-term LVEF recovery.
Conclusions
Our cohort of 17 severely ill patients received acute immunosuppressive therapy and showed a rapid LVEF recovery, short duration of ECMO support, and low mortality rate. Our suggested scheme of investigation and treatment is presented. These results bring more cases of successfully treated FVM with immunosuppression and ECMO to the literature, which might stimulate further prospective trials or a registry.
Résumé
Contexte
La myocardite virale fulminante (MVF) est une cause rare de choc cardiogénique, un état associé à des taux élevés de morbidité et de mortalité. L’activation inappropriée du système immunitaire entraîne une inflammation grave du myocarde. Le recours à un traitement immunosuppresseur aigu en cas de MVF a donc gagné en popularité et a fait l’objet de nombreuses études rétrospectives.
Méthodologie
Nous avons effectué une revue exhaustive de la littérature et comparé nos observations avec les résultats de notre examen rétrospectif de tous les cas de MVF traités dans un même centre entre 2009 et 2019, afin d’évaluer l’effet possible d’une immunosuppression aiguë par des immunoglobulines administrées par voie intraveineuse et/ou par une corticothérapie à forte dose chez les patients présentant une MVF.
Résultats
Nous rapportons les cas de 17 patients dont l’âge moyen était de 46 ± 15 ans et qui avaient une fraction d’éjection ventriculaire gauche (FEVG) moyenne de 15 ± 9 % à l’admission. Chez 14 (82 %) d’entre eux, la FEVG aiguë s’est rétablie à une valeur ≥ 45 % dans les 74 ± 49 heures (3,1 jours) en moyenne après l’administration d’un traitement immunosuppresseur. Un soutien par oxygénation extracorporelle par membrane (ECMO) a dû être administré à 35 % (6/17) des patients, pendant 126 ± 37 heures en moyenne. Le taux global de mortalité s’établissait à 12 % (2/17). Aucun patient n’a eu besoin d’assistance ventriculaire gauche de façon prolongée ni d’une transplantation cardiaque. La FEVG a fini par se rétablir complètement chez tous les patients qui ont survécu.
Conclusions
Les 17 patients gravement malades de notre cohorte qui ont reçu un traitement immunosuppresseur aigu ont vu leur FEVG se rétablir rapidement, n’ont eu besoin d’ECMO que pendant une courte période et ont affiché un faible taux de mortalité. Nous présentons notre algorithme d’investigation et de traitement. Nos résultats s’ajoutent à ceux d’autres études témoignant de l’efficacité du traitement de la MVF par immunosuppression et ECMO, ce qui pourrait stimuler la réalisation de nouveaux essais prospectifs ou l’établissement d’un registre.
Myocarditis is an inflammation of the myocardium induced by multiple factors with the most common etiology being viral infection.1, 2, 3, 4, 5, 6 A broad spectrum of clinical presentations exists in acute myocarditis, ranging from mild subclinical to severe life-threatening disease.7 Acute myocarditis with significant hemodynamic compromise requiring pharmacological or mechanical circulatory support (MCS) after the recent onset of symptoms is classified as fulminant myocarditis.7,8
Fulminant viral myocarditis (FVM) is caused by severe lymphocytic myocardium infiltration in the context of a recent viral infection with severe local inflammation, an increase in systemic inflammatory mediators, and myonecrosis. Pathogenesis of acute and chronic viral myocarditis shares a common pathway of inappropriately activated immune system and a certain degree of inflammatory cell infiltrate after an initial viral insult to the myocardial cells.9 The key role of autoimmune response was shown in experimental models.4,10 However, immunosuppression failed to show consistent benefits in large studies on acute or chronic myocarditis but none of them included the fulminant presentation.11, 12, 13, 14, 15
In patients with FVM, mortality rates between 7% and 45% have been reported16, 17, 18, 19, 20 and a need for short-term MCS up to 60%.16,18 Because of the higher morbidity and mortality rates of FVM reported in recent literature compared with previous studies, immunosuppressive therapy gained in popularity and was described in numerous studies of varying methodological robustness.21, 22, 23, 24, 25, 26
Methods
The objective of this study was to evaluate the effect of acute immunosuppression with intravenous immunoglobulins (IVIG) and/or high dose corticosteroids on myocardial recovery, use of MCS, and mortality in patients with FVM at our centre.
A retrospective chart review was carried out for the complete cohort of patients with a diagnosis of acute myocarditis between January 2009 and December 2019 at Institut Universitaire de Cardiologie et Pneumologie de Québec (Quebec Heart and Lungs Institute). Clinical, laboratory, and echocardiography data were reviewed to identify only cases of FVM. The institutional ethical board approved the design of the study.
Definition of FVM
Patients had the classical FVM presentation defined by a viral prodrome of less than 2 weeks and subsequent cardiogenic shock due to acute myocarditis with elevated troponin levels and left ventricular systolic dysfunction with left ventricular ejection fraction (LVEF) < 40%. Cardiogenic shock was defined by significant hypotension or signs of hypoperfusion requiring hemodynamic support.
Inclusion criteria
-
•
Adult patients aged ≥ 18 years.
-
•
Clinical diagnosis of acute myocarditis with ≤ 2 weeks of symptoms.
-
•
Evidence of active inflammation (fever, increased white blood cell count, elevated c-reactive protein, and/or sedimentation time).
-
•
Evidence of myocardial damage: elevated troponin level.
-
•
Hypotension (systolic blood pressure < 90 mm Hg or mean blood pressure < 65 mm Hg).
-
•
Need for hemodynamic support (pharmacological and/or mechanical) justified by objective clinical or laboratory signs of hypoperfusion: persistent oliguria (< 20 mL/h), hyperlactatemia (> 2 mmol/L), acute renal insufficiency (serum creatinine increase > 30 μmol/L or > 1.5 times baseline serum creatinine), hepatic dysfunction (> 3 times the upper limit of normal of transaminase level), altered level of consciousness, or cold extremities.
Exclusion criteria
-
•
Dilated left ventricle defined as a left ventricular end-diastolic dimension using 2-dimensional guided linear measurements > 52 mm in women and > 58 mm in men.27
-
•
Coronary atherosclerotic disease (any history of ischemic heart disease or known coronary obstruction ≥ 50% in at least 1 main artery).
-
•
Giant cell myocarditis or eosinophilic myocarditis.
-
•
Previous cardiomyopathy of any type.
-
•
Postpartum onset < 6 months.
-
•
Systemic inflammatory disease.
Pathology
Histopathological confirmation of lymphocytic myocardial infiltrate was not mandatory for inclusion. In the patients for whom endomyocardial biopsy (EMB) was deemed necessary, revised Dallas criteria were used for histopathologic diagnosis.28,29 Only patients with definite active myocarditis were considered to have a positive biopsy.
Active research of viral etiology using EMB polymerase chain reaction (PCR) analysis is not routine at our institution. However, PCR analysis for influenza and respiratory syncytial viruses was done in all patients. On a case-by-case basis, a more extensive search for specific viral etiology was done by the infectious disease specialist.
Echocardiography
Patients underwent transthoracic echocardiography performed using a GE Vivid E95 (GE Vingmed Ultrasound, Horten, Norway) or Philips iE33 (Philips, Eindhoven, Netherlands) system. Two principal investigators (P.Y.T., M.S.) reviewed and confirmed all measurements according to the American Society of Echocardiography’s guidelines on chamber quantification.27,30 LVEF was determined using Simpson’s biplane method of disks. Two-dimensional guided linear measurement in the parasternal long-axis view was used for diameter and thickness.
Cardiac magnetic resonance
Two different cardiac magnetic resonance (CMR) scanners were used during our study: the Philips Achieva 1.5T (Philips Medical Systems, Best, Netherlands) until the end of September 2016 and Siemens 3T MAGNETOM Skyra (Siemens Medical Solutions USA, Inc, Malvern, PA) since.
Diagnostic CMR criteria for acute myocarditis were the Lake Louise Consensus Criteria defined by the presence of at least 2 of the following criteria: regional or global myocardial signal intensity increase in T2-weighted images, increased global myocardial early gadolinium enhancement ratio between myocardium and skeletal muscle in gadolinium-enhanced T1-weighted images, and nonischemic regional distribution in inversion recovery-prepared gadolinium-enhanced T1-weighted images (late gadolinium enhancement).31 Most recent mapping techniques were not available during the study because of the lack of local validation of these techniques for clinical use during the study period.
Clinical management
Clinical management was at the discretion of the treating physician with the support of advanced heart failure and intensive care specialists. Pharmacological hemodynamic support consisted of noradrenaline, adrenaline, and milrinone. Intra-aortic balloon pump, extracorporeal membrane oxygenation (ECMO), and left ventricular assist device (VAD) were readily available for all patients included in this study. All patients with persisting or deteriorating signs of hypoperfusion, or severe hemodynamic instability despite inotropic support were considered for MCS with ECMO. The usual dose of IVIG is 1 g per kilogram repeated every 24 hours for 3-5 doses. With a corresponding volume of 10 mL per gram given in slow perfusion of 12 hours, it represents a volume of 500-1200 mL per day depending on the patient’s weight. This volume load was proactively considered in the diuretic management to prevent and treat lung edema.
Results
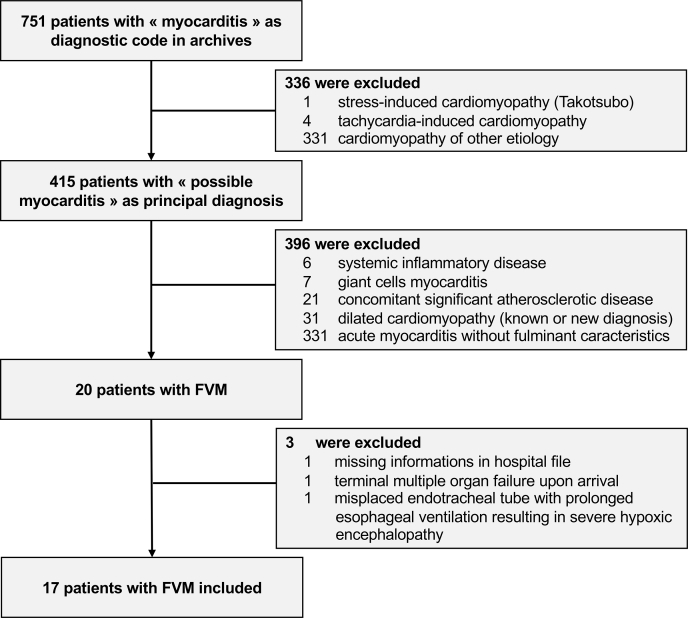
Seventeen patients with FVM were included in our analysis (Fig. 1). We excluded 7 patients with confirmed giant-cell myocarditis and 6 patients with systemic inflammatory disease associated with autoimmune myocarditis.
Figure 1.
Flow chart of patient selection. FVM, fulminant viral myocarditis.
As shown in Table 1, 53% of our cohort were female and the mean age was 46 ± 15 years. The mean duration of the viral prodrome was 5 ± 3 days before FVM diagnosis. Patients had a mean LVEF at admission of 15 ± 9% and a left ventricular end-diastolic diameter of 47 ± 5 mm. All patients had at least mildly increased ventricular wall thickness with a mean of 12 ± 2 mm. Myocarditis diagnosis was confirmed by positive EMB (n = 6) and/or CMR (n = 8) in 12 of 17 patients. All patients who underwent EMB (n = 6) had histopathological criteria for definite active lymphocytic myocarditis. Of the 11 remaining patients without EMB, 5 had identified viral pathogens. Two patients underwent both diagnostic confirmation tests. For the remaining 5 patients without a confirmatory test, 3 had positive PCR for influenza and 2 patients needed ECMO before CMR or EMB could be done. Coronary angiograms were performed in 8 patients and all were normal. All patients required pharmacological hemodynamic support and 41% (7/17) needed MCS. Of these, 6 required ECMO after a mean time from FVM diagnosis of 28 ± 27 hours and for a mean duration of 126 ± 37 hours. Thirteen patients (76%) needed invasive mechanical ventilation for a mean duration of 168 ± 93 hours. Subgroup data associated with treatment and outcomes is presented in Supplemental Table S1.
Table 1.
Clinical characteristics and echocardiographic parameters
| Patient | Sex | Age, years | Identified pathogen | EMB | CMR | Initial LVEF, % | LVEDD, mm | LVESD, mm | IVS thickness, mm | LVPW thickness, mm |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 48 | – | + | - | 20 | 58 | 43 | 11 | 11 |
| 2 | F | 56 | – | + | - | 20 | 45 | 35 | 11 | 13 |
| 3 | M | 44 | – | + | + | 10 | 47 | 35 | 13 | 15 |
| 4 | F | 28 | – | + | + | 35 | 52 | 42 | 12 | 13 |
| 5 | F | 37 | – | + | - | 5 | 49 | 44 | 8 | 10 |
| 6 | M | 31 | Rhinovirus | - | + | 30 | 50 | 40 | 10 | 12 |
| 7 | M | 37 | – | - | + | 20 | 54 | 42 | 13 | 14 |
| 8 | M | 63 | – | - | - | 5 | 46 | 37 | 13 | 12 |
| 9 | F | 72 | Influenza A | - | - | 10 | 46 | 37 | 11 | 10 |
| 10 | F | 70 | – | - | + | 10 | 49 | 35 | 15 | 13 |
| 11 | M | 37 | – | - | + | 25 | 49 | 40 | 12 | 12 |
| 12 | F | 45 | – | - | + | 10 | 45 | 36 | 9 | 11 |
| 13 | F | 60 | Influenza B | - | - | 20 | 37 | 29 | 15 | 15 |
| 14 | M | 58 | WN virus | + | - | 10 | 47 | 43 | 12 | 14 |
| 15 | M | 27 | – | - | + | 15 | 45 | 38 | 12 | 10 |
| 16 | F | 35 | Influenza A | - | - | 5 | 48 | 41 | 10 | 10 |
| 17 | F | 31 | Hantavirus | - | - | 10 | 37 | 33 | 11 | 10 |
| Summary | 9∗ | 46 | 6 | 6 | 8 | 15 | 47 | 38 | 12 | 12 |
| % or ± SD | 53% | ± 15 | 35% | 35% | 47% | ± 9 | ± 5 | ± 4 | ± 2 | ± 2 |
+, indicates patients that underwent the diagnostic exam. -, indicates patients who have not undergone the diagnostic exam.
CMR, cardiac magnetic resonance; EMB, endomyocardial biopsy; IVS, interventricular septum; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic diameter; LVPW, left ventricular posterior wall; WN, West Nile.
Indicates number of female patients.
Immunosuppressive treatment consisted of combination therapy with IVIG and high-dose methylprednisolone in 59% of patients (n = 10), IVIG only in 35% (n = 6), and methylprednisolone only in 6% (n = 1). Treatment with IVIG and methylprednisolone was received after a mean time from onset of the cardiogenic shock of 29 and 22 hours, respectively. The mean cumulative dose for IVIG was 3.1 ± 1.0 g per kilogram and for methylprednisolone was 2955 ± 1150 mg.
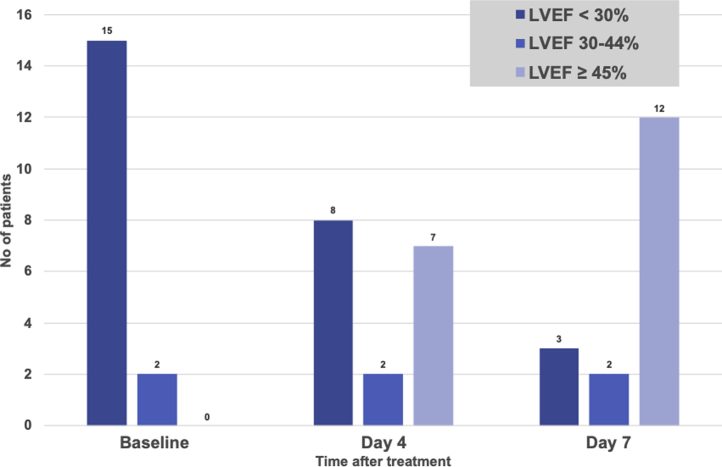
As shown in Table 2, acute recovery as defined by LVEF ≥ 45% was achieved in 82% (14/17) of patients after an average time of 74 hours (3.1 days) after the first dose of immunosuppressive treatment. Five of 6 patients who required ECMO support demonstrated acute LVEF recovery after an average time of 99 hours (4.1 days) after diagnosis compared with 60 hours (2.5 days) for the non-ECMO patients. Figure 2 shows the LVEF evolution in the first week. The overall mean LVEF achieved at 14 days was 50%. All survivors achieved complete recovery between 1 and 10 months.
Table 2.
Hemodynamic support, immunosuppressive treatment, and acute and long-term left ventricular function recovery
| Patient | MV duration, h | IABP duration, h | ECMO duration, h | Time from diagnosis to ECMO, h | Methylprednisolone |
IVIG |
LVEF at admission, % | Time from treatment to LVEF ≥ 45%, h | Maximal LVEF |
Maximum long-term LVEF, % | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total dose, mg | Time, h∗ | Total dose, g/kg | Time, h∗ | At day 7, % | At day 14, % | ||||||||
| 1 | 226 | - | - | - | - | - | 2 | 95 | 20 | 45 | 50 | 50 | 50 |
| 2 | 359 | - | - | - | 2000 | 46 | - | - | 20 | 20 | 45 | 60 | 70 |
| 3 | - | 69 | - | - | 3000 | 34 | 3 | 39 | 10 | 90 | 45 | 65 | 65 |
| 4 | 62 | - | - | - | 3000 | 5 | 2 | 17 | 35 | 7 | 45 | 65 | 65 |
| 5 | 110 | - | - | - | 1500 | 30 | 3 | 8 | 5 | - | 20 | 25 | 50 |
| 6 | 66 | - | - | - | 3000 | 4 | 2 | 6 | 30 | 84 | 45 | 45 | 55 |
| 7 | 110 | - | - | - | - | - | 2 | 13 | 20 | 156 | 50 | 50 | 55 |
| 8 | 240 | - | 155 | 5 | - | - | 3 | 134 | 5 | 47 | 50 | 50 | 70 |
| 9 | - | - | - | - | 3000 | 8 | 3 | 9 | 10 | 16 | 50 | 50 | 60 |
| 10† | 175 | - | 82 | 55 | 4000 | 74 | 4 | 74 | 10 | 108 | 35 | 50 | Death at day 11 |
| 11 | - | - | - | - | 1000 | 14 | 3 | 12 | 25 | 44 | 50 | 50 | 60 |
| 12 | 28 | - | - | - | - | - | 3 | 13 | 10 | - | 20 | 20 | 60 |
| 13 | 168 | - | 75 | 6 | 4000 | 0 | 4 | 2 | 20 | 58 | 55 | 75 | 75 |
| 14† | 216 | - | 140 | 67 | 3000 | 7 | 5 | 7 | 10 | - | 20 | 20 | Death at day 9 |
| 15 | - | - | - | - | - | - | 3 | 3 | 15 | 78 | 55 | 55 | 60 |
| 16 | 265 | - | 148 | 27 | - | - | 2 | 21 | 5 | 149 | 40 | 60 | 60 |
| 17 | 158 | - | 156 | 9 | 5000 | 18 | 5 | 16 | 10 | 133 | 60 | 65 | 65 |
| Mean | 168 | 69 | 126 | 28 | 2955 | 22 | 3,1 | 29 | 15 | 74 | 43 | 50 | 61 |
| ± SD | 93 | N/A | 37 | 27 | 1150 | 23 | 1 | 38 | 9 | 49 | 12 | 16 | 7 |
ECMO, extracorporeal membrane oxygenation; IABP, intra-aortic balloon pump; IVIG, intravenous immunoglobulins; LVEF, left ventricular ejection fraction; MV, mechanical ventilation; N/A, not applicable.
indicates time between the fulminant viral myocarditis diagnosis criteria fulfilment and the specific treatment.
indicates decreased patient.
Figure 2.
Left ventricular ejection fraction (LVEF) evolution in the acute phase.
The mortality rate was 12% (2/17). One patient died of mesenteric ischemia after embolization of an intraventricular thrombus on day 11, 4 days after successful weaning of ECMO, and 2 days after the complete normalization of LVEF. The second patient developed rapid and refractory multiple organ failure despite ECMO support that led to death on day 9 of hospitalization. Atypical West Nile virus infection with very severe cardiac inflammation was identified at autopsy. No patient needed VAD or heart transplant (HTx). All surviving patients showed stabilization or improvement after complete weaning of pharmacological or mechanical support and were discharged from the hospital. This left ventricular function recovery was durable, as shown in a mean follow-up period of 4.1 years.
Adverse effects associated with IVIG were reported in 2 patients. One patient suffered from self-limited aseptic meningitis with headaches that lasted < 48 hours. One patient developed immune hemolysis with direct Coombs test conversion. No adverse effect attributed to high-dose methylprednisolone was identified.
Selected studies that have described outcomes in FVM patients with and without immunosuppressive therapy are presented in Tables 3 and 4.
Table 3.
Descriptive studies that have described morbidity and mortality in patients with fulminant myocarditis
| Reference | Study years | Study type | Fulminant |
Overall mortality |
Overall MCS use |
IABP |
ECMO or short-term VAD |
HTx or long-term VAD |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | |||
| Lieberman, et al.7 | 1983-1988 | Retrospective, single-centre, n = 35 | 4 | 11 | 1 | 25 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| McCarthy et al.32 | 1984-1997 | Retrospective, single-centre, n = 147 | 15 | 10 | 1 | 7 | 2 | 13 | n.s. | n.s. | n.s. | n.s. | 0 | 0 |
| Lee et al.20 | 1990-2004 | Retrospective, single-centre, n = 35 | 11 | 31 | 5 | 45 | 7 | 64 | 5 | 45 | 2 | 18 | 0 | 0 |
| Freixa et al.19 | 2000-2007 | Prospective, single-centre, n = 185 | 15 | 8 | 4 | 27 | 8 | 53 | 3 | 20 | 5 | 33 | 2 | 13 |
| Ammirati et al.18 | 2001-2016 | Retrospective, 2 centres, n = 130∗ | 34 | 26 | 4 | 12 | 21 | 62 | 19 | 56 | 15 | 44 | 1 | 3 |
| Xu et al.17 | 2012-2016 | Retrospective, single-centre, n = 73 | 73 | 100 | 10 | 14 | 20 | 27 | 20 | 27 | 0 | 0 | n.s. | n.s. |
| Summary (available data) | n = 605 | 152 | 25 | 25 | 16 | 58 | 38 | 47 | 31 | 22 | 14 | 3 | 2 | |
ECMO, extracorporeal membrane oxygenation; HTx, heart transplantation; IABP, intra-aortic balloon pump; MCS, mechanical circulatory support; n.s., not significant; VAD, ventricular assist device.
Subgroup of patients with symptoms onset < 2 weeks. Overall mortality represents the mortality at discharge or 30 days.
Table 4.
Studies that have described outcomes in patients with fulminant myocarditis treated with acute immunosuppressive therapy
| Reference | Study years | Study type | Fulminant |
Immunosuppression |
Immunosuppressive regimen | Overall mortality |
MCS |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||||
| Chau et al.56 | 1995-2005 |
Retrospective, single-centre, n = 8 | 7 |
88 | 6 |
86 | Methylprednisolone 10 mg/kg q 24 hours for 3 doses | 0 | 0 | 3 | 43 |
| IVIG 1-2 g/kg divided in 2-3 daily doses | |||||||||||
| 4/7 Steroid only; 2/7 steroid with IVIG | |||||||||||
| Goland et al.55 | 1998-2004 |
Retrospective, single-centre, n = 6 | 6 |
100 | 6 |
100 | IVIG 2 g/kg total dose | 0 | 0 | 0 | 0 |
| 1 g/kg q 24 hours for 2 doses (3/6) | |||||||||||
| 2 g/kg in 24-hour perfusion (2/6) | |||||||||||
| 400 mg/kg q 24 hours for 5 doses (1/6) | |||||||||||
| Manins et al.54 | 2009 |
Retrospective, single-centre, n = 5 | 5 |
100 | 5 |
100 | ATG 10 mg/kg q 24 hours for 2-4 days | 0 | 0 | 3 | 60 |
| Aiming T-lymphocytes CD3 count < 100 × 106/L | |||||||||||
| Yu et al.53 | 2001-2010 |
Retrospective, single-centre, n = 58 | 58 |
100 | 32 |
55 | IVIG 400 mg/kg q 24 hours for 5 doses |
2 | 6 | 10 | 31 |
| Summary | n = 77 | 76 | 99 | 49 | 64 | 2 | 4 | 16 | 33 | ||
Overall mortality represents acute mortality.
ATG, antithymocyte globulins; IVIG, intravenous immunoglobulins; MCS, mechanical circulatory support; q, every.
Discussion
In this cohort, immunosuppressive therapy with IVIG and/or high dose methylprednisolone was associated with rapid LVEF recovery, short duration of ECMO support, and low mortality rate. The results of our retrospective study suggest that acute immunosuppression might influence the natural evolution of FVM compared with the actual published literature, and therefore, may be considered in carefully selected patients with FVM in addition to proactive ECMO support when indicated.
Recent literature clearly supports higher morbidity and mortality rates patients with fulminant myocarditis (FM) than what has been described earlier.16, 17, 18, 19, 20 Knowledge about FM has dramatically increased since Lieberman et al. described the first clinicopathologic classification of myocarditis on the basis of EMB and reported 4 cases of FM with a mortality rate of 25%.7 In 2000, McCarthy et al. suggested that FM has an excellent long-term prognosis of 93% transplantation-free survival at 11 years, compared with acute myocarditis in their retrospective cohort of 147 cases of which only 15 patients met FM criteria.32 Of those 15 patients, only 2 required left VAD. However, the study was subject to a selection bias because patients were included on the basis of their biopsy results. This could have led to the under-representation of the most severe cases of FM that often do not have the time to be confirmed with biopsy. A more recent large study confirmed the poor short-term prognosis of patients with FM with a rate of in-hospital death or heart transplantation of more than 25% compared with none in the nonfulminant myocarditis group.18 Moreover, the risk of long-term left ventricular dysfunction with LVEF < 55% was more than 3 times higher in the FM group (29% vs 9%).18 Therefore, therapies that promote left ventricular recovery and prevent irreversible myocardial damage are the mainstay in the approach to FVM.
Diagnosis confirmation
Because complete imaging with CMR or pathologic evaluations with EMB is often limited in patients with rapidly deteriorating disease that needs advanced and invasive support, FVM is mainly a clinical diagnosis. It is well known that EMB might help guide a specific treatment depending on the histology results, which might have an effect on the outcomes.1 According to guidelines, it is a class I recommendation to perform EMB in all patients that correspond to FVM criteria as defined by new-onset heart failure of < 2 weeks’ duration associated with normal-sized left ventricle and hemodynamic compromise.33 However, because of its limited availability and invasive character, EMB might not be possible to perform in critically ill patients. 28
Using strict clinical criteria, we are confident that we only included patients with FVM. Moreover, we present cases of high-risk FVM that are usually under-represented in the biopsy-proven registries. This is illustrated with a 76% need for mechanical ventilation and a 41% use of MCS in our cohort. Accordingly, only 6 (35%) patients underwent EMB. All patients for whom we managed to identify a typical pathogen or who required prompt use of ECMO were not considered for this invasive technique. EMB for suspected FVM with a typical presentation is not routine because of the precarity of most patients. In accordance with our study, a larger study with severely ill patients requiring ECMO had a rate of EMB of < 30%.34
CMR imaging has an increasingly important role in the diagnostic confirmation of acute myocarditis. The Lake Louise criteria as used in our study shows good diagnostic accuracy of > 80% in a recent meta-analysis.35 Newer advanced techniques, especially parametric mapping techniques combined with late gadolinium enhancement, have shown higher diagnostic accuracy > 90%, which fairly compares with EMB.35,36 Most recent expert recommendations place CMR as a key evaluation tool for patients with suspected myocarditis with the potential to avoid exposing patients to invasive procedures such as EMB. 37
In light of the previous considerations, we suggest that EMB should be done mostly in FVM patients with severe hemodynamic instability, atypical presentation, or to rule out giant-cell myocarditis. However, because a significant proportion of patients will need prompt MCS use, the diagnosis of FVM using strict clinical criteria has an important role. For stabilized patients or after acute recovery, CMR may be used to confirm the diagnosis.
Mortality
Our experience from a high-volume tertiary centre with a reference population of > 2.5 million people is that FVM myocarditis presentation might have an improved prognosis in the contemporary era when treated aggressively with immunosuppression and MCS. Although the patients in our cohort were severely ill, as supported by a higher need for ECMO support, we report a lower mortality rate than the most important descriptive studies presented in Table 3.7,17, 18, 19, 20,32 The 2 patients who died in our study required ECMO. One died of refractory left ventricular dysfunction with severe multiple organ failure. The other patient died of a left ventricular thrombus embolization in the superior mesenteric artery after complete and rapid recovery of left ventricular function. None of our patients needed VAD or HTx.
MCS
ECMO has a main role in the paradigm shift of the treatment approach for FVM. Aggressive hemodynamic support with ECMO leaves time for the reduction of the severe systemic inflammation that directly and reversibly alters myocardial systolic function. The most robust studies that evaluated ECMO in patients with fulminant myocarditis are presented in Supplemental Table S2.34,38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52 Those studies represent the sickest subgroups of fulminant myocarditis patients and suggest that at least a third of patients supported by ECMO will die of the disease.
Ammirati et al. recently concluded that MCS or HTx should be considered early in hemodynamically unstable patients with acute myocarditis and efforts should be made to anticipate its necessity.18 It is now well recognized that most deaths occur early after the presentation, therefore proactive ECMO use is essential in patients with refractory cardiogenic shock as a bridge to recovery. Severe hypotension, systemic hypoperfusion, or refractory arrhythmias are the most frequent consequences that lead to advanced support.
Our results suggest that the prompt use of MCS with ECMO might promote the rapid recovery of LVEF. Rapid ECMO installation (time from diagnosis to ECMO use of 28 hours) in combination with acute immunosuppressive treatment might explain the short mean duration of ECMO use in our cohort (126 hours) vs that in previously published studies (206 hours).
Immunosuppressive treatment
Table 4 shows the main studies on acute immunosuppressive therapy that we selected after an extensive review of the literature and only the studies with clear inclusion criteria for FVM were retained.53, 54, 55, 56 In summary, those studies suggest an estimated average mortality rate of < 5% in patients with fulminant myocarditis treated with aggressive immunosuppressive therapy. Of note, a total of 27 patients who did not receive immunosuppressive treatment were included in 2 of those studies.53,56 Seven patients died in this control group, suggesting a mortality rate of 26%.
Recently, Huang et al. published a meta-analysis, mostly on the basis of pediatric studies, that suggests that IVIG therapy can reduce in-hospital mortality, promote left ventricular function recovery, and increase long-term survival in patients with fulminant myocarditis. 57
More than half of our patients received a high dose of methylprednisolone and all but 1 patient received IVIG within a mean time from hemodynamic instability of 22 and 29 hours, respectively. This reflects a rapid recognition of the disease that resulted in prompt targeted treatment, which could have contributed to the high rate of acute LVEF recovery.
On the basis of the previously cited studies and the results of our case series, early acute immunosuppressive therapy with IVIG and/or high dose methylprednisolone may be considered for selected patients with FVM.
Inflammation and cytokines: the rationale for immunosuppressive treatment
Multiple pathophysiological hypotheses for FVM were described in recent years and the direct effect of cytokines on cardiomyocytes seems to play a major role in the rapid reversibility of the disease.58 Interleukin-1 and tumour necrosis factor α were shown to have a reversible negative inotropic effect, impaired β-adrenergic receptor function, and endothelial dysfunction.22,59,60 The rapid rise and fall of cytokine levels due to an inappropriately activated inflammatory cascade might explain the transient profound systolic dysfunction without left ventricular dilatation or necrosis. This hypothesis stresses the importance of aggressive MCS until recovery and explains why acute immunosuppression therapy might be beneficial in FVM.
Moreover, it is well studied that cytokines contribute to the recruitment of lymphocytes, particularly T cells, and other inflammatory cells in the myocardium that ultimately maintain prolonged inflammation with subsequent risk of myonecrosis and fibrosis.4,22,54,61 A subclass of T cells, T regulatory cells, has beneficial anti-inflammatory properties by negative regulation of the immune response. B lymphocytes have a central role in the inappropriate humoral response to viral infection with the production of autoantibodies that target myocardium.62
Various immunosuppressive regimens were studied in the past decades, and their role in recent-onset idiopathic or inflammatory cardiomyopathy remains highly controversial.12,15,59,63, 64, 65 Of note, those large studies did not evaluate the specific treatment of fulminant myocarditis.
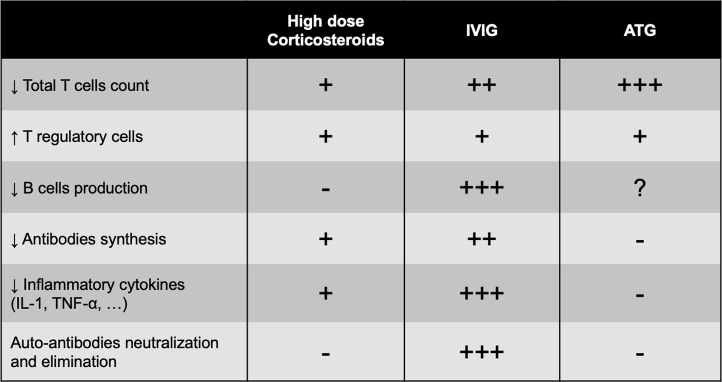
Figure 3 illustrates the mechanisms of action of the most frequently used acute immunosuppressive treatments as described in clinical and fundamental laboratory studies.54,59, 60, 61,66, 67, 68, 69, 70, 71
Figure 3.
Mechanisms of immunosuppressive agents frequently used in fulminant myocarditis. +, mild effect. ++, moderate effect. +++, strong effect. -, no significant effect. ?, unknown effect. ATG, antithymocyte globulin; IL, interleukin; IVIG, intravenous immunoglobulins; TNF, tumour necrosis factor.
Proposed treatment scheme
In light of the previously described considerations, Supplemental Figure S1 represents our suggested treatment and investigation scheme for patients suspected to have FVM. Considering the potential adverse effects of immunosuppression, we strongly recommend that IVIG be given only after the administration of appropriate antibiotics in patients suspected to have concomitant bacterial infection or septic shock. High-dose methylprednisolone can also accentuate serious systemic infections, therefore we recommend that it should not be used in those patients. Our approach is more aggressive than what is suggested by a recent scientific statement on FVM by the American Heart Association, which was mainly on the basis of studies without a clear definition of fulminant myocarditis or inclusion of patients with acute and subacute presentations.72 However, we consider that our study stresses the importance of giving more attention to such a proactive algorithm before concluding that immunosuppressive treatment is ineffective. Nonetheless, we must emphasize that in patients without acute recovery and requiring prolonged inotropic or mechanical support, which is atypical for FVM, EMB should be done to eliminate alternate causes of myocarditis with specific treatments before considering heart transplantation or durable support.
Limitations
Our study presents the usual limitations of a small retrospective single-centre experience. However, a prospective study to evaluate acute immunosuppressive therapy in the setting of FVM would be difficult to realize, mainly because of the very low incidence of the disease and the likely reluctance to use a placebo. Because of the widespread use of immunosuppressive treatment for FVM in our centre in the past decade, we lack a control group that could have allowed us to confirm and quantify the benefits of immunosuppression. Therefore, we cannot draw firm conclusions about the efficacy of the suggested treatment regimen but our results are hypothesis-generating. Finally, the local aggressive weaning strategy could have played a role in the short duration of ECMO.
Conclusions
Our cohort of 17 severely ill patients received acute immunosuppressive therapy and showed a rapid LVEF recovery, short duration of ECMO support, and low mortality rate. Our suggested scheme of investigation and treatment is presented. These results bring more cases of successfully treated FVM with immunosuppression in the literature, which might stimulate further prospective trials or a registry.
Acknowledgements
The authors thank Jean François Turgeon for language editing and proofreading.
Funding Sources
Dr Massot’s fellowship is supported by a research grant from the Fundación Alfonso Martín Escudero (Madrid, Spain).
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: The study protocol was approved by the IUCPQ institutional review board and ethical committees.
See page 300 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2020.10.017.
Supplementary Material
References
- 1.Caforio A.L., Pankuweit S., Arbustini E. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648, 2648a-d. doi: 10.1093/eurheartj/eht210. [DOI] [PubMed] [Google Scholar]
- 2.Magnani J.W., Dec G.W. Myocarditis: current trends in diagnosis and treatment. Circulation. 2006;113:876–890. doi: 10.1161/CIRCULATIONAHA.105.584532. [DOI] [PubMed] [Google Scholar]
- 3.Kindermann I., Barth C., Mahfoud F. Update on myocarditis. J Am Coll Cardiol. 2012;59:779–792. doi: 10.1016/j.jacc.2011.09.074. [DOI] [PubMed] [Google Scholar]
- 4.Feldman A.M., McNamara D. Myocarditis. N Engl J Med. 2000;343:1388–1398. doi: 10.1056/NEJM200011093431908. [DOI] [PubMed] [Google Scholar]
- 5.Ellis C.R., Di Salvo T. Myocarditis: basic and clinical aspects. Cardiol Rev. 2007;15:170–177. doi: 10.1097/CRD.0b013e31806450c4. [DOI] [PubMed] [Google Scholar]
- 6.Comarmond C., Cacoub P. Myocarditis in auto-immune or auto-inflammatory diseases. Autoimmun Rev. 2017;16:811–816. doi: 10.1016/j.autrev.2017.05.021. [DOI] [PubMed] [Google Scholar]
- 7.Lieberman E.B., Hutchins G.M., Herskowitz A., Rose N.R., Baughman K.L. Clinicopathoiogic description of myocarditis. J Am Coll Cardiol. 1991;18:1617–1626. doi: 10.1016/0735-1097(91)90493-s. [DOI] [PubMed] [Google Scholar]
- 8.Ginsberg F., Parrillo J.E. Fulminant myocarditis. Crit Care Clin. 2013;29:465–483. doi: 10.1016/j.ccc.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Liu P., Martino T., Opavsky M.A., Penninger J. Viral myocarditis: balance between viral infection and immune response. Can J Cardiol. 1996;12:935–943. [PubMed] [Google Scholar]
- 10.Nakamura H., Yamamura T., Umemoto S. Autoimmune response in chronic ongoing myocarditis demonstrated by heterotopic cardiac transplantation in mice. Circulation. 1996;94:3348–3354. doi: 10.1161/01.cir.94.12.3348. [DOI] [PubMed] [Google Scholar]
- 11.Stanton C., Mookadam F., Cha S. Greater symptom duration predicts response to immunomodulatory therapy in dilated cardiomyopathy. Int J Cardiol. 2008;128:38–41. doi: 10.1016/j.ijcard.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 12.Frustaci A., Russo M.A., Chimenti C. Randomized study on the efficacy of immunosuppressive therapy in patients with virus-negative inflammatory cardiomyopathy: the TIMIC study. Eur Heart J. 2009;30:1995–2002. doi: 10.1093/eurheartj/ehp249. [DOI] [PubMed] [Google Scholar]
- 13.Mason J.W., O’Connell J.B., Herskowitz A. A clinical trial of immunosuppressive therapy for myocarditis. The Myocarditis Treatment Trial Investigators. N Engl J Med. 1995;333:269–275. doi: 10.1056/NEJM199508033330501. [DOI] [PubMed] [Google Scholar]
- 14.McNamara D.M., Rosenblum W.D., Janosko K.M. Intravenous immune globulin in the therapy of myocarditis and acute cardiomyopathy. Circulation. 1997;95:2476–2478. doi: 10.1161/01.cir.95.11.2476. [DOI] [PubMed] [Google Scholar]
- 15.McNamara D.M., Holubkov R., Starling R.C. Controlled trial of intravenous immune globulin in recent-onset dilated cardiomyopathy. Circulation. 2001;103:2254–2259. doi: 10.1161/01.cir.103.18.2254. [DOI] [PubMed] [Google Scholar]
- 16.Ammirati E., Veronese G., Brambatti M. Fulminant versus acute nonfulminant myocarditis in patients with left ventricular systolic dysfunction. J Am Coll Cardiol. 2019;74:299–311. doi: 10.1016/j.jacc.2019.04.063. [DOI] [PubMed] [Google Scholar]
- 17.Xu M., Jiang T., Zhou Y., Yang X. Influence of echocardiographic measurements and renal impairments on the prognosis of fulminant myocarditis. Medicine (Baltimore) 2018;97 doi: 10.1097/MD.0000000000009812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ammirati E., Cipriani M., Lilliu M. Survival and left ventricular function changes in fulminant versus nonfulminant acute myocarditis. Circulation. 2017;136:529–545. doi: 10.1161/CIRCULATIONAHA.117.026386. [DOI] [PubMed] [Google Scholar]
- 19.Freixa X., Sionis A., Castel A. Low troponin-I levels on admission are associated with worse prognosis in patients with fulminant myocarditis. Transplant Proc. 2009;41:2234–2236. doi: 10.1016/j.transproceed.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 20.Lee C.H., Tsai W.C., Hsu C.H. Predictive factors of a fulminant course in acute myocarditis. Int J Cardiol. 2006;109:142–145. doi: 10.1016/j.ijcard.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Hu H., Ma F., Wei X., Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2021;42:206. doi: 10.1093/eurheartj/ehaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abe S., Okura Y., Hoyano M. Plasma concentrations of cytokines and neurohumoral factors in a case of fulminant myocarditis successfully treated with intravenous immunoglobulin and percutaneous cardiopulmonary support. Circ J. 2004;68:1223–1226. doi: 10.1253/circj.68.1223. [DOI] [PubMed] [Google Scholar]
- 23.Segawa T., Arita Y., Akari T., Hasegawa S. Fulminant myocarditis. BMJ Case Rep. 2018;2018 doi: 10.1136/bcr-2017-223973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakashima H., Umeyama Y., Minami K. Successive immunosuppressive treatment of fulminant myocarditis that is refractory to mechanical circulatory support. Am J Case Rep. 2013;14:116–119. doi: 10.12659/AJCR.889109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kato S., Morimoto S., Hiramitsu S. Successful high-dose intravenous immunoglobulin therapy for a patient with fulminant myocarditis. Heart Vessels. 2007;22:48–51. doi: 10.1007/s00380-006-0923-3. [DOI] [PubMed] [Google Scholar]
- 26.Takeda Y., Yasuda S., Miyazaki S. High-dose immunoglobulin G therapy for fulminant myocarditis. Jpn Circ J. 1998;62:871–872. doi: 10.1253/jcj.62.871. [DOI] [PubMed] [Google Scholar]
- 27.Lang R.M., Badano L.P., Mor-Avi V. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Leone O., Veinot J.P., Angelini A. 2011 Consensus statement on endomyocardial biopsy from the Association for European Cardiovascular Pathology and the Society for Cardiovascular Pathology. Cardiovasc Pathol. 2012;21:245–274. doi: 10.1016/j.carpath.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Aretz H.T., Billingham M.E., Edwards W.D. Myocarditis. A histopathologic definition and classification. Am J Cardiovasc Pathol. 1987;1:3–14. [PubMed] [Google Scholar]
- 30.Lang R.M., Bierig M., Devereux R.B. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Friedrich M.G., Sechtem U., Schulz-Menger J. Cardiovascular magnetic resonance in myocarditis: a JACC white paper. J Am Coll Cardiol. 2009;53:1475–1487. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCarthy R.E., 3rd, Boehmer J.P., Hruban R.H. Long-term outcome of fulminant myocarditis as compared with acute (nonfulminant) myocarditis. N Engl J Med. 2000;342:690–695. doi: 10.1056/NEJM200003093421003. [DOI] [PubMed] [Google Scholar]
- 33.Cooper L.T., Baughman K.L., Feldman A.M. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. J Am Coll Cardiol. 2007;50:1914–1931. doi: 10.1016/j.jacc.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Lorusso R., Centofanti P., Gelsomino S. Venoarterial extracorporeal membrane oxygenation for acute fulminant myocarditis in adult patients: a 5-year multi-institutional experience. Ann Thorac Surg. 2016;101:919–926. doi: 10.1016/j.athoracsur.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 35.Lagan J., Schmitt M., Miller C.A. Clinical applications of multi-parametric CMR in myocarditis and systemic inflammatory diseases. Int J Cardiovasc Imaging. 2018;34:35–54. doi: 10.1007/s10554-017-1063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kotanidis C.P., Bazmpani M.A., Haidich A.B. Diagnostic accuracy of cardiovascular magnetic resonance in acute myocarditis: a systematic review and meta-analysis. JACC Cardiovasc Imaging. 2018;11:1583–1590. doi: 10.1016/j.jcmg.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 37.Ferreira V.M., Schulz-Menger J., Holmvang G. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72:3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 38.Chong S.Z., Fang C.Y., Fang H.Y. Associations with the in-hospital survival following extracorporeal membrane oxygenation in adult acute fulminant myocarditis. J Clin Med. 2018;7:452. doi: 10.3390/jcm7110452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ting M., Wang C.H., Tsao C.I. Heart transplantation under mechanical circulatory support for acute fulminant myocarditis with cardiogenic shock: 10 years’ experience of a single center. Transplant Proc. 2016;48:951–955. doi: 10.1016/j.transproceed.2015.12.109. [DOI] [PubMed] [Google Scholar]
- 40.Diddle J.W., Almodovar M.C., Rajagopal S.K., Rycus P.T., Thiagarajan R.R. Extracorporeal membrane oxygenation for the support of adults with acute myocarditis. Crit Care Med. 2015;43:1016–1025. doi: 10.1097/CCM.0000000000000920. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura T., Ishida K., Taniguchi Y. Prognosis of patients with fulminant myocarditis managed by peripheral venoarterial extracorporeal membranous oxygenation support: a retrospective single-center study. J Intensive Care. 2015;3:5. doi: 10.1186/s40560-014-0069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ishida K., Wada H., Sakakura K. Long-term follow-up on cardiac function following fulminant myocarditis requiring percutaneous extracorporeal cardiopulmonary support. Heart Vessels. 2013;28:86–90. doi: 10.1007/s00380-011-0211-8. [DOI] [PubMed] [Google Scholar]
- 43.Wu M.Y., Lee M.Y., Lin C.C. Resuscitation of non-postcardiotomy cardiogenic shock or cardiac arrest with extracorporeal life support: the role of bridging to intervention. Resuscitation. 2012;83:976–981. doi: 10.1016/j.resuscitation.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 44.Chung S.Y., Sheu J.J., Lin Y.J. Outcome of patients with profound cardiogenic shock after cardiopulmonary resuscitation and prompt extracorporeal membrane oxygenation support. Circ J. 2012;76:1385–1392. doi: 10.1253/circj.cj-11-1015. [DOI] [PubMed] [Google Scholar]
- 45.Mirabel M., Luyt C.E., Leprince P. Outcomes, long-term quality of life, and psychologic assessment of fulminant myocarditis patients rescued by mechanical circulatory support. Crit Care Med. 2011;39:1029–1035. doi: 10.1097/CCM.0b013e31820ead45. [DOI] [PubMed] [Google Scholar]
- 46.Gariboldi V., Grisoli D., Tarmiz A. Mobile extracorporeal membrane oxygenation unit expands cardiac assist surgical programs. Ann Thorac Surg. 2010;90:1548–1552. doi: 10.1016/j.athoracsur.2010.06.091. [DOI] [PubMed] [Google Scholar]
- 47.Chen Y.S., Yu H.Y., Huang S.C. Extracorporeal membrane oxygenation support can extend the duration of cardiopulmonary resuscitation. Crit Care Med. 2008;36:2529–2535. doi: 10.1097/CCM.0b013e318183f491. [DOI] [PubMed] [Google Scholar]
- 48.Asaumi Y., Yasuda S., Morii I. Favourable clinical outcome in patients with cardiogenic shock due to fulminant myocarditis supported by percutaneous extracorporeal membrane oxygenation. Eur Heart J. 2005;26:2185–2192. doi: 10.1093/eurheartj/ehi411. [DOI] [PubMed] [Google Scholar]
- 49.Maejima Y., Yasu T., Kubo N. Long-term prognosis of fulminant myocarditis rescued by percutaneous cardiopulmonary support device. Circ J. 2004;68:829–833. doi: 10.1253/circj.68.829. [DOI] [PubMed] [Google Scholar]
- 50.Aoyama N., Izumi T., Hiramori K. National survey of fulminant myocarditis in Japan: therapeutic guidelines and long-term prognosis of using percutaneous cardiopulmonary support for fulminant myocarditis (special report from a scientific committee) Circ J. 2002;66:133–144. doi: 10.1253/circj.66.133. [DOI] [PubMed] [Google Scholar]
- 51.Kato S., Morimoto S., Hiramitsu S. Use of percutaneous cardiopulmonary support of patients with fulminant myocarditis and cardiogenic shock for improving prognosis. Am J Cardiol. 1999;83:623–625, a610. doi: 10.1016/s0002-9149(98)00931-x. [DOI] [PubMed] [Google Scholar]
- 52.Kawahito K., Murata S., Yasu T. Usefulness of extracorporeal membrane oxygenation for treatment of fulminant myocarditis and circulatory collapse. Am J Cardiol. 1998;82:910–911. doi: 10.1016/s0002-9149(98)00503-7. [DOI] [PubMed] [Google Scholar]
- 53.Yu D.Q., Wang Y., Ma G.Z. Intravenous immunoglobulin in the therapy of adult acute fulminant myocarditis: a retrospective study. Exp Ther Med. 2014;7:97–102. doi: 10.3892/etm.2013.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manins V., Parle N., Dembo L., O’Driscoll G. Anti-thymocyte globulin as an adjunct to treatment of fulminant lymphocytic myocarditis. J Heart Lung Transplant. 2009;28:1211–1214. doi: 10.1016/j.healun.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 55.Goland S., Czer L.S.C., Siegel R.J. Intravenous immunoglobulin treatment for acute fulminant inflammatory cardiomyopathy: series of six patients and review of literature. Can J Cardiol. 2008;24:571–574. doi: 10.1016/s0828-282x(08)70638-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chau E.M., Chow W.H., Chiu C.S., Wang E. Treatment and outcome of biopsy-proven fulminant myocarditis in adults. Int J Cardiol. 2006;110:405–406. doi: 10.1016/j.ijcard.2005.07.082. [DOI] [PubMed] [Google Scholar]
- 57.Huang X., Sun Y., Su G., Li Y., Shuai X. Intravenous immunoglobulin therapy for acute myocarditis in children and adults. Int Heart J. 2019;60:359–365. doi: 10.1536/ihj.18-299. [DOI] [PubMed] [Google Scholar]
- 58.Liu P.P., Mason J.W. Advances in the understanding of myocarditis. Circulation. 2001;104:1076–1082. doi: 10.1161/hc3401.095198. [DOI] [PubMed] [Google Scholar]
- 59.Kishimoto C., Shioji K., Kinoshita M. Treatment of acute inflammatory cardiomyopathy with intravenous immunoglobulin ameliorates left ventricular function associated with suppression of inflammatory cytokines and decreased oxidative stress. Int J Cardiol. 2003;91:173–178. doi: 10.1016/s0167-5273(03)00002-0. [DOI] [PubMed] [Google Scholar]
- 60.Castellano G., Affuso F., Di Conza P., Fazio S. Myocarditis and dilated cardiomyopathy: possible connections and treatments. J Cardiovasc Med (Hagerstown) 2008;9:666–671. doi: 10.2459/JCM.0b013e3282f3e9c2. [DOI] [PubMed] [Google Scholar]
- 61.Kawai C. From myocarditis to cardiomyopathy: mechanisms of inflammation and cell death: learning from the past for the future. Circulation. 1999;99:1091–1100. doi: 10.1161/01.cir.99.8.1091. [DOI] [PubMed] [Google Scholar]
- 62.Kaya Z., Afanasyeva M., Wang Y. Contribution of the innate immune system to autoimmune myocarditis: a role for complement. Nat Immunol. 2001;2:739–745. doi: 10.1038/90686. [DOI] [PubMed] [Google Scholar]
- 63.Mason J.W. Immunopathogenesis and treatment of myocarditis: the United States Myocarditis Treatment Trial. J Card Fail. 1996;2(4 suppl):S173–S177. doi: 10.1016/s1071-9164(96)80074-1. [DOI] [PubMed] [Google Scholar]
- 64.Jones S.R., Herskowitz A., Hutchins G.M., Baughman K.L. Effects of immunosuppressive therapy in biopsy-proved myocarditis and borderline myocarditis on left ventricular function. Am J Cardiol. 1991;68:370–376. doi: 10.1016/0002-9149(91)90834-8. [DOI] [PubMed] [Google Scholar]
- 65.Parrillo J.E., Cunnion R.E., Epstein S.E. A prospective, randomized, controlled trial of prednisone for dilated cardiomyopathy. N Engl J Med. 1989;321:1061–1068. doi: 10.1056/NEJM198910193211601. [DOI] [PubMed] [Google Scholar]
- 66.Chen H.S., Wang W., Wu S.N., Liu J.P. Corticosteroids for viral myocarditis. Cochrane Database Syst Rev. 2013;10:CD004471. doi: 10.1002/14651858.CD004471.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kraaij M.D., van der Kooij S.W., Reinders M.E. Dexamethasone increases ROS production and T cell suppressive capacity by anti-inflammatory macrophages. Mol Immunol. 2011;49:549–557. doi: 10.1016/j.molimm.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 68.Robinson J.L., Hartling L., Crumley E., Vandermeer B., Klassen T.P. A systematic review of intravenous gamma globulin for therapy of acute myocarditis. BMC Cardiovasc Disord. 2005;5:12. doi: 10.1186/1471-2261-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takada H., Kishimoto C., Hiraoka Y. Therapy with immunoglobulin suppresses myocarditis in a murine coxsackievirus B3 model. Antiviral and anti-inflammatory effects. Circulation. 1995;92:1604–1611. doi: 10.1161/01.cir.92.6.1604. [DOI] [PubMed] [Google Scholar]
- 70.Maisch B., Pankuweit S. Standard and etiology-directed evidence-based therapies in myocarditis: state of the art and future perspectives. Heart Fail Rev. 2013;18:761–795. doi: 10.1007/s10741-012-9362-7. [DOI] [PubMed] [Google Scholar]
- 71.Kishimoto C., Abelmann W.H. Monoclonal antibody therapy for prevention of acute coxsackievirus B3 myocarditis in mice. Circulation. 1989;79:1300–1308. doi: 10.1161/01.cir.79.6.1300. [DOI] [PubMed] [Google Scholar]
- 72.Kociol R.D., Cooper L.T., Fang J.C. Recognition and initial management of fulminant myocarditis: a scientific statement from the American Heart Association. Circulation. 2020;141:e69–92. doi: 10.1161/CIR.0000000000000745. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.