Abstract
Surface modification can play a crucial role in enhancing cell adhesion to synthetic polymer-based scaffolds in tissue engineering applications. Here, we report a novel approach for layer-by-layer (LbL) fabrication of nanometer-size fibronectin and gelatin (FN-G) layers on electrospun fibrous poly(carbonate urethane)urea (PCUU) scaffolds. Alternate immersions into the solutions of fibronectin and gelatin provided thickness-controlled FN-G nano-layers (PCUUFN-G) which maintained the scaffold’s 3D structure and width of fibrous bundle of PCUU as evidenced by scanning electron miscroscopy. The PCUUFN-G scaffold improved cell adhesion and proliferation of bladder smooth muscles (BSMCs) when compared to uncoated PCUU. The high affinity of PCUUFN-G for cells was further demonstrated by migration of adherent BSMCs from culture plates to the scaffold. Moreover, the culture of UROtsa cells, human urothelium-derived cell line, on PCUUFN-G resulted in an 11–15 μm thick multilayered cell structure with cell-to-cell contacts although many UROtsa cells died without forming cell connections on PCUU. Together these results indicate that this approach will aid in advancing the technology for engineering bladder tissues in vitro. Because FN-G nano-layers formation is based on nonspecific physical adsorption of fibronectin onto polymer and its subsequent interactions with gelatin, this technique may be applicable to other polymer-based scaffold systems for various tissue engineering/regenerative medicine applications.
Keywords: scaffold, extracellular matrix, layer-by-layer, smooth muscle cell, artificial tissue
INTRODUCTION
In the field of regenerative medicine, proper design of scaffolds for seeding cells has become an important aspect of engineering of functional tissues.1 To date, scaffolds of various materials, including hydrogels,2,3 ceramics,4,5 biomacromolecules,6,7 and synthetic polymers,8,9 have been developed. Synthetic polymers are a particularly attractive option because their mechanical properties and chemical functionality can be easily manipulated by changing the molecular structure of polymer chains. Moreover, various preparation methods,10 such as salt leaching,11,12 electrospinning,13,14 gas-foaming,15,16 and 3D printing17 allow creation of a wide variety of 3D structures. Because the surface of certain polymer-based scaffolds does not support cell adhesion without modification, surface-coating with collagen18 or hydroxyapatite19,20 has been employed to promote attachment of cells to the scaffolds. Such molecules, however, tend to form a coating with uncontrolled thickness, which alters the surface structure18 and physical properties of the scaffolds.19 To address this issue, the aim of the present study is to explore a novel approach for coating a synthetic polymer-based scaffold with a layer-by-layer (LbL) assembly of fibronectin and gelatin to form nanometer-sized ECM thin layers without compromising the structure of the scaffold.
The LbL technique has been utilized to fabricate nanometer-size polymer layers on a variety of substrates through alternate immersion into interactive polymer solutions.21 In general, repeated immersion of a substrate in a single polymer solution leads to saturation of the surface, which prevents further attachment of the polymer to the substrate. The LbL approach instead provides a fresh active surface by alternate immersion in complementary polymers interactive to each other to form nano-layers of polymers with controllable thickness. Previously, LbL assembly of fibronectin-gelatin (FN-G) nano-layers employing the ligand-receptor interaction between two molecules by the use of RGD peptide sequence in fibronectin and RGD receptor in gelatin,22 have emerged as an attractive option to cover the surfaces of various substrates and live cells.23–26 More specifically, Matsusaki et al. have demonstrated that FN-G nano-layers on cells supports cell-cell adhesion which allows for fabrication of a variety of 3D multilayered tissue constructs.24 We hypothesized that this LbL application of FN-G nano-layers would be applicable to coating of polymer scaffolds because fibronectin is a glycoprotein27,28 that exhibits nonspecific physical adsorption to various polymer chains. In addition, due to interactions of fibronectin with α5β1 integrin receptors on cell surfaces,29 FN-G nano-layers could provide an adhesive surface for cells without disruption of the scaffold’s 3D structure.
The long-term goal of the present study is to fabricate a functional urinary bladder tissue that consists of smooth muscle and urothelial cells as well as vasculature for repair and regeneration of dysfunctional organs. The seminal work by Atala et al. demonstrated the clinical feasibility of fabricating an artificial urinary bladder with autologous cell-seeded polylglycolic acid-based scaffolds.30 To date, however, treating bladder diseases using an engineered neo-bladder remains challenging and has not received wide acceptance because successful clinical outcomes are still limited31 although a number of pre-clinical studies with various biomaterials have been reported.32 In the present study, we chose electrospun fibrous poly(carbonate urethane)urea (PCUU) to fabricate a scaffold with FN-G nano-layers (Fig. 1). Polyurea-based elastomers including PCUU have shown potential as tissue-engineering scaffolds to aid the repair and reconstruction of mechanically active soft tissue,33 and it was recently reported that PCUU scaffolds exhibited high extensibility and stability against enzymatic degradation.34 To demonstrate that FN-G nano-layers coating enhances PCUU scaffolds, we examined adhesion and cell proliferation of bladder smooth muscle cells (BSMCs) and formation of a multilayered structure by cells from a urothelial cell line (UROtsa cells) in vitro.
FIGURE 1.

LbL nano-ECM coating of electrospun fibrous poly(carbonate urethane)urea (PCUU) scaffold. Fibrous PCUU scaffold (pictured, A, and illustrated, B) was coated with fibronectin (FN) and gelatin (G) by layer-by-layer (LbL) technique (C) allowing for efficient adhesion of cells to the scaffold (D).
MATERIALS AND METHODS
Synthesis of PCUU
PCUU was synthesized by a two-step solvent synthesis method using poly(1,6-hexamethylene carbonate) diol (PHC, Mn = 2000, Sigma), 1,4-diisocyanatobutane (BDI, Sigma), and putrescine (Sigma) as previously described.33 Briefly, PHC was completely dissolved in DMSO in a 3-neck flask with argon protection and then BDI was added to the solution, following 40 μL of Sn(Oct)2. The flask was placed in an oil bath at 70°C. After 3 h, the prepolymer solution was cooled at room temerature and then a putrescine/DMSO solution was dropwise into the agitated solution. The final polymer solution concentration was controlled to be ~4% (w/v). Then the flask was placed in the oil bath and kept at 50°C overnight. The polymer was precipitated in an excess volume of cool deionized water and then dried in a vacuum at 60°C for 3 days.
Preparing a fibrous PCUU scaffold by electrospinning
The PCUU scaffolds were prepared using conventional electrospinning methods as previously described.35 Briefly, PCUU was dissolved at either 5 or 12 wt % in hexafluroiso-propanol (HFIP) under mechanical stirring. The PCUU solution and cell culture medium (DMEM supplemented with 10% fetal bovine serum (FBS) and 5% penicillin–streptomycin) were added independently at ~0.98–1.2 mL h−1 (depending on ambient relative humidity) using two different syringe pumps (Harvard Apparatus) located at 17 and 4.5 cm, respectively, from the mandrel. The cell culture medium was mixed to delay binding between fibers in order to avoid alternation of the mechanical properties.36 High voltage generators (Gamma High Voltage Research) were used to charge the PCUU solution at 12 kV, the cell culture medium at 9 kV, and their target at −7 kV. The electrospinning was performed at a rotational speed of ~200 rpm with a 5 cm motor translation pattern while minimal scaffold wetting during fabrication was maintained. To obtain a 5 cm × 8 cm PCUU sheet of 0.1–0.2 mm thickness, the approximate fabrication time was 2 h. The PCUU scaffold was sterilized under UV light overnight, and then immersed in sterile cell culture medium for 24 h prior to use to remove any residual HFIP solvent.
Preparations of FN-G nano-layers on PCUU scaffold
For preparation of FN-G nano-layers, PCUU scaffolds (thickness: 0.1–0.2 mm) were immersed for 2 min in each of 0.04 mg mL−1 fibronectin (Mw = 4.6 × 105, isoelectric point: pH = 4.5–4.9 Sigma–Aldrich) and 0.04 mg mL−1 gelatin (Mw = 1.0 × 105, isoelectric point: pH = 5.6–6.1, Wako) in 50 mM Tris-HCl (pH = 7.4). After each immersion step, the PCUU was washed with 50 mM Tris-HCl (pH = 7.4) for 1 min. After 9 or 21 alternating steps of immersion in fibronectin and gelatin, (FN/G)4FN or (FN/G)10FN nano-layers were formed on the scaffold.26 PCUU scaffolds coated with (FN/G)4FN nano-layers (PCUUFN-G) were used for cell culture throughout the experiments reported here.
Fluorescence imaging of FN-G nano-layers on PCUU scaffolds
For visualization of fibronectin and gelatin in fluorescence microscopy, rhodamine-labeled fibronectin (Rh-FN; Mw = 2.5 × 105, Cytoskelton) was obtained from a commercial source, and gelatin was labeled with fluorescein isothiocyanate (FITC) as reported previously.37 In brief, 1.2 mg of FITC was added to 20 mL of gelatin solution (2.0 mg mL−1, Mw = 1.0 × 105, Wako) in Tris-HCl buffer (50 mM, pH = 7.4) and then incubated overnight. Unreacted FITC was removed by dialysis with excess amount of water. Each PCUU scaffold was coated with a 9-layer ((Rh-FN/FITC-G)4Rh-FN).23 The samples were imaged using confocal laser microscopy (Nikon, λex = 488 nm for FITC, λex = 550 nm for rhodamine). To obtain cross sections, the samples were immersed in Neg-50 (Thermo) and slowly frozen until solid before cryosectioning at a thickness of 7–10 μm. The sections were imaged using epifluorescence microscopy (Nikon). To determine the amount of fibronectin and gelatin in the LbL coating,25 separate specimens were prepared by alternating immersion in fluorescent and nonfluorescent solutions of fibronectin and gelatin (Rh-FN/G, and FN/FITC-G) and fluorescence intensity was measured using a microplate reader (BioTek, λex = 550 nm, λem = 590 nm for rhodamine and λex = 488 nm, λem = 530 nm for FITC) after each immersion step. Fluorescence measurements on specimens without coating (0 layer) for each wavelength were used as the reference value to be subtracted from measurements for the coated specimens to account for potential autofluorescence of the PCUU scaffold itself. A PCUU scaffold immersed in a solution of Rh-FN repeatedly without immersion in a gelatin solution served as a control.
Scanning electron microscopy of the surfaces of PCUU scaffolds
To analyze the surface structures of scaffolds, PCUU scaffolds with no layer, (FN/G)4FN nano-layers, and (FN/G)10FN nano-layers were lyophilized overnight and observed using a variable pressure scanning electron microscope (SEM; SU6600, Hitachi) operated under 15 kV.
Cell cultures
Bladder smooth muscle cells (BSMCs) were harvested from the bladders of female Sprague–Dawley rats according to our established protocol38 and cultured in RPMI (Invitrogen) supplemented with 10% fetal bovine serum (FBS; Hyclone) and 1% penicillin–streptomycin (Invitrogen) under standard culture conditions (humidified, 37°C, 5% CO2, and 95% air). BSMCs at passage 3–7 were used throughout the experiments. UROtsa cells which are a cell line derived from normal human urothelium and established previously as a model cell type for studying urothelial functions39 were donated by Dr. Naoki Yoshimura, University of Pittsburgh. These cells were cultured in DMEM (Invitrogen) supplemented with 10% FBS under standard conditions.
Seeding of BSMCs on PCUU scaffolds
BSMCs (1 × 104 cells suspended in culture medium) were seeded on PCUU or PCUUFN-G (3.5 mm × 3.5 mm) resting at the bottom of cell-culture inserts (BD Biosciences, 0.9 cm2, 0.4-mm pore size) in 24-well plates and cultured under standard conditions for 2 days. In separate experiments, BSMCs (1 × 104 cells) were seeded directly in individual wells of 24-well plates for 2 days and unseeded PCUU or PCUUFN-G was placed on top of BSMC for 5 more days of culture. The presence of BSMCs on the PCUU and the PCUUFN-G was confirmed by immunostaining with primary antibodies for α-actin (Santa Cruz) and Alexa 488-tagged secondary antibody and nuclear staining using 4’,6-diamidino-2-phenylindole (DAPI).
Analysis of glucose metabolisms by BSMCs
Glucose metabolism of BSMCs in culture medium was quantified as an index of cell proliferation of BSMCs on PCUU scaffolds. Following an initial 2-day culture period, the culture medium was replaced by fresh RMPI with 10% FBS (glucose 2.17 mg mL−1) every day for the next 2 days. Differences in the concentrations of glucose in the conditioned supernatant medium were measured using an enzymatic glucose biosensor (appending glucose oxidase), in which an oxidization product, hydrogen peroxide, was detected by a platinum electrode (Biochemistry Analyzer).
Multilayered urothelial cell culture on PCUU scaffolds
To fabricate multilayered cells with the scaffold, 4 × 105 UROtsa cells were seeded onto PCUU or PCUUFN-G (3.5 mm × 3.5 mm) and cultured in DMEM (Gibco) supplemented with 1% FBS for 1 day. Viability of UROtsa cells on a PCUU or a PCUUFN-G was confirmed using fluorescence microscopy and the LIVE/DEAD® staining kit (Molecular Probes) following the manufacturer’s instructions. For histological examination, UROtsa cells on scaffolds were fixed with 4% formaldehyde (Aldrich) in PBS for 2 days and subjected to a series of sucrose solutions in increasing concentrations (5% for 4 h, 10% for 4 h, and 20% overnight). The samples were immersed in Neg-50 (Thermo) and frozen slowly until solid prior to cryosectioning to obtain 7–10 μm sections. Dried sections were stained with hematoxylin and eosin (H&E), and cells were visualized using brightfield light microscopy.
Statistical analysis
All quantitative data were statistically analyzed using t test, with p values <0.05 were considered statistically significant.
RESULTS
Fluorescence microscopy of FN-G nano-layers on PCUU scaffold
To visualize the FN-G nano-layers on PCUU, the LbL procedure was performed using rhodamine-labeled fibronectin (Rh-FN) and FITC-labeled gelatin (FITC-G). Low magnification fluorescence microscopy images of the PCUU with (Rh-FN/FITC-G)4Rh-FN nano-layers revealed red fluorescence of Rh-FN [Fig. 2(A)] and green fluorescence of FITC-G [Fig. 2(B)] on PCUU. In confocal laser scanning microscopy images of the scaffold, fibrous structures in both red and green fluorescence were clearly visible [Fig. 2(C)]. Moreover, cross-sectional images of PCUU with (Rh-FN/FITC-G)4Rh-FN revealed fluorescence of Rh-FN and FITC-G not only on the outer surface of the scaffold, but also on the interior [Fig. 2(D)].
FIGURE 2.
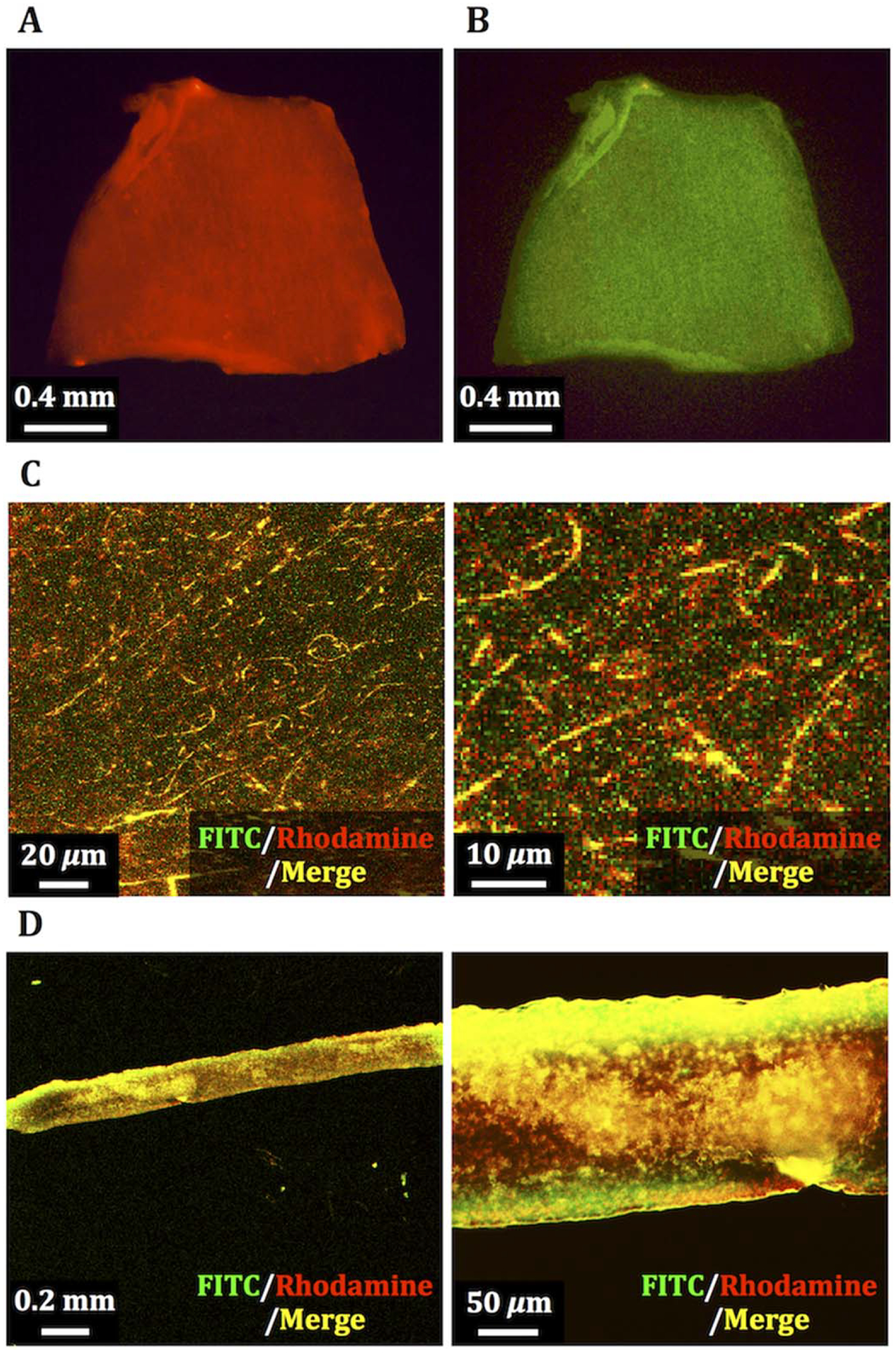
Fluorescence images of PCUU scaffold with FN-G nano-layers composed of rhodamine-labeled fibronectin (Rh-FN) and FITC-labeled gelatin (FITC-G). Fluorescence derived from rhodamine (A) and FITC (B) on a whole scaffold, laser confocal micrographs of fibrous network in a magnified view (C), and cross-sectional images (D) of the PCUU scaffold.
Quantification of the fluorescence of FN-G nano-layers through LbL coating
To estimate the amount of adsorptions of fibronectin and gelatin, we quantified the fluorescence intensities of Rh-FN and FITC-G after each coating step of the LbL process. When we prepared FN-G nano-layers by alternate immersion of PCUU in Rh-FN and gelatin without fluorescence labeling, a stepwise increase of red fluorescence was observed [Fig. 3(A, red)]. Similarly, FN-G nano-layers prepared with non-fluorescent fibronectin and FITC-G showed a stepwise increase in green fluorescence [Fig. 3(B)]. However, when a PCUU scaffold was immersed in a solution of Rh-FN repeatedly without the gelatin steps in between, fluorescence intensity remained low and constant after two immersions [Fig. 3(A, blue)].
FIGURE 3.

LbL coating of fluorescence-labeled fibronectin and gelatin on PCUU scaffold. Changes in fluorescence intensity of rhodamine-labeled fibronectin (Rh-FN) under LbL coating with alternating Rh-FN and unlabeled gelatin (A, red); multiple immersions in Rh-FN without gelatin (A, blue); and FITC-labeled gelatin (FITC-G) through LbL coating with alternating unlabeled FN and FITC-G (B).
SEM microscopy of the surfaces of PCUU
After layering gelatin and fibronectin four or ten times each to fabricate (FN/G)4FN or (FN/G)10FN nano-layers, respectively, on the electrospun fibrous PCUU, we performed scanning electron microscopy (SEM) to examine the surface structures. SEM images revealed that not only the surfaces of both PCUU with (FN/G)4FN [Fig. 4(B)], but also PCUU with and (FN/G)10FN [Fig. 4(C)], displayed 3D fibrous networks similar to untreated PCUU scaffold [Fig. 4(A)].
FIGURE 4.
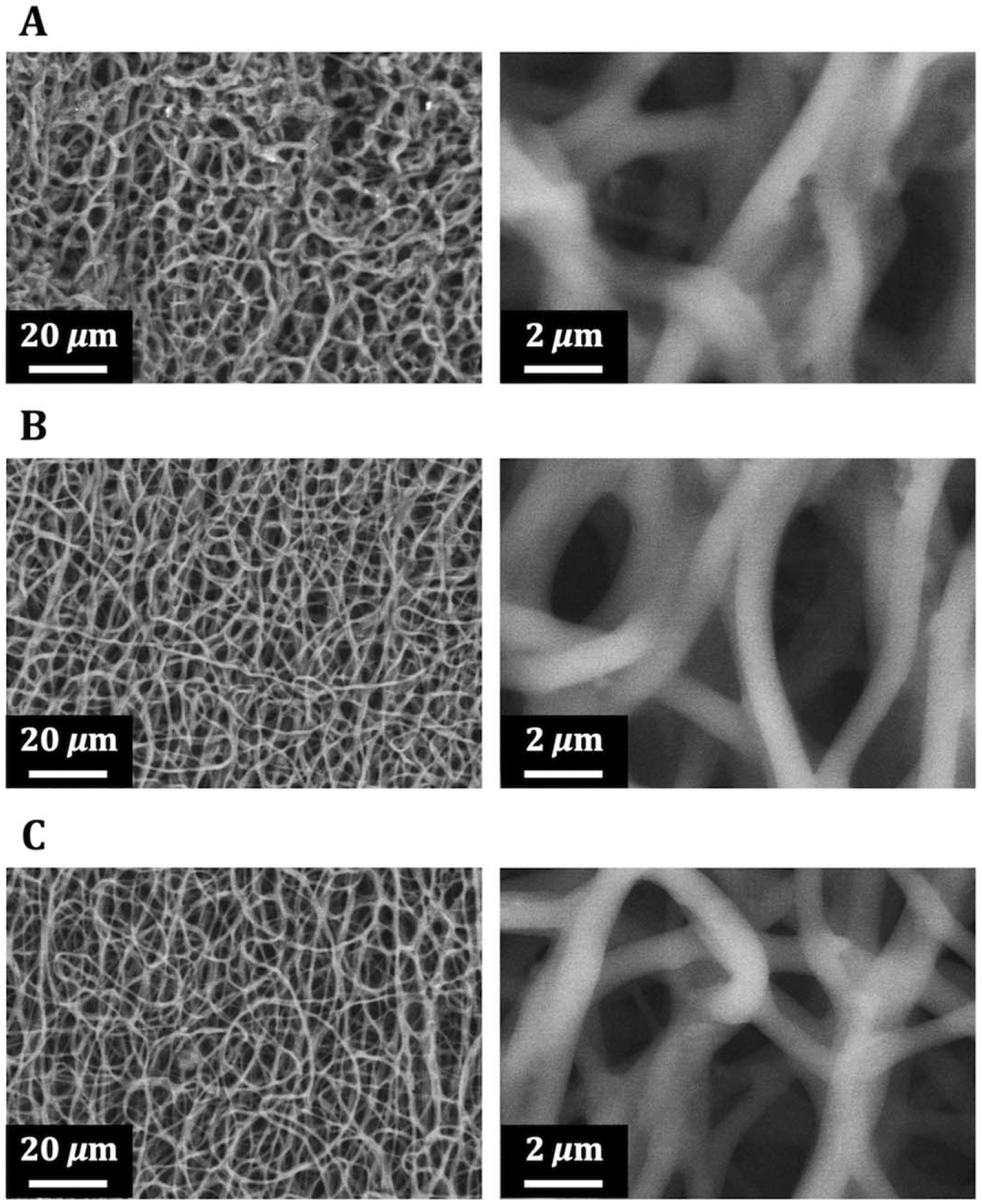
Scanning electron microscopy images of PCUU scaffolds. The surface images of scaffolds with no coating (A), (FN-G)4-FN nano-layers (B), and (FN-G)10-FN nano-layers (C) exhibited similar fiber diameters.
BSMC adhesion and proliferation on PCUUFN-G scaffolds
BSMCs were cultured on PCUU or PCUUFN-G scaffolds to evaluate the effects of FN-G nano-layers on early cellular responses. As shown in fluorescence images, many aggregated BSMCs exhibited disrupted cell morphology on the surface of PCUU scaffolds 2 days after seeding [Fig. 5(A)]. However, when cultured on PCUUFN-G scaffolds, BSMCs exhibited spread cell morphology [Fig. 5(B)]. In some images, blue fluorescence background could be observed due to adsorption of DAPI molecules onto the scaffolds. To assess cell proliferation of BSMCs after adhesion to the scaffolds, we quantified their glucose consumption by measuring the reduction in glucose concentrations of conditioned media. BSMCs cultured on PCUU exhibited 137 and 155 μg day−1 of metabolic activity of glucose in 2 and 3 days after seeding, respectively [Fig. 5(C)]. In contrast, BSMCs on PCUUFN-G metabolized glucose at 373 and 813 μg day−1 after 2 and 3 days, respectively [Fig. 5(C)]. In addition, a significant (p < 0.05) increase in glucose metabolism by BSMCs on PCUUFN-G between day 2 and 3 was observed. The FN-G nano-layers are expected to remain stable during the culture period as previous studies demonstrated that FN-G nano-layers are stable for at least 7 days in cell culture.23 The high affinity of PCUUFN-G for cells was further confirmed by observing the migration of adherent BSMC from tissue culture plastic to scaffolds. We placed an unseeded PCUU or PCUUFN-G scaffold on top of a monolayer of BSMCs adhering to the bottom of a well plate and cultured for 5 days [Fig. 6(A)]. The result of immunostaining for α-actin revealed that while only aggregations of round-shaped cells were observed on PCUU [Fig. 6(B)], BSMCs on PCUUFN-G displayed typical spread-cell morphology [Fig. 6(C)].
FIGURE 5.

BSMCs seeded on PCUU and PCUUFN-G scaffolds. Immunostaining for α-actin (green) and DAPI nuclear staining (blue) of BSMCs on PCUU (A) and PCUUFN-G (B) revealed different cell morphology. Quantification of glucose metabolism (C) after 2- (blue) and 3-day (orange) incubation indicated greater cell proliferation by the cells on PCUUFN-G than those on PCUU. *P < 0.01, **P < 0.05; t test. Data are mean ± standard deviation (n = 3).
FIGURE 6.

Migration of adherent BSMCs to PCUUFN-G scaffold. Placing unseeded scaffolds on top of adherent BSMCs (A) allowed cell migration from a culture plate to scaffolds. Immunostaining for α-actin (green) and DAPI nuclear staining (blue) after 5 days of culture demonstrated adhesion and spreading of BSMCs on PCUUFN-G (C), but not on PCUU (B).
Multilayering of UROtsa cells on PCUU scaffolds
After 24 h of culture, H&E-stained cross-sections of PCUU showed UROtsa cells poorly adhered to the scaffold with undeveloped cell-to-cell connections [Fig. 7(A)]. In contrast, the culture of the same number of UROtsa cells on PCUUFN-G revealed an 11–15 μm thick multilayered cell structure with cell-to-cell contacts [Fig. 7(B)], which corresponds to two to three layers (the thickness of one cell layer is 4.4–6.3 μm).23,25,40 This was further confirmed by LIVE/DEAD assay to evaluate the viability of UROtsa cells on the scaffolds. Although many UROtsa cells died without forming cell connections on PCUU [Fig. 7(C)], the cells cultured on PCUUFN-G remained viable, developing tight connections [Fig. 7(D)].
FIGURE 7.

Multilayered UROtsa cells on PCUU and PCUUFN-G scaffolds. Images of H&E stained cross sections (A and B) and live/dead staining (C and D) of UROtsa cells revealed tightly packed multilayers and high viability of these cells on PCUUFN-G (B and D), but not on PCUU (A and C).
DISCUSSION
The long-term goal of the present study is to fabricate a functional, multi-cellular tissue construct for repair and regeneration of the urinary bladder. In pursuit of this goal, we chose electrospun fibrous PCUU scaffold for culturing bladder smooth muscle and urothelial cells. Polyurethane-based elastomers9,41 have been investigated as materials for fabrication of mechanically active soft tissues, such as blood vessels,42 cardiac wall,43 cardiac valves,44 skin,45 and urinary bladder.46 In a previous unpublished study of our laboratory, uniaxial and planar biaxial tensile testing demonstrated that the PCUU scaffolds exhibited mechanical behaviors similar to that of native bladder tissue.47 It was also observed that BSMCs would detach from PCUU scaffolds when they were subjected to stretching to simulate the in vivo mechanical loading conditions of the urinary bladder,47 indicating that there was relatively weak adhesion of the cells to the polymer surface. Thus, we hypothesized that application of the FN-G coating would enhance adhesion and subsequent function of BSMCs seeded on PCUU scaffolds. We here prepared fibrous PCUU scaffolds by the electron spinning method and utilized LbL assembly of FN-G nano-layers to investigate the effect of the ECM coating on BSMC and urothelial cell functions.
The results of the present study provided evidence that FN-G nano-layers could be prepared not only on the outer surface, but also in the interior space of fibrous PCUU scaffold by alternate immersions into the solutions of fibronectin and gelatin [Fig. 2(D)]. As revealed by the quantification of fluorescence from rhodamine-labeled fibronectin and FITC-labeled gelatin (Fig. 3), without the alternating gelatin layers, accumulation of fibronectin on the surface of PCUU fibers was limited likely due to saturation. In contrast, the LbL approach allowed increasing adsorption of fibronectin to the PCUU scaffold proportionally to the number of alternate immersion cycles at least up to 10 times in the present study. These results are in agreement with previous reports of the LbL assembly of FN-G layers on cell surface.25 Interestingly, unlike other reported methods for coating scaffolds,18,19 the LbL preparation of FN-G nano-layers hardly changed the fiber surface structure of PCUU as evidenced by SEM observations of the present study (Fig. 4). Together these results clearly demonstrated that FN-G layers were successfully fabricated on PCUU scaffolds as nanometer scale ultrathin coating of each fiber with unmodified 3D structure. It was also reported previously that fibronectin alone was not as effective as (FN/G)4FN thick nano-layers in enhancing cell adhesion because fibronectin quickly degraded enzymatically25 which, in turn, could expose the underlying substrate.48 Thus, we concluded that combination of gelatin as complementary layers for fibronectin via LbL assembly not only allowed for preparation of ultrathin nano-layers on scaffolds with controllable thickness, but was also necessary for improving cell adhesion.
Previously, Hong et al. demonstrated in vitro that rat aortic smooth muscle cells adhered and exhibited well-spread cell morphology on a porous PCUU scaffold prepared by salt leaching.34 However, in the present study using fibrous PCUU, culturing BSMCs on electrospun scaffold resulted in incomplete adhesion of these cells [Fig. 5(A)] despite the presence of FBS in the culture medium. The discrepancy may be due to the smaller surface area and fewer serum protein adsorption to the fibrous scaffold compared to the previous reports.49,50 As shown in the immuno-stained BSMCs on PCUUFN-G exhibiting spread cell morphology [Fig. 5(B)], FN-G nano-layers could compensate for these limitations with fibrous PCUU scaffold by providing BSMC adhesion sites. In addition, the measurements of glucose metabolism of BSMCs [Fig. 5(C)] indicate that the effect of FBS on cell proliferation is enhanced by the presence of FN-G nano-layers. Moreover, the results of indirect cell seeding indicated that PCUUFN-G is superior to uncoated PCUU for culturing of BSMCs. Specifically, it was observed that adherent BSMCs at the bottom of a culture plate migrated to PCUUFN-G scaffold that was simply placed on top [Fig. 6(C)]. This facile way to seed cells can protect BSMCs from the potential damage associated with trypsin treatment. Furthermore, from the glucose consumption data [Fig. 5(C)], we estimated ~3.9 × 104 and 8.6 × 104 BSMCs were present on PCUUFN-G (based on an estimate that glucose metabolism by each BSMC is ~9.5 ng day−1)51 after 2 and 3 days of culture, respectively. Because these scaffolds were initially seeded with 1 × 104 cells each, these results indicate that BSMCs doubled in number each day. This contrasted glucose metabolism of BSMCs on PCUU corresponding to 1.4 × 104 and 1.6 × 104 cells, indicating that BSMCs on PCUU without FN-G nano-layers barely proliferated. Taken together, it can be expected from the results of the present study that when implanted in vivo host cells may migrate and proliferate to populate the scaffold for a faster regeneration of the tissue.
For the engineered bladder tissue to be functional, a multi-cellular structure must be constructed because bladder wall tissue in vivo exhibits complicated structure composed of transitional (superficial, intermediate, and basal) urothelial cells lining the smooth muscle layer that is innervated and vascularized.52 Thus, fabrication of multilayered urothelial tissue on top of BSMC-seeded PCUUFN-G should yield a more realistic structure, and has considerable appeal for reconstruction of the bladder.32,53 Taking into account this ideal model for bladder tissue, we examined the affinity of the PCUUFN-G scaffold with UROtsa cells (urothelial cell line). As revealed by the H&E stained cross section image, multilayered UROtsa cells corresponding to two to three layers on PCUUFN-G developed connections between cells [Fig. 7(B)], while the cells seeded on PCUU exhibited weak attachment to the scaffold and to each other with undeveloped cell-to-cell connections [Fig. 7(A)]. By using LIVE/DEAD assay, remarkable effect of FN-G nano-layers on PCUU to enhance the viability of UROtsa cells was also unveiled, showing the importance of FN-G nano-layers on scaffolds in fabrication of multilayered UROtsa cells.
CONCLUSION
Here, we presented a novel approach for coating fibrous PCUU scaffold with nanometer-sized layers of fibronectin and gelatin by the LbL technique. The enhanced cell adhesion and proliferation of BSMCs and formation of multilayered urotheilum on PCUUFN-G achieved in the present work indicate that this approach will aid in advancing the technology for engineering bladder tissues in vitro. Moreover, our observation that adherent BSMCs migrated from a culture plate to attach to PCUUFN-G suggests that the scaffold with FN-G nano-layers has great potential to attract host cells for faster integration when implanted. Because FN-G nano-layers formation is based on nonspecific physical adsorption of fibronectin onto polymer as an initiating layer and its subsequent molecular interactions with gelatin, this technique may be applicable to other polymer-based scaffold systems for various tissue engineering/regenerative medicine applications.
ACKNOWLEDGMENTS
The authors thank the staff of Clemson Light Imaging Facility and Electron Microscopy Laboratory for their technical support.
Contract grant sponsor: NIH-COBRE; contract grant number: P20 GM103444
Contract grant sponsors: Fuji Film Endowment Funds; University of Tokyo Graduate Program for Leaders in Life Innovation (GPLLI)
REFERENCES
- 1.Bhatia SK Tissue engineering for clinical applications. Biotechnol J 2010;5:1309–1323. [DOI] [PubMed] [Google Scholar]
- 2.Fedorovich NE, Alblas J, de Wijn JR, Hennink WE, Verbout AJ, Dhert WJ Hydrogels as extracellular matrices for skeletal tissue engineering: State-of-the-art and novel application in organ printing. Tissue Eng 2007;13:1905–1925. [DOI] [PubMed] [Google Scholar]
- 3.Hunt JA, Chen R, van Veen T, Bryan N Hydrogels for tissue engineering and regenerative medicine. J Mater Chem B 2014;2:5319–5338. [DOI] [PubMed] [Google Scholar]
- 4.Vanderleyden E, Mullens S, Luyten J, Dubruel P Implantable (bio)-polymer coated titanium scaffolds: A review. Curr Pharm Des 2012;18:2576–2590. [DOI] [PubMed] [Google Scholar]
- 5.Habraken WJEM Wolke JGC, Jansen JA Ceramic composites as matrices and scaffolds for drug delivery in tissue engineering. Adv Drug Deliv Rev 2007;59:234–248. [DOI] [PubMed] [Google Scholar]
- 6.Benders KEM, van Weeren PR, Badylak SF, Saris BFD, Dhert WJA, Malda J Extracellular matrix scaffolds for cartilage and bone regeneration. Trends Biotechnol 2013;31:169–176. [DOI] [PubMed] [Google Scholar]
- 7.Rajangam T, An SS Fibrinogen and fibrin based micro and nano scaffolds incorporated with drugs, proteins, cells and genes for therapeutic biomedical applications. Int J Nanomed 2013;8:3641–3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gentile P, Chiono V, Carmagnola I, Hatton PV An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int J Mol Sci 2014;15:3640–3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sartori S, Chiono V, Tonda-Turo C, Mattu C, Ciardelli G Biomimetic polyurethanes in nano and regenerative medicine. J Mater Chem B 2014;2:5128–5144. [DOI] [PubMed] [Google Scholar]
- 10.Chung HJ, Park TG Surface engineered and drug releasing prefabricated scaffolds for tissue engineering. Adv Drug Deliv Rev 2007;59:249–262. [DOI] [PubMed] [Google Scholar]
- 11.Nasri-Nasrabadi B, Mehrasa M, Rafienia M, Bonakdar S, Behzad T, Gavanji S Porous starch/cellulose nanofibers composite prepared by saltleaching technique for tissue engineering. Biomed Eng Carbohydr Polym 2014;108:232–238. [DOI] [PubMed] [Google Scholar]
- 12.Cho YS, Hong MW, Kim YY, Cho Y-S Assessment of cell proliferation in salt-leaching using powder (SLUP) scaffolds with penetrated macro-pores. J Appl Polym Sci 2014;131:40240. [Google Scholar]
- 13.Sill TJ, von Recum HA Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2014;29:1989–2006. [DOI] [PubMed] [Google Scholar]
- 14.Ingavle GC, Leach JK Advancements in electrospinning of polymeric nanofibrous scaffolds for tissue engineering. Tissue Eng Part B 2014;20:277–293. [DOI] [PubMed] [Google Scholar]
- 15.Dehghani F, Annabi N Engineering porous scaffolds using gas-based techniques. Curr Opin Biotechnol 2011;22:661–666. [DOI] [PubMed] [Google Scholar]
- 16.Sauceau M, Fages J, Common A, Nikitine C, Rodier E New challenges in polymer foaming: A review of extrusion processes assisted by supercritical carbon dioxide. Prog Polym Sci 2011;36: 749–766. [Google Scholar]
- 17.Peltola SM, Melchels FPW, Grijpma DW, Kellomäki M A review of rapid prototyping techniques for tissue engineering purposes. Ann Med 2008;40:268–280. [DOI] [PubMed] [Google Scholar]
- 18.Chen G, Ushida T, Tateishi T Scaffold design for tissue engineering. Macromol Biosci 2002;2:67–77. [Google Scholar]
- 19.Yunos DM, Bretcanu O, Boccaccini AR Polymer-bioceramic composites for tissue engineering scaffolds. J Mater Sci 2008;43: 4433–4442. [Google Scholar]
- 20.Rezwan K, Chen QZ, Blaker JJ, Boccaccini AR Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006;27:3413–3431. [DOI] [PubMed] [Google Scholar]
- 21.Tang Z, Wang Y, Podsiadlo P, Kotov NA Biomedical applications of layer-by-layer assembly: From biomimetics to tissue engineering. Adv Mater 2006;18:3203–3224. [Google Scholar]
- 22.Ruoslahti E, Pierschbacher MD New perspectives in cell adhesion: RGD and integrins. Nature 1987;23:491–497. [DOI] [PubMed] [Google Scholar]
- 23.Nishiguchi A, Yoshida H, Matsusaki M, Akashi M Rapid construction of three-dimensional multilayered tissues with endothelial tube networks by the cell-accumulation technique. Adv Mater 2011;23:3506–3510. [DOI] [PubMed] [Google Scholar]
- 24.Matsusaki M, Ajiro H, Kida T, Serizawa T, Akashi M Layer-by-layer assembly through weak interactions and their biomedical applications. Adv Mater 2012;24:454–474. [DOI] [PubMed] [Google Scholar]
- 25.Matsusaki M Development of three-dimensional tissue models based on hierarchical cell manipulation using nanofilms. Bull Chem Soc Jpn 2012;85:401–414. [Google Scholar]
- 26.Nishiguchi A, Matsusaki M, Asano Y, Shimoda H, Akashi M Effects of angiogenic factors and 3D-microenvironments on vascularization within sandwich cultures. Biomaterials 2014;35:4739–4748. [DOI] [PubMed] [Google Scholar]
- 27.Yamada KM Cell surface interactions with extracellular materials. Annu Rev Biochem 1983;52:761–799. [DOI] [PubMed] [Google Scholar]
- 28.Magnusson MK, Mosher DF Fibronectin: Structure, assembly, and cardiovascular implications. Arterioscler Thromb Vasc Biol 1998; 18:1363–1370. [DOI] [PubMed] [Google Scholar]
- 29.Ruoslahti E, Pierschbacher M New perspectives in cell adhesion: RGD and integrins. Science 1987;238:491–497. [DOI] [PubMed] [Google Scholar]
- 30.Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet 2006;367:1241–1246. [DOI] [PubMed] [Google Scholar]
- 31.Joseph DB, Borer JG, De Filippo RE, Hodges SJ, McLorie GA Autologous cell seeded biodegradable scaffold for augmentation cystoplasty: Phase II study in children and adolescents with spina bifida. J Urol 2014;191:1389–1395. [DOI] [PubMed] [Google Scholar]
- 32.Simaioforidis V, de Jonge P, Sloff M, Oosterwijk E, Geutjes P, Feitz WFJ Ureteral tissue engineering: Where are we and how to proceed? Tissue Eng Part B 2013;19:413–419. [DOI] [PubMed] [Google Scholar]
- 33.Danielsson C, Ruault S, Simonet M, Neuenschwander P, Frey P Polyesterurethane foam scaffold for smooth muscle cell tissue engineering. Biomaterials 2006;27:1410–1415. [DOI] [PubMed] [Google Scholar]
- 34.Hong Y, Guan J, Fujimoto KL, Hashizume R, Pelinescu AL, Wagner WR Tailoring the degradation kinetics of poly(ester carbonate urethane)urea thermoplastic elastomers for tissue engineering scaffolds. Biomaterials 2010;31:4249–4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amoroso NJ, D’Amore A, Hong Y, Wagner WR, Sacks MS Elastomeric electrospun polyurethane scaffolds: The interrelationship between fabrication conditions, fiber topology, and mechanical properties. Adv Mater 2011;23:106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stankus JJ, Guan J, Fujimoto K, Wagner WR Microintegrating smooth muscle cells into a biodegradable, elastomeric fiber matrix. Biomaterials 2006;27:735–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhavasa MD, Tiwari SB, Amiji MM Formulation optimization for the nanoparticles-in-microsphere hybrid oral delivery system using factorial design. J Controll Release 2006;110:422–430. [DOI] [PubMed] [Google Scholar]
- 38.Stover J, Nagatomi J Cyclic pressure stimulates DNA synthesis through the PI3K/Akt signaling pathway in rat bladder smooth muscle cells. Ann Biomed Eng 2007;35:1585–1594. [DOI] [PubMed] [Google Scholar]
- 39.Rossi MR, Masters JRW, Park S, Todd JH, Garrett SH, Sens MA, Somji S, Nath J, Sens DA The immortalized UROtsa cell line as a potential cell culture model of human urothelium. Environ Health Perspect 2001;109:801–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuanyuan Z, Anthony A Urothelial cell culture. Methods Mol Biol 2013;1037:27–43. [DOI] [PubMed] [Google Scholar]
- 41.Guan J, Wagner WR Synthesis, characterization and cytocompatibility of polyurethaneurea elastomers with designed elastase sensitivity. Biomacromolecules 2005;6:2833–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hofmann A, Ritz U, Verrier S, Eglin D, Alini M, Fuchs S, Kirkpatrick C, Rommens PM The effect of human osteoblasts on proliferation and neo-vessel formation of human umbilical vein endothelial cells in a long-term 3D co-culture on polyurethane scaffolds. Biomaterials 2008;29:4217–4226. [DOI] [PubMed] [Google Scholar]
- 43.Fromstein JD, Zandstra PW, Alperin C, Rockwood D, Rabolt JF, Woodhouse KA Seeding bioreactor-produced embryonic stem cell-derived cardiomyocytes on different porous, degradable, polyurethane scaffolds reveals the effect of scaffold architecture on cell morphology. Tissue Eng Part A 2008;14:369–378. [DOI] [PubMed] [Google Scholar]
- 44.Hayashida K, Kanda K, Yaku H, Ando J, Nakayama Y Development of an in vivo tissue-engineered, autologous heart valve (the biovalve): Preparation of a prototype model. J Thorac Cardiovasc Surg 2007;134:152–159. [DOI] [PubMed] [Google Scholar]
- 45.Li B, Davidson JM, Guelcher SA The effect of the local delivery of platelet-derived growth factor from reactive two-component polyurethane scaffolds on the healing in rat skin excisional wounds. Biomaterials 2009;30:3486–3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park KD, Kwon IK, Kim YH Tissue engineering of urinary organs. Yonsei Med J 2000;41:780–788. [DOI] [PubMed] [Google Scholar]
- 47.Sivaraman S Investigating Polymer based Scaffolds for Urinary Bladder Tissue Engineering. PhD Dissertation, Clemson University, 2015. Available at: http://tigerprints.clemson.edu/all_dissertations/1509. [Google Scholar]
- 48.Wittmer CR, Phelps JA, Saltzam WM, van Tassel PR Fibronectin terminated multilayer films: Protein adsorption and cell attachment studies. Biomaterials 2007;28:851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sheets K, Wang J, Meehan S, Sharma P, Ng C, Khan M, Koons B, Behkam B, Nain AS Cell-fiber interactions on aligned and suspended nanofiber scaffolds. J Biomater Tissue Eng 2013;3:355–368. [Google Scholar]
- 50.Murphy CM, O’Brien FJ, Little DG, Schindeler A Cell-scaffold interactions in the bone tissue engineering triad. Eur Cell Mater 2013;26:120–132. [DOI] [PubMed] [Google Scholar]
- 51.Hardin CD, Roberts TM Differential regulation of glucose and glycogen metabolism in vascular smooth muscle by exogenous substrates. J Mol Cell Cardiol 1997;29:1207–1216. [DOI] [PubMed] [Google Scholar]
- 52.Orabi H, Bouhout S, Morissette A, Rousseau A, Chabaud S, Bolduc S Tissue engineering of urinary bladder and urethra: Advances from bench to patients. Sci World J 2013; 154564–154513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gloeckner DC, Sacks MS, Fraser MO, Somogyi GT, de Groat WC, Chancellor MB Passive biaxial mechanical properties of the rat bladder wall after spinal cord injury. J Urol 2002;167:2247–2252. [PubMed] [Google Scholar]


