Abstract
Surgical anatomy training in a dedicated research laboratory and attendance to focused “hands-on” dissection courses are of high educational importance in order to acquire and maintain surgical expertise in skull base surgery, both for young and more experienced surgeons. Nevertheless, transitioning surgical skills and anatomic knowledge from the laboratory to the operative room it is not free of challenges, especially during skull base approaches where the three-dimensional surgical orientation can be quite complex. We present a “step-by-step” and “side-by-side” surgical anatomy report on a translabyrinthine approach that was practiced in the laboratory then performed in the operative room by the surgical team, and we compare surgical anatomy exposures while discussing intraoperative techniques, nuances and challenges, both in the laboratory and the operative room.
Keywords: Skull base surgery, Translabyrinthine approach, Vestibular schwannomas
Abbreviations: TL, translabyrinthine; OR, operative room; EMG, electromyogram
1. Introduction
The translabyrinthine (TL) approach is a transpetrosal presigmoid surgical route used for the operative treatment of symptomatic or enlarging vestibular schwannomas (and more rarely other tumors involving the cerebello-pontine angle) when non-serviceable preoperative hearing is documented. The concept of developing a surgical route to the internal acoustic canal and posterior fossa through the temporal bone has been evolving for over a century, and the indications, risks and benefits of the TL approach are widely known and accepted in current surgical practice.
Transitioning surgical skills and anatomic knowledge from surgical anatomy textbooks and laboratory training to the operative room is challenging, especially when facing the complex anatomy of lateral approaches to the skull base (Matsushima et al., 2019, Rhoton, 2007). The acquisition of a good understanding of three-dimensional anatomy and surgical orientation within the operative field can be quite complex. Periods of focused “hands-on” training in a dissection laboratory may be needed before a satisfactory level of knowledge with a particular approach can be reached. Fully equipped dissection laboratories may not be available at every teaching institution, therefore we present an illustrative report of the surgical steps performed in a dissection laboratory during a TL approach, adding a “side-by-side” intraoperative documentation of the procedure, with the final goal of facilitating surgeons-in-training understanding of surgical anatomy and related challenges (as if the actual surgery was to be performed in the laboratory). Conversely, more experienced surgeons may find this illustrative presentation with dissection images useful to refresh and maintain their anatomical knowledge, in preparation for a surgical case.
2. Material and methods
Under general anesthesia, the patient is positioned supine with the head rotated toward the contralateral side (Fig. 1). The patient should be safely secured to the operative room (OR) bed, as rotation toward and away from the surgeon is usually needed during the procedure. Comprehensive neuro-monitoring (somatosensory evoked potentials, facial nerve electromyography (EMG), brain stem evoked potentials and lower cranial nerve EMG) is established and monitored throughout the procedure. The ear pinna is gently taped anteriorly to protect neuro-monitoring equipment and facilitate the opening.
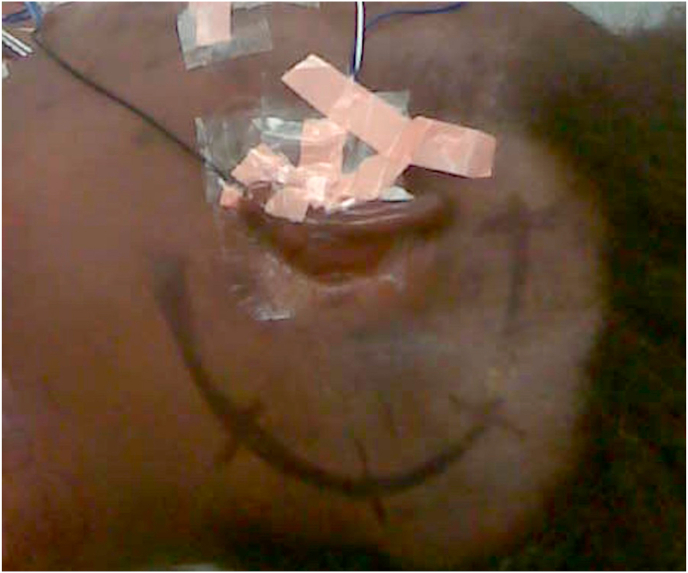
Fig. 1.
Left TL, supine position with head rotated. Skin incision is marked. 1a: (Lab) periosteum is dissected away from the mastoid that is now exposed. Spine of Henle is visualized. 1 b:(OR) mastoid, posterior temporal and suboccipital regions are exposed, periosteum has been dissected away.
Following are the surgical steps that can be practiced “hands-on” in the dissection Laboratory, correlated with intraoperative matching images from an illustrative case.
Cutaneous Opening. A curvilinear retro-auricular incision is favored, as at closure the incision line will lie 1–2 cm away from the mastoidectomy cavity, thus minimizing the risks of postoperative cerebrospina fluid (CSF) leak. When extended exposure of the subtemporal or suboccipital regions is needed, as it is the case during combined approaches or when a partial labyrinthectomy petrous apicectomy is planned, larger incisions are utilized. Once the skin is opened, the cutaneous flap is reflected and secured anteriorly (Fig. 1a and b). The bulk of the posterior part of the temporal muscle can be mobilized anteriorly with the use of monopolar cautery. Particular care is placed not to extend the exposure too anteriorly, as an aggressive dissection toward the spine of Henle could lead to damage to the external auricular canal. The tip of the mastoid and its groove are identified, the digastric muscle is protected, and coagulation deeper to its skull base attachment is avoided (to protect the facial nerve at it emerges from underneath the muscle).
Challenges in the lab: Depending on the age and status of the anatomic specimen, the skin may be difficult to mobilize and not easily pliable. Plan for a larger opening than the one that what will be needed in the OR.
Challenges in the OR: The skin opening should to be tailored depending on the thickness of the subcutaneous pannus. In obese patients, the anterior mobilization of the skin flap may be challenging when a narrow-based incision is carried out. The use of monopolar electrocautery near the region of the external auditory canal should be minimized to avoid the risk of cutaneous, or worse, cartilaginous injury.
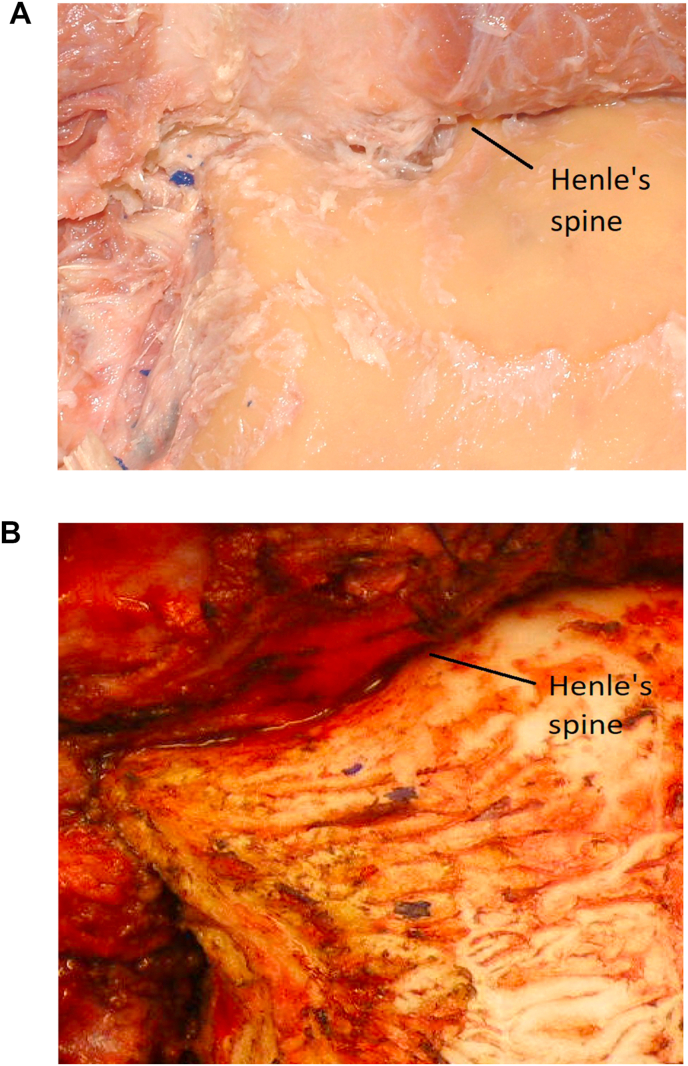
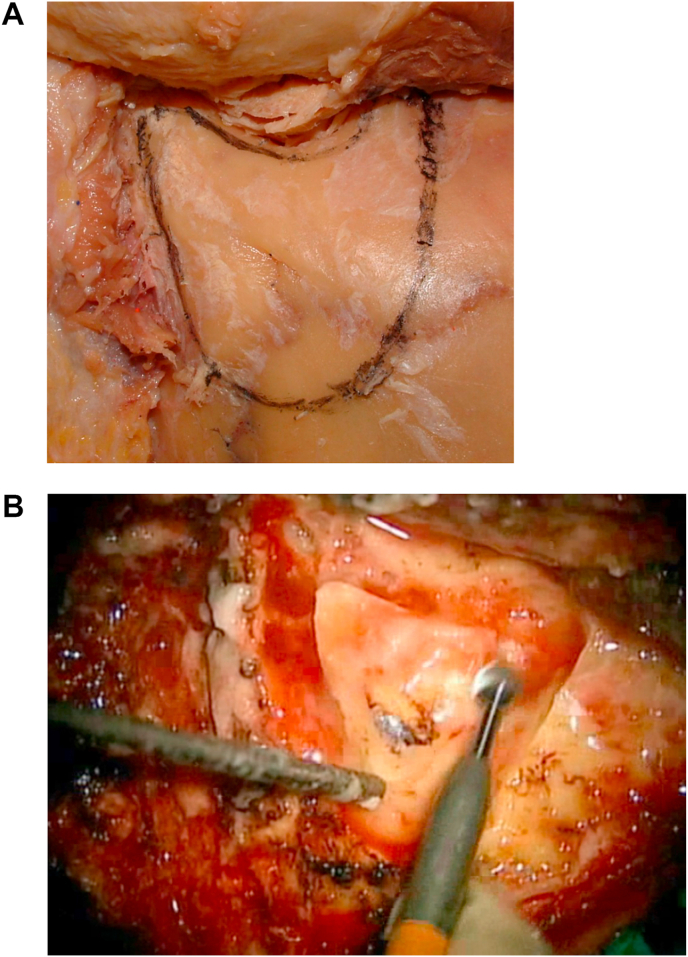
Identify the “Approach Triangle.” At this point, using a cutting round burr under continuous irrigation, a mastoidectomy is started. We like to identify an “approach triangle” defined superiorly by the level of the posterior root of the zygomatic arch (which approximately sets a landmark for the middle fossa floor and, more posteriorly, the level of the transverse sinus), anteriorly by the petrous bone behind the spine of Henle, and posteriorly by the asterion (which approximately sets a landmark for the posterior border of the sigmoid sinus). The posterior border of this “approach triangle” is then extended as needed, as the true location of the sigmoid sinus is progressively identified during the mastoidectomy (Fig. 2a and b).
Challenges in the lab: The periosteum tends to be strictly adherent to the calvarium and its complete removal may be needed to visualize sutures and landmarks, while planning the approach triangle.
Challenges in the OR: The drill may tend to slip over the mastoid if the periosteum is not completely mobilized before the mastoidectomy is started. The copious use of irrigation helps removing the bony dust during the initial steps of the mastoidectomy.
Fig. 2.
2a: (Lab) “Approach Triangle” has been outlined. 2 b:(OR) Mastoidectomy within the “Approach Triangle” is started.
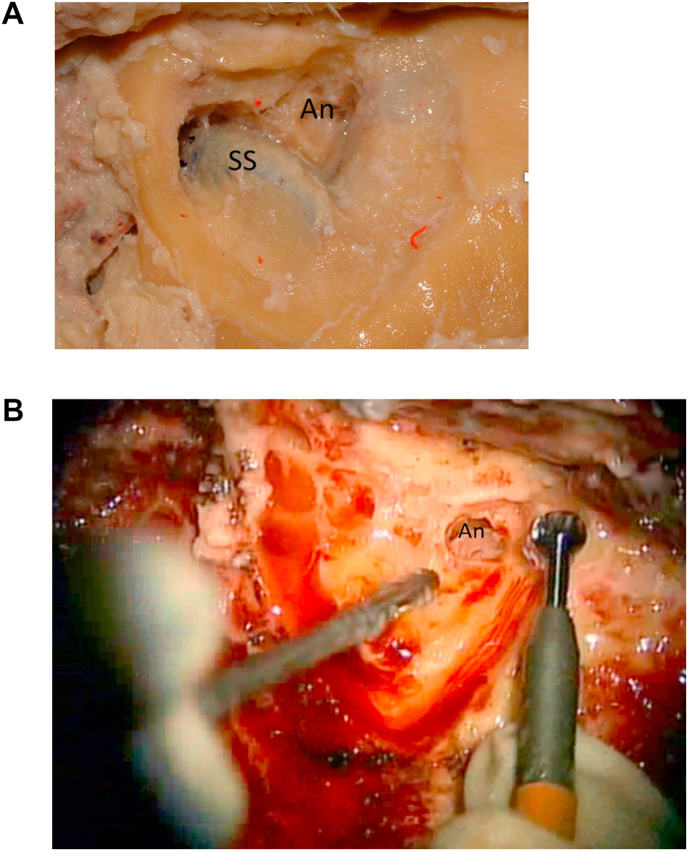
Identify the Antrum. The mastoid cells are carefully opened and the larger cell (antrum) is identified (Fig. 3a and b). A large round cutting burr is used during these initial steps as the risks of inadvertently opening the dura is usually minimized by using larger diameter drill bits.
Fig. 3.
3a: (Lab) Sigmoid sinus (SS) is located and the antrum (An) is opened. 3 b: (OR) The antrum (An) is opened.
Enter the Aditus ad Antrum. Once the mastoid antrum is open, this large cell is unroofed and drilling is continued anteriorly, following the pneumatization of the mastoid toward the middle ear. The antrum leads to the aditus ad antrum which then leads to the middle ear more anteriorly and superiorly. Here, the short process of the incus can be located as it points toward the genu of the facial nerve (tympanic tract). This is the first important landmark used to establish course, depth and direction of the facial nerve as it enters the mastoid (Fig. 4a and b). The incus can be gently palpated to confirm its exposure/localization, as it can be easily mobilized using a Peinfield #4 or a Rhoton micro-instrument. Visualization of the short process of the incus usually requires minimal rotation of the patient away from the surgeon, as it lies laterally within the middle ear cavity. Dislocation and removal of the incus and ossicles are usually carried out later during the closure phase.
Challenges in the lab: Some specimens may not be suitable for a TL approach. The presence of a compact mastoid or a prominent and anteriorly located sigmoid sinus are relative contraindications to this approach. Differently than in a clinical setting, CT scans of the cadaveric specimens are not usually performed, therefore complete pre-dissection planning cannot be achieved in the laboratory.
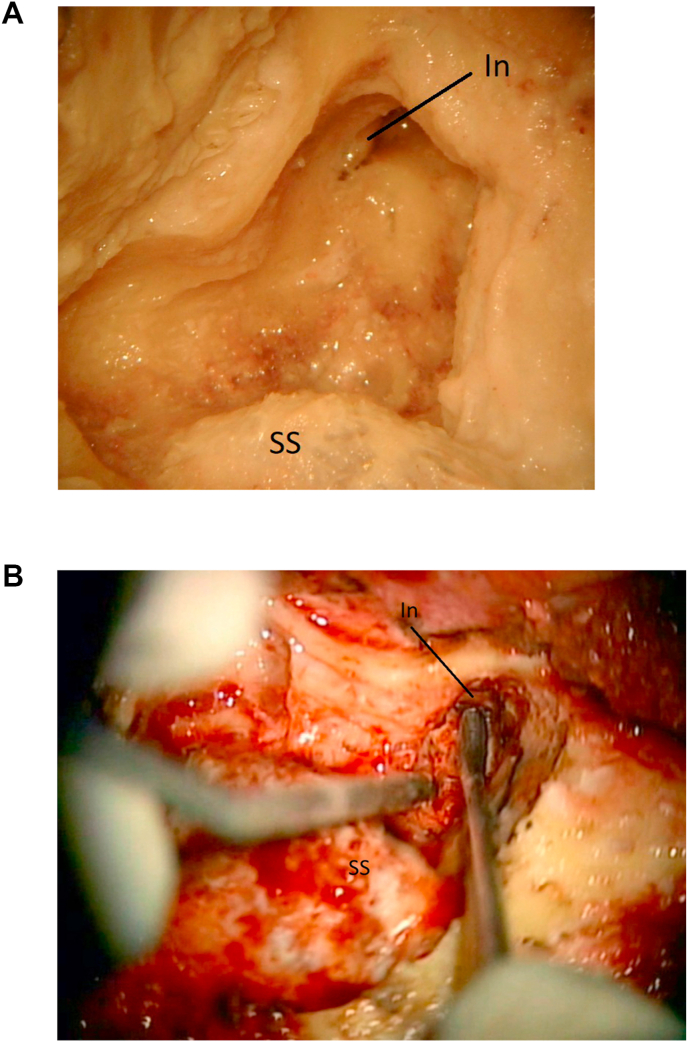
Fig. 4.
4a: (Lab) The short process of the incus (In) is visualized. The sigmoid sinus (SS) is marked. 4 b: (OR) The aditus ad antrum has been opened and the short process of the incus (In) is found. Sigmoid sinus (SS) is marked for orientation.
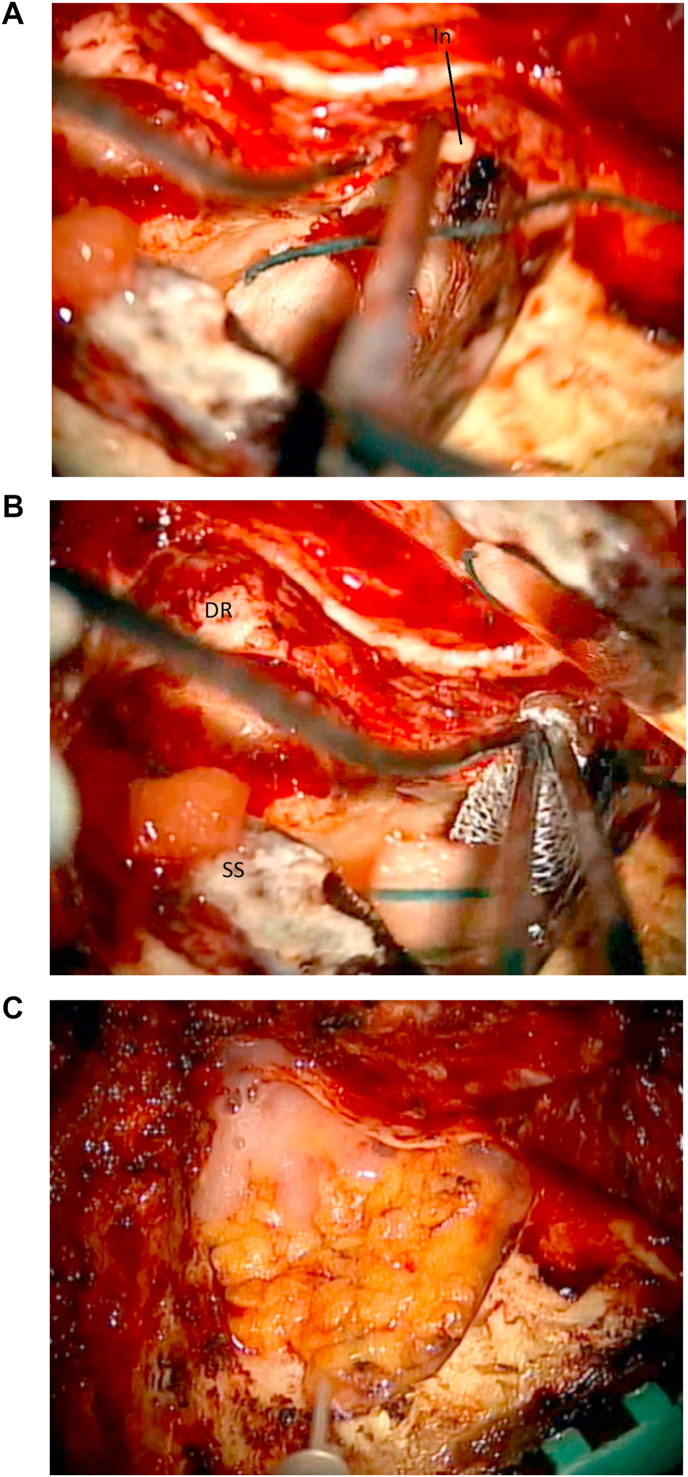
Define the Digastric Ridge. The digastric ridge is a prominence of compact bone found anterior to the sigmoid sinus near the mastoid tip. On the skull base, it corresponds to the digastric muscle groove and approximately defines the point where the facial nerve leaves the mastoid. This is the second important landmark used to establish course, depth and direction of the facial nerve within the mastoid (Fig. 5a and b). The use of a smaller burr may be needed during this step, and particular care is applied not to injury the nearby sigmoid sinus or jugular bulb.
Challenges in the lab: The absence of prominent vascularization and lack of penetration of injected silicone in the small vessels may render the visualization of the facial nerve at the digastric crest challenging. Look for the cortical bony ridge using a properly sized burr.
Challenges in the OR: Depending on the prominence of the sigmoid sinus, the drilling may have to be carried out very near to it. In this case the use of a larger burr can minimize the risk of sharp injury to the venous sinus. Exposure of the facial nerve should be avoided, as it significantly increases the risks of direct and/or secondary injury to the nerve due to its devascularization.
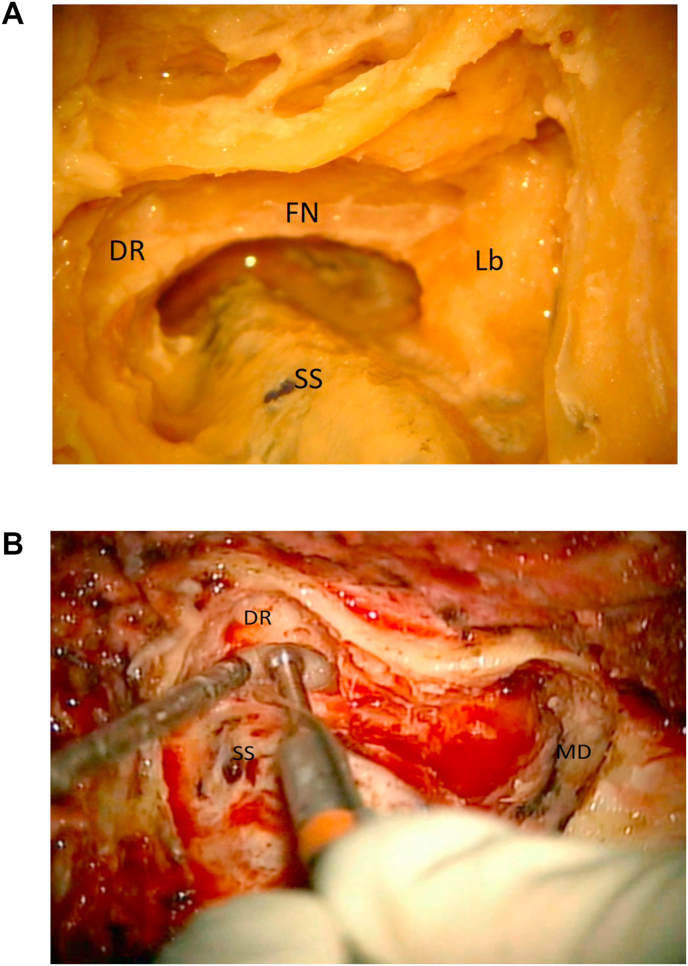
Fig. 5.
5a: (Lab) The digastric ridge (DR) is located. The course of the facial nerve (FN), the sigmoid sinus (SS) and the bony labyrinth (Lb) are marked. 5 b: (OR) The digastric ridge (DR) is located in front of the sigmoid sinus (SS). Middle fossa dura (MD) is exposed.
Expose the Middle Fossa Dura. The middle floor dura is exposed, carefully drilling down the mastoid cells near the superior border of the “approach triangle” (Fig. 6a and b). The dura may be gently coagulated, as its retraction may facilitate bony removal from it and minimize the risks of durotomy during the drilling.
Challenges in the lab and OR: Durotomy at this stage of the dissection/approach is possible. As noted above, dural retraction after gentle coagulation may facilitate its dissection from the surrounding bone. This will also facilitate the subtemporal dissection needed to locate the arcuate eminence (that serves as an inconstant landmark for the superior semicircular canal), when performing the labyrhintectomy.
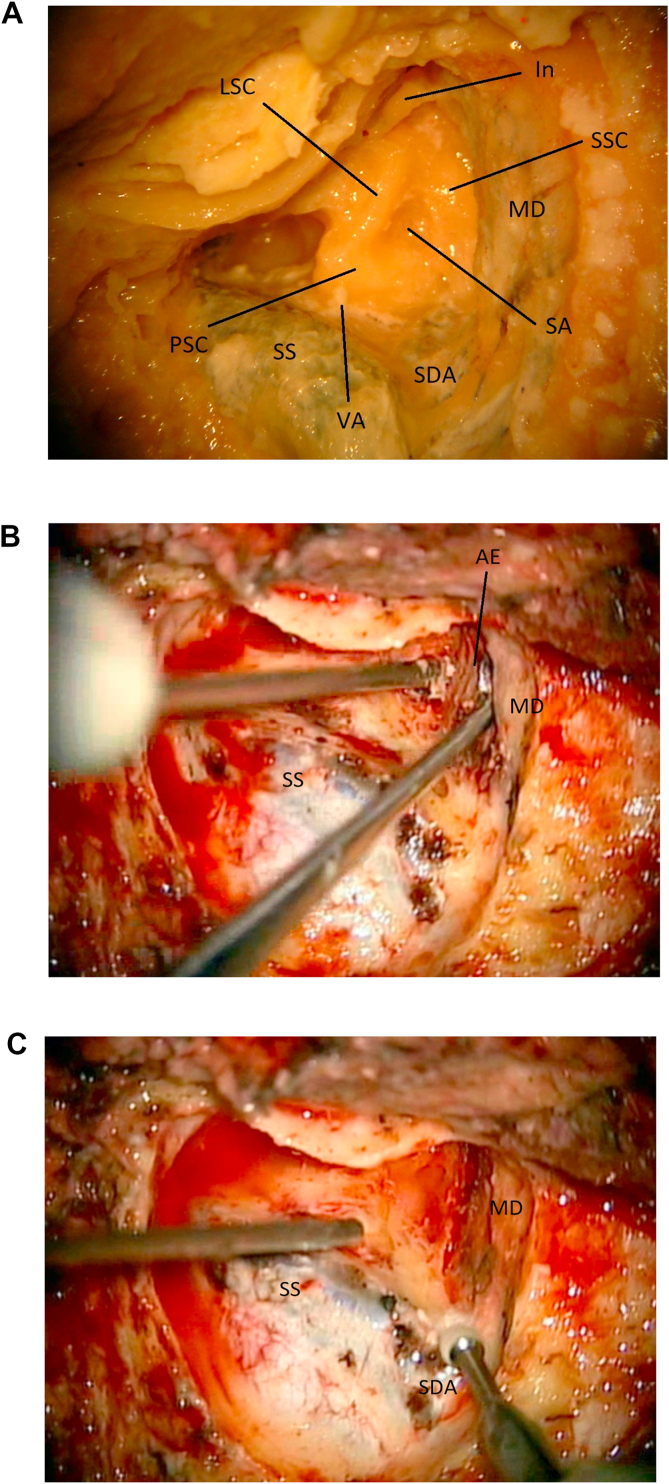
Fig. 6.
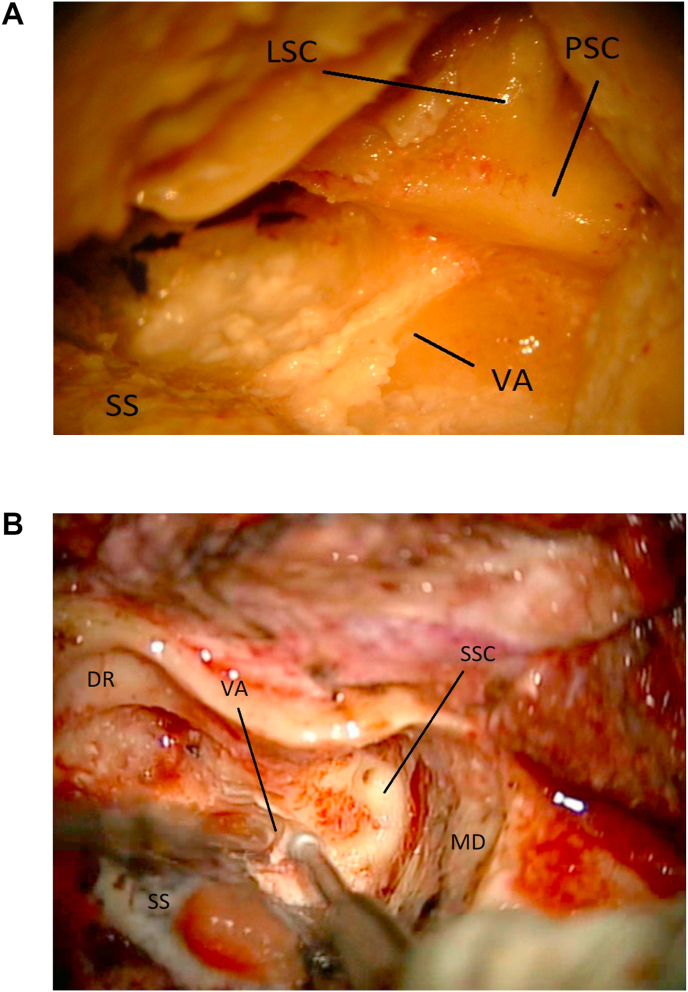
6a: (Lab) Laboratory image of the exposed anatomy once the mastoidectomy is completed. The labyrinth is visualized. Location of the subarcuate artery is shown (SA). Superior semicircular canal (SSC). Lateral semicircular canal (LSC). Posterior semicircular canal (PSC). Sino-dural angle (SDA). Incus (In). Middle fossa dura (MD). Vestibular Aqueduct (VA). Sigmoid sinus (SS). 6 b: (OR). The arcuate eminence (AE) is located by gently elevating the middle fossa dura (MD). Sigmoid sinus (SS). 6c: (OR) The sino-dural angle (SDA) is exposed. Sigmoid sinus (SS). Middle fossa dura (MD).
Define the Sino-Dural Angle, the Sigmoid Sinus and the Jugular Bulb. Once the atrium is located, dissection is carried out posteriorly. The sino-dural angle is defined as the location where the dura of the middle fossa and the pre-sigmoid dura meet the venous angle formed by the sigmoid and transverse sinuses (Fig. 6a and c). The cells overlying the sigmoid sinus are then carefully drilled away (using a coarse diamond drill bit) and the venous sinus is followed down from the transverse sinus to the tip of the mastoid. A thin layer of bone is usually left over the sigmoid sinus for protection at this stage. The presigmoid mastoid cells, deeper to the digastric ridge, are carefully drilled away to expose the region of the jugular bulb (Fig. 7). Depending on the level of involvement of the lower cranial nerves by the pathology at hand, exposure of this region can be enhanced. Generally, when treating lesions confined in the superior portion of the cerebellopontine angle, significant exposure of this region is not needed. In these cases the jugular bulb does not need to be exposed.
Challenges in the lab: Depending on age and status of the anatomical specimen as well as quality of the venous silicone injection, durotomies leading to a loss of the surgical plane during the dissection may be common. In such cases, it may be wise to continue the dissection away from the durotomy until the surgical anatomy at hand has been recognized. Depending on size and preparation, the jugular bulb may be found only partially injected, therefore prone to injury during this step. The use of an appropriate size burr can facilitate its exposure during this step.
Challenges in the OR: Minor venous oozing from the bone surrounding the dural sinus is common and is usually easily controlled with the gentle use of cold saline irrigation. When needed, the use of Gelfoam followed by gentle pressure with a cottonoid is sufficient to stop any continuous or tedious bleeding. Coagulation over the sinus dura is avoided, as it is usually un-effective. In case of large durotomy over the sinus, the use of Avitene is avoided and known maneuvers to decrease the negative venous pressure (and associated risk of venous embolism) are carried out. If needed, the sinus can be repaired directly with the use of temporal muscle fascia following the well-knows microsurgical techniques, although this is, luckily, a rare complication to be encountered.
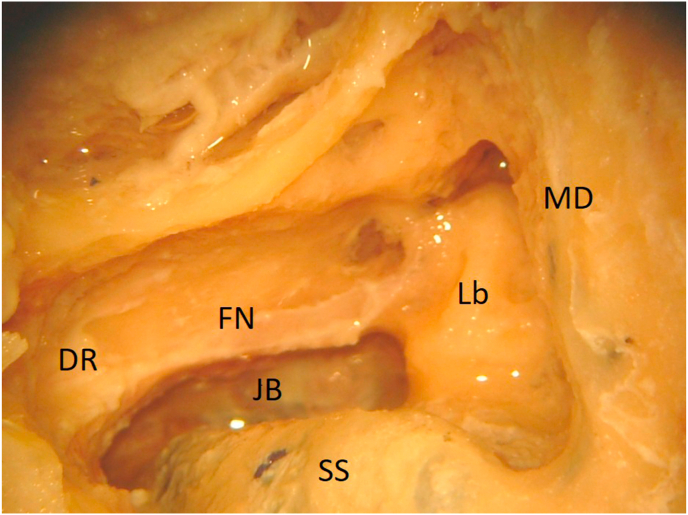
Fig. 7.
(Lab) The jugular bulb (JB) is exposed. Labyrinth (Lb). Middle fossa dura (MD). Sigmoid sinus (SS). Facial nerve (FN). Digastric ridge (DR).
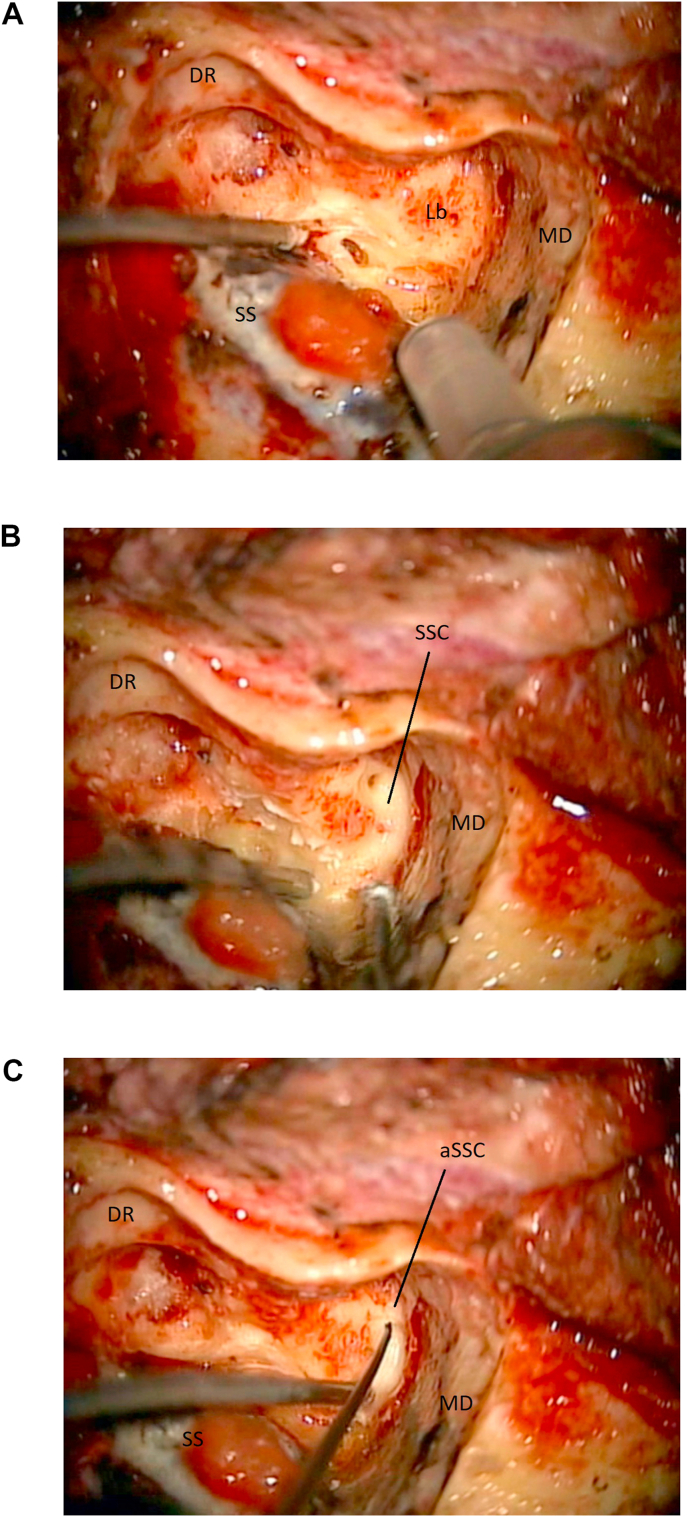
Locate the Bony Labyrinth. Once landmarks to define course and depth of the petrous portion of the facial nerve have been identified, the retro-facial cells can be opened and the bony labyrinth can be exposed (Fig. 6, Fig. 8a). Once the semicircular canals are identified, and their anatomical orientation is confirmed, a labyrinthectomy can be carried out. Selective skeletonization of all the semicircular canals is not needed. The location of the ampullas of the superior and posterior semicircular canals should be noted, as they may serve as landmarks to guide the exposure of the internal auditory canal (IAC). The bony and membranous labyrinths are gently opened and drilled away, keeping portions of the two above-mentioned ampullas in place, to serve as landmarks for the IAC (Fig. 8c).
Challenges in the lab: In light of the compact nature of the bony labyrinth, it is easy for unexperienced surgeons to end up “molding” their own labyrinthine block, thus creating a fictitious anatomy. To avoid this pitfall, once a semicircular canal is located, drilling and exposing the membranous canal within it can lead to a correct anatomical orientation, as the direction of the membranous canal can now be traced and followed during the dissection (Fig. 8a and b). We recommend to start the exposure from the superior semicircular canal, as the arcuate eminence may be located by gently elevating the middle fossa dura, thus adding another surgical landmark to the dissection (the arcuate eminence generally defines the location of the superior semicircular canal) (Fig. 6b).
Challenges in the OR: The subarcuate artery can also be utilized to define the location of the superior semicircular canal (Fig. 6a). A small amount of bleeding from it can be controlled by simply continuing the dissection with a diamond burr or by the use of cold saline irrigation (or minimal coagulation). Where present, exposure of the arcuate eminence within the middle fossa can also be utilized to confirm depth and location of the superior semicircular canal.
Fig. 8.
8a: (OR) The labyrinth (Lb) is located at the end of the mastoidectomy. Middle fossa dura (MD). Sigmoid sinus (SS). Digastric ridge (DR). 8 b: (OR) The labyrinthectomy is carried out. Here the superior semicircular (SSC) and its membranous labyrinth are opened. Middle fossa dura (MD). 8c: (OR) The ampulla of the superior semicircular canal (aSSC) is opened and kept as landmark for the fundus. Middle fossa dura (MD). Sigmoid sinus (SS). Digastric ridge (DR).
Locate and Cut the Vestibular Aqueduct. Once the labyrinth has been partly opened, the posterior fossa dura near the posterior semicircular canal is gently dissected away from it. The vestibular aqueduct is here located as a thin fold of dura diving underneath the posterior semicircular canal. The aqueduct is then cut, so that dural dissection can be continued deeper toward the IAC (Fig. 9a and b).
Fig. 9.
9a: (Lab) The vestibular aqueduct/endolymphatic sac (VA) are marked after a partial labyrinthectomy. Sigmoid sinus (SS). Lateral semicircular canal (LSC). Posterior semicircular canal (PSC). 9 b: (OR) The vestibular aqueduct (VA) is unroofed while completing the labyrinthectomy. Middle fossa dura (MD). Sigmoid sinus (SS). Digastric ridge (DR). Superior semicircular canal (SSC).
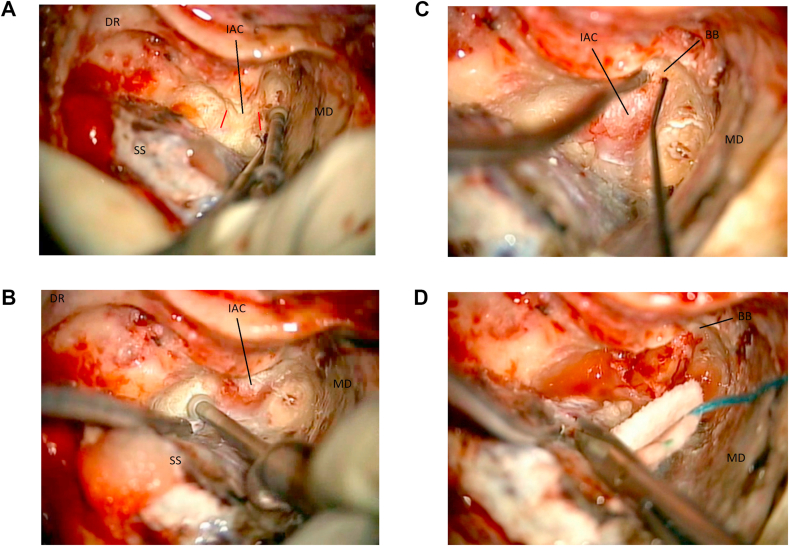
Expose the IAC. Drilling of the bony labyrinth continues on a direction parallel to an imaginary line that connects the ampullas of the superior and posterior semicircular canals (Fig. 10a and b). Drilling in a direction perpendicular to the orientation of the IAC long axis should be avoided. When the labyrinthectomy is completed, any residual shell of bone left over the dura is removed and the IAC is identified.
Challenges in the lab: Depending on age and status of the specimen, the neurovascular bundle within the IAC can be very thin and therefore prone to injury if aggressive drilling is carried out in this region. Dissection and drilling should be carried out keeping parallel to the long axis of the IAC. This minimizes risks of entering the IAC before completing its unroofing.
Challenges in the OR: Depending on the extent of the intracanalicular pathology, the IAC can be more or less enlarged. Direct drilling near the fundus should be avoided. Dural exposure of this region can be carried out with small Kerrison roungeurs, after appropriate direct nerve stimulation is performed.
Fig. 10.
10a: (OR) The dura of the internal acoustic canal (IAC) is skeletonized. The orientation of the canal is outlined in red. Middle fossa dura (MD). Sigmoid sinus (SS). Digastric ridge (DR). 10 b: (OR) The internal acoustic canal (IAC) is exposed. Middle fossa dura (MD). Sigmoid sinus (SS). Digastric ridge (DR). 10c: (OR) lateral exposure of the internal acoustic canal (IAC) with direct stimulation (nerve stimulator is seen in the field). The vertical crest/Bill’s bar (BB) is located. Middle fossa dura (MD). 10 d: (OR) The posterior fossa dura is opened and the end of the TL approach. Middle fossa dura (MD). Bill bar (BB).
Locate the Facial Nerve at the Fundus. At the fundus of the IAC a vertical crest of cortical bone (Bill’s Bar) is separating the superior vestibular nerve (and its superior ampullary branch) from the facial nerve. Intraoperative neuro-monitoring with very low amplitude direct nerve stimulation is utilized to guide the dissection in the OR (Fig. 10c). This is the more challenging step, both in the laboratory and in the OR, as the facial nerve in this location is usually thin and exposed, therefore prone to injury. Alternatively, the drilling may be carried out starting from the infero-posterior wall of the IAC, to locate the inferior vestibular nerve. Here the nerve is separated from the superior vestibular nerve by another crest of cortical bone, the transverse crest. Once the superior vestibular nerve is located, and after the ampulla has been completely removed, its superior ampullary branch can be followed as it leads to the Bill’s bar and the facial nerve.
Challenges in the lab: It is sometimes hard to define Bill’s bar (as neuro-monitoring cannot be utilized). In case of difficulty locating the vertical crest once the IAC has been skeletonized in the laboratory, the dura of the canal can be opened and the vestibular and facial nerves located and followed toward the porus. From here Bill’s bar can be located using the nerves as landmarks (Fig. 11a).
Challenges in the OR: Any direct coagulation near the fundus should be avoided and any bleeding controlled with gentle irrigation or the use of Gelfoam. Very low amplitude direct nerve stimulation is utilized to help locating and confirming the course of the facial nerve.
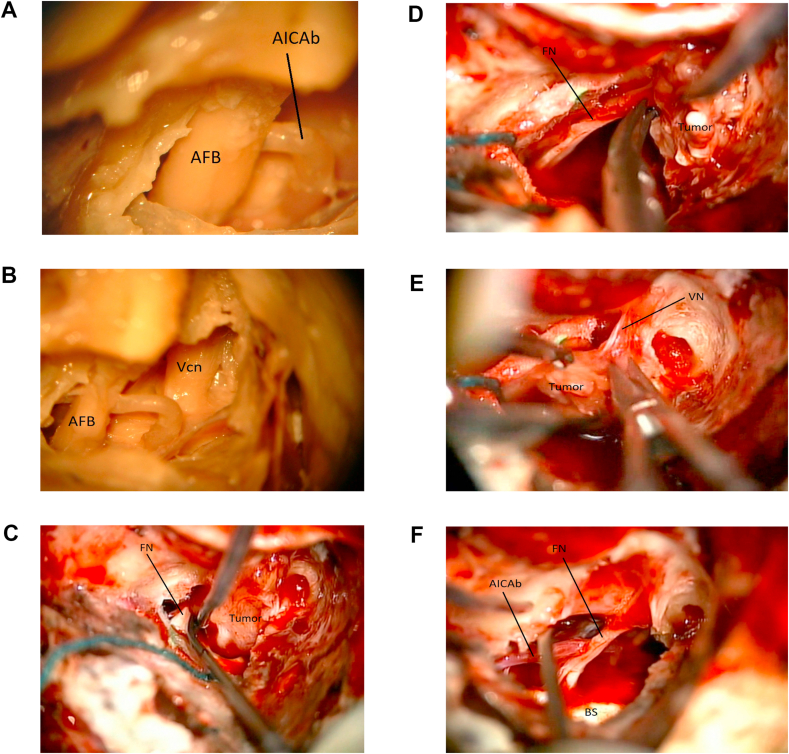
Fig. 11.
11a: (Lab) Dissection images of the acoustic-facial bundle (AFB) within the IAC. A branch of the Anterior inferior cerebellar artery is seen (AICAb). 11 b: (Lab) Dissection image after removal of portion of the middle fossa dura. Acoustic-facial bundle (AFB). Trigeminal nerve (Vcn). 11c: (OR) Intraoperative image. A vestibular schwannoma (Tumor) is being dissected away from the facial nerve (FN) near the brainstem. 11 d: (OR) Intraoperative image. The vestibular schwannoma (Tumor) is being dissected away from the facial nerve (FN) more laterally. 11e: (OR) Intraoperative image. The vestibular schwannoma (Tumor) is being dissected away from the vestibular nerve (VN). 11f: (OR) Intraoperative image. The tumor has been removed. Brainstem (BS). Anterior inferior cerebellar artery branch (AICAb). Facial nerve (FN).
Dural Opening. The dural opening is carried out parallel and in front of the sigmoid sinus, extending it toward the IAC as needed (Fig. 10d). Depending on the pathology at hand, a second incision can be made over the middle fossa dura, so that the superior petrosal sinus can be coagulated and cut. In selected cases (sych as meningiomas or hemangioblastomas) this sinus can be hypertrophic and may be the source of significant bleeding if not properly exposed and ligated/coagulated. Sectioning the tentorium will then allow a supra and infratentorial exposure (Fig. 11b). Once the dura is opened, the tumor can be located and dissected away from the acoustic-facial bundle. In the presented illustrative case of the surgical resection of a left Vestibular Schwannoma, the facial nerve had been previously located at the fundus and its cisternal portion was exposed at the brainstem, following known microsurgical techniques (Fig. 11c and d). Upon confirmation of the lack of response to direct stimulation, the superior vestibular nerve was cut to remove the distal part of the tumor within the IAC (Fig. 11e). Anatomical and functional integrity of the facial nerve was then confirmed by microscopic visualization and direct very low amplitude stimulation (Fig. 11f).
Closure. The ossicles are removed, and the middle ear is gently packed with Surgicel (Fig. 12a and b). This step is of great importance to avoid postoperative CSF leak, as a watertight dural closure is not feasible. Abdominal fat graft is utilized to fill the mastoid cavity and fibrin glue is applied (Fig. 12c). The myocutaneous flap is then sutured close and a semi-compressive dressing is applied.
Fig. 12.
12a: (OR) The incus (In) and the ossicles are removed from the middle ear before final closure. 12 b: (OR) The middle ear is gently packed with Surgicel to avoid postoperative CSF leak. Sigmoid sinus (SS). Digastric ridge (DR). 12c: (OR) Abdominal fat graft and fibrin glue are utilized to fill the mastoidectomy cavity before myocutaneous closure.
Intraoperative images for the illustrative case presented here were obtained during a TL approach that was selected for the resection of a left-sided 1.5 cm Vestibular Schwannoma, in a patient with non-serviceable preoperative hearing. This patient declined radiosurgery treatment. Tumoral resection was complete and no postoperative deficits or complications were recorded.
3. Discussion
The TL approach was initially conceptualized by Rudolph Panse at the turn of the 20th century, and in 1911 Franciscus Hubertus Quix performed the first TL approach for a vestibular schwannoma (Nguyen-Huynh et al., 2007; Quix, 1912). Subsequent attempts at this approach were associated with significant morbidity and mortality, and Quix’s initial attempt was found at a later autopsy, to have only removed the intracanalicular portion of a large tumor extending into the posterior fossa (Nguyen-Huynh et al., 2007). At the same time, in his 1917 monograph “Tumors of the Nervus Acusticus and the Syndrome of the Cerebellopontine Angle,” Harvey Cushing was publishing his advancements in the treatment of vestibular schwannomas using the suboccipital approach (Cushing, 1917). Cushing’s work documented significant advances, decreasing the mortality after resection of vestibular schwannomas from 80% to 4% by 1931. He also became a critic of the TL approach, arguing that it would not provide an adequate working corridor, as he published in the otolaryngology literature: “there is no possible route more dangerous or difficult than this one” (Cushing, 1921).
Due to early failures and criticisms, the TL approach was abandoned until the 1960s when William House resurrected the concept of an approach through the mastoid, labyrinth, and internal auditory canal for the surgical access to vestibular schwannomas (House, 1964). With technical advancements (drills, lighting, magnification, and anesthesia), Dr. House was able to minimize the complications faced by his predecessors and propelled the TL approach to its current position as a well-recognized skull base approach to treat vestibular schwannomas (House and Belal, 1980).
Surgeons have continued to improve upon the TL approach in the attempt to maximize surgical exposure while minimizing associated morbidity. The TL approach has been combined with the retrosigmoid suboccipital, the infratemporal, and the far lateral approaches, with the goals of enhancing surgical access to, respectively, the posterior fossa, the middle fossa, and the foramen magnum (Shen et al., 2004; Spetzler et al., 1991, 1992). In the past, and before the widespread use of radiosurgery techniques, the TL exposure has also been extended to transcochlear routes with transposition of the facial nerve, thus maximizing ventral exposure (Spetzler et al., 1991, 1992; House and Hitselberger, 1976). A modification of the TL procedure, the partial labyrinthectomy petrous apicectomy approach (PLPA), has also been introduced in the attempt to maintain hearing preservation in selected cases (Hirsch et al., 1993).
Classically the TL approach has been utilized (and over the years refined) by neuro-otologists (Ammar et al., 2012), with the involvement of neurosurgeons often reserved for the intradural steps of the procedure, once dural exposure and mapping of the facial nerve at the distal porus had been completed. Progressive collaboration and advancement of multiple disciplines over the past years has brought neuro-otologists and neurosurgeons together to form multispecialty surgical teams and/or centers of excellence for specialized care and skull base surgery. In these centers, team members’ knowledge and expertise is shared and advanced, with the final goal of providing modern and individualized multidisciplinary medical and surgical care, as well as promoting the continuous training of students and residents in the subspecialty field.
At the George Washington University, via a continued and cooperative effort between neuro-otologists and neurosurgeons, a three-decade experience with the use of skull base techniques and complex cranial base approaches has been developed, and residents and research fellows undergo periods of focused surgical anatomy training at the local H. Ammerman Microsurgery Laboratory.
Attendance to neuroanatomy dissection courses and periods of focused training and research in a surgical dissection laboratory, have proven to be of invaluable importance for the training of young surgeons and for maintaining or acquiring surgical expertise in a particular subspecialty field (Rhoton, 2007). Despite the advances that recently introduced virtual reality platforms can offer to surgeons-in-training and experienced neurosurgeons (Tai et al., 2019), periods of neuro-anatomy research and focused “hands-on” surgical anatomy courses continue to be attended by surgeons who seek to maintain or expand their surgical knowledge while practicing new skills and techniques (Rhoton and Tedeschi, 2008). Many residency programs have integrated periods of laboratory training in their teaching curriculum and at the George Washington University students, residents, fellows as well as visiting surgeons can attend neurosurgery dissection courses and periods of focused surgical anatomy training at the H. Ammerman Microsurgery Laboratory. Despite the presence of such a facility, tutors, materials and proper equipment, carrying out a surgical dissection in preparation to a particularly challenging surgery requires time, diligence, patience, mentoring as well as appropriate funding. Transitioning the skills, proficiency and knowledge acquired during a laboratory training to the operative room it is not free of challenges, as setting, anatomy, expectations, stress level, and many other variables greatly impact on the final delivery of care to our surgical patients. Intra-operative surgical anatomy may significantly differ from patient to patient and/or be distorted by the pathology at hand. Also, unless dissection is carried out on fresh specimens, visualization and consistency of the anatomical regions exposed in the laboratory may greatly differ from what is experienced in the OR. Aside from acquiring an advanced knowledge of human anatomy and practicing surgical skills, “hands-on” dissection exercises facilitate the acquisition of a three-dimensional understanding of the surgical anatomy needed when preparing for a skull base procedure. We believe that young surgeons interested in skull base surgery should be trained to learn and perform every steps of the TL approach (as well as other lateral approaches) so that the surgical expertise among team members (neuro-otologists and skull base neurosurgeons) can be to a certain degree interchangeable. Economic constraints, allocation of adequate and safe working spaces, need for basic equipment and technical support from ancillary services, as well as strict compliance to current regulations, are all challenges that need to be overcome to allow such programs to exist and expand.
4. Conclusions
We described the surgical steps to be performed in a dissection laboratory while practicing a TL approach and provided “side-by-side” intraoperative documentation of such a procedure with an illustrative surgical case. Time spent in the dissection laboratory was found to be essential in understanding the approach-related surgical anatomy and in acquiring a three-dimensional orientation of the surgical landmarks and critical structures, by both attending surgeons and neurosurgery residents. Fully equipped dissection laboratories may not available at every teaching institution around the globe, therefore illustrative “side-by-side” reports may help surgeons in training understand the surgical anatomy of various approaches and related challenges. Conversely, more experienced surgeons may find this illustrative presentation useful to refresh and maintain their anatomical knowledge, in preparation for a surgical case.
Along with all of the other training opportunities offered by the addition of novel tools such as virtual reality platforms, attendance to neuroanatomy dissection courses and periods of focused “hands-on” training in a surgical anatomy laboratory remain of invaluable importance to acquire, maintain and advance surgical skills and expertise in neurosurgery.
Footnotes
Peer review under responsibility of PLA General Hospital Department of Otolaryngology Head and Neck Surgery.
References
- Ammar M.B., Piccirillo E., Topsakal V., Taibah A., Sanna M. Surgical results and technical refinements in translabyrinthine excision of vestibular schwannomas: the gruppo otologico experience. Neurosurgery. Jun 2012;70(6):1481–1491. doi: 10.1227/NEU.0b013e31824c010f. [DOI] [PubMed] [Google Scholar]
- Cushing H. WB Saunders Co; Philadelphia, PA: 1917. Tumors of the Nervus Acusticus and the Syndrome of the Cerebellopontine Angle. [Google Scholar]
- Cushing H. Further concerning the acoustic neuromas. Laryngoscope. 1921;31:209–228. [Google Scholar]
- Hirsch B.E., Cass S.P., Sekhar L.N., Wright D.C. Translabyrinthine approach to skull base tumors with hearing preservation. Am. J. Otol. 1993;14:533–543. [PubMed] [Google Scholar]
- House W.F. Transtemporal bone microsurgical removal of acoustic neuromas. Report of cases. Arch. Otolaryngol. 1964;80:617–667. [PubMed] [Google Scholar]
- House W.F., Belal A., Jr. Translabyrinthine surgery: anatomy and pathology. Am. J. Otol. 1980;1:189–198. [PubMed] [Google Scholar]
- House W.F., Hitselberger W.E. The transcochlear approach to the skull base. Arch. Otolaryngol. 1976;102:334–342. doi: 10.1001/archotol.1976.00780110046004. [DOI] [PubMed] [Google Scholar]
- Matsushima T., Lister J.R., Matsushima K., de Oliveira E., Timurkaynak E., Peace D.A., Kobayashi S. The history of rhoton’s. Lab. Neurosurg Rev. Mar. 2019;42(1):73–83. doi: 10.1007/s10143-017-0902-4. [DOI] [PubMed] [Google Scholar]
- Nguyen-Huynh A.T., Jackler R.K., Pfister M., Tseng J. The aborted early history of the translabyrinthine approach: A victim of suppression or technical prematurity? Otol. Neurotol. 2007;28:269–279. doi: 10.1097/MAO.0b013e31802b3264. [DOI] [PubMed] [Google Scholar]
- Quix F. Ein Fall von translabyrintharisch operiertem Tumor acusticus. Verh Dtsch Otoil Ges. 1912;21:245–255. [Google Scholar]
- Rhoton A.L., Jr. The cerebrum. Anatomy. Neurosurgery. Jul 2007;61(1 Suppl. l):37–118. doi: 10.1227/01.NEU.0000255490.88321.CE. [DOI] [PubMed] [Google Scholar]
- Rhoton A.L., Tedeschi H. Microsurgical anatomy of acoustic neuroma. Neurosurgery clinics of north. Am. April. 2008;19(2):145–174. doi: 10.1016/j.nec.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Shen T., Friedman R.A., Brackmann D.E., Slattery W.H., 3rd, Hitselberger W.E., Schwartz M.S. The evolution of surgical approaches for posterior fossa meningiomas. Otol. Neurotol. 2004;25:394–397. doi: 10.1097/00129492-200405000-00031. [DOI] [PubMed] [Google Scholar]
- Spetzler R.F., Daspit C.P., Pappas C.T. Combined approach for lesions involving the cerebellopontine angle and skull base: experience with 30 cases. Skull Base Surg. 1991;1:226–234. doi: 10.1055/s-2008-1057102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spetzler R.F., Daspit C.P., Pappas C.T. The combined supra- and infratentorial approach for lesions of the petrous and clival regions: experience with 46 cases. J. Neurosurg. 1992;76:588–599. doi: 10.3171/jns.1992.76.4.0588. [DOI] [PubMed] [Google Scholar]
- Tai A.X., Sack K.D., Herur-Raman A., Jean W.C. The benefits of limited orbitotomy on the supraorbital approach: an anatomic and morphometric study in virtual reality. Oper Neurosurg (Hagerstown) 2019 Jul 23 doi: 10.1093/ons/opz201. [DOI] [PubMed] [Google Scholar]