Abstract
The insulin-like growth factors (IGFs) are polypeptides with similar sequences with insulin. These factors regulate cell growth, development, maturation, and aging via different processes including the interplay with MAPK, Akt, and PI3K. IGF signaling participates in the pathogenesis of neoplasia, insulin resistance, diabetes mellitus, polycystic ovarian syndrome, cerebral ischemic injury, fatty liver disease, and several other conditions. Recent investigations have demonstrated the interplay between non-coding RNAs and IGF signaling. This interplay has fundamental roles in the development of the mentioned disorders. We designed the current study to search the available data about the role of IGF-associated non-coding RNAs in the evolution of neoplasia and other conditions. As novel therapeutic strategies have been designed for modification of IGF signaling, identification of the impact of non-coding RNAs in this pathway is necessary for the prediction of response to these modalities.
Keywords: IGF, miRNA, lncRNA, expression, disorders
Background
The insulin-like growth factors (IGFs) are involved in growth and developmental processes and are evolutionarily conserved among several species (Rosenzweig, 2020). The functions of IGFs are mediated through two receptor tyrosine kinases and receptors for IGF1 and insulin. Besides, several IGF binding proteins selectively inhibit IGF1 or IGF2. IGF1 receptors have been shown to be up-regulated in tumors, thus participating in the tumorigenesis, resistance to therapies, and facilitation of metastasis in various cancer kinds (Rosenzweig, 2020). IGF1 receptors are known inducers of the Akt and mitogen-activated protein kinase (MAPK) (Pollak, 2008). Besides, IGF signaling is involved in the pathogenesis of insulin resistance and other disorders (Rosenzweig, 2020). The contribution of IGF in the pathogenesis of a wide assortment of human disorders including neoplasia and other disorders is explained by its influence on energy metabolism and cell growth (Pollak, 2008). IGF1 acts downstream of the growth hormone and through activation of MAPK and PI3K pathways and anabolism, it promotes growth and maturation of almost all tissues. Therefore, it is also involved in the aging process (Wrigley et al., 2017). Figure 1 depicts an overview of Insulin-like growth factor (IGF) signal transduction and two downstream signaling pathways: PI3K/AKT and MAPK/ERK. The IGF signaling network is composed of three receptor tyrosine kinases (IGF1R, IGF2R, and INSR), three ligands (insulin, IGF1, and IGF2), and six serum insulin-like growth factor binding proteins (IGFBP). Figure 1 shows the IGF signal transduction and its downstream effectors.
FIGURE 1.
A schematic diagram of the insulin-like growth factor (IGF) signal transduction and main downstream effects. Both IGF-1 and IGF-2 could bind with plasma membrane IGF-1R and IGF-1/Insulin hybrid receptor leading to autophosphorylation of this target receptor in the intracellular β-subunits and thus triggering the catalytic function of the IGF-1R, while insulin binds only to the Insulin-R. IGFBPs could regulate the bioavailability of both IGF-1 and IGF-2 signaling cascades. The bioavailability of IGF-2 could also be regulated via binding to the IGF-2R which results in receptor-triggered internalization and endosomal degradation of IGF-2 in the lysosomes. Phosphorylated β-subunits could in turn create docking sites for the adaptor proteins IRS-1/2, and Shc that modulate the activation of two signaling pathways: PI3K/AKT and MAPK/ERK. Activation of the PI3K and AKT pathway leads to modulation of a variety of cell signaling cascades, such as regulation of TSC1/2 to suppress mTORC1 complex and modulation of 4EB-P1 and S6K1/2 phosphorylation, promoting cell survival via activation or suppression of major effectors like the Foxo transcription factors, BCL-2, BAD, and P27, upregulation of transformation of glucose to glycogen through suppression of GSK-3β, increasing protein synthesis, as well as suppressing apoptosis and autophagy. Activation of AKT family of kinases via PDK1 and mTORC2 leads to the phosphorylation at Thr308 and Ser473, respectively. Besides, docking of Grb2 to the phosphorylated IGF-1R β subunits could trigger the Ras/Raf/MEK/ERK axis. Shc binding to activated IGF-1R leads to stimulation of the MAPK/ERK cascade which regulates a kinase signaling pathway and eventually results in promoting cellular proliferation via enhancing transcription factors activities including ELK1.
Recent investigations have verified the influence of regulatory non-coding RNAs on IGF signaling (Chen B. et al., 2019). Most investigations in this regard have focused on long non-coding RNAs (lncRNAs) and microRNAs (miRNAs) (Chen B. et al., 2019). LncRNAs are transcripts with sizes of more than 200 nucleotides which are principally produced by RNA polymerase II. These transcripts have various functions in the modulation of genomic structure, chromatin configuration, mRNA stability, alternative splicing, and enhancement or inhibition of transcription. The other types of regulatory non-coding RNAs, i.e., miRNAs mainly influence gene expression at the post-transcriptional phase via binding with the 3′ UTR of their specific targets. Both classes of non-coding RNAs participate in the pathogenesis of human diseases. We designed the current study to search the available data about the role of IGF-associated non-coding RNAs in the evolution of neoplasia and other conditions.
IGF-Associated miRNAs in Human Disorders
Several IGF-associated miRNAs have been dysregulated in neoplastic conditions. For instance, experiments in ovarian cancer cells have shown that miR−19a−3p suppresses the levels of IGF binding protein−3 (IGFBP−3), thus promoting the growth and migration of these cells. Notably, the expression of this miRNA can be modulated by NF−κB (Bai et al., 2019). Shastri et al. have demonstrated the inhibitory effects of the miR-29 family on IGF-1. Members of the miR-30 family can inhibit both IGF-1 and IGF-1R. Notably, calorie restriction has resulted in the over-expression of miR-29 and miR-30 in the normal liver and the liver being metastasized by breast cancer cells, indicating a possible role for dietary modifications in the management of liver metastases (Shastri et al., 2020). In nasopharyngeal squamous cell carcinoma cells, miR-30a inhibitor could reverse IGF-I-associated epithelial-mesenchymal transition (EMT). The IGF-1R/Src/miR-30a/E-cadherin axis has been identified as an important pathway in the regulation of EMT in these cells (Wang et al., 2016). miR-99a is another miRNA that can inhibit proliferation, migration, and invasion of breast carcinoma via suppression of IGF-1R (Xia et al., 2016). Being up-regulated in hepatocellular carcinoma cells, miR-155 can increase expression of IGF-II and IGF-1R, while decreasing IGFBP-3 expression. Through these pathways, miR-155 can increase proliferation, migration, and clonogenicity of hepatocellular carcinoma cells (El Tayebi et al., 2015). In the same type of cancer, miR-342-3p can inhibit cell proliferation through the suppression of the IGF-1R-associated Warburg effect (Liu et al., 2018d). In colorectal cancer cells, the oncogenic protein IGF2BP2 has a functional interaction with miR-195 through which it regulates RAF1 expression and participates in the carcinogenic process (Ye S. et al., 2016). Meanwhile, miR-197 can inhibit the expression of IGFBP3 through binding with its 3′-UTR, hence enhancing cell migratory potential and invasion of colorectal cancer cells (Zhou et al., 2018). Supplementary Table 1 reviews the results of studies that displayed the role of IGF-associated miRNAs in the neoplastic conditions.
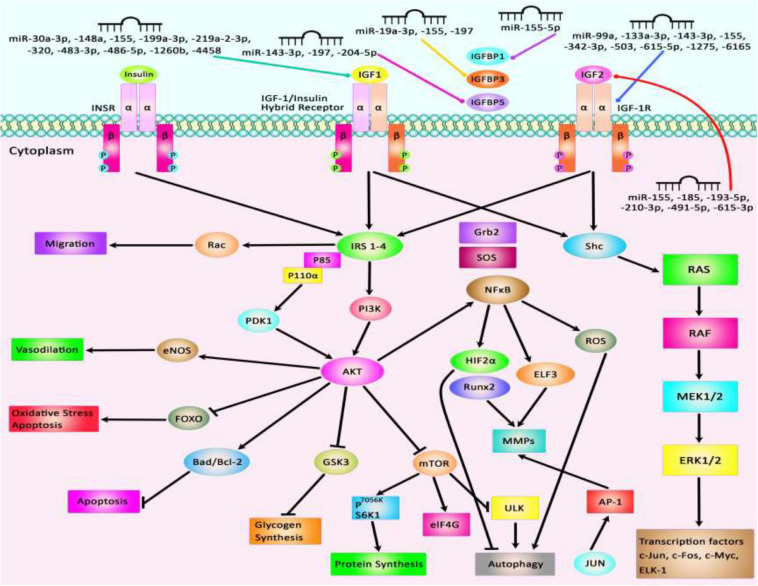
The interaction between IGF-related proteins and miRNAs has been also assessed in non-neoplastic conditions. For instance, IGF-1 is targeted by miR-17. This miRNA has been over-expressed in ox-LDL treated human umbilical vascular endothelial cells (HUVECs) in association with down-regulation of IGF-1. Up-regulation of miR-17 has enhanced cell viability and suppressed the apoptosis of ox-LDL exposed cells. Such effects have been accompanied by down-regulation of Bax and Caspase3 expressions, while up-regulation of Bcl-2, suggesting a role for miR-17 as a biomarker for coronary heart disease (Chen Z. et al., 2019). Expression of miR-30a-3p has been elevated in the placenta samples of women with preeclampsia. This miRNA has been shown to regulate the expression of IGF-1, therefore influencing the invasive capacity and apoptosis of trophoblasts (Niu et al., 2018). Over-expression of miR-129 has suppressed proliferation and migration of Schwan cells and axonal outgrowth of dorsal root ganglion neurons through modulation of several targets including IGF-1 (Zhu H. et al., 2018). Yang et al. have reported up-regulation of miR-143-3p in synovial tissues of patients with rheumatoid arthritis compared with those affected with osteoarthritis. Down-regulation of miR-143-3p has inhibited cell proliferation, enhanced apoptosis, and reduced production of inflammatory cytokines. miR-143-3p has been shown to target IGF1R and IGFBP5 and regulate the Ras/p38 MAPK axis (Liu et al., 2018c). In colonic smooth muscle cells, miR-155 has been shown to down-regulate IGF-1 levels. Up-regulation of miR-155 has increased apoptosis of these cells and reduced the thickness of the related tissue in the diabetic mice, suggesting the role of this miRNA in the aggravation of colonic dysmotility (Shen et al., 2020). Figure 2 illustrates the IGF signaling cascade modulating by dysregulated miRNAs in various human diseases as well as cancers. Table 1 reviews the role of IGF-associated miRNAs in non-neoplastic conditions.
FIGURE 2.
Several proteins in the IGF signaling pathway such as IGF1, IGF2, IGF-1R, and IGFBPs are regulated by miRNAs. Through modulation of these proteins, miRNAs can affect several cellular processes such as apoptosis, autophagy, protein synthesis, response to oxidative stress, and cell migration.
TABLE 1.
IGF-associated miRNAs in non-malignant disorders.
| Type of disease | microRNA | P-value | Animal | Clinical samples (human) | Cell lines | Target | Pathway | Function | References |
| Coronary Heart Disease (CHD) | miR-17 | <0.01 | – | – | HUVECs | IGF-1, Caspase-3, Bax, Bcl-2 | – | Overexpression of miR-17 via targeting IGF-1 could promote the proliferation and inhibit apoptosis of HUVECs. | Chen Z. et al., 2019 |
| Diabetic Retinopathy (DR) | miR-18b | <0.01 | – | – | HRECs | IGF-1 | AKT, MEK, ERK | Downregulation of miR-18b by targeting IGF-1 could increase the proliferation of HRECs exposed to VEGF secretion and normal glucose. | Wu et al., 2016 |
| Polycystic Ovary | miR-19b | <0.01 | – | PCOS (n = 18), normally menstruating women (n = 10) | KGN | IGF-1, CDK1, Cyclin-D1 | – | Downregulation of miR-19b by targeting IGF-1 could enhance ovarian GCs proliferation in PCOS. | Zhong et al., 2018 |
| Preeclampsia (PE) | miR-30a-3p | <0.05 | – | PE (n = 25), normal pregnant women (n = 20) | HTR-8/SVneo, JEG-3 | IGF-1 | – | Overexpression of miR-30a-3p via targeting IGF-1 could induce the apoptosis of trophoblast HTR-8/SVneo cells. | Niu et al., 2018 |
| Polycystic Ovary Syndrome (PCOS) | miR-99a | <0.05 | – | 15 pairs of married women with PCOS and a control group of women without PCOS | COV434 | IGF-1R | – | Overexpression of miR-99a by targeting IGF-1R could reduce the proliferation and promote apoptosis of human granulosa cells (GCs). | Geng et al., 2019 |
| Peripheral Nerve Injury (PNI) | miR-129 | <0.01 | Male SD rats | – | SCs, 293T | IGF-1 | – | Overexpression of miR-129 by targeting IGF-1 could suppress the proliferation and migration of SCs, and axonal outgrowth of DRG neurons in PNI. | Zhu H. et al., 2018 |
| Rheumatoid Arthritis (RA) | miR-129-5p | <0.05 | – | RA (n = 15), healthy controls (n = 12) | FLSs | IGF-1R, Caspase-3/8 | Src/ERK/Egr-1 | Overexpression of miR-129-5p by targeting IGF-1R and activating Src/ERK/Egr-1 signaling could inhibit cell proliferation and induce apoptosis of RA cells. | Zhang Y. et al., 2019 |
| Idiopathic Pulmonary Fibrosis (IPF) | miR-130b-3p | <0.05 | – | 4 IPF patients and 3 normal lung tissues | A549, ATII, MRC5 | IGF-1 | – | Downregulation of miR-130b-3p by enhancing IGF-1 production from the epithelium of the lung could activate fibroblasts to increase the proliferation, migration ability, and expression of collagen I of fibroblasts in co-culture systems. | Li S. et al., 2016 |
| Diabetic Retinopathy (DR) | miR-142-5p | <0.001 | Male SD rats | – | HRECs, 293T | IGF-1, VEGF | PI3K, ERK, AKT, VEGF | Inhibition of miR-142-5p via blocking the IGF-1/p-IGF-1R pathway could promote HREC proliferation in response to DR conditions. | Qiao et al., 2020 |
| Rheumatoid Arthritis (RA) | miR-143-3p | <0.01 | – | 5 pairs of RA and normal control | MH7A | IGF-1R, IGFBP-5, TNF-α, Bax, Bcl-2, Caspase-3 | Ras/p38 MAPK | Downregulation of miR-142-3p could reduce cell proliferation and promotes cell apoptosis by targeting IGF-1R and IGFBP-5. | Liu et al., 2018c |
| Fracture Healing | miR-148a | <0.0 1 | Male Wistar rats | – | 293T, rBMSCs | IGF-1, Runx2, OCN, OPN | – | Downregulation of miR−148a by targeting IGF-1 could promote the expression of osteogenesis−related proteins and regulate bone BMSCs−mediated fracture healing. | Liu et al., 2018a |
| Diabetes Mellitus | miR-155 | <0.05 | – | – | SMCs | IGF-1 | – | Overexpression of miR-155 by regulating the IGF-1 could decrease the thickness of colonic smooth muscle tissues in diabetic mice and also could increase apoptosis of colonic SMCs. | Shen et al., 2020 |
| Non-alcoholic Fatty Liver Disease (NAFLD) | miR-190b | <0.05 | Male C57Bl/6 mice | 15 pairs of NAFLD and NNTs | L02 | IGF-1, ADAMTS9 | IRS2/AKT | Downregulation of miR-190b by directly targeting IGF-1 and ADAMTS9 could regulate lipid metabolism and insulin signaling pathway in vitro and could reduce the hepatic steatosis and insulin resistance in vivo. | Xu et al., 2018 |
| Diabetic Cardiomyopathy | miR-193-5p | <0.05 | Wistar rat | – | MMEC | IGF-2 | – | Downregulation of miR-193a-5p by inhibiting IGF-2 could reduce cell migration and proliferation in type 2 diabetic cardiomyopathy. | Yi et al., 2017 |
| Estrogen-Mediated Autophagy | miR-199a-3p | <0.05 | – | – | MLO-Y4, MC3T3-E1 | IGF-1 | mTOR | Overexpression of miR-199a-3p by targeting IGF-1 and inhibiting the mTOR signaling pathway could induce autophagy in osteocyte-like MLO-Y4 cells. | Fu et al., 2018 |
| Atherosclerosis | miR-210-3p | <0.01 | Male C57BL/6J mice | – | THP-1 | IGF-2, TNF-α, MCP-1, IL-6, iNOS | NF-kB | Overexpression of miR-210-3p by inhibiting the IGF-2/IGF-2R axis could inhibit the expression of CD36 and NF-κB, then led to a reduction in the inflammatory response of macrophages and lipid accumulation in atherosclerosis. | Qiao et al., 2020 |
| Spinal Cord Injury (SCI) | miR-219a-2-3p | <0.01 | Female SD rats | – | NSCs, PC12 | IFG-1, BAX, Bcl-2, Beclin-1, Caspase-3 | NF-kB | Exposure of exosomes derived from NSCs to IGF-1 via the miR-219a-2-3p-dependent pathway could suppress the nerve inflammation, inhibit apoptosis, and promote nerve regeneration after the SCI. | Moharamoghli et al., 2019 |
| Acute Myocardial Infarction (AMI) | miR-320 | <0.05 | Female Wistar rats | – | – | IGF-1, ASK1, Bcl-2, Bax, Caspase-3 | p38, JNK | Downregulation of miR-320 via increasing IGF-1 could suppress cardiomyocyte apoptosis. | Song et al., 2016 |
| Cerebral I/R Injury | miR-320 | <0.01 | Male C57BL mice | – | PC12 | IGF-1 | – | overexpression of miR-320 by targeting IGF-1 could enhance brain infarction volume and edema volume in MCAO/R mice. | Liang et al., 2018 |
| Polycystic Ovary Syndrome (PCOS) | miR-323-3p | <0.05 | – | 20 pairs of PCOS lesion and normal ovarian cortex tissue samples | KGN, CCs | IGF-1, AR, AMHR-II, CYP19A, EGFR, GATA-4 | – | Downregulation of miR-323-3p by targeting IGF-1 could enhance apoptosis and increase the steroidogenesis in KGN cells. | Wang et al., 2019b |
| PCOS | miR-483 | <0.001 | – | 20 pairs of PCOS lesion and normal ovarian cortex tissue samples | KGN | IGF-1, CCNB1, CCND1, CDK2 | – | Overexpression of miR-483 possibly by targeting IGF-1 could inhibit KGN cell viability and proliferation and induces cell cycle arrest. | Xiang et al., 2016 |
| Rheumatoid Arthritis (RA) | miR-483-3p | <0.001 | – | Synovial tissues from RA patients (n = 10), healthy controls (n = 6) | HFLS, HFLS-RA | IGF-1 | – | Overexpression of miR-483-3p via targeting IGF-1 could promote cell proliferation, the G0/G1-to-S phase transition, and suppress apoptosis in RA FLSs. | Wang Y. et al., 2020 |
| Acute Myocardial Infarction (AMI) | miR-483-3p | <0.05 | – | 6 pairs of AMI patients and normal volunteers | H9c2 | IGF-1, Bax, Bcl-2, Caspase-3, Caspase-9 | – | Overexpression of miR-483-3p by targeting IGF-1 could promote apoptosis in the AMI model. | Sun et al., 2018 |
| Congenital Heart Disease | miR-486-5p | <0.01 | – | – | H9C2 | IGF-1, Bcl-2, Bax, Caspase-3, Caspase-9, | – | Downregulation of miR-486-5p by targeting IGF-1 could increase cardiomyocyte growth in hypoxic conditions. | Fan et al., 2019 |
| Coronary Heart Disease (CHD) | miR-503 | <0.05 | – | – | H9c2 | IGF-1R, Cyto-C, c-PARP, Caspase3 | PI3K/AKT | Overexpression of miR-503 by inhibiting the PI3K/AKT pathway via targeting IGF-1R could accelerate hypoxia-induced injury. | Zhu W. et al., 2018 |
| Lumbar Disc Degeneration (LDD) | miR-4458 | <0.05 | – | 24 LDD samples and 22 normal controls | SV40 | IGF-1 | PI3K/AKT | Overexpression of miR-4458 by decreasing both total IGF-1R and phosphorylated IGF-1R could lead to a decrease of phosphorylated AKT. Also, miR-4458 by suppressing the PI3K/AKT pathway via inhibiting IGF-1R could promote the development of LDD. | Liu Z. Q. et al., 2016 |
Overexpression of IGF-associated miRNAs namely miR-30a-3p, miR-155, miR-199a-3p, and miR-486-5p has important roles in different conditions such as preeclampsia, hepatocellular carcinoma, estrogen-mediated autophagy, and congenital heart disease (El Tayebi et al., 2015; Fu et al., 2018; Niu et al., 2018; Fan et al., 2019). Besides, dysregulation of miR-210-3p, miR-491-5p, and miR-615-3p contributes to the pathogenesis of atherosclerosis, colorectal carcinoma, and non-small lung cancer through modulation of IGF2 expression level (Liu j. et al., 2019; Lu et al., 2019; Qiao et al., 2020). Besides, aberrant expressions of miR-204-5p, miR-197, and miR-155-5p participate in the pathogenesis of papillary thyroid carcinoma, colorectal cancer, and non-small lung cancer through affecting expressions of IGFBP5, IGFBP3, and IGFBP1, respectively (Ling et al., 2015; Liu L. et al., 2015; Zheng et al., 2018). miR-99a, miR-503, and miR-1275 contribute to the pathogenesis of polycystic ovary syndrome, coronary heart disease, and hepatocellular carcinoma by affecting IGF-1R levels (Fawzy et al., 2015; Zhu W. et al., 2018; Geng et al., 2019). Figure 2 summarizes the role of a number of IGF-associated miRNAs in human disorders including cancers.
IGF-Associated lncRNAs in Human Disorders
Several lncRNAs have functional links with IGF-related proteins. Wang et al. have demonstrated over-expression of circ_0014130 in Non-Small Cell Lung Carcinoma tissues and cells. Down-regulation of this cirCRNA has suppressed cell proliferation and enhanced cell apoptosis in these cells. Circ_0014130 has functional interactions with miR-142-5p and IGF-1. Small interfering RNA-mediated circ_0014130 silencing has enhanced IGF-1 levels through up-regulation of miR-142-5p (Wang M. et al., 2020). Another study in this kind of cancer has shown up-regulation of HOXA-AS2 in the tumor samples. HOXA-AS2 silencing has decreased the expression of IGF2. Therefore, HOXA-AS2 promotes the migratory and invasive capacities of lung cancer cells by enhancing IGF2 expression (Zheng et al., 2019). In cervical cancer cells, the expression of linc00319 has been increased. Linc00319 silencing has suppressed cell proliferation, invasion, and migration of cervical cancer cells. This lncRNA interacts with miR-147a to modulate the expression of IGF1R (Ma et al., 2020). DBH-AS1 is another oncogenic lncRNA in hepatocellular carcinoma. Up-regulation of this lncRNA has been associated with the down-regulation of miR-138. DBH-AS1 knockdown and miR-138 up-regulation have decreased cell viability, repressed colony formation, and increased cell apoptosis. DBH-AS1 enhanced tumor growth and activated FAK/Src/ERK axis by modulating the expression of miR-138 (Bao et al., 2018). H19 is another up-regulated lncRNA in melanoma. H19 silencing has increased the sensitivity of melanoma cells to cisplatin, suppressed colony formation, and enhanced apoptosis of cisplatin-resistant melanoma cells. This lncRNA regulates IGF-1 expression through modulation of miR-18b expression (An et al., 2020). Honda et al. have assessed the methylation pattern of the H19 differentially methylated region (DMR), loss of heterozygosity, and allelic expression of IGF2 in hepatoblastoma. They reported associations between biallelic IGF2 expression and hypermethylation of H19 DMR. On the other hand, the monoallelic expression of IGF2 has been correlated with normal methylation of this region. They also reported over-expression of IGF2 and predominance of the embryonic P3 transcript in most hepatoblastoma with retention of imprinting (Honda et al., 2008). Table 2 summarizes the role of IGF-associated lncRNAs in cancers.
TABLE 2.
IGF-associated lncRNAs in cancers (NNTs: nearby normal tissues).
| Type of Cancer | lncRNA | P-value | Animal | Clinical Samples (Human) | Cell Lines | Target | Pathway | Function | References |
| NSCLC | circ_0014130 | <0.01 | – | 20 pairs of NSCLC and NNTs | H1299, A549, BEAS-2B | miR-142-5p, IGF-1 | – | Downregulation of circ_0014130 via upregulating miR-142-5p and downregulating IGF-1 expression could inhibit NSCLC cell proliferation and promotion of cell apoptosis. | Wang M. et al., 2020 |
| NSCLC | HOXA-AS2 | <0.01 | – | 63 pairs of NSCLC and NNTs | SPCA1, A549, PC-9, H1975, 16HBE | IGF-2 | – | HOXA-AS2 via upregulating IGF-2 could promote cell migration and invasion in NSCLC. | Zheng et al., 2019 |
| Cervical Cancer (CC) | LINC00319 | <0.01 | – | 60 pairs of CC and NNTs | Ect1/E6E7, HeLa, SiHa, Caski, C33A, Me180 | IGF-1R, miR-147a, Vimentin, MMP2, p21, E-cadherin | – | Downregulation of Linc00319 via targeting the miR-147a and IGF-1R could inhibit CC cell proliferation, invasion, and migration. | Ma et al., 2020 |
| Melanoma | DBH-AS1 | <0.001 | – | 62 pairs of M and NNTs | A375, HaCaT, A875 | IGF-1R, miR-223-3p, EGFR, GLUT1 | AKT | Overexpression of DBH-AS1 via miR-223-3p/EGFR/AKT axis could enhance the glycolytic activity and reduce cancer progression. | Bao et al., 2018 |
| Melanoma | H19 | <0.01 | – | 30 pairs of M and NNTs | HEMa, A375, WM35, M8, SK-MEL-2, A2508 | miR-18b, IGF1 | – | H19 via acting as a molecular sponge of miR-18b can regulate IGF-1 expression and sensitivity of melanoma cells to DDP. | An et al., 2020 |
| Hepatoblastoma | H19 | <0.05 | – | HB (n = 54), normal controls (n = 5) | HuH6, HepG2 | IGF-2, PLAG1, CTNNB1 | – | There was an association between biallelic IGF2 expression and hypermethylation of H19 DMR. | Honda et al., 2008 |
| Follicular Thyroid Cancer (FTC) | H19 | <0.05 | Male nude mice | 45 pairs of FTC and NNTs | FTC-133, FTC-238, Nthy-ori 3-1 | IGF2BP1, IGF-1, SOCS3, Pax5, miR-29-3p | JAK/STAT | H19 via the IGF1/JAK/STAT axis could suppress metastasis of FTC. | Xu et al., 2019 |
| Breast Cancer (BCa) | IRAIN | <0.05 | – | – | MDA-MB-31 | IGF-1R | – | IRAIN via targeting IGF1R could alter the phenotypes of MDA-MB-231 tumor cells. | Pian et al., 2018 |
| Bladder Cancer | circVANGL1 | <0.001 | BALB/c nude mice | 60 pairs of BLC and NNTs | SV−HUC, T24, EJ, J82, RT−4, UM−UC−3, TCC | miR−1184, IGFBP2 | – | Downregulation of circVANGL1 via inhibiting IGFBP−2 could inhibit cell invasion, migration, and growth. | Yang D. et al., 2020 |
| Breast cancer (BCa) | NR2F1-AS1 | <0.0001 | Female NOD/SCID mice | – | MDA-MB-231, MCF-7, HUVECs | IGF-1 | ERK | Overexpression of NR2F1-AS1 by increasing miR-338-3p and activating IGF-1and ERK pathway could enhance the HUVEC proliferation, tube formation, and migration ability in BCa cells. | Zhang et al., 2020 |
| BCa | SNHG7 | <0.05 | – | TCGA database | MCF7, T47D, MDA-MB-231, MCF10A | IGF-1 | MAPK | The silencing of SNHG7 could lead to cell cycle arrest in G0/G1. A negative feedback loop between SNHG7 and IGF-1 could regulate transcript levels and proliferation in BCa cells. | Boone et al., 2019 |
| Colorectal Cancer (CRC) | circRUNX1 | <0.001 | Male BALB/c nude mice | 52 pairs of CRC and NNTs | SW480, SW620, HCT116, HT29, LoVo, RKO | miR-145-5p, IGF-1 | – | Overexpression of circRUNX1 via miR-145-5p/IGF-1 Signaling could enhance cell proliferation and migration and also inhibit apoptosis and metastasis in CRC cells. | Chen et al., 2020 |
| CRC | LINRIS | <0.01 | female BALB/c nude mice | 60 pairs of CRC and NNTs | LOVO, CCD841, RKO, CW2, SW1116, SW480, SW620, DLD-1, HCT116, HT29, COLO205 | IGF2BP2, | – | LINRIS by stabilizing IGF2BP2 could promote the aerobic glycolysis in CRC cells. | Wang et al., 2019e |
| Colon Cancer | NEAT1 | <0.001 | – | 10 pairs of CRC and NNTs | SW620 HT-29, HCT 116, LoVo, SW480, NCM460 | IGF-2, miR-185-5p | – | Overexpressed NEAT1 via the miR-185-5p/IGF-2 axis could promote invasion and migration of colon cancer cells. | Zhuang et al., 2020 |
| Pancreatic Cancer (PaC) | AFAP1-AS1 | <0.01 | 63 pairs of PaC and NNTs | AsPC-1, BxPC-3, PANC-1, PaCa-2, SW1990, HPDE6c7 | IGF-1R | – | Downregulation of AFAP1-AS1 by upregulating the IGF1R oncogene via sequestration of miR-133a could suppress the tumor cell growth and invasion in PaC. | Chen B. et al., 2018 | |
| PaC | HOTAIR | <0.05 | BALB/c nude mice | 25 pairs of PaC and NNTs | BXPC3, 293T, CFPAC-1, Panc-1, L3.6pl | IGF-2, miR-663b, Caspase-3, Caspase-9 | – | HOTAIR by inhibiting miR-663b via upregulating IFG-2 could promote PaC cell proliferation. | Cai et al., 2016 |
| Renal Cell Carcinoma (RCC) | HOTTIP | <0.01 | – | TCGA database, 57 pairs of RCC and NNTs | A-498, 786-O, Caki-1, Caki-2, ACHN, HK-2, 293T | IGF-2, hsa-miR-615-3p | – | HOTTIP by regulating the miR-615/IGF-2 axis could promote RCC progression. | Wang Q. et al., 2018 |
| Glioma | linc01023 | <0.001 | nude mice | Glioma (n = 169), normal brain tissues (NBTs, n = 30) | U87, U251, NHA | IGF-1R | AKT | Knockdown of linc01023 by regulating the IGF-1R/AKT axis could restrain glioma proliferation, migration, and invasion. | Yu et al., 2019 |
| Hepatocellular carcinoma (HCC) | DLEU1 | <0.01 | Male BALB/c nude mice | 56 pairs of HCC and NNTs | SMMC-7721, Hep3B, HepG2, Huh-7, LO2 | IGF-1R, miR-133a, E-cadherin, N-cadherin Vimentin | PI3K/AKT | DLEU1 by sponging miR−133a to regulate IGF−1R expression through the PI3K/AKT pathway could promote HCC progression. | Zhang W. et al., 2019 |
| Tongue Squamous Cell Carcinoma (TSCC) | THOR | <0.05 | – | 55 TSCC and 31 NNTs | Tca-8113 and Cal-27 | IGF2BP1, IGF-2, Cyclin-E1, Cyclin-D1, p21, p27, | MEK-ERK | THOR by stabilizing IGF2BP1 could increase TSCC cell proliferation. | Wang et al., 2019a |
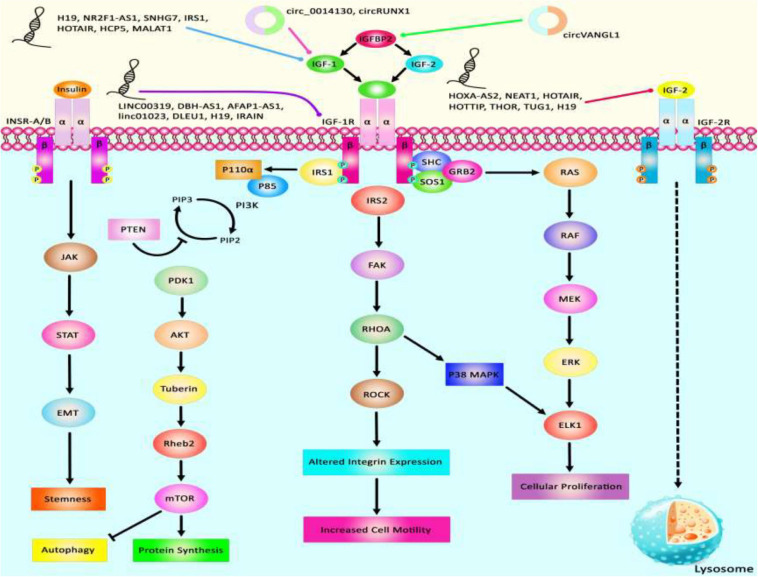
Via regulation of the IGF−1 signaling pathway, H19 can modulate proliferation and apoptosis of male germline stem cells. H19 silencing has reduced the cell quantities in the seminiferous tubule (Lei et al., 2019). Expression of the lncRNA 150Rik has been enhanced in renal tissue of animal models of diabetic nephropathy and in mesangial cells cultured in hyperglycemic media. This lncRNA regulates mesangial cell proliferation through interacting with miR-451, thus regulating the IGF1R/p38MAPK axis (Zhang et al., 2018). LncIRS1 has been shown to act as a molecular sponge for miR−15a, miR−15b−5p, and miR−15c−5p to modulate the expression of IRS1 a downstream target of the IGF1-R. Up-regulation of lncIRS1 has enhanced IRS1 expression and increased phosphorylation of AKT as an important element in the IGF-1 pathway. LncIRS1 can also regulate the expression of atrophy−associated genes and affect muscle atrophy (Li Z. et al., 2019). TUG1 is an up-regulated lncRNA in ox-LDL-exposed vascular smooth muscle cell (VSMC) and HUVEC. Its silencing has suppressed proliferation and enhanced apoptosis in ox-LDL-exposed VSMC but has exerted opposite effects in HUVEC. miR-148b has been identified as a target of TUG1 in these cells. In turn, miR-148b has been shown to target IGF2. Therefore, TUG1 enhances IGF2 levels by sequestering miR-148b (Wu X. et al., 2020). HCP5 is a lncRNA that is involved in the pathogenesis of polycystic ovarian syndrome (PCOS). Down-regulation of this lncRNA inhibits cell proliferation via inducing cell cycle arrest at the G1 phase and stimulating the mitochondrial apoptotic route. miR-27a-3p has been recognized as a direct target of HCP5. This miRNA can bind with IGF-1. Therefore, HCP5 can be involved in the development of PCOS via modulating the miR-27a-3p/IGF-1 axis (Luo L.-H. et al., 2020). Figure 3 represents the dysregulation of various types of lncRNAs which have a remarkable role in negatively modulating IGF1, IGF2, IGFBP2, and IGF-1R through the IGF signaling pathway in different human cancers. Table 3 summarizes the information about the role of IGF-associated lncRNAs in non-neoplastic conditions.
FIGURE 3.
A schematic summary of lncRNAs that target IGF signaling cascade. IGF1, IGF2, IGFBP2, and IGF-1R are among proteins that are regulated by lncRNAs. Abnormal levels of lncRNAs can affect the carcinogenesis process by influencing autophagy, cell proliferation, protein synthesis, and stemness.
TABLE 3.
IGF-associated lncRNAs in non-malignant disorders.
| Type of disorder | lncRNA | P-value | Animal | Clinical Samples (Human) | Cell Lines | Target | Pathway | Function | References |
| – | H19 | <0.05 | Bovin | – | GC1, mGSCs | IGF-1, IGF-1R, C-MYC, PNCA, p53, Caspase-3, | AKT, ERK | H19 via the IGF−1 pathway could regulate the proliferation of bovine male germline stem cells. | Lei et al., 2019 |
| Diabetic Nephropathy (DN) | 150Rik | <0.01 | Male db/db mice | – | – | miR-451/IGF1R, Ago2 | p38 MAPK | Overexpression of 150Rik via miR-451/IGF-1R/p38 MAPK pathway could promote mesangial cell proliferation in DN. | Zhang et al., 2018 |
| Skeletal Muscle Atrophy | IRS1 | <0.05 | Hypertrophic (WRR) and leaner broilers (XH) | – | DF-1 | IGF1 | PI3K/AKT | Overexpression of lncIRS1 via increasing miR-15 to activate IGF1-PI3K/AKT signaling could promote muscle proliferation, differentiation, and muscle mass. | Li Z. et al., 2019 |
| Atherosclerosis | TUG1 | <0.001 | – | – | HUVEC, VSMC | IGF-2, miR-148b, Bax, Bcl-2, PCNA | – | TUG1 by regulating the miR-148b/IGF2 axis could regulate apoptosis and proliferation in ox-LDL-stimulated HUVEC and VSMC. | Wu X. et al., 2020 |
| Polycystic Ovary Syndrome (PCOS) | HOTAIR | <0.01 | Rat | – | Granulosa cells | IGF-1, miR-130a | – | Downregulation of HOTAIR by reducing the expression of IGF-1 via miR-130a could alleviate PCOS in rats. | Jiang et al., 2020 |
| Polycystic Ovarian Syndrome (PCOS) | HCP5 | <0.001 | – | – | KGN | IGF-1, miR-27a-3p, caspase-9, Bax, Bcl-2 | – | Downregulation of HCP5 via the IGF-1/miR-27a-3p axis could induce apoptosis and also could suppress cell proliferation by arresting cell cycle progression at the G1 phase. | Luo L.-H. et al., 2020 |
| Preeclampsia (PE) | MALAT1 | <0.01 | – | 30 pairs of PE and matched normal pregnant women | HTR-8/SVneoc, JEG-3 | miR-206, IGF-1 | PI3K/AKT | Downregulation of MALAT1 via knockdown of IGF-1 and upregulating miR-206 could suppress the trophoblast cell migration and invasion. | Wu H. Y. et al., 2020 |
| PE | MALAT1 | <0.01 | – | 30 patients with PE and 30 normal samples | HTR-8/SVneoc, JEG-3 | IGF-1, miR-206 | PI3K/AKT | MALAT1 via regulating the miR-206/IGF-1 axis through the PI3K/AKT pathway could regulate trophoblast cell migration and invasion. | Wu H. Y. et al., 2020 |
| Liver Fibrosis | H19 | <0.01 | Male Sprague-Dawley rats | – | HSC-T6 | IGF1R, MeCP2, a-SMA, Col1A1, miR-200a | – | H19 by targeting the MeCP2/IGF1R axis control hepatic stellate cell proliferation. | Yang et al., 2016 |
| Endometriosis | H19 | =0.035 | – | – | Human endometrial stromal cells | IGF-1R, let-7 | – | Downregulation of H19 via the let-7/IGF-1R axis could reduce the proliferation of endometrial stromal cells. | Ghazal et al., 2015 |
LncRNAs that regulate the expression of IGF1, IGF2, IGF-1R, and IGFBPs can participate in the pathogenesis of human disorders. H19, NR2F1-AS1, and SNHG7 participate in the development of melanoma and breast cancer through modulation of IGF1 (Boone et al., 2019; An et al., 2020; Zhang et al., 2020). NEAT1, THOR, and HOTTIP via targeting IGF2 affect carcinogenic processes in colorectal cancer, tongue squamous cell carcinoma, and renal cell carcinoma (Wang Q. et al., 2018; Yang et al., 2019; Zhuang et al., 2020). Additionally, downregulation of circVANGL1 through suppressing the expression level of IGFBP2 could attenuate breast cancer cell invasion, migration, and proliferation (Yang D. et al., 2020). Also, IRAIN, Linc00319, and DLEU1 through negatively regulating IGF-1R could cause breast cancer, cervical cancer, and hepatocellular carcinoma (Pian et al., 2018; Zhang W. et al., 2019; Ma et al., 2020). Figure 3 summarizes the role of these IGF-associated lncRNAs in human disorders.
Diagnostic/Prognostic Values of IGF-Associated miRNAs/lncRNAs in Cancers
A number of miRNAs and lncRNAs which are functionally linked with IGF signaling have potential applications as diagnostic/prognostic markers in cancers. Zhuang et al. have demonstrated high accuracy of NEAT1 levels in distinguishing colon cancer tissues from normal ones (area under the receiver operating curve = 0.89) (Zhuang et al., 2020). Expression levels of the IGF-associated miRNAs miR-485-5p and miR-155-5p have been associated with the survival of patients with lung cancer and Wilms tumor, respectively (Huang et al., 2018; Luo X. et al., 2020). Also, Linc00319, H19, AFAP1-AS1, SNHG7, HOTTIP, linc01023, DLEU1, and NEAT1 have been identified as prognostic markers in diverse kinds of cancer (Table 4).
TABLE 4.
Prognostic values of miRNAs/lncRNAs in cancers (NNTs: nearby normal tissues).
| Sample number | Kaplan-Meier analysis | Univariate/multivariate cox regression | References |
| 87 pairs of lung cancer and NNTs | Downregulation of miR-485-5p was correlated with poor prognosis in NSCLC. | – | Huang et al., 2018 |
| 87 pairs of WT tissues and paracarcinoma kidney tissues | miR-155-5p and IGF2 level did not correlate with the survival time of WT patients. | – | Luo X. et al., 2020 |
| 60 pairs of cervical cancer samples and NNTs | Higher Linc00319 expression level was related to the low survival rate | – | Ma et al., 2020 |
| 44 pairs of resected OSCC and NNTs | Downregulation of miR-375 is correlated with tumor progression and poor prognosis of OSCC patients. | – | Zhang et al., 2017 |
| 60 pairs of HCC tissues and NNTs | Reduced expression of miR-505 was correlated with the worse prognosis of HCC patients. | Tumor size, Lymph node metastasis, and TNM stage were correlated with prognosis. | Ren et al., 2019 |
| 150 pairs of HCC tissues and NNTs | Downregulation of miR-216b was correlated with poor prognosis in HCC. | – | Liu F. Y. et al., 2015 |
| 62 paired HCC samples and NNTs | Downregulation of miR-29a-3p was correlated with poor prognosis in HCC. | – | Wang X. et al., 2017 |
| 80 pairs of CRC and NNTs | The decreased miR-491-5p expression level was associated with poor overall survival in CRC patients. | Differentiation level and TNM stage were correlated with prognosis. | Lu et al., 2019 |
| 30 pairs of malignant melanoma tissues and NNTs | Lower expression of lncRNA H19 was associated with better overall survival. | – | Men et al., 2020 |
| 89 pairs of LGG tissues and NNTs | Low expression of miR-138 was associated with poor prognosis in LGG patients. | – | Yang Y. et al., 2020 |
| 90 pairs of GC tissues and NNTs | Downregulation of miR-598 was correlated with poor prognosis in GC. | – | Liu et al., 2018c |
| 63 pairs of PC and NNTs | Upregulation of AFAP1-AS1 was correlated with poor overall survival in patients with PC. | – | Chen B. et al., 2018 |
| 40 pairs of glioblastoma tissues and non-tumor tissues | Lower expression of miR-15b was associated with a shorter survival rate. | – | Wang J. et al., 2017 |
| TCGA database | Higher expression of SNHG7 was associated with a lower OS rate. | – | Boone et al., 2019 |
| TCGA database, 57 pairs of RCC and NNTs | Higher expression of HOTTIP was associated with lower OS and DFS rates. | Higher expression of HOTTIP was associated with pathological grade, tumor size, and TNM stage. | Wang Q. et al., 2018 |
| Glioma (n = 169), NBTs (n = 30) | Higher expression of linc01023 was associated with a lower OS rate. | – | Yu et al., 2019 |
| 56 pairs of HCC and NNTs | Higher expression of DLEU1 was associated with a lower OS rate. | – | Zhang W. et al., 2019 |
| 10 pairs of CRC and NNTs | Higher expression of NEAT1 was associated with a lower OS rate. | – | Zhuang et al., 2020 |
Importance of IGF-Associated Pathways in Response to Chemotherapy
IGF-associated molecules have been involved in the resistance of cancer cells to chemotherapeutic agents. In some cases, miRNAs or lncRNAs have been identified as molecules that mediate this phenotype. For instance, H19 silencing has enhanced the sensitivity of cancer cells to cisplatin and increased apoptosis of cisplatin-resistant melanoma cells through modulation of IGF1 expression (An et al., 2020). In a number of ovarian cancer cell lines, IGF-2 expression has been higher in Taxol-resistant cells compared with chemosensitive cell lines. Transient IGF2 silencing has enhanced Taxol sensitivity in these cells. However, IGF1R blocking did not affect the chemosensitivity of these cells. These results have supported the role of IGF-2 as a possible therapeutic target in drug-resistant ovarian cancer (Brouwer-Visser et al., 2014). IGF-1 has been shown to confer resistance to docetaxel in prostate cancer cells. IGF-I treatment has reduced expression of miR-143 expression, while enhanced expression IGF-1R and IRS1, direct targets of this miRNA. Up-regulation of miR-143 has stopped IGF-I-associated resistance to docetaxel, reduced expressions of IGF-I, IRS1, and VEGF in these cells (Niu et al., 2017). Table 5 reviews the importance of IGF-related pathways in response to chemotherapy.
TABLE 5.
Importance of IGF-associated pathways in response to chemotherapy.
| Type of cancer or disease | microRNA/lncRNA | Animal | Clinical Samples (Human) | Cell lines | Target | Pathway | Function | References |
| Melanoma | H19 | – | 30 pairs of M and NNTs | HEMa, A375, WM35, M8, SK-MEL-2, A2508 | miR-18b, IGF1 | – | Downregulation of H19 could increase the sensitivity of melanoma cells to DDP. | An et al., 2020 |
| Ovarian cancer (OC) | – | Female athymic nude mice (Harlan) | 489 cases of high grade serous OC | A2780, HEY, NIH: OVCAR-8, HET-T30, A2780-T15, HEY-B20, HEY-Epo8, OVCAR-8-D30 | IGF-2 | AKT, ERK | Downregulation of IGF-2 could activate taxol sensitivity in drug-resistant OC. | Brouwer-Visser et al., 2014 |
| OC | – | – | – | HEY, OVCAR-8, SKOV-3, BG-1, A2780, HEY-T30 | IGF-1R | PI3K, ERK | Overexpression of the IGF-1 could induce cisplatin resistance of OC cells. | Eckstein et al., 2009 |
| OC | – | – | 134 pairs of OC and NNTs | A2780, HEY, HEY-T30 | IGF2 | AKT | Downregulation of IGF-2 could reduce drug-resistant OC cells to taxol. | Huang et al., 2010 |
| OC | – | – | 212 pairs of OC and NNTs | – | IGF-II | – | IGF-II and its SNP could be associated with elevated risks of disease progression and death in epithelial OC. | Lu et al., 2013 |
| Breast Cancer (BCa) | – | – | – | Hs578T, Hs578T/PTX | IGFBP-3, Caspase-3 | – | Paclitaxel could increase the endogenous IGFBP-3 production, then induce apoptosis of Hs578T human BCa cells. | Fowler et al., 2000 |
| BCa | – | – | – | MCF-7, CAMA-1, MDA-MB-361, HCC1954, BT474, MDA-MB-453, UACC893, HCC70, MDA-MB-435S, ZR75-30, HCC1419, SKBR-3, BT549, T47D, ZR75-1, MDA-MB-231 | IGF-1R, IRS-1 | PI3K/AKT | NVPAEW541 treatment via Inhibiting IGF-1R and the PI3K/AKT pathway could lead to inhibit cell growth and increase the effect of chemotherapeutic drugs. | Mukohara et al., 2009 |
| BCa | – | – | 24 BC patients and 16 healthy women | – | IGF-1 | – | Adjuvant cyclophosphamide, methotrexate, and 5-fluorouracil could decrease the plasma concentration of IGF-1 in premenopausal BCa women. | Kajdaniuk et al., 2001 |
| BCa | – | – | 73 pairs of BCa and NNTs | Py230, 4T1, MDA-MB231 | IGF-1/2 | – | Downregulation of IGF1/2 in combination with paclitaxel could reduce tumor cell proliferation and lung metastasis in pre-clinical BCa models. | Ireland et al., 2018 |
| Triple-Negative Breast Cancer (TNBC) | – | Female BALB/c nude | – | MDA-MB-468, HCC1806 | GFBP-3, EGFR, S1P, SphK-1, Caspase-3 | – | Inhibition of EGFR and SphK could lead to a block of IGFBP-3-dependent signaling and inhibit cell proliferation in TNBC. | Julovi et al., 2018 |
| Pancreatic Cancer | – | Female Fox Chase SCID Beige mice | – | KP-4, BxPC-3, Capan-2, CFPAC-1, HPAF-II, SU8686, SW1990, AsPC1, PANC1 | IGF-1R, ErbB3 | PI3K/AKT | IGF-1 and HRG by targeting the PI3K/AKT pathway could reduce pancreatic cancer cell sensitivity to gemcitabine or paclitaxel. | Camblin et al., 2018 |
| Prostate Cancer | miR-143 | Male BALB/cA-nu nude mice | – | PC-3, DU145, 293T, DU145/DTX, PC-3/DTX | IGF-1/R, VEGF | – | Overexpression of miR-143 could reduce IGF-1-induced chemoresistance to docetaxel treatment and inhibit tumor growth in vivo. | Niu et al., 2017 |
| Prostate Adenocarcinoma (PaC) | – | – | – | DU145, PC-3, DU145/DTX, PC-3/DTX | IGF-I, IGFBP-2 | PTEN | Downregulation of IGFBP-2 could increase the sensitivity of Cap cells to docetaxel. | Uzoh et al., 2011 |
| CRC | miR-143 | – | 62 pairs of CRC and NNTs | – | IGF-1R | – | Upregulation of miR-143 by targeting IGF-1R could inhibit cell proliferation, migration, and also increase chemo-sensitivity to oxaliplatin. | Qian et al., 2013 |
| CRC | – | – | – | WiDr, SW620, HMEC-1, | IGF-1, HIF-1α | – | IGF-1 could increase the cell viability of stromal and cancer cells in response to chemotherapy in CRC. | Volkova et al., 2014 |
| CRC | miR-497 | 131 pairs of CRC and NNTs | HCT116, HCT28, LoVo, Colon205, SW480, SW620, CRL-1831 | IGF-1R | PI3 K/AKT | Overexpression of miR-497 via inhibiting IGF1-R activity could increase sensitivity to apoptosis induced by chemotherapeutic drugs in CRC cells. | Guo et al., 2013 | |
| Adrenocortical Carcinoma (ACC) | – | – | 17 ACCs and 6 normal adrenal tissue samples samples | H295R, HAC15 | IGF-2, IRA, IGF-1R, IGF-2R | mTOR | Linsitinib treatment by IGF pathway could inhibit cell growth in the H295R and HAC15 cell lines | De Martino et al., 2019 |
| Gastric Cancer | – | – | 3 pairs of GC and NNTs | – | IGFBP-3, ICAM-1, VCAM-1, p65, NF-kB, IkB | – | Overexpression of IGFBP-3 could increase cell growth inhibition via suppressing the NF-kB activity by regulating ICAM-1 and VCAM-1 in GC cells. | Kim and Lee, 2015 |
| Gastrointestinal Stromal Tumor (GIST) | – | – | – | GIST-882, GIST-T1, GIST-882/imatinib, GIST-T1/imatinib | IGF-1R, | – | Knockdown of CCDC26 by regulating IGF-1R could induce imatinib resistance in GIST cells. | Xie et al., 2019 |
| Esophageal Squamous Cell Carcinoma (ESCC) | – | – | – | SLMT-1, SLMT-1/CDDP1R, SLMT-1R-pcMV3, SLMT-1R-IGFBP5 | IGFBP5 | – | Downregulation of IGFBP5 could induce cisplatin-resistance in ESCC cells. | Chan et al., 2018 |
| Brain Tumor | – | – | – | MCH-BT-31, MCH-BT-39, MCH-BT-30, MCH-BT-52, HTB-14 | IGF-1R, IGF-I, IGF-II, P-gp | PKC | Tamoxifen treatment could reduce PKC activity and IGF-II expression in brain tumor cells. | Ramachandran et al., 2004 |
| Glioma | – | – | – | U-87MG, KNS-42 | CPP32, Bcl-2 | p53 | Overexpression of IGF-I by increasing the expression of Bcl-2 and decreasing the activity of CPP32 could decrease apoptosis in glioma cells. | Yin et al., 2005 |
| Non-Small-Cell Lung Carcinoma (NSCLC) | – | Nude mice | NSLC (n = 14), patients without cancer (n = 9) | SCC-25, HeLa, SCC-25/CP, KB-3-1, KB-CP, 2008/CP, IGROV1/CP, A2780/CP, PC-9/CDDP, PC-14/CDDP, SBC-3/CDDP | IGFBP7, MKP3 | MAPK Erk1/2, MEK/Erk, Stat3 | Downregulation of IGFBP7 could increase cellular resistance to cisplatin. | Okamura et al., 2012 |
| NSCLC | – | – | – | SKMES1, SKMES, SKLu-1, Calu-3, H1299, H460, H157, HCC44, A549, H1975 | IGF-1, IGF-1R, VEGF | AKT, MAPK | AVE1642 treatment by targeting IGF-1 could increase the paclitaxel-mediated anti-tumor effect. | Spiliotaki et al., 2011 |
| NSCLC | miR-223 | Male nude mice | – | PC-9, PC-9/ER, PC-9/ER-EV | IGF-1R | AKT/S6 | Overexpression of miR-223 by relating IGF-1R could inhibit tumor growth in nude mice and also increase the sensitivity to erlotinib. | Zhao et al., 2016 |
| NSCLC | – | – | – | NCI-H460, H1299, A549 | IGF-1, Chk2, Chk1 | p53 | Overexpression of IGF-1 could recover cisplatin-derived inhibition of proliferation and apoptosis in NSCLC cells. | Jeon et al., 2008 |
| NSCLC | – | Female athymic nude mice | – | H460 | IGF-1, IGFBP-3 | – | Targeting of the IGF-1 receptor using siRNA could result in the sensitization of cisplatin-R-cells to cisplatin and radiation. | Sun et al., 2012 |
| NSCLC | – | – | – | A549, A549/PTX | IGF-1, SphK1, Vimentin, Fibronectin, N-cadherin, E-cadherin | ERK, AKT | IGF-1 treatment via activating SphK1, ERK, and AKT could decrease the sensitivity of A549 cells to paclitaxel. | Wu et al., 2019 |
| NSCLC | LUCAT1 | – | – | A549/DDP, A549 | IGF-2 | – | Overexpression of LUCAT1 by regulating IGF-2 could promote the cisplatin resistance in NSCLC. | Wang et al., 2019c |
| Cardiac Toxicity | – | – | – | H9c2 | IGF-1R, IGFBP-3 | p53 | Doxorubicin via inhibiting IGF-1R and upregulating IGFBP-3 through p53 could lead to resistance to IGF-1 that may contribute to doxorubicin-initiated apoptosis. | Fabbi et al., 2015 |
IGF Signaling Pathway in Tumorigenesis and Progression of Chemotherapeutic Drug Resistance Providing the New Concepts in Cancer Therapy
One of the major impediments to current cancer remedy endeavors is the induction of drug resistance by tumors. Despite recent improvements in diagnostic methods and surgical interventions, many aggressive tumors have a poor response to adjuvant or neoadjuvant chemotherapy and radiation. The IGF signaling axis has been detected to have a pivotal role in the progression and development of a variety of tumors (Denduluri et al., 2015). The IGF-1R is involved in various human cancers, such as ovarian, breast, pancreatic, glioma, hepatocellular, lymphoma, and non-small lung cancers. In some cases, its anti-apoptotic attributes strengthen cancerous cells to resist the cytotoxic characteristics of chemotherapeutic agents or radiotherapy (Beauchamp et al., 2009; Dool et al., 2011; Awasthi et al., 2012; Zhou, 2015). Zhou et al. demonstrated that the IGF-1R kinase inhibitor nVp-ADW742 combined with temozolomide could trigger inhibition of P38, GSK3β, and AKT phosphorylation along with a considerable reduction in the intracellular expression levels of Bcl-2, P38, and GSK3β, thereby resulting in promoting response to chemotherapeutic drug temozolomide in medulloblastoma to a large extent (Zhou et al., 2011). Also, Vewinger et al. have illustrated that the IGF signaling pathway has an important role in HGNET-BCOR brain tumor since IGF-1R could be a significant target to improve the sensitivity of vinca alkaloids, vinblastine, doxorubicin, ceritinib, and actinomycin D as efficient drugs in patients affected with this kind of brain tumor. As a consequence, utilizing the off-target IGF1R suppressor ceritinib may pave the way for the remedy of tumor cells driven by IGF1R and IGF2 (Vewinger et al., 2019). In another study, Valerie et al. have indicated that the activity of histone deacetylase inhibitors (HDACi) has reduced in Ewing sarcoma patients. Drug combinations of temozolomide with the dual ALK and IGF-1R inhibitor, AZD3463 could suppress AKT and STAT3 to promote the cytotoxic impacts of temozolomide, and thereby decreasing cell proliferation and enhancing apoptosis via cleavage of PARP and caspase-3 indicating that AKT and STAT3 activation could be modulated by ALK and IGF-1R signaling pathway (Sampson et al., 2015). Additionally, Refolo et al. have figured out that the combined treatment with regorafenib, vitamin K1, and two IGF-1R tyrosine kinase inhibitors GSK1838705A or OSI-906 could strengthen antitumor effects of the target drug, improving their actions and decreasing their toxicity to a large extent. Therefore, both IGF1-R inhibitors could enhance the pro-apoptotic and antiproliferative impacts of regorafenib and VK1 in hepatocellular carcinoma downregulating both MAPK and PI3K/AKT signaling pathways (Refolo et al., 2017). Supplementary Table 2 summarizes the results of various studies that indicate utilizing IGF-1R drug inhibitors with the aim of suppressing the anti-apoptotic properties of IGFR which cause cancerous cells to resist the cytotoxicproperties of chemotherapeutic drugs or radiotherapy.
Epigenetic Regulation of IGF-I, IGF-II, IGF-1R, and IGFBPS of IGF Axis in a Variety of Human Cancers
Accumulating evidence indicates that dysregulation of epigenetic systems has an important role in cancer pathogenesis resulting in overexpression of altered target genes as well as malignant cellular transformation. Since the IGF axis could contribute to cancer progression and invasion, it is now widely accepted that aberrant methylation of IGFBP7, IGFBP-4, IGFBP-3, IGF-1R, IGF-1, and IGF-II promoters could be a potential factor in various common human cancers (Qian et al., 2011; Sato et al., 2011; Bolomsky et al., 2015; Ye P. et al., 2016). Beeghly et.al have demonstrated that differential promoter P2 and P3 methylation patterns of the IGF-II gene could be remarkably related to promoting the risk of disease progression in epithelial ovarian cancer, especially hypermethylation of P2 could be associated with unpleasant symptoms of this serious disease (Beeghly et al., 2007). Additionally, another research indicated that epigenetic alterations in the IGF signaling pathway could play an effective role in the emergence of hepatocellular carcinoma. Therefore, considerable demethylation and upregulation of IGFBP3 via employing 5-Aza-2′- deoxycytidine and trichostatin A therapy results in attenuating cell proliferation and decreasing colony formation in HCC cells (Han et al., 2015). Chang et al. have illustrated that hypermethylation of the IGFBP-3 promoter which dramatically suppressed the expression level of this target gene could be substantially related to poor prognosis among Non-Small Cell Lung Carcinoma patients. Therefore, utilizing demethylation agents to upregulate the expression of IGFBP-3 could pave the way for providing a pivotal remedial procedure for these patients (Chang et al., 2002). Besides, Dar et al. have discovered that epigenetic silencing of IGFBP3 via hypermethylation of its promoter in human melanoma cells. Upregulation of IGFBP3 through applying 5AZA treatment resulting in inhibiting cancer cell survival, triggering tumor cell death, decrease colony formation and invasion, inducing expression of the pro-apoptotic genes containing PUMA, p21, and BAX as well as caspase 3 cleavage and downregulating phosphorylation of AKT (Dar et al., 2010). Furthermore, Schayek et al. have indicated that hypermethylation of AR promoter in metastatic prostate cancer cells results in downregulation of IGF1R expression levels which indicates the fact that the IGF1R gene has been detected s a downstream target for AR action. Employing 5-Aza treatment could trigger demethylation of AR promoter and as a consequence the expression level of IGF1R could increase significantly which may consider as a promising therapy in human prostate cancer (Schayek et al., 2010). An overview of promoter methylation and epigenetic modulation of various genes relevant to the IGF signaling pathway in different human cancers is represented in Supplementary Table 3.
Applying Remedial Crispr and siRNA State-Of-The-Art Genome Editing Systems to Manipulate the IGF Signaling Pathway in Various Human Cancers
It is now accepted that gene silencing via CRISPR-Cas9 and small interfering RNA (siRNA) is becoming an inevitable gene-editing tool in biological research, especially to repair genetic defects via editing or knock out various genes related to the IGF signaling pathway. Via applying a CRISPR/Cas9 or siRNA genome editing tool, it could be possible to knock out or edit ectopic expression of various genes related to IGF signaling cascade through which we could be able to improve response to chemotherapeutic agents as well as attenuating tumor cell survival, proliferation, invasion, angiogenesis, and metastasis of different kinds to a large extent (Singh et al., 2008; Brouwer-Visser et al., 2014; Hussmann et al., 2017; Strub et al., 2018). Liu et al. have detected that knockdown of IGF2BP1 expression level through applying a CRISPR/Cas9 genome editing system could play a crucial role in repressing the expression levels of IGF2, Gli1, CD44, and Myc in skin SCC cells through which tumor cell proliferation and survival were suppressed considerably. Likewise, via utilizing siRNA-mediated knockout of IGF2BP1-bound lncRNA THOR, skin SCC cell growth could be suppressed dramatically (Liu et al., 2018e). In addition, another research demonstrated that silencing IGF1R expression through employing a CRISPR/Cas9 genome editing system leads to functional endpoint mechanism for TKI resistance in a targetable direction MET-amplification, and thereby resulting in improving response to treatment via suppressing resistance to Erlotinib in Non-Small Cell Lung Carcinoma cells and inhibiting epithelial-mesenchymal transition in tumor cells (Hussmann et al., 2017). Besides, Strub et al. have demonstrated that via applying a CRISPR–Cas9 screen targeting chromatin regulators the histone deacetylase SIRT6 haploinsufficiency could play an effective role in upregulating IGFBP2 expression level through promoting chromatin availability, H3K56 acetylation at the IGFBP2 locus, and overexpression of IGF-1R function as well as downstream AKT signaling cascade. Additionally, elevating the IGFBP2 expression could lead to attenuate sensitivity to MAPK signaling inhibitors, and thereby increasing BRAFV600E melanoma cell survival via triggering IGF-1R/AKT signaling pathway. Thus, incorporating a clinically suitable IGF-1Ri with BRAFi could pave the way for promoting the sensitivity of SIRT6 haploinsufficient melanoma cells (Strub et al., 2018). Besides, another research indicated that POU2F3 can be expressed particularly in variant SCLC cancers that have the insufficient expression of neuroendocrine markers and markers of a chemosensory lineage. They applied domain-focused CRISPR screening as a suitable procedure to identify POU2F3 as a significant transcription factor in a subset of SCLC cells and to display other important associations in POU2F3-expressing SCLC lines, containing the lineage TFs SOX9 and ASCL2 and IGF1R. Besides, this strategy shed light on the fact that upregulation of IGFBP5 through employing lentivirus in POU2F3high SCLC lines could suppress tumor cell growth remarkably (Wu et al., 2018). Baade Rø et al. have illustrated that there are an extreme intricacy and interaction between the chemokine and cytokine network triggering migration. They have detected the positive relevance among the degree of cytokine-induced migration and phosphorylation of PAK. PAK phosphorylation was considerably elevated when tumor cells were triggered by combinations of SDF-1a, IGF-1, and HGF which could play an effective role in promoting myeloma cell migration to the large extent. Therefore, via utilizing small interfering RNA, the expression of PAK was downregulated leading to attenuating cytokine-driven migration (Rø et al., 2013). Another study detected that silencing expression of IGFBP-6 or IGF-I or IGF-II through applying siRNA mechanism as well as knockdown IGF-1R activity on fibroblasts could lead to altering fibroblast mobilization, attenuating tumor invasion and TME remodeling through the IGFs/IGF-1R axis in breast epithelial cells which can be considered as a helpful tool for pivotal therapeutic of breast cancer related to dysregulation of IGF signaling pathway (De Vincenzo et al., 2019). Additionally, Brouwer-Visser et.al indicated that suppressing the expression level of IGF2 in ovarian cancer cells via employing RNA interference technology could elevate paclitaxel sensitivity and could restore sensitivity to both microtubule-stabilizing and destabilizing agents (Brouwer-Visser et al., 2014). A summary of clinical researches with the aim of editing or knocking down aberrant expression of different target genes relevant to IGF signaling pathway in various human cancers via employing CRISPR/Cas9 and siRNA gene-editing tools are demonstrated in Tables 6, 7, respectively.
TABLE 6.
Pre-clinical studies employing the CRISPR/Cas9 system with the aim of editing or knocking down various target genes related to the IGF signaling pathway in different human cancers.
| Type of cancer or disease | Target | In vitro | Cell line | Animal | In vivo | CRISPR | Vector | Treatment | Pathway | Function | References |
| Diffuse large B-cell lymphoma (DLBCL) | YAP | + | LY1, LY3 | 5-week-old female SCID beige mice | + | Knockout | Lentiviral | Doxorubicin | Hippo-YAP | Knockdown of YAP expression level through a CRISPR/Cas9 genome editing system accompanied with suppression of the expression level of IGF-1R leading to the activation of downstream targets CTGF and CYR61, and thereby could remarkably inhibit tumor cell proliferation and cause cell cycle arrest in DLBCL cells. | Zhou et al., 2020 |
| BRAF V600E melanoma | SIRT6 | + | SKMel-239 | 6-week-old female athymic mice (NCrnu/nu) | + | Screening (targeting ∼140 chromatin factors) | Lentiviral | Dabrafenib, Trametinib | IGF-1R/AKT | Employing CRISPRCas9 screen targeting chromatin regulators illuminate that SIRT6 haploinsufficiency could upregulate IGFBP2 expression level as well as attenuate sensitivity to MAPKi, and thereby enhancing BRAFV600E melanoma cell survival via triggering IGF-1R/AKT signaling pathway. | Strub et al., 2018 |
| BRAF V600E melanoma | PTRF | + | MM121224 | – | – | Knockout (targeting exon 1) | Lentiviral | Vemurafenib, Encorafenib | TGFβ, MAPK, IGF | Proteomic analysis of CRISPR/Cas derived PTRF knockouts demonstrated that two markers (PTRF and IGFBP7), which are considerably overexpressed, have an effective contribution to MAPKi resistance and EMT as well as promoting cell adhesion and sphere formation in melanoma cells harboring BRAF mutations. | Paulitschke et al., 2019 |
| Skin squamous cell carcinoma (SCC) | IGF2BP1 | + | A431 | SCID mice | + | Knockout | Lentiviral | – | – | Knockdown of IGF2BP1 expression level via a CRISPR/Cas9 genome editing system could downregulate the expression levels of IGF2, Gli1, CD44, and Myc, and thereby attenuating proliferation and survival of skin SCC cells. | Liu et al., 2018e |
| Breast cancer (BCa) | IRAIN | + | MDA-MB-231 | – | – | Insertion a strong CMV promoter in front of IRAIN to upregulate IRAIN lncRNA via inducing homologous recombination | Lentiviral | – | IGF1R | Via employing CRISPRCas9 gene-editing system IRAIN could compete in cis with the overlapping IGF1R promoter, and thereby suppress the IGF1R signaling cascade that in turn attenuate tumor cell proliferation and metastasis in BCa cells. | Pian et al., 2018 |
| Colorectal cancer (CRC) | CXCR4 | + | HT115, COLO201 | – | – | Knockout | Plasmid | – | IGF1R | Knockdown of CXCR4 expression level via applying a CRISPR/Cas9 genome editing system in CRC cells could inhibit tumor angiogenesis triggered via IGF1R with the help of SDF-1 in the tumor microenvironment. | Zheng et al., 2017 |
| Ewing sarcoma (EWS) | PAPPA | + | EW8 | 6–8-week-old male NSG mice | + | Knockout | Not available | – | IGF-1 | Knockdown of PAPPA expression level via applying a CRISPR/Cas9 genome editing system could overwhelmingly attenuate immune evasion in EWS cells triggered by PAPPA via reinforcement of IGF-1 signaling. | Heitzeneder et al., 2019 |
| Glioblastoma (GBM) | ERN1, IGFBP3, IGFBP5 | + | U251 | – | – | Knockout | Plasmid | 12ADT | IGF-1, IRE1α | Inhibition of the expression levels of ERN1, IGFBP3, and IGFBP5 via applying a CRISPR/Cas9 genome editing system could promote sensitivity to 12ADT in GBM cells. | Rodvold et al., 2020 |
| GBM | IGF2BP1 | + | A172 | – | – | Knockout | Lentiviral | – | – | Knockdown of IGF2BP1 expression level via applying a CRISPR/Cas9 genome editing system leading to upregulation of miR-4500 in GBM cells, and thereby suppressing tumor cell growth and metastasis to a large extent. | Li Z.-W. et al., 2019 |
| Liver cancer stem cells (liver CSCs) | β-Catenin | + | Huh7 | – | – | Knockout (targeting exon 1 and 5) | Lentiviral | – | Wnt/β-catenin, IGF/MEK/ERK | Inhibition of the expression level of β-catenin via applying a CRISPR/Cas9 genome editing system demonstrated that IGF/MEK/ERK triggers Tcf7l1 phosphorylation and ubiquitination and controlling its suppression independent of β-catenin in liver CSCs. | Shan et al., 2019 |
| Lung cancer (LC) | Nrf2 | + | A549 | 11–12-week-old female C.B-17 SCID.beige mice | + | Knockout | Lentiviral | – | IGF1R | Suppression of the expression level of Nrf2 via applying a CRISPR/Cas9 genome editing system illustrated that ERBB3 and IGF1R signaling pathway accompanied by thioredoxin and peroxiredoxin proteins play an effective role in KEAP1-mutant cancer cells. | Vartanian et al., 2019 |
| Non-Small Cell Lung Carcinoma (NSCLC) | IGF1R | + | HCC827 | – | – | Knockout (targeting exon 2 leading to a deletion of 101 bp) | Plasmid | Erlotinib | IGF1R | Knockdown of IGF1R expression level via applying a CRISPR/Cas9 genome editing system could promote the responsiveness of NSCLC cells to Erlotinib, and thereby suppressing EMT. | Hussmann et al., 2017 |
| Oral squamous cell carcinoma (OSCC) | IGF1R | + | SCC-4 | – | – | Knockout | Lentiviral | – | PI3K-AKT, hedgehog | Knockdown of IGF-1 expression level via applying a CRISPR/Cas9 genome editing system could suppress the activation of AKT and Hedgehog signaling pathways, and thereby inhibiting cell proliferation, migration, and tumor aggressiveness in OSCC cells. | Ferreira Mendes et al., 2020 |
| Osteosarcoma (OS) | IGF1, IGFBP3 | + | U2OS | – | – | Knockout | Not available | Graphene Oxide nanoparticles | IGF1, IGFBP3 | Knockdown of IGF1 and IGFBP3 expression level via applying a CRISPR/Cas9 genome editing system could promote apoptosis in OS cells which in turn leading to downregulating the expression level of ROS and Nrf-2, and thereby enhancing the sensitivity of Graphene Oxide in tumor cells. | Burnett et al., 2020 |
| Prostate cancer (PCa) | MDA-9/syntenin | + | ARCaPM | Athymic nude mice | + | Knockout | Plasmid | – | STAT3 | Knockdown of MDA-9/syntenin expression level via applying a CRISPR/Cas9 genome editing system could downregulate the expression levels of MMP-2 and MMP-9 and inhibit STAT3 activation as well as suppressing pro-angiogenic factors containing IGFBP-2, VEGF-A, IL-8, and IL-6, and thereby attenuating invasion in PCa cells. | Das et al., 2018 |
| Renal cell carcinoma (RCC) | THOR | + | 786-O | 5–6-week-old female nude mice | + | Knockout | Plasmid | – | – | Knockdown of THOR expression level via applying a CRISPR/Cas9 genome editing system could suppress the expression levels of IGF2BP1-regulated genes, containing IGF2, Myc, and GLI1, and thereby inhibiting proliferation and viability of RCC cells. | Zhu W. et al., 2018 |
TABLE 7.
Pre-clinical researches applying the siRNA silencing mechanism to edit or knockdown aberrant expression of target genes relevant to the IGF signaling pathway in various human cancers.
| Type of cancer or disease | Target | In vitro | Cell line | Animal | In vivo | siRNA | Vector | Treatment | Pathway | Function | References |
| Breast cancer (BCa) | IGF-1R | + | EMT6, C4HD | BALB/c females | + | Inhibition of IGF-1R expression | Cytomegalovirus (CMV) | – | IGF-1R, AKT, ERK | Attenuating tumor cell proliferation, Suppressing phosphorylation of downstream signaling cascades ERK and AKT, Triggering secretion of proinflammatory cytokines IFN-γ and TNF-α, and blocking G0/G1 cell cycle. | Durfort et al., 2012 |
| BCa | IGF-II | + | MCF-7 | – | – | Inhibition of IGF-II expression | Not available | Resveratrol (RSV) | PI3K/AKT, MAPK/ERK | Enhancing progression and chemoresistance in BCa cells via negatively regulating Bcl-2 and Bcl-XL. | Singh et al., 2008 |
| BCa | IGF-1R | + | SKBR3 | – | – | Inhibition of IGF-1R expression | Plasmid | Docetaxel | IGF-1R | Utilizing the MUC1 Apt-conjugated CH NPs with the aim of co-delivery of Docetaxel and IGF-1R siRNA remarkably inhibiting the expression levels of IGF-1R, MMP9, STAT3, and VEGF. | Jafari et al., 2019 |
| BCa | IGFBP-6, IGF-I, IGF-II, IGF-1R | + | MCF10A-MycER MCF10A-MycON, MCF10A-MycOFF | – | – | Inhibition of IGFBP-6, IGF-1R IGF-I, and IGF-II expression | Not available | – | IGFs/IGF-1R | Downregulation of IGFBP-6 or IGF-I or IGF-II expression levels via siRNAs in breast epithelial cells or knockdown IGF-1R activity on fibroblasts could play an effective role in changing fibroblast mobilization, suppressing TME remodeling and tumor invasion via the IGFs/IGF-1R axis. | De Vincenzo et al., 2019 |
| Triple-negative breast cancer (TNBC) | IGF-1R | + | MDA-MB-231, BT-549 | – | – | Inhibition of IGF-1R expression | Not available | – | PI3K-Akt | IGF-1R knockdown via NVP-AEW541, 3-MA, and Atg7 siRNA could induce TNBC cell-protective autophagy and thereby attenuating the efficacy of IGF-1R-modulating therapeutic agents in tumor cells. | Wu W. et al., 2017 |
| Ovarian cancer (OC) | IGF2 | + | HEY-T30 | – | – | Inhibition of IGF2 expression | Not available | Paclitaxel | IGF | Suppression of the IGF signaling pathway via siRNA could promote sensitivity to paclitaxel in OC cells. | Huang et al., 2010 |
| OC | IGF2 | + | HEY-T30 | 6–8-week-old female athymic nude mice | + | Inhibition of IGF2 expression | Plasmid | Paclitaxel | IGF | IGF2 knockdown via siRNA leading to the suppression of paclitaxel resistance in OC cells. | Brouwer-Visser et al., 2014 |
| Colorectal cancer (CRC) | IGF-1R | + | SW480 | – | – | Inhibition of IGF-1R expression | Plasmid | 5-Fluorouracil | IGF-1R | Inhibiting CRC cell proliferation and promoting chemosensitization to 5-FU. | Yavari et al., 2010 |
| CRC | IGF-1R | + | SW480 | – | – | Inhibition of IGF-1R expression | Not available | – | IGF-1R | Utilizing radioconjugate of IGF-1R siRNA, p-SCN-Bn-DTPA, and 177Lu as radiopharmaceutical to suppress CRC cell proliferation caused by upregulation of IGF-1R via triggering apoptosis. | Fathi et al., 2013 |
| CRC | IGF-1R, IR-A | + | SW480 | – | – | Inhibition of IGF-1R and IR-A expression | Plasmid | – | IGF-1R | Inhibiting IR-A expression causing a concomitant promotion of IGF-1R activation through IGF-I and IGF-II, decreasing the formation of IGF-1R: IR-A hybrid receptors, and enhancing IGF-1R homodimer formation in CRC cells. | Brierley et al., 2010 |
| CRC | IGF-1R, PIAS3 | + | HT29, HT29-OxR, DLD-1-OxR | – | – | Inhibition of IGF-1R, PIAS3 expression | Not available | Ganitumab, NVP-AEW541, Dasatinib, FOLFOX, CAPOX, FOLFIRI, Oxaliplatin | IGF-1R, AKT, Wnt | Upregulation of PIAS3 could contribute to promoting the expression level of IGF-1R that in turn leading to Wnt pathway activation and thus causing resistance to chemotherapeutic agents. IGF-1R and PIAS3 knockdown via siRNAs leading to the chemotherapy sensitivity in CRC cells. | Codony-Servat et al., 2017 |
| CRC | IGF-1R | + | HCT116 | – | – | Inhibition of IGF-1R expression | Plasmid | 5-fluorouracil, Cisplatin | IGF-1R, MEK/ERK, PI3K/AKT | IGF-1R knockdown via siRNA could lead to upregulation of miR-497 and activation of PI3K/AKT signaling pathway, which in turn promoting the sensitivity of CRC cells to the chemotherapeutic drugs 5-fluorouracil and cisplatin. | Guo et al., 2013 |
| Gastric carcinoma (GC) | AKT, ERK1, ERK2 | + | MGC803, SGC-7901 | – | – | Inhibition of AKT, ERK1, and ERK2 expression | Plasmid | – | AKT/ERK | Upregulation of IGF-I could trigger EMT in gastric cancer cells which is accompanied by enhancing ZEB2 expression level. Thus, AKT, ERK1, and ERK2 knockdown via siRNA could reverse IGF-I-induced ZEB2 up-regulation and EMT via promoting the expression of miR-200c. | Li et al., 2014 |
| Pancreatic cancer (PC) | FAK | + | Panc-1, MiaPaca-2 | – | – | Inhibition of FAK expression | Adenoviral | – | IGF-1R, FAK | Dual knockdown of FAK and IGF-1R via TAE 226 and siRNA could lead to remarkable suppression of cell viability, reducing ERK and AKT phosphorylation levels, and promoting apoptosis in PC cells which in turn resulting in caspase-3 activation as well as ADP-ribose and PARP cleavage in tumor cells. | Liu et al., 2008 |
| PC | PTEN | + | BxPC-3, SW1990, AsPC-1, MIA PaCa-2, PANC-1 | – | – | Inhibition of PTEN expression | Not available | – | IGF-1, PI3K/AKT, NFκB | IGF-1 could trigger tumor cell growth and invasiveness of PC cells leading to promoting activation of PI3K/AKT/NFκB signaling as well as downregulating phosphorylation of PTEN. PTEN knockdown via siRNA could increase PI3K/AKT/NFκB pathway activation and increasing tumor cell proliferation and invasion. | Ma et al., 2010 |
| Prostate adenocarcinoma (PaC) | IGFBP-2 | + | DU145, PC3 | – | – | Inhibition of IGFBP-2 expression | Not available | Docetaxel | – | Downregulation of IGFBP-2 via siRNA modulating promotion of PTEN activity as well as sensitivity to docetaxel in CaP cells. | Uzoh et al., 2011 |
| Acute myeloid leukemia (AML) | IGF-1R, IR, PI3K | + | U937 | – | – | Inhibition of the class Ia PI3K isoforms p110β and p110δ | Plasmid | – | PI3K/AKT, ERK | Targeting isoforms p110β and p110δ via RNAi could reduce AKT activation through IGF-I or insulin, indicating that both PI3K isoforms contributing to the upregulation of IGF-1R or IR in AML cells and improving the sensitivity of tumor cells to chemotherapeutical drugs. | Doepfner et al., 2007 |
| Ewing’s sarcoma (ES) | IGF-1R, EWS/FLI-1 | + | TC-71 | – | – | Inhibition of IGF-1R and EWS/FLI-1 expression | Not available | R1507 | IGF, AKT | Transferring EWS/FLI-1 siRNA leading to upregulation of IGF-BP3 levels and downregulation of IGF-1 and IGF-2 levels and following that reducing p-Akt levels, thereby suppressing signaling via p-IGF-1R. As a result, triggering apoptosis and proliferation inhibition in ES cells. | Huang et al., 2011 |
| Lung Cancer (LC) | IGF-1R | + | A549 | – | – | Inhibition of IGF-1R expression | Lomustine-loaded chitosan nanoparticles (ChiNPs) | Doxorubicin | IGF-1R, STAT3 | The IGF-1R siRNA/DOX co-delivery system loaded chitosan nanoparticles play an effective role in reducing mmp9, STAT3, and VEGF in tumor cells. | Shali et al., 2018 |
| LC | IGF-1R | + | H460 | 5–6-week-old female athymic nude mice | + | Inhibition of IGF-1R expression | Not available | Cisplatin | IGF-1R | IGF-1R knockdown via siRNA could upregulate the expression level of IGFBP-3 in tumor drug resistance cells and lead to promoting the sensitivity of LC cells to cisplatin and radiation. | Sun et al., 2012 |
| Non-small cell lung carcinoma (NSCLCs) | IGFBP7 | + | PC-9, PC-14 | – | – | Inhibition of IGFBP7 expression | Not available | Cisplatin | – | IGFBP7 knockdown via siRNA could play an effective role in promoting resistance to cisplatin as well as upregulating the expression level of MKP-3 in NSCLCs. | Okamura et al., 2012 |
| NSCLCs | IRS-1 | + | H1299 | – | – | Inhibition of IRS-1 expression | Not available | Cisplatin | DSBs repair and checkpoint | IRS-1 knockdown via siRNA indicating that IRS-1 and ATM expression levels are downregulated by IGF-1 that could contribute to promoting cisplatin resistance in NSCLC cells and blocking the activation of DSBs repair and checkpoint pathways as well as cisplatin-induced γH2AX formation. | Jeon et al., 2008 |
| Renal cell carcinoma (RCC) | IGF-2, HOTTIP | + | A-498, 786-O | – | – | Inhibition of IGF-2, HOTTIP expression | Plasmid | – | IGF-2 | LncRNA HOTTIP could contribute as a miR-615 sponge which negatively modulates its target IGF-2. There is a positive association between the expression of HOTTIP and IGF-2 in tumor cells. HOTTIP knockdown via siRNA could remarkably suppress cell growth and carcinogenesis, and promote apoptosis in RCC cells. | Wang Q. et al., 2018 |
| Esophageal squamous cell carcinoma (ESCC) | IGFBP5 | + | SLMT-1 | – | – | Inhibition of IGFBP5 expression | Not available | Cisplatin | IGF | IGFBP5 knockdown via siRNA indicating Cisplatin resistance in ESCC cells, and thereby upregulation of IGFBP5 could play an important role in promoting sensitivity of tumor cells to chemotherapeutic agents. | Chan et al., 2018 |
| Hepatocellular carcinoma (HCC) | IGF-1R | + | Huh7, Hep3B | 6-week-old BALB/c nude mice | + | Inhibition of IGF-1R expression | Lentiviral | – | IGF-1R | IGF-1R knockdown via lentivirus-mediated RNAi could remarkably suppress tumor cell growth and apoptosis through attenuating the expression level of midkine in HCC cells. | Bie et al., 2016 |
Discussion
IGFs and the related signal transduction networks partake in the pathogenesis of cancers, diabetes complications, atherosclerosis, PCOS, and other disorders. Meanwhile, these signaling pathways are regulated by hundreds of miRNAs and lncRNAs. Several members of IGF signaling including IGF-I, IGF-II, IGF-1R, and IGFBP-3 are targets of regulation by miRNAs and lncRNAs. Therefore, understanding the complex interplay between these factors is a necessary step in the design of appropriate therapeutic options for these conditions. The importance of this task has been further underscored by the availability of several IGF-modifying modalities including receptor-specific antibodies, inhibitors of receptor kinases, and activators of AMP-activated protein kinases (Pollak, 2008). In addition to these types of therapeutics, a number of alternative medicines act by affecting the expression of IGF-related non-coding RNAs. For instance, bufothionine induces gastric cancer cell apoptosis via up-regulating miR-133a-3p which sponges IGF1R and regulates PI3K/Akt associated production of reactive oxygen species (Hu Z. H. et al., 2020).
The data presented above indicate that most of the IGF-associated lncRNAs exert their roles via modulation of miRNAs. Examples of lncRNA/miRNA interactions in the IGF-related pathways are circ_0014130/miR-142-5p, Linc00319/miR-147a, TUG1/miR-148b, H19/miR-18b, HCP5/miR-27a-3p and DBH-AS1/miR-138. The association between lncRNAs/miRNAs and the IGF system has importance in regenerative medicine as well. IGF1R signaling has been shown to partake in the preservation of stem cell features and improvement of efficiency of stem cell therapy, as IGF1R-expressing stem cells exhibit strong pluripotent or multipotent features (Teng et al., 2018). Therefore, the lncRNA/miRNA-mediated regulation of IGF1R signaling might offer putative modalities for maintaining stem cell features and enhancing the effects of these therapeutics in clinical settings.
IGF-related miRNAs and lncRNAs can be used as potential markers for forecasting the prognosis of cancer. Moreover, expression levels of these transcripts can be used as diagnostic markers for neoplastic conditions. The importance of IGF signaling in the modulation of response of melanoma, ovarian cancer, breast cancer, pancreatic cancer, prostate cancer, colorectal cancer, and several other cancers to chemotherapeutic agents has been validated. Some lncRNAs and miRNAs such as H19, LUCAT1, miR-143, miR-497, and miR-223 are involved in this process. However, the role of other transcripts should be assessed in the upcoming researches. Based on the role of IGF-related miRNAs and lncRNAs in the modulation of response o chemotherapeutic agents, these transcripts are putative targets for the improvement of the response of cancer cells to these agents.
Besides, promoter methylation of IGF-1R, IGF-1, IGF-II, and especially IGFBP-3 in various regions could be associated with cancer prognosis (Supplementary Table 3). Methylation patterns of these promoters are important for the regulation of their expression and could have pivotal clinical implications in various cancers. Re-expression of IGFBP-3 will be really helpful in curing the majority of aggressive tumors and can solve the problem of intratumoral heterogeneity.
Furthermore, employing CRISPR-Cas9 or siRNAs gene editing tools with the aim of knockdown of ectopic expression of target genes including IGF1R, IGF1, IGF2, IGFBP3, and IGFBP-6 can play an important role in attenuating the tumorigenesis characteristics as well as improving response to treatment in various human cancer cells. Utilizing this effective method will pave the way for future clinical advancement.
Conclusion
The advent of novel genome editing modalities and clarification of the role of epigenetic factors including both genomic marks and non-coding RNAs have raised the possibility of management of human cancers particularly neoplastic disorders with novel therapeutics. Meanwhile, concomitant assessment of expression profile and genomic marks of IGF-related genes using high throughput methods would facilitate appropriate stratification of patients with regards to possible response to each therapeutic option. Further investigations are needed to appraise the clinical application of novel therapeutic modalities that target IGF signaling and related lncRNAs.
Availability of Data and Materials
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
Author Contributions
MT and SG-F supervised the study, wrote the draft, and edited the submission. HS, AA, and MM performed the data collection, designed the tables and figures. All of the authors are contributed equally and fully aware of submission.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
- IGFs
insulin-like growth factors
- MAPK
mitogen-activated protein kinase
- IGFBP
insulin-like growth factor binding proteins
- lncRNAs
long non-coding RNAs
- miRNAs
microRNAs
- IGFBP-3
IGF binding protein-3
- EMT
epithelial-mesenchymal transition
- NNTs
nearby normal tissues
- GBM
Glioblastoma
- OC
ovarian cancer
- OS
osteosarcoma
- HCC
hepatocellular carcinoma
- BCa
breast cancer
- NPC
nasopharyngeal carcinoma
- GC
gastric cancer
- NSCLC
non-small cell lung carcinoma
- LGG
low-grade gliomas
- WT
wilms tumor
- RB
retinoblastoma
- OSCC
oral squamous cell carcinoma
- CRC
colorectal cancer
- RCC
renal cell carcinoma
- ULM
uterine leiomyoma
- PTC
papillary thyroid carcinoma
- EC
endometrial carcinoma
- M
melanoma
- SCCHN
squamous cell carcinoma of head & neck
- HUVECs
human umbilical vascular endothelial cells
- CHD
coronary heart disease
- DR
diabetic retinopathy
- PE
preeclampsia
- PCOS
polycystic ovary syndrome
- PNI
peripheral nerve injury
- RA
rheumatoid arthritis
- IPF
idiopathic pulmonary fibrosis
- NAFLD
non-alcoholic fatty liver disease
- SCI
spinal cord injury
- AMI
acute myocardial infarction
- LDD
lumbar disc degeneration
- DMR
differentially methylated region
- PaC
pancreatic cancer
- TSCC
tongue squamous cell carcinoma
- VSMC
vascular smooth muscle cell
- TNBC
triple-negative breast cancer
- PaC
prostate adenocarcinoma
- ACC
adrenocortical carcinoma
- GIST
gastrointestinal stromal tumor
- HDACi
histone deacetylase inhibitors
- NICTH
non-islet cell tumor hypoglycemia
- HB
hepatoblastoma
- LA
lung adenocarcinoma
- MM
multiple myeloma
- EOC
epithelial ovarian cancer
- DLBCL
diffuse large b-cell lymphoma
- EWS
ewing sarcoma
- liver CSCs
liver cancer stem cells.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2021.634512/full#supplementary-material
IGF-associated miRNAs in cancers (NNTs: nearby normal tissues).
Role of different drug inhibitors in suppressing the IGF-1R activity and attenuating tumorigenesis as well as drug resistance in various human cancer cells and promoting response to treatment.
Epigenetic regulation of different genes associated with the IGF signaling pathway in human cancers.
References
- Adachi Y., Ohashi H., Imsumran A., Yamamoto H., Matsunaga Y., Taniguchi H., et al. (2014). The effect of IGF-I receptor blockade for human esophageal squamous cell carcinoma and adenocarcinoma. Tumor Biol. 35 973–985. 10.1007/s13277-013-1131-2 [DOI] [PubMed] [Google Scholar]
- Afshar S., Najafi R., Sedighi Pashaki A., Sharifi M., Nikzad S., Gholami M. H., et al. (2018). MiR-185 enhances radiosensitivity of colorectal cancer cells by targeting IGF1R and IGF2. Biomed. Pharmacother. 106 763–769. 10.1016/j.biopha.2018.07.002 [DOI] [PubMed] [Google Scholar]
- Alyoussef A. (2020). The therapeutic effects of blocking IGF-R1 on mice model of skin cancer. J. Dermatol. Treat. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- An L. F., Huang J. W., Han X., Wang J. (2020). Downregulation of lncRNA H19 sensitizes melanoma cells to cisplatin by regulating the miR-18b/IGF1 axis. Anticancer Drugs 31 473–482. 10.1097/cad.0000000000000888 [DOI] [PubMed] [Google Scholar]
- Awasthi N., Zhang C., Ruan W., Schwarz M. A., Schwarz R. E. (2012). BMS-754807, a small-molecule inhibitor of insulin-like growth factor-1 receptor/insulin receptor, enhances gemcitabine response in pancreatic cancer. Mol. Cancer Ther. 11 2644–2653. 10.1158/1535-7163.mct-12-0447 [DOI] [PubMed] [Google Scholar]
- Bai R., Cui Z., Ma Y., Wu Y., Wang N., Huang L., et al. (2019). The NF-kappaB-modulated miR-19a-3p enhances malignancy of human ovarian cancer cells through inhibition of IGFBP-3 expression. Mol. Carcinog. 58 2254–2265. 10.1002/mc.23113 [DOI] [PubMed] [Google Scholar]
- Bao J., Chen X., Hou Y., Kang G., Li Q., Xu Y. (2018). LncRNA DBH-AS1 facilitates the tumorigenesis of hepatocellular carcinoma by targeting miR-138 via FAK/Src/ERK pathway. Biomed. Pharmacother. 107 824–833. 10.1016/j.biopha.2018.08.079 [DOI] [PubMed] [Google Scholar]
- Beauchamp M.-C., Knafo A., Yasmeen A., Carboni J. M., Gottardis M. M., Pollak M. N., et al. (2009). BMS-536924 sensitizes human epithelial ovarian cancer cells to the PARP inhibitor, 3-aminobenzamide. Gynecol. Oncology 115 193–198. 10.1016/j.ygyno.2009.07.009 [DOI] [PubMed] [Google Scholar]
- Beck O., Paret C., Russo A., Burhenne J., Fresnais M., Steimel K., et al. (2020). Safety and activity of the combination of ceritinib and dasatinib in osteosarcoma. Cancers 12:793. 10.3390/cancers12040793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeghly A., Katsaros D., Wiley A., de la Longrais I. R., Prescott A., Chen H., et al. (2007). IGF-II promoter methylation and ovarian cancer prognosis. J. Cancer Res. Clin. Oncol. 133 713–723. 10.1007/s00432-007-0211-3 [DOI] [PubMed] [Google Scholar]
- Bie C. Q., Liu X. Y., Cao M. R., Huang Q. Y., Tang H. J., Wang M., et al. (2016). Lentivirus-mediated RNAi knockdown of insulin-like growth factor-1 receptor inhibits the growth and invasion of hepatocellular carcinoma via down-regulating midkine expression. Oncotarget 7:79305. 10.18632/oncotarget.13027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biernacka K., Uzoh C., Zeng L., Persad R., Bahl A., Gillatt D., et al. (2013). Hyperglycaemia-induced chemoresistance of prostate cancer cells due to IGFBP2. Endocr Relat. Cancer 20 741–751. 10.1530/erc-13-0077 [DOI] [PubMed] [Google Scholar]
- Bolomsky A., Hose D., Schreder M., Seckinger A., Lipp S., Klein B., et al. (2015). Insulin like growth factor binding protein 7 (IGFBP7) expression is linked to poor prognosis but may protect from bone disease in multiple myeloma. J. Hematol. Oncol. 8:10. 10.1186/s13045-014-0105-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone D. N., Warburton A., Som S., Lee A. V. (2019). A negative feedback loop between Insulin-like Growth Factor signaling and the lncRNA SNHG7 tightly regulates transcript levels and proliferation. bioRxiv [Preprint]. 10.1101/709352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley G., Macaulay S., Forbes B., Wallace J., Cosgrove L., Macaulay V. (2010). Silencing of the insulin receptor isoform A favors formation of type 1 insulin-like growth factor receptor (IGF-1R) homodimers and enhances ligand-induced IGF-1R activation and viability of human colon carcinoma cells. Endocrinology 151 1418–1427. 10.1210/en.2009-1006 [DOI] [PubMed] [Google Scholar]
- Brouwer-Visser J., Lee J., McCullagh K., Cossio M. J., Wang Y., Huang G. S. (2014). Insulin-like growth factor 2 silencing restores taxol sensitivity in drug resistant ovarian cancer. PLoS One 9:e100165. 10.1371/journal.pone.0100165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett M., Abuetabh Y., Wronski A., Shen F., Persad S., Leng R., et al. (2020). Graphene oxide nanoparticles induce apoptosis in wild-type and CRISPR/Cas9-IGF/IGFBP3 knocked-out osteosarcoma cells. J. Cancer 11:5007. 10.7150/jca.46464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H., An Y., Chen X., Sun D., Chen T., Peng Y., et al. (2016). Epigenetic inhibition of miR-663b by long non-coding RNA HOTAIR promotes pancreatic cancer cell proliferation via up-regulation of insulin-like growth factor 2. Oncotarget 7:86857. 10.18632/oncotarget.13490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camblin A. J., Pace E. A., Adams S., Curley M. D., Rimkunas V., Nie L., et al. (2018). Dual inhibition of IGF-1R and ErbB3 enhances the activity of gemcitabine and nab-paclitaxel in preclinical models of pancreatic cancer. Clin. Cancer Res. 24 2873–2885. 10.1158/1078-0432.ccr-17-2262 [DOI] [PubMed] [Google Scholar]
- Carrasco-Garcia E., Martinez-Lacaci I., Mayor-López L., Tristante E., Carballo-Santana M., García-Morales P., et al. (2018). PDGFR and IGF-1R inhibitors induce a G2/M arrest and subsequent cell death in human glioblastoma cell lines. Cells 7:131. 10.3390/cells7090131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan D., Zhou Y., Chui C. H., Lam K. H., Law S., Chan A. S.-C. (2018). Expression of insulin-like growth factor binding protein-5 (IGFBP5) reverses cisplatin-resistance in esophageal carcinoma. Cells 7:143. 10.3390/cells7100143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. S., Wang L., Liu D., Mao L., Hong W. K., Khuri F. R., et al. (2002). Correlation between insulin-like growth factor-binding protein-3 promoter methylation and prognosis of patients with stage I non-small cell lung cancer. Clin. Cancer Res. 8 3669–3675. [PubMed] [Google Scholar]
- Chang Y. S., Wang L., Suh Y.-A., Mao L., Karpen S. J., Khuri F. R., et al. (2004). Mechanisms underlying lack of insulin-like growth factor-binding protein-3 expression in non-small-cell lung cancer. Oncogene 23 6569–6580. 10.1038/sj.onc.1207882 [DOI] [PubMed] [Google Scholar]
- Chen B., Li J., Chi D., Sahnoune I., Calin S., Girnita L., et al. (2019). Non-Coding RNAs in IGF-1R signaling regulation: the underlying pathophysiological link between diabetes and cancer. Cells 8:1638. 10.3390/cells8121638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B., Li Q., Zhou Y., Wang X., Zhang Q., Wang Y., et al. (2018). The long coding RNA AFAP1-AS1 promotes tumor cell growth and invasion in pancreatic cancer through upregulating the IGF1R oncogene via sequestration of miR-133a. Cell Cycle 17 1949–1966. 10.1080/15384101.2018.1496741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Deng T., Li X., Cai W. (2019). MiR-193b inhibits the growth and metastasis of renal cell carcinoma by targeting IGF1R. Artif. Cells Nanomed. Biotechnol. 47 2058–2064. 10.1080/21691401.2019.1620251 [DOI] [PubMed] [Google Scholar]
- Chen P. H., Cheng C. H., Shih C. M., Ho K. H., Lin C. W., Lee C. C., et al. (2016). The Inhibition of microRNA-128 on IGF-1-activating mTOR signaling involves in temozolomide-induced glioma cell apoptotic death. PLoS One 11:e0167096. 10.1371/journal.pone.0167096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Pan X., Sheng Z., Yan G., Chen L., Ma G. (2019). miR-17 regulates the proliferation and apoptosis of endothelial cells in coronary heart disease via targeting insulin-like-growth factor 1. Pathol. Res. Pract. 215:152512. 10.1016/j.prp.2019.152512 [DOI] [PubMed] [Google Scholar]
- Chen Z., Yang H., Nie Y., Xing Y. (2018). miR-145 regulates the proliferation and apoptosis of Y79 human retinoblastoma cells by targeting IGF-1R. Int. J. Clin. Exp. Pathol. 11 4331–4338. [PMC free article] [PubMed] [Google Scholar]
- Chen Z. L., Li X. N., Ye C. X., Chen H. Y., Wang Z. J. (2020). Elevated levels of circRUNX1 in colorectal cancer promote cell growth and metastasis via miR-145-5p/IGF1 Signalling. Onco Targets Ther. 13 4035–4048. 10.2147/ott.s254133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y.-L., Hur S.-M., Kim J.-Y., Kim J.-H., Lee D.-K., Choe J., et al. (2015). Specific activation of insulin-like growth factor-1 receptor by ginsenoside Rg5 promotes angiogenesis and vasorelaxation. J. Biol. Chem. 290 467–477. 10.1074/jbc.m114.603142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu S., Gu J., Feng L., Liu J., Zhang M., Jia X., et al. (2014). Ginsenoside Rg5 improves cognitive dysfunction and beta-amyloid deposition in STZ-induced memory impaired rats via attenuating neuroinflammatory responses. Int. Immunopharmacol. 19 317–326. 10.1016/j.intimp.2014.01.018 [DOI] [PubMed] [Google Scholar]
- Codony-Servat J., Cuatrecasas M., Asensio E., Montironi C., Martínez-Cardús A., Marín-Aguilera M., et al. (2017). Nuclear IGF-1R predicts chemotherapy and targeted therapy resistance in metastatic colorectal cancer. Br. J. Cancer 117 1777–1786. 10.1038/bjc.2017.279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen B., McLarty K., Kersemans V., Reilly R. M. (2008). The level of insulin growth factor-1 receptor expression is directly correlated with the tumor uptake of 111In-IGF-1 (E3R) in vivo and the clonogenic survival of breast cancer cells exposed in vitro to trastuzumab (Herceptin). Nuclear Med. Biol. 35 645–653. 10.1016/j.nucmedbio.2008.05.010 [DOI] [PubMed] [Google Scholar]
- Cortes-Sempere M., De Miguel M., Pernia O., Rodriguez C., de Castro Carpeno J., Nistal M., et al. (2013). IGFBP-3 methylation-derived deficiency mediates the resistance to cisplatin through the activation of the IGFIR/Akt pathway in non-small cell lung cancer. Oncogene 32 1274–1283. 10.1038/onc.2012.146 [DOI] [PubMed] [Google Scholar]
- Cunningham M. P., Thomas H., Marks C., Green M., Fan Z., Modjtahedi H. (2008). Co-targeting the EGFR and IGF-1R with anti-EGFR monoclonal antibody ICR62 and the IGF-1R tyrosine kinase inhibitor NVP-AEW541 in colorectal cancer cells. Int. J. Oncol. 33 1107–1113. [PubMed] [Google Scholar]
- Dang X., Li X., Wang L., Sun X., Tian X. (2017). MicroRNA-3941 targets IGF-1 to regulate cell proliferation and migration of breast cancer cells. Int. J. Clin. Exp. Pathol. 10 7650–7660. [PMC free article] [PubMed] [Google Scholar]
- Dar A. A., Majid S., Nosrati M., De Semir D., Federman S., Kashani-Sabet M. (2010). Functional modulation of IGF-binding protein-3 expression in melanoma. J. Investig. Dermatol. 130 2071–2079. 10.1038/jid.2010.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das F., Dey N., Bera A., Kasinath B. S., Ghosh-Choudhury N., Choudhury G. G. (2016). MicroRNA-214 reduces insulin-like growth factor-1 (IGF-1) receptor expression and downstream mTORC1 signaling in renal carcinoma cells. J. Biol. Chem. 291 14662–14676. 10.1074/jbc.m115.694331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S. K., Pradhan A. K., Bhoopathi P., Talukdar S., Shen X.-N., Sarkar D., et al. (2018). The MDA-9/Syntenin/IGF1R/STAT3 axis directs prostate cancer invasion. Cancer Res. 78 2852–2863. 10.1158/0008-5472.can-17-2992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Caceres I. I., Cortes-Sempere M., Moratilla C., Machado-Pinilla R., Rodriguez-Fanjul V., Manguan-Garcia C., et al. (2010). IGFBP-3 hypermethylation-derived deficiency mediates cisplatin resistance in non-small-cell lung cancer. Oncogene 29 1681–1690. 10.1038/onc.2009.454 [DOI] [PubMed] [Google Scholar]
- De Martino M. C., van Koetsveld P. M., Feelders R. A., de Herder W. W., Dogan F., Janssen J., et al. (2019). IGF and mTOR pathway expression and in vitro effects of linsitinib and mTOR inhibitors in adrenocortical cancer. Endocrine 64 673–684. 10.1007/s12020-019-01869-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vincenzo A., Belli S., Franco P., Telesca M., Iaccarino I., Botti G., et al. (2019). Paracrine recruitment and activation of fibroblasts by c-Myc expressing breast epithelial cells through the IGFs/IGF-1R axis. Int. J. Cancer 145 2827–2839. 10.1002/ijc.32613 [DOI] [PubMed] [Google Scholar]
- Denduluri S. K., Idowu O., Wang Z., Liao Z., Yan Z., Mohammed M. K., et al. (2015). Insulin-like growth factor (IGF) signaling in tumorigenesis and the development of cancer drug resistance. Genes Dis. 2 13–25. 10.1016/j.gendis.2014.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L., Wang L., Guo F. (2017). microRNA188 acts as a tumour suppressor in glioma by directly targeting the IGF2BP2 gene. Mol. Med. Rep. 16 7124–7130. 10.3892/mmr.2017.7433 [DOI] [PubMed] [Google Scholar]
- Doepfner K., Spertini O., Arcaro A. (2007). Autocrine insulin-like growth factor-I signaling promotes growth and survival of human acute myeloid leukemia cells via the phosphoinositide 3-kinase/Akt pathway. Leukemia 21 1921–1930. 10.1038/sj.leu.2404813 [DOI] [PubMed] [Google Scholar]
- Dool C. J., Mashhedi H., Zakikhani M., David S., Zhao Y., Birman E., et al. (2011). IGF1/insulin receptor kinase inhibition by BMS-536924 is better tolerated than alloxan-induced hypoinsulinemia and more effective than metformin in the treatment of experimental insulin-responsive breast cancer. Endocrine Relat. Cancer 18:699. 10.1530/erc-11-0136 [DOI] [PubMed] [Google Scholar]
- Durfort T., Tkach M., Meschaninova M. I., Rivas M. A., Elizalde P. V., Venyaminova A. G., et al. (2012). Small interfering RNA targeted to IGF-1R delays tumor growth and induces proinflammatory cytokines in a mouse breast cancer model. PLoS One 7:e29213. 10.1371/journal.pone.0029213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein N., Servan K., Hildebrandt B., Politz A., von Jonquieres G., Wolf-Kummeth S., et al. (2009). Hyperactivation of the insulin-like growth factor receptor I signaling pathway is an essential event for cisplatin resistance of ovarian cancer cells. Cancer Res. 69 2996–3003. 10.1158/0008-5472.can-08-3153 [DOI] [PubMed] [Google Scholar]
- Economou M. A., Andersson S., Vasilcanu D., All-Ericsson C., Menu E., Girnita A., et al. (2008). Oral picropodophyllin (PPP) is well tolerated in vivo and inhibits IGF-1R expression and growth of uveal melanoma. Investig. Ophthalmol. Vis. Sci. 49 2337–2342. 10.1167/iovs.07-0819 [DOI] [PubMed] [Google Scholar]
- El Tayebi H. M., Waly A. A., Assal R. A., Hosny K. A., Esmat G., Abdelaziz A. I. (2015). Transcriptional activation of the IGF-II/IGF-1R axis and inhibition of IGFBP-3 by miR-155 in hepatocellular carcinoma. Oncol. Lett. 10 3206–3212. 10.3892/ol.2015.3725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbi P., Spallarossa P., Garibaldi S., Barisione C., Mura M., Altieri P., et al. (2015). Doxorubicin impairs the insulin-like growth factor-1 system and causes insulin-like growth factor-1 resistance in cardiomyocytes. PLoS One 10:e0124643. 10.1371/journal.pone.0124643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J., Shi S., Qiu Y., Zheng Z., Yu L. (2019). MicroRNA-486-5p down-regulation protects cardiomyocytes against hypoxia-induced cell injury by targeting IGF-1. Int. J. Clin. Exp. Pathol. 12 2544–2551. [PMC free article] [PubMed] [Google Scholar]
- Fathi M., Taghikhani M., Ghannadi-Maragheh M., Yavari K. (2013). Demonstration of dose dependent cytotoxic activity in SW480 colon cancer cells by 177Lu-labeled siRNA targeting IGF-1R. Nuclear Med. Biol. 40 529–536. 10.1016/j.nucmedbio.2012.05.001 [DOI] [PubMed] [Google Scholar]
- Fawzy I. O., Hamza M. T., Hosny K. A., Esmat G., El Tayebi H. M., Abdelaziz A. I. (2015). miR-1275: a single microRNA that targets the three IGF2-mRNA-binding proteins hindering tumor growth in hepatocellular carcinoma. FEBS Lett. 589 2257–2265. 10.1016/j.febslet.2015.06.038 [DOI] [PubMed] [Google Scholar]
- Fei H.-D., Yuan Q., Mao L., Chen F.-L., Cui Z.-H., Tao S., et al. (2017). Assessment of GSK1904529A as a promising anti-osteosarcoma agent. Oncotarget 8:49646. 10.18632/oncotarget.17911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira Mendes J. M., de Faro Valverde L., Torres Andion Vidal M., Paredes B. D., Coelho P., Allahdadi K. J., et al. (2020). Effects of IGF-1 on proliferation, angiogenesis, tumor stem cell populations and activation of AKT and hedgehog pathways in oral squamous cell carcinoma. Int. J. Mol. Sci. 21:6487. 10.3390/ijms21186487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler C. A., Perks C. M., Newcomb P. V., Savage P. B., Farndon J. R., Holly J. M. (2000). Insulin-like growth factor binding protein-3 (IGFBP-3) potentiates paclitaxel-induced apoptosis in human breast cancer cells. Int. J. Cancer 88 448–453. [DOI] [PubMed] [Google Scholar]
- Franks S. E., Jones R. A., Briah R., Murray P., Moorehead R. A. (2016). BMS-754807 is cytotoxic to non-small cell lung cancer cells and enhances the effects of platinum chemotherapeutics in the human lung cancer cell line A549. BMC Res. Notes 9:134. 10.1186/s13104-016-1919-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J., Hao L., Tian Y., Liu Y., Gu Y., Wu J. (2018). miR-199a-3p is involved in estrogen-mediated autophagy through the IGF-1/mTOR pathway in osteocyte-like MLO-Y4 cells. J. Cell. Physiol. 233 2292–2303. 10.1002/jcp.26101 [DOI] [PubMed] [Google Scholar]
- Gable K. L., Maddux B. A., Penaranda C., Zavodovskaya M., Campbell M. J., Lobo M., et al. (2006). Diarylureas are small-molecule inhibitors of insulin-like growth factor I receptor signaling and breast cancer cell growth. Mol. Cancer Ther. 5 1079–1086. 10.1158/1535-7163.mct-05-0397 [DOI] [PubMed] [Google Scholar]
- Gebeshuber C. A., Martinez J. (2013). miR-100 suppresses IGF2 and inhibits breast tumorigenesis by interfering with proliferation and survival signaling. Oncogene 32 3306–3310. 10.1038/onc.2012.372 [DOI] [PubMed] [Google Scholar]
- Geng Y., Sui C., Xun Y., Lai Q., Jin L. (2019). MiRNA-99a can regulate proliferation and apoptosis of human granulosa cells via targeting IGF-1R in polycystic ovary syndrome. J. Assist. Reprod. Genet. 36 211–221. 10.1007/s10815-018-1335-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- George B., George S. K., Shi W., Haque A., Shi P., Eskandari G., et al. (2019). Dual inhibition of IGF-1R and ALK as an effective strategy to eradicate NPM-ALK+ T-cell lymphoma. J. Hematol. Oncol. 12:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazal S., McKinnon B., Zhou J., Mueller M., Men Y., Yang L., et al. (2015). H19 lnc RNA alters stromal cell growth via IGF signaling in the endometrium of women with endometriosis. EMBO Mol. Med. 7 996–1003. 10.15252/emmm.201505245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigek C. O., Leal M. F., Lisboa L. C. F., Silva P. N. O., Chen E. S., Lima E. M., et al. (2010). Insulin-like growth factor binding protein-3 gene methylation and protein expression in gastric adenocarcinoma. Growth Hormone IGF Res. 20 234–238. 10.1016/j.ghir.2010.02.005 [DOI] [PubMed] [Google Scholar]
- Guan J., Zhou Y., Mao F., Lin Y., Shen S., Zhang Y., et al. (2018). MicroRNA320a suppresses tumor cell growth and invasion of human breast cancer by targeting insulinlike growth factor 1 receptor. Oncol. Rep. 40 849–858. [DOI] [PubMed] [Google Scholar]
- Guo S. T., Jiang C. C., Wang G. P., Li Y. P., Wang C. Y., Guo X. Y., et al. (2013). MicroRNA-497 targets insulin-like growth factor 1 receptor and has a tumour suppressive role in human colorectal cancer. Oncogene 32 1910–1920. 10.1038/onc.2012.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J.-J., Xue D.-W., Han Q.-R., Liang X.-H., Xie L., Li S., et al. (2015). Induction of apoptosis by IGFBP3 overexpression in hepatocellular carcinoma cells. Asian Pac. J. Cancer Prevent. 15 10085–10089. 10.7314/apjcp.2014.15.23.10085 [DOI] [PubMed] [Google Scholar]
- Hanafusa T., Shinji T., Shiraha H., Nouso K., Iwasaki Y., Yumoto E., et al. (2005). Functional promoter upstream p53 regulatory sequence of IGFBP3 that is silenced by tumor specific methylation. BMC Cancer 5:9. 10.1186/1471-2407-5-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa T., Yumoto Y., Nouso K., Nakatsukasa H., Onishi T., Fujikawa T., et al. (2002). Reduced expression of insulin-like growth factor binding protein-3 and its promoter hypermethylation in human hepatocellular carcinoma. Cancer Lett. 176 149–158. 10.1016/s0304-3835(01)00736-4 [DOI] [PubMed] [Google Scholar]
- Hassanlou M., Soltani B. M., Medlej A., Kay M., Mowla S. J. (2020). Hsa-miR-6165 downregulates insulin-like growth factor-1 receptor (IGF-1R) expression and enhances apoptosis in SW480 cells. Biol. Chem. 401 477–485. 10.1515/hsz-2018-0421 [DOI] [PubMed] [Google Scholar]
- He Y., Zhang J., Zheng J., Du W., Xiao H., Liu W., et al. (2010). The insulin-like growth factor-1 receptor kinase inhibitor, NVP-ADW742, suppresses survival and resistance to chemotherapy in acute myeloid leukemia cells. Oncol. Res. Featuring Preclin. Clin. Cancer Ther. 19 35–43. 10.3727/096504010x12828372551821 [DOI] [PubMed] [Google Scholar]
- Heitzeneder S., Sotillo E., Shern J. F., Sindiri S., Xu P., Jones R., et al. (2019). Pregnancy-associated plasma protein-A (PAPP-A) in Ewing sarcoma: role in tumor growth and immune evasion. JNCI J. Natl. Cancer Instit. 111 970–982. 10.1093/jnci/djy209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson A. E. W., Haluska P., Schneider P. A., Loegering D. A., Peterson K. L., Attar R., et al. (2009). Expression of insulin receptor isoform A and insulin-like growth factor-1 receptor in human acute myelogenous leukemia: effect of the dual-receptor inhibitor BMS-536924 in vitro. Cancer Res. 69 7635–7643. 10.1158/0008-5472.can-09-0511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda S., Arai Y., Haruta M., Sasaki F., Ohira M., Yamaoka H., et al. (2008). Loss of imprinting of IGF2 correlates with hypermethylation of the H19 differentially methylated region in hepatoblastoma. Br. J. Cancer 99 1891–1899. 10.1038/sj.bjc.6604754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X., Huang F., Macedo L. F., Harrington S. C., Reeves K. A., Greer A., et al. (2011). Dual IGF-1R/InsR inhibitor BMS-754807 synergizes with hormonal agents in treatment of estrogen-dependent breast cancer. Cancer Res. 71 7597–7607. 10.1158/0008-5472.can-11-1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G.-F., Wang C., Hu G.-X., Wu G., Zhang C., Zhu W., et al. (2020). AZD3463, an IGF-1R inhibitor, suppresses breast cancer metastasis to bone via modulation of the PI3K-Akt pathway. Ann. Transl. Med. 8:336. 10.21037/atm.2020.02.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Yang Z., Bao D., Ni J. S., Lou J. (2019). miR-455-5p suppresses hepatocellular carcinoma cell growth and invasion via IGF-1R/AKT/GLUT1 pathway by targeting IGF-1R. Pathol. Res. Pract. 215:152674. 10.1016/j.prp.2019.152674 [DOI] [PubMed] [Google Scholar]
- Hu Z. H., Wang G. J., Li R. X., Zhu T. Y., Wang Z. Y., Ding H. X., et al. (2020). Upregulation of miR-133a-3p enhances Bufothionine-induced gastric cancer cell death by modulating IGF1R/PI3K/Akt signal pathway mediated ER stress. Life Sci. 259:118180. 10.1016/j.lfs.2020.118180 [DOI] [PubMed] [Google Scholar]
- Huang F., Chang H., Greer A., Hillerman S., Reeves K. A., Hurlburt W., et al. (2015). IRS2 copy number gain, KRAS and BRAF mutation status as predictive biomarkers for response to the IGF-1R/IR inhibitor BMS-754807 in colorectal cancer cell lines. Mol. Cancer Ther. 14 620–630. 10.1158/1535-7163.mct-14-0794-t [DOI] [PubMed] [Google Scholar]
- Huang G. S., Brouwer-Visser J., Ramirez M. J., Kim C. H., Hebert T. M., Lin J., et al. (2010). Insulin-like growth factor 2 expression modulates Taxol resistance and is a candidate biomarker for reduced disease-free survival in ovarian cancer. Clin. Cancer Res. 16 2999–3010. 10.1158/1078-0432.ccr-09-3233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H. J., Angelo L. S., Rodon J., Sun M., Kuenkele K.-P., Parsons H. A., et al. (2011). R1507, an anti-insulin-like growth factor-1 receptor (IGF-1R) antibody, and EWS/FLI-1 siRNA in Ewing’s sarcoma: convergence at the IGF/IGFR/Akt axis. PLoS One 6:e26060. 10.1371/journal.pone.0026060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R. S., Zheng Y. L., Li C., Ding C., Xu C., Zhao J. (2018). MicroRNA-485-5p suppresses growth and metastasis in non-small cell lung cancer cells by targeting IGF2BP2. Life Sci. 199 104–111. 10.1016/j.lfs.2018.03.005 [DOI] [PubMed] [Google Scholar]
- Hussmann D., Madsen A. T., Jakobsen K. R., Luo Y., Sorensen B. S., Nielsen A. L. (2017). IGF1R depletion facilitates MET-amplification as mechanism of acquired resistance to erlotinib in HCC827 NSCLC cells. Oncotarget 8:33300. 10.18632/oncotarget.16350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland L., Santos A., Campbell F., Figueiredo C., Hammond D., Ellies L. G., et al. (2018). Blockade of insulin-like growth factors increases efficacy of paclitaxel in metastatic breast cancer. Oncogene 37 2022–2036. 10.1038/s41388-017-0115-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari R., Zolbanin N. M., Majidi J., Atyabi F., Yousefi M., Jadidi-Niaragh F., et al. (2019). Anti-Mucin1 Aptamer-conjugated Chitosan nanoparticles for targeted co-delivery of Docetaxel and IGF-1R siRNA to SKBR3 metastatic breast cancer cells. Iranian Biomed. J. 23:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson M. J., Beckler A. D., Taniguchi L. E., Allak A., VanWagner L. B., Lee N. G., et al. (2011). Activation of the insulin-like growth factor-1 receptor induces resistance to epidermal growth factor receptor antagonism in head and neck squamous carcinoma cells. Mol. Cancer Ther. 10 2124–2134. 10.1158/1535-7163.mct-11-0294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon J. H., Kim S. K., Kim H. J., Chang J., Ahn C. M., Chang Y. S. (2008). Insulin-like growth factor-1 attenuates cisplatin-induced gammaH2AX formation and DNA double-strand breaks repair pathway in non-small cell lung cancer. Cancer Lett. 272 232–241. 10.1016/j.canlet.2008.07.011 [DOI] [PubMed] [Google Scholar]
- Jeon S. H., Yoo J. K., Kim C. M., Lim E. S., Lee S. J., Lee J. M., et al. (2018). The novel hsa-miR-12528 regulates tumourigenesis and metastasis through hypo-phosphorylation of AKT cascade by targeting IGF-1R in human lung cancer. Cell Death Dis. 9:493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia T., Ren Y., Wang F., Zhao R., Qiao B., Xing L., et al. (2020). MiR-148a inhibits oral squamous cell carcinoma progression through ERK/MAPK pathway via targeting IGF-1R. Biosci. Rep. 40:BSR20182458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B., Xue M., Xu D., Song J., Zhu S. (2020). Down-regulated lncRNA HOTAIR alleviates polycystic ovaries syndrome in rats by reducing expression of insulin-like growth factor 1 via microRNA-130a. J. Cell. Mol. Med. 24 451–464. 10.1111/jcmm.14753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julovi S. M., Martin J. L., Baxter R. C. (2018). Nuclear insulin-like growth factor binding protein-3 as a biomarker in triple-negative breast cancer xenograft tumors: effect of targeted therapy and comparison with chemotherapy. Front. Endocrinol. 9:120. 10.3389/fendo.2018.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajdaniuk D., Marek B., Kos-Kudla B. (2001). Influence of adjuvant chemotherapy with cyclophosphamide, methotrexate and 5-fluorouracil on plasma melatonin and chosen hormones in breast cancer premenopausal patients. J. Clin. Pharm. Ther. 26 297–301. [DOI] [PubMed] [Google Scholar]
- Kawasaki T., Nosho K., Ohnishi M., Suemoto Y., Kirkner G. J., Fuchs C. S., et al. (2007). IGFBP3 promoter methylation in colorectal cancer: relationship with microsatellite instability, CpG island methylator phenotype, p53. Neoplasia 9 1091–1098. 10.1593/neo.07760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. S., Lee D. Y. (2015). Insulin-like growth factor binding protein-3 enhances etoposide-induced cell growth inhibition by suppressing the NF-kappaB activity in gastric cancer cells. Mol. Cell Biochem. 403 107–113. [DOI] [PubMed] [Google Scholar]
- Kim S. T., Jang H.-L., Lee J., Park S. H., Park Y. S., Lim H. Y., et al. (2015). Clinical significance of IGFBP-3 methylation in patients with early stage gastric cancer. Transl. Oncol. 8 288–294. 10.1016/j.tranon.2015.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger D. T., Alexi X., Opdam M., Schuurman K., Voorwerk L., Sanders J., et al. (2020). IGF-1R pathway activation as putative biomarker for linsitinib therapy to revert tamoxifen resistance in ER-positive breast cancer. Int. J. Cancer 146 2348–2359. 10.1002/ijc.32668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küffer S., Gutting T., Belharazem D., Sauer C., Michel M. S., Marx A., et al. (2018). Insulin-like growth factor 2 expression in prostate cancer is regulated by promoter-specific methylation. Mol. Oncol. 12 256–266. 10.1002/1878-0261.12164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurio N., Shimo T., Fukazawa T., Takaoka M., Okui T., Hassan N. M. M., et al. (2011). Anti-tumor effect in human breast cancer by TAE226, a dual inhibitor for FAK and IGF-1R in vitro and in vivo. Exp. Cell Res. 317 1134–1146. 10.1016/j.yexcr.2011.02.008 [DOI] [PubMed] [Google Scholar]
- Lamhamedi-Cherradi S.-E., Menegaz B. A., Ramamoorthy V., Vishwamitra D., Wang Y., Maywald R. L., et al. (2016). IGF-1R and mTOR blockade: novel resistance mechanisms and synergistic drug combinations for Ewing sarcoma. JNCI J. Natl. Cancer Instit. 108:djw182. 10.1093/jnci/djw182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson E. A., Zhang X., Crocker J. T., Wang W.-L., Klibanski A. (2009). Hypoglycemia from IGF2 overexpression associated with activation of fetal promoters and loss of imprinting in a metastatic hemangiopericytoma. J. Clin. Endocrinol. Metab. 94 2226–2231. 10.1210/jc.2009-0153 [DOI] [PubMed] [Google Scholar]
- Lei Q., Pan Q., Li N., Zhou Z., Zhang J., He X., et al. (2019). H19 regulates the proliferation of bovine male germline stem cells via IGF-1 signaling pathway. J. Cell. Physiol. 234 915–926. 10.1002/jcp.26920 [DOI] [PubMed] [Google Scholar]
- Li B., Ge L., Li M., Wang L., Li Z. (2016). miR-448 suppresses proliferation and invasion by regulating IGF1R in colorectal cancer cells. Am. J. Transl. Res. 8 3013–3022. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Li H., Xu L., Li C., Zhao L., Ma Y., Zheng H., et al. (2014). Ubiquitin ligase Cbl-b represses IGF-I-induced epithelial mesenchymal transition via ZEB2 and microRNA-200c regulation in gastric cancer cells. Mol. Cancer 13:136. 10.1186/1476-4598-13-136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Geng J., Xu X., Huang X., Leng D., Jiang D., et al. (2016). miR-130b-3p modulates epithelial-mesenchymal crosstalk in lung fibrosis by targeting IGF-1. PLoS One 11:e0150418. 10.1371/journal.pone.0150418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Li Y., Lu H. (2017). miR-1193 suppresses proliferation and invasion of human breast cancer cells through directly targeting IGF2BP2. Oncol. Res. 25 579–585. 10.3727/97818823455816x14760504645779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Wang K., Song N., Hou K., Che X., Zhou Y., et al. (2019). Activation of IGF-1R pathway and NPM-ALK G1269A mutation confer resistance to crizotinib treatment in NPM-ALK positive lymphoma. Investig. New Drugs 38 599–609. 10.1007/s10637-019-00802-7 [DOI] [PubMed] [Google Scholar]
- Li Z., Cai B., Abdalla B. A., Zhu X., Zheng M., Han P., et al. (2019). LncIRS1 controls muscle atrophy via sponging miR-15 family to activate IGF1-PI3K/AKT pathway. J. Cachexia Sarcopenia Muscle 10 391–410. 10.1002/jcsm.12374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z.-W., Xue M., Zhu B.-X., Yue C.-L., Chen M., Qin H.-H. (2019). microRNA-4500 inhibits human glioma cell progression by targeting IGF2BP1. Biochem. Biophys. Res. Commun. 513 800–806. 10.1016/j.bbrc.2019.04.058 [DOI] [PubMed] [Google Scholar]
- Liang L., Wang J., Yuan Y., Zhang Y., Liu H., Wu C., et al. (2018). MicRNA-320 facilitates the brain parenchyma injury via regulating IGF-1 during cerebral I/R injury in mice. Biomed. Pharmacother. 102 86–93. 10.1016/j.biopha.2018.03.036 [DOI] [PubMed] [Google Scholar]
- Ling J., Jiang L., Zhang C., Dai J., Wu Q., Tan J. (2015). Upregulation of miR-197 inhibits cell proliferation by directly targeting IGFBP5 in human uterine leiomyoma cells. In Vitro Cell Dev. Biol. Anim. 51 835–842. 10.1007/s11626-015-9887-x [DOI] [PubMed] [Google Scholar]
- Liu F. Y., Zhou S. J., Deng Y. L., Zhang Z. Y., Zhang E. L., Wu Z. B., et al. (2015a). MiR-216b is involved in pathogenesis and progression of hepatocellular carcinoma through HBx-miR-216b-IGF2BP2 signaling pathway. Cell Death Dis. 6:e1670. 10.1038/cddis.2015.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Su H., Wang X., Hao W. (2018a). MiR-148a regulates bone marrow mesenchymal stem cells-mediated fracture healing by targeting insulin-like growth factor 1. J. Cell. Biochem. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Liu J., Jia Y., Jia L., Li T., Yang L., Zhang G. (2019). MicroRNA 615-3p Inhibits the Tumor Growth and Metastasis of NSCLC via Inhibiting IGF2. Oncol. Res. 27 269–279. 10.3727/096504018x15215019227688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Wang J., Li X., Ma J., Shi C., Zhu H., et al. (2015b). MiR-204-5p suppresses cell proliferation by inhibiting IGFBP5 in papillary thyroid carcinoma. Biochem. Biophys. Res. Commun. 457 621–626. 10.1016/j.bbrc.2015.01.037 [DOI] [PubMed] [Google Scholar]
- Liu M. D., Wu H., Wang S., Pang P., Jin S., Sun C. F., et al. (2018b). MiR-1275 promotes cell migration, invasion and proliferation in squamous cell carcinoma of head and neck via up-regulating IGF-1R and CCR7. Gene 646 1–7. 10.1016/j.gene.2017.12.049 [DOI] [PubMed] [Google Scholar]
- Liu N., Yang H., Wang H. (2018c). miR-598 acts as a tumor suppressor in human gastric cancer by targeting IGF-1R. Onco Targets Ther. 11 2911–2923. 10.2147/ott.s166597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Hu Y., Ma L., Du M., Xia L., Hu Z. (2015c). miR-425 inhibits melanoma metastasis through repression of PI3K-Akt pathway by targeting IGF-1. Biomed. Pharmacother. 75 51–57. 10.1016/j.biopha.2015.08.010 [DOI] [PubMed] [Google Scholar]
- Liu T.-J., LaFortune T., Honda T., Ohmori O., Hatakeyama S., Meyer T., et al. (2007). Inhibition of both focal adhesion kinase and insulin-like growth factor-I receptor kinase suppresses glioma proliferation in vitro and in vivo. Mol. Cancer Ther. 6 1357–1367. 10.1158/1535-7163.mct-06-0476 [DOI] [PubMed] [Google Scholar]
- Liu W., Bloom D. A., Cance W. G., Kurenova E. V., Golubovskaya V. M., Hochwald S. N. (2008). FAK and IGF-1R interact to provide survival signals in human pancreatic adenocarcinoma cells. Carcinogenesis 29 1096–1107. 10.1093/carcin/bgn026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Kang L., Han J., Wang Y., Shen C., Yan Z., et al. (2018d). miR-342-3p suppresses hepatocellular carcinoma proliferation through inhibition of IGF-1R-mediated Warburg effect. Onco Targets Ther. 11 1643–1653. 10.2147/ott.s161586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. Y., Tang S. H., Wu S. L., Luo Y. H., Cao M. R., Zhou H. K., et al. (2015d). Epigenetic modulation of insulin-like growth factor-II overexpression by hepatitis B virus X protein in hepatocellular carcinoma. Am. J. Cancer Res. 5:956. [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Zhu S. T., Wang X., Deng J., Li W. H., Zhang P., et al. (2016). MiR-100 inhibits osteosarcoma cell proliferation, migration, and invasion and enhances chemosensitivity by targeting IGFIR. Technol. Cancer Res. Treat. 15 N40–N48. [DOI] [PubMed] [Google Scholar]
- Liu Z. Q., Fu W. Q., Zhao S., Zhao X. (2016). Regulation of insulin-like growth factor 1 receptor signaling by microRNA-4458 in the development of lumbar disc degeneration. Am. J. Transl. Res. 8 2309–2316. [PMC free article] [PubMed] [Google Scholar]
- Liu Z., He F., OuYang S., Li Y., Ma F., Chang H., et al. (2019). miR-140-5p could suppress tumor proliferation and progression by targeting TGFBRI/SMAD2/3 and IGF-1R/AKT signaling pathways in Wilms’ tumor. BMC Cancer 19:405. 10.1186/s12885-019-5609-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Wu G., Lin C., Guo H., Xu J., Zhao T. (2018e). IGF2BP1 over-expression in skin squamous cell carcinoma cells is essential for cell growth. Biochem. Biophys. Res. Commun. 501 731–738. 10.1016/j.bbrc.2018.05.057 [DOI] [PubMed] [Google Scholar]
- Lu L., Cai M., Peng M., Wang F., Zhai X. (2019). miR-491-5p functions as a tumor suppressor by targeting IGF2 in colorectal cancer. Cancer Manag. Res. 11 1805–1816. 10.2147/cmar.s183085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Risch E., Deng Q., Biglia N., Picardo E., Katsaros D., et al. (2013). An insulin-like growth factor-II intronic variant affects local DNA conformation and ovarian cancer survival. Carcinogenesis 34 2024–2030. 10.1093/carcin/bgt168 [DOI] [PubMed] [Google Scholar]
- Luo L.-H., Rao L., Luo L.-F., Chen K., Ran R.-Z., Liu X.-L. (2020). Long non-coding RNA NKILA inhibited angiogenesis of breast cancer through NF-κB/IL-6 signaling pathway. Microvasc. Res. 129:103968. 10.1016/j.mvr.2019.103968 [DOI] [PubMed] [Google Scholar]
- Luo X., Dong J., He X., Shen L., Long C., Liu F., et al. (2020). MiR-155-5p exerts tumor-suppressing functions in Wilms tumor by targeting IGF2 via the PI3K signaling pathway. Biomed. Pharmacother. 125:109880. 10.1016/j.biopha.2020.109880 [DOI] [PubMed] [Google Scholar]
- Ma J., Sawai H., Matsuo Y., Ochi N., Yasuda A., Takahashi H., et al. (2010). IGF-1 mediates PTEN suppression and enhances cell invasion and proliferation via activation of the IGF-1/PI3K/Akt signaling pathway in pancreatic cancer cells. J. Surg. Res. 160 90–101. 10.1016/j.jss.2008.08.016 [DOI] [PubMed] [Google Scholar]
- Ma Z., Cai Y., Zhang L., Tian C., Lyu L. (2020). LINC00319 promotes cervical cancer progression via targeting miR-147a/IGF1R pathway. Cancer Biother. Radiopharm. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Martinez-Quetglas I., Pinyol R., Dauch D., Torrecilla S., Tovar V., Moeini A., et al. (2016). IGF2 is up-regulated by epigenetic mechanisms in hepatocellular carcinomas and is an actionable oncogene product in experimental models. Gastroenterology 151 1192–1205. 10.1053/j.gastro.2016.09.001 [DOI] [PubMed] [Google Scholar]
- Mata R., Palladino C., Nicolosi M. L., Lo Presti A. R., Malaguarnera R., Ragusa M., et al. (2016). IGF-I induces upregulation of DDR1 collagen receptor in breast cancer cells by suppressing MIR-199a-5p through the PI3K/AKT pathway. Oncotarget 7 7683–7700. 10.18632/oncotarget.6524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Men Y., Ye L., Risgaard R. D., Promes V., Zhao X., Paukert M., et al. (2020). Astroglial FMRP deficiency cell-autonomously up-regulates miR-128 and disrupts developmental astroglial mGluR5 signaling. Proc. Natl. Acad. Sci. U.S.A. 117 25092–25103. 10.1073/pnas.2014080117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moharamoghli M., Hassan-Zadeh V., Dolatshahi E., Alizadeh Z., Farazmand A. (2019). The expression of GAS5, THRIL, and RMRP lncRNAs is increased in T cells of patients with rheumatoid arthritis. Clin. Rheumatol. 38 3073–3080. 10.1007/s10067-019-04694-z [DOI] [PubMed] [Google Scholar]
- Moritake H., Saito Y., Sawa D., Sameshima N., Yamada A., Kinoshita M., et al. (2019). TAE226, a dual inhibitor of focal adhesion kinase and insulin-like growth factor-I receptor, is effective for Ewing sarcoma. Cancer Med. 8 7809–7821. 10.1002/cam4.2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser C., Schachtschneider P., Lang S. A., Gaumann A., Mori A., Zimmermann J., et al. (2008). Inhibition of insulin-like growth factor-I receptor (IGF-1R) using NVP-AEW541, a small molecule kinase inhibitor, reduces orthotopic pancreatic cancer growth and angiogenesis. Eur. J. Cancer 44 1577–1586. 10.1016/j.ejca.2008.04.003 [DOI] [PubMed] [Google Scholar]
- Mukohara T., Shimada H., Ogasawara N., Wanikawa R., Shimomura M., Nakatsura T., et al. (2009). Sensitivity of breast cancer cell lines to the novel insulin-like growth factor-1 receptor (IGF-1R) inhibitor NVP-AEW541 is dependent on the level of IRS-1 expression. Cancer Lett. 282 14–24. 10.1016/j.canlet.2009.02.056 [DOI] [PubMed] [Google Scholar]
- Niu X. B., Fu G. B., Wang L., Ge X., Liu W. T., Wen Y. Y., et al. (2017). Insulin-like growth factor-I induces chemoresistence to docetaxel by inhibiting miR-143 in human prostate cancer. Oncotarget 8 107157–107166. 10.18632/oncotarget.22362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Z.-R., Han T., Sun X.-L., Luan L.-X., Gou W.-L., Zhu X.-M. (2018). MicroRNA-30a-3p is overexpressed in the placentas of patients with preeclampsia and affects trophoblast invasion and apoptosis by its effects on IGF-1. Am. J. Obstetr. Gynecol. 218 249.e1–249.e12. [DOI] [PubMed] [Google Scholar]
- Ohshima-Hosoyama S., Hosoyama T., Nelon L. D., Keller C. (2010). IGF-1 receptor inhibition by picropodophyllin in medulloblastoma. Biochem. Biophys. Res. Commun. 399 727–732. 10.1016/j.bbrc.2010.08.009 [DOI] [PubMed] [Google Scholar]
- Okamura J., Huang Y., Moon D., Brait M., Chang X., Kim M. S. (2012). Downregulation of insulin-like growth factor-binding protein 7 in cisplatin-resistant non-small cell lung cancer. Cancer Biol. Ther. 13 148–155. 10.4161/cbt.13.3.18695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani H., Yamamoto H., Takaoka M., Sakaguchi M., Soh J., Jida M., et al. (2015). TAE226, a bis-anilino pyrimidine compound, inhibits the EGFR-mutant kinase including T790M mutant to show anti-tumor effect on EGFR-mutant non-small cell lung cancer cells. PLoS One 10:e0129838. 10.1371/journal.pone.0129838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Øy G. F., Slipicevic A., Davidson B., Solberg Faye R. M., Mælandsmo G., Flørenes V. A. (2010). Biological effects induced by insulin-like growth factor binding protein 3 (IGFBP-3) in malignant melanoma. Int. J. Cancer 126 350–361. 10.1002/ijc.24727 [DOI] [PubMed] [Google Scholar]
- Pan Y.-H., Jiao L., Lin C.-Y., Lu C.-H., Li L., Chen H.-Y., et al. (2018). Combined treatment with metformin and gefitinib overcomes primary resistance to EGFR-TKIs with EGFR mutation via targeting IGF-1R signaling pathway. Biol. Targets Ther. 12:75. 10.2147/btt.s166867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulitschke V., Eichhoff O., Gerner C., Paulitschke P., Bileck A., Mohr T., et al. (2019). Proteomic identification of a marker signature for MAPK i resistance in melanoma. EMBO J. 38:e95874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pian L., Wen X., Kang L., Li Z., Nie Y., Du Z., et al. (2018). Targeting the IGF1R pathway in breast cancer using antisense lncRNA-mediated promoter cis competition. Mol. Ther. Nucleic Acids 12 105–117. 10.1016/j.omtn.2018.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak M. (2008). Insulin and insulin-like growth factor signalling in neoplasia. Nat. Rev. Cancer 8 915–928. 10.1038/nrc2536 [DOI] [PubMed] [Google Scholar]
- Premkumar D. R., Jane E. P., Pollack I. F. (2010). Co-administration of NVP-AEW541 and dasatinib induces mitochondrial-mediated apoptosis through Bax activation in malignant human glioma cell lines. Int. J. Oncol. 37 633–643. [DOI] [PubMed] [Google Scholar]
- Qi B., Zhang R., Sun R., Guo M., Zhang M., Wei G., et al. (2019). IGF-1R inhibitor PQ401 inhibits osteosarcoma cell proliferation, migration and colony formation. Int. J. Clin. Exp. Pathol. 12:1589. [PMC free article] [PubMed] [Google Scholar]
- Qian B., Katsaros D., Lu L., Canuto E. M., Benedetto C., Beeghly-Fadiel A., et al. (2011). IGF-II promoter specific methylation and expression in epithelial ovarian cancer and their associations with disease characteristics. Oncol. Rep. 25 203–213. [PMC free article] [PubMed] [Google Scholar]
- Qian X., Yu J., Yin Y., He J., Wang L., Li Q., et al. (2013). MicroRNA-143 inhibits tumor growth and angiogenesis and sensitizes chemosensitivity to oxaliplatin in colorectal cancers. Cell Cycle 12 1385–1394. 10.4161/cc.24477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao X. R., Wang L., Liu M., Tian Y., Chen T. (2020). MiR-210-3p attenuates lipid accumulation and inflammation in atherosclerosis by repressing IGF2. Biosci. Biotechnol. Biochem. 84 321–329. 10.1080/09168451.2019.1685370 [DOI] [PubMed] [Google Scholar]
- Rahmoon M. A., Youness R. A., Gomaa A. I., Hamza M. T., Waked I., El Tayebi H. M., et al. (2017). MiR-615-5p depresses natural killer cells cytotoxicity through repressing IGF-1R in hepatocellular carcinoma patients. Growth Fact. 35 76–87. 10.1080/08977194.2017.1354859 [DOI] [PubMed] [Google Scholar]
- Ramachandran C., Khatib Z., Petkarou A., Fort J., Fonseca H. B., Melnick S. J., et al. (2004). Tamoxifen modulation of etoposide cytotoxicity involves inhibition of protein kinase C activity and insulin-like growth factor II expression in brain tumor cells. J. Neurooncol. 67 19–28. 10.1023/b:neon.0000021738.77612.1b [DOI] [PubMed] [Google Scholar]
- Refolo M. G., D’Alessandro R., Lippolis C., Carella N., Cavallini A., Messa C., et al. (2017). IGF-1R tyrosine kinase inhibitors and Vitamin K1 enhance the antitumor effects of Regorafenib in HCC cell lines. Oncotarget 8:103465. 10.18632/oncotarget.21403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regel I., Eichenmüller M., Joppien S., Liebl J., Häberle B., Müller-Höcker J., et al. (2012). IGFBP3 impedes aggressive growth of pediatric liver cancer and is epigenetically silenced in vascular invasive and metastatic tumors. Mol. Cancer 11 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren L., Yao Y., Wang Y., Wang S. (2019). MiR-505 suppressed the growth of hepatocellular carcinoma cells via targeting IGF-1R. Biosci. Rep. 39:BSR20182442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rø T. B., Holien T., Fagerli U.-M., Hov H., Misund K., Waage A., et al. (2013). HGF and IGF-1 synergize with SDF-1α in promoting migration of myeloma cells by cooperative activation of p21-activated kinase. Exp. Hematol. 41 646–655. 10.1016/j.exphem.2013.03.002 [DOI] [PubMed] [Google Scholar]
- Rodvold J. J., Xian S., Nussbacher J., Tsui B., Waller T. C., Searles S. C., et al. (2020). IRE1α and iGf signaling predict resistance to an endoplasmic reticulum stress-inducing drug in glioblastoma cells. Sci. Rep. 10 1–12. 10.1515/ersc-2018-0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig S. A. (2020). The continuing evolution of insulin-like growth factor signaling. F1000Research 9:F1000FacultyRev–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo A., Paret C., Alt F., Burhenne J., Fresnais M., Wagner W., et al. (2019). Ceritinib-Induced regression of an insulin-like growth factor-driven neuroepithelial brain tumor. Int. J. Mol. Sci. 20:4267. 10.3390/ijms20174267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbatini P., Korenchuk S., Rowand J. L., Groy A., Liu Q., Leperi D., et al. (2009). GSK1838705A inhibits the insulin-like growth factor-1 receptor and anaplastic lymphoma kinase and shows antitumor activity in experimental models of human cancers. Mol. Cancer Ther. 8 2811–2820. 10.1158/1535-7163.mct-09-0423 [DOI] [PubMed] [Google Scholar]
- Sampson V. B., Vetter N. S., Kamara D. F., Collier A. B., Gresh R. C., Kolb E. A. (2015). Vorinostat enhances cytotoxicity of SN-38 and temozolomide in ewing sarcoma cells and activates STAT3/AKT/MAPK pathways. PLoS One 10:e0142704. 10.1371/journal.pone.0142704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Sakaeda M., Ishii J., Kashiwagi K., Shimoyamada H., Okudela K., et al. (2011). Insulin-like growth factor binding protein-4 gene silencing in lung adenocarcinomas. Pathol. Int. 61 19–27. 10.1111/j.1440-1827.2010.02612.x [DOI] [PubMed] [Google Scholar]
- Schayek H., Bentov I., Sun S., Plymate S. R., Werner H. (2010). Progression to metastatic stage in a cellular model of prostate cancer is associated with methylation of the androgen receptor gene and transcriptional suppression of the insulin-like growth factor-I receptor gene. Exp. Cell Res. 316 1479–1488. 10.1016/j.yexcr.2010.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shali H., Shabani M., Pourgholi F., Hajivalili M., Aghebati-Maleki L., Jadidi-Niaragh F., et al. (2018). Co-delivery of insulin-like growth factor 1 receptor specific siRNA and doxorubicin using chitosan-based nanoparticles enhanced anticancer efficacy in A549 lung cancer cell line. Artif. Cells Nanomed. Biotechnol. 46 293–302. 10.1080/21691401.2017.1307212 [DOI] [PubMed] [Google Scholar]
- Shan J., Shen J., Wu M., Zhou H., Feng J., Yao C., et al. (2019). Tcf7l1 acts as a suppressor for the self-renewal of liver cancer stem cells and is regulated by IGF/MEK/ERK signaling independent of β-catenin. Stem Cells 37 1389–1400. 10.1002/stem.3063 [DOI] [PubMed] [Google Scholar]
- Shastri A. A., Saleh A., Savage J. E., DeAngelis T., Camphausen K., Simone N. L. (2020). Dietary alterations modulate the microRNA 29/30 and IGF-1/AKT signaling axis in breast Cancer liver metastasis. Nutr. Metab. 17:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X., Zhao Z., Yang B. (2020). MicroRNA-155 promotes apoptosis of colonic smooth muscle cells and aggravates colonic dysmotility by targeting IGF-1. Exp. Ther. Med. 19 2725–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X., Teng F. (2015). Down-regulated miR-28-5p in human hepatocellular carcinoma correlated with tumor proliferation and migration by targeting insulin-like growth factor-1 (IGF-1). Mol. Cell. Biochem. 408 283–293. 10.1007/s11010-015-2506-z [DOI] [PubMed] [Google Scholar]
- Simpson A. D., Soo Y. W. J., Rieunier G., Aleksic T., Ansorge O., Jones C., et al. (2020). Type 1 IGF receptor associates with adverse outcome and cellular radioresistance in paediatric high-grade glioma. Br. J. Cancer 122 624–629. 10.1038/s41416-019-0677-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin S. T., Li Y., Liu M., Ma S., Guan X.-Y. (2019). TROP-2 exhibits tumor suppressive functions in cervical cancer by dual inhibition of IGF-1R and ALK signaling. Gynecol. Oncol. 152 185–193. 10.1016/j.ygyno.2018.10.039 [DOI] [PubMed] [Google Scholar]
- Singh S. K., Moretta D., Almaguel F., De Leon M., De Leon D. D. (2008). Precursor IGF-II (proIGF-II) and mature IGF-II (mIGF-II) induce Bcl-2 and Bcl-XL expression through different signaling pathways in breast cancer cells. Growth Fact. 26:92. 10.1080/08977190802057258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C. L., Liu B., Diao H. Y., Shi Y. F., Zhang J. C., Li Y. X., et al. (2016). Down-regulation of microRNA-320 suppresses cardiomyocyte apoptosis and protects against myocardial ischemia and reperfusion injury by targeting IGF-1. Oncotarget 7 39740–39757. 10.18632/oncotarget.9240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R. X.-D., Chen Y., Zhang Z., Bao Y., Yue W., Wang J.-P., et al. (2010). Estrogen utilization of IGF-1-R and EGF-R to signal in breast cancer cells. J. Steroid Biochem. Mol. Biol. 118 219–230. 10.1016/j.jsbmb.2009.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiliotaki M., Markomanolaki H., Mela M., Mavroudis D., Georgoulias V., Agelaki S. (2011). Targeting the insulin-like growth factor I receptor inhibits proliferation and VEGF production of non-small cell lung cancer cells and enhances paclitaxel-mediated anti-tumor effect. Lung Cancer 73 158–165. 10.1016/j.lungcan.2010.11.010 [DOI] [PubMed] [Google Scholar]
- Strub T., Ghiraldini F. G., Carcamo S., Li M., Wroblewska A., Singh R., et al. (2018). SIRT6 haploinsufficiency induces BRAF V600E melanoma cell resistance to MAPK inhibitors via IGF signalling. Nat. Commun. 9 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H., Cai J., Xu L., Liu J., Chen M., Zheng M., et al. (2018). miR-483-3p regulates acute myocardial infarction by transcriptionally repressing insulin growth factor 1 expression. Mol. Med. Rep. 17 4785–4790. [DOI] [PubMed] [Google Scholar]
- Sun Y., Zheng S., Torossian A., Speirs C. K., Schleicher S., Giacalone N. J., et al. (2012). Role of insulin-like growth factor-1 signaling pathway in cisplatin-resistant lung cancer cells. Int. J. Radiat. Oncol. Biol. Phys. 82 e563–e572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai B.-J., Yao M., Zheng W.-J., Shen Y.-C., Wang L., Sun J.-Y., et al. (2019). Alteration of oncogenic IGF-II gene methylation status associates with hepatocyte malignant transformation. Hepatob. Pancreatic Dis. Int. 18 158–163. 10.1016/j.hbpd.2019.01.003 [DOI] [PubMed] [Google Scholar]
- Tan X., Fan S., Wu W., Zhang Y. (2015). MicroRNA-26a inhibits osteosarcoma cell proliferation by targeting IGF-1. Bone Res. 3:15033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S. H., Yang D. H., Huang W., Zhou H. K., Lu X. H., Ye G. (2006). Hypomethylated P4 promoter induces expression of the insulin-like growth factor-II gene in hepatocellular carcinoma in a Chinese population. Clin. Cancer Res. 12 4171–4177. 10.1158/1078-0432.ccr-05-2261 [DOI] [PubMed] [Google Scholar]
- Teng C.-F., Jeng L.-B., Shyu W.-C. (2018). Role of insulin-like growth factor 1 receptor signaling in stem cell stemness and therapeutic efficacy. Cell Transpl. 27 1313–1319. 10.1177/0963689718779777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa M., Shinozaki F., Motoyoshi Y., Sugiyama T., Yamamoto S., Sueishi M. (2014). Picropodophyllin and sorafenib synergistically suppress the proliferation and motility of hepatocellular carcinoma cells. Oncol. Lett. 8 2023–2026. 10.3892/ol.2014.2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torng P.-L., Lin C.-W., Chan M. W., Yang H.-W., Huang S.-C., Lin C.-T. (2009). Promoter methylation of IGFBP-3 and p53 expression in ovarian endometrioid carcinoma. Mol. Cancer 8:120. 10.1186/1476-4598-8-120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu C., Wang F., Wan J. (2018). MicroRNA-381 inhibits cell proliferation and invasion in endometrial carcinoma by targeting the IGF-1R. Mol. Med. Rep. 17 4090–4098. [DOI] [PubMed] [Google Scholar]
- Uzoh C. C., Holly J. M., Biernacka K. M., Persad R. A., Bahl A., Gillatt D., et al. (2011). Insulin-like growth factor-binding protein-2 promotes prostate cancer cell growth via IGF-dependent or -independent mechanisms and reduces the efficacy of docetaxel. Br. J. Cancer 104 1587–1593. 10.1038/bjc.2011.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartanian S., Lee J., Klijn C., Gnad F., Bagniewska M., Schaefer G., et al. (2019). ERBB3 and IGF1R signaling are required for Nrf2-dependent growth in KEAP1-mutant lung cancer. Cancer Res. 79 4828–4839. 10.1158/0008-5472.can-18-2086 [DOI] [PubMed] [Google Scholar]
- Vewinger N., Huprich S., Seidmann L., Russo A., Alt F., Bender H., et al. (2019). IGF1R is a potential new therapeutic target for HGNET-BCOR brain tumor patients. Int. J. Mol. Sci. 20:3027. 10.3390/ijms20123027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkova E., Robinson B. A., Willis J., Currie M. J., Dachs G. U. (2014). Marginal effects of glucose, insulin and insulin-like growth factor on chemotherapy response in endothelial and colorectal cancer cells. Oncol. Lett. 7 311–320. 10.3892/ol.2013.1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Mehren M., George S., Heinrich M. C., Schuetze S. M., Yap J. T., Jain Q. Y., et al. (2020). Linsitinib (OSI-906) for the treatment of adult and pediatric wild-type gastrointestinal stromal tumors, a SARC phase II study. Clin. Cancer Res. 26 1837–1845. 10.1158/1078-0432.ccr-19-1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Yan X., Ji L. Y., Ji X. T., Wang P., Guo S. W., et al. (2017). miR-139 Functions as An Antioncomir to Repress Glioma Progression Through Targeting IGF-1 R, AMY-1, and PGC-1beta. Technol. Cancer Res. Treat. 16 497–511. 10.1177/1533034616630866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Liu H., Tian L., Wang F., Han L., Zhang W., et al. (2017). miR-15b inhibits the progression of glioblastoma cells through targeting insulin-like growth factor receptor 1. Horm. Cancer 8 49–57. 10.1007/s12672-016-0276-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Luan T., Zhou S., Lin J., Yang Y., Liu W., et al. (2019a). LncRNA HCP5 promotes triple negative breast cancer progression as a ceRNA to regulate BIRC3 by sponging miR-219a-5p. Cancer Med. 8 4389–4403. 10.1002/cam4.2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Shi J., Jiang H., Xu K., Huang Z. (2020). Circ_0014130 Participates in the Proliferation and Apoptosis of Nonsmall Cell Lung Cancer Cells via the miR-142-5p/IGF-1 Axis. Cancer Biother. Radiopharm. 35 233–240. 10.1089/cbr.2019.2965 [DOI] [PubMed] [Google Scholar]
- Wang Q., Wu G., Zhang Z., Tang Q., Zheng W., Chen X., et al. (2018). Long non-coding RNA HOTTIP promotes renal cell carcinoma progression through the regulation of the miR-615/IGF-2 pathway. Int. J. Oncol. 53 2278–2288. [DOI] [PubMed] [Google Scholar]
- Wang Q., Zhang Y., Zhu J., Zheng H., Chen S., Chen L., et al. (2020). IGF-1R inhibition induces MEK phosphorylation to promote survival in colon carcinomas. Signal Transd. Target. Ther. 5 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Li H., Guo X., Wang Z., Liang S., Dang C. I. G. F.-I. (2016). Induces epithelial-to-mesenchymal transition via the IGF-1R-Src-MicroRNA-30a-E-cadherin pathway in nasopharyngeal carcinoma cells. Oncol. Res. 24 225–231. 10.3727/096504016x14648701447931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Liu Y., Lv M., Xing Q., Zhang Z., He X., et al. (2019b). miR-323-3p regulates the steroidogenesis and cell apoptosis in polycystic ovary syndrome (PCOS) by targeting IGF-1. Gene 683 87–100. 10.1016/j.gene.2018.10.006 [DOI] [PubMed] [Google Scholar]
- Wang W., Dong M., Zhang W., Liu T. (2019c). Long noncoding LUCAT1 promotes cisplatin resistance of non-small cell lung cancer by promoting IGF-2. Eur. Rev. Med. Pharmacol. Sci. 23 5229–5234. [DOI] [PubMed] [Google Scholar]
- Wang X., Liu S., Cao L., Zhang T., Yue D., Wang L., et al. (2017). miR-29a-3p suppresses cell proliferation and migration by downregulating IGF1R in hepatocellular carcinoma. Oncotarget 8 86592–86603. 10.18632/oncotarget.21246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Hou L., Yuan X., Xu N., Zhao S., Yang L., et al. (2020). miR-483-3p promotes cell proliferation and suppresses apoptosis in rheumatoid arthritis fibroblast-like synoviocytes by targeting IGF-1. Biomed. Pharmacother. 130:110519. 10.1016/j.biopha.2020.110519 [DOI] [PubMed] [Google Scholar]
- Wang Y., Jia L., Wang B., Diao S., Jia R., Shang J. (2019d). MiR-495/IGF-1/AKT signaling as a novel axis is involved in the epithelial-to-mesenchymal transition of oral squamous cell carcinoma. J. Oral Maxillofac. Surg. 77 1009–1021. 10.1016/j.joms.2018.12.021 [DOI] [PubMed] [Google Scholar]
- Wang Y., Lu J.-H., Wu Q.-N., Jin Y., Wang D.-S., Chen Y.-X., et al. (2019e). LncRNA LINRIS stabilizes IGF2BP2 and promotes the aerobic glycolysis in colorectal cancer. Mol. Cancer 18 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. G., Fukazawa T., Nishikawa T., Watanabe N., Sakurama K., Motoki T., et al. (2008). TAE226, a dual inhibitor for FAK and IGF-1R, has inhibitory effects on mTOR signaling in esophageal cancer cells. Oncol. Rep. 20 1473–1477. [PubMed] [Google Scholar]
- Wang Z., Liu G., Mao J., Xie M., Zhao M., Guo X., et al. (2019f). IGF-1R inhibition suppresses cell proliferation and increases radiosensitivity in nasopharyngeal carcinoma cells. Mediat. Inflamm. 2019:5497467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Zhao Z., Yang Y., Luo M., Zhang M., Wang X., et al. (2018). MiR-99b-5p and miR-203a-3p function as tumor suppressors by targeting IGF-1R in gastric cancer. Sci. Rep. 8:10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warshamana-Greene G. S., Litz J., Buchdunger E., García-Echeverría C., Hofmann F., Krystal G. W. (2005). The insulin-like growth factor-I receptor kinase inhibitor, NVP-ADW742, sensitizes small cell lung cancer cell lines to the effects of chemotherapy. Clin. Cancer Res. 11 1563–1571. 10.1158/1078-0432.ccr-04-1544 [DOI] [PubMed] [Google Scholar]
- Warshamana-Greene G. S., Litz J., Buchdunger E., Hofmann F., García-Echeverría C., Krystal G. W. (2004). The insulin-like growth factor-I (IGF-I) receptor kinase inhibitor NVP-ADW742, in combination with STI571, delineates a spectrum of dependence of small cell lung cancer on IGF-I and stem cell factor signaling. Mol. Cancer Ther. 3 527–536. [PubMed] [Google Scholar]
- Watanabe N., Takaoka M., Sakurama K., Tomono Y., Hatakeyama S., Ohmori O., et al. (2008). Dual tyrosine kinase inhibitor for focal adhesion kinase and insulin-like growth factor-I receptor exhibits anticancer effect in esophageal adenocarcinoma in vitro and in vivo. Clin. Cancer Res. 14 4631–4639. 10.1158/1078-0432.ccr-07-4755 [DOI] [PubMed] [Google Scholar]
- Wen Y. Y., Liu W. T., Sun H. R., Ge X., Shi Z. M., Wang M., et al. (2017). IGF-1-mediated PKM2/beta-catenin/miR-152 regulatory circuit in breast cancer. Sci. Rep. 7:15897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrigley S., Arafa D., Tropea D. (2017). Insulin-like growth factor 1: at the crossroads of brain development and aging. Front. Cell. Neurosci. 11:14. 10.3389/fncel.2017.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Liu J., Wu Z., Wu X., Yao X. (2017). MicroRNA-184 inhibits cell proliferation and metastasis in human colorectal cancer by directly targeting IGF-1R. Oncol. Lett. 14 3215–3222. 10.3892/ol.2017.6499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. Y., Wang X. H., Liu K., Zhang J. L. (2020). LncRNA MALAT1 regulates trophoblast cells migration and invasion via miR-206/IGF-1 axis. Cell Cycle 19 39–52. 10.1080/15384101.2019.1691787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. H., Wang Y. H., Wang W., Shen W., Sang Y. Z., Liu L., et al. (2016). MiR-18b suppresses high-glucose-induced proliferation in HRECs by targeting IGF-1/IGF1R signaling pathways. Int. J. Biochem. Cell. Biol. 73 41–52. 10.1016/j.biocel.2016.02.002 [DOI] [PubMed] [Google Scholar]
- Wu W., Chen F., Cui X., Yang L., Chen J., Zhao J., et al. (2018). LncRNA NKILA suppresses TGF-β-induced epithelial–mesenchymal transition by blocking NF-κB signaling in breast cancer. Int. J. Cancer 143 2213–2224. 10.1002/ijc.31605 [DOI] [PubMed] [Google Scholar]
- Wu W., Ma J., Shao N., Shi Y., Liu R., Li W., et al. (2017). Co-targeting IGF-1R and autophagy enhances the effects of cell growth suppression and apoptosis induced by the IGF-1R inhibitor NVP-AEW541 in triple-negative breast cancer cellsA. PLoS One 12:e0169229. 10.1371/journal.pone.0169229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Wu Q., Zhou X., Huang J. (2019). SphK1 functions downstream of IGF-1 to modulate IGF-1-induced EMT, migration and paclitaxel resistance of A549 cells: a preliminary in vitro study. J. Cancer 10 4264–4269. 10.7150/jca.32646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Zheng X., Cheng J., Zhang K., Ma C. (2020). LncRNA TUG1 regulates proliferation and apoptosis by regulating miR-148b/IGF2 axis in ox-LDL-stimulated VSMC and HUVEC. Life Sci. 243:117287. 10.1016/j.lfs.2020.117287 [DOI] [PubMed] [Google Scholar]
- Xia M., Li H., Wang J. J., Zeng H. J., Wang S. H. (2016). MiR-99a suppress proliferation, migration and invasion through regulating insulin-like growth factor 1 receptor in breast cancer. Eur. Rev. Med. Pharmacol. Sci. 20 1755–1763. [PubMed] [Google Scholar]
- Xiang Y., Song Y., Li Y., Zhao D., Ma L., Tan L. (2016). miR-483 is down-regulated in polycystic ovarian syndrome and inhibits KGN cell proliferation via targeting insulin-like growth factor 1 (IGF1). Med. Sci. Monit. 22 3383–3393. 10.12659/msm.897301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J., He C., Zhong Y., Liu L., Xiao Y., Liu W. (2019). miR-223 regulates proliferation and apoptosis by targeting insulin-like growth factor 1 receptor (IGF1R) in osteosarcoma cells. Am. Sci. Publish. 9 459–466. 10.1166/mex.2019.1516 [DOI] [Google Scholar]
- Xie R., Wang M., Zhou W., Wang D., Yuan Y., Shi H., et al. (2019). Long non-coding RNA (LncRNA) UFC1/miR-34a contributes to proliferation and migration in breast cancer. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 25:7149. 10.12659/msm.917562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G., Yuan Y., Wang M., Yang J., Wang H., Sun Y., et al. (2019). LncRNA H19 suppresses metastasis of follicular thyroid carcinoma via the IGF1/JAK/STAT pathway. STAT Pathway [Epub ahead of print]. [Google Scholar]
- Xu M., Zheng X. M., Jiang F., Qiu W. Q. (2018). MicroRNA-190b regulates lipid metabolism and insulin sensitivity by targeting IGF-1 and ADAMTS9 in non-alcoholic fatty liver disease. J. Cell. Biochem. 119 5864–5874. 10.1002/jcb.26776 [DOI] [PubMed] [Google Scholar]
- Yang D., Qian H., Fang Z., Xu A., Zhao S., Liu B., et al. (2020). Silencing circular RNA VANGL1 inhibits progression of bladder cancer by regulating miR-1184/IGFBP2 axis. Cancer Med. 9 700–710. 10.1002/cam4.2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Fu G., Liu F., Hu C., Lin J., Tan Z., et al. (2019). LncRNA THOR promotes tongue squamous cell carcinomas by stabilizing IGF2BP1 downstream targets. Biochimie 165 9–18. 10.1016/j.biochi.2019.06.012 [DOI] [PubMed] [Google Scholar]
- Yang J.-J., Liu L.-P., Tao H., Hu W., Shi P., Deng Z.-Y., et al. (2016). MeCP2 silencing of LncRNA H19 controls hepatic stellate cell proliferation by targeting IGF1R. Toxicology 359 39–46. 10.1016/j.tox.2016.06.016 [DOI] [PubMed] [Google Scholar]
- Yang Y., Liu X., Cheng L., Li L., Wei Z., Wang Z., et al. (2020). Tumor suppressor microRNA-138 suppresses low-grade glioma development and metastasis via regulating IGF2BP2. Onco Targets Ther. 13 2247–2260. 10.2147/ott.s232795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Song T., Xiong G., Wu Z., Li Q., Xia H., et al. (2018). Combination of peripheral blood mononuclear cell miR-19b-5p, miR-221, miR-25-5p, and hypertension correlates with an increased heart failure risk in coronary heart disease patients. Anatolian J. Cardiol. 20:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavari K., Taghikhani M., Maragheh M. G., Mesbah-Namin S. A., Babaei M. H. (2010). Downregulation of IGF-1R expression by RNAi inhibits proliferation and enhances chemosensitization of human colon cancer cells. Int. J. Colorect. Dis. 25 9–16. 10.1007/s00384-009-0783-2 [DOI] [PubMed] [Google Scholar]
- Ye P., Qu C.-F., Hu X.-L. (2016). Impact of IGF-1, IGF-1R, and IGFBP-3 promoter methylation on the risk and prognosis of esophageal carcinoma. Tumor Biol. 37 6893–6904. 10.1007/s13277-015-4489-5 [DOI] [PubMed] [Google Scholar]
- Ye S., Song W., Xu X., Zhao X., Yang L. (2016). IGF2BP2 promotes colorectal cancer cell proliferation and survival through interfering with RAF-1 degradation by miR-195. FEBS Lett. 590 1641–1650. 10.1002/1873-3468.12205 [DOI] [PubMed] [Google Scholar]
- Yi F., Shang Y., Li B., Dai S., Wu W., Cheng L., et al. (2017). MicroRNA-193-5p modulates angiogenesis through IGF2 in type 2 diabetic cardiomyopathy. Biochem. Biophys. Res. Commun. 491 876–882. 10.1016/j.bbrc.2017.07.108 [DOI] [PubMed] [Google Scholar]
- Yin D., Tamaki N., Parent A. D., Zhang J. H. (2005). Insulin-like growth factor-I decreased etoposide-induced apoptosis in glioma cells by increasing bcl-2 expression and decreasing CPP32 activity. Neurol. Res. 27 27–35. 10.1179/016164105x18151 [DOI] [PubMed] [Google Scholar]
- Youness R. A., El-Tayebi H. M., Assal R. A., Hosny K., Esmat G., Abdelaziz A. I. (2016). MicroRNA-486-5p enhances hepatocellular carcinoma tumor suppression through repression of IGF-1R and its downstream mTOR, STAT3 and c-Myc. Oncol. Lett. 12 2567–2573. 10.3892/ol.2016.4914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M., Yu S., Gong W., Chen D., Guan J., Liu Y. (2019). Knockdown of linc01023 restrains glioma proliferation, migration and invasion by regulating IGF-1R/AKT pathway. J. Cancer 10:2961. 10.7150/jca.31004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L., Jarrett C., Brown K., Gillespie K. M., Holly J. M., Perks C. M. (2013). Insulin-like growth factor binding protein-3 (IGFBP-3) plays a role in the anti-tumorigenic effects of 5-Aza-2′-deoxycytidine (AZA) in breast cancer cells. Exp. Cell Res. 319 2282–2295. 10.1016/j.yexcr.2013.06.011 [DOI] [PubMed] [Google Scholar]
- Zhang B., Li Y., Hou D., Shi Q., Yang S., Li Q. (2017). MicroRNA-375 Inhibits Growth and Enhances Radiosensitivity in Oral Squamous Cell Carcinoma by Targeting Insulin Like Growth Factor 1 Receptor. Cell Physiol. Biochem. 42 2105–2117. 10.1159/000479913 [DOI] [PubMed] [Google Scholar]
- Zhang Q., Li T., Wang Z., Kuang X., Shao N., Lin Y. (2020). lncRNA NR2F1-AS1 promotes breast cancer angiogenesis through activating IGF-1/IGF-1R/ERK pathway. J. Cell. Mol. Med. 24 8236–8247. 10.1111/jcmm.15499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Liu S., Liu K., Liu Y. (2019). Long non-coding RNA deleted in lymphocytic leukaemia 1 promotes hepatocellular carcinoma progression by sponging miR-133a to regulate IGF-1R expression. J. Cell. Mol. Med. 23 5154–5164. 10.1111/jcmm.14384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Sun Y., Peng R., Liu H., He W., Zhang L., et al. (2018). The long noncoding RNA 150Rik promotes mesangial cell proliferation via miR-451/IGF1R/p38 MAPK signaling in diabetic nephropathy. Cell Physiol. Biochem. 51 1410–1428. 10.1159/000495590 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Yan N., Wang X., Chang Y., Wang Y. (2019). MiR-129-5p regulates cell proliferation and apoptosis via IGF-1R/Src/ERK/Egr-1 pathway in RA-fibroblast-like synoviocytes. Biosci. Rep. 39:BSR20192009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F. Y., Han J., Chen X. W., Wang J., Wang X. D., Sun J. G., et al. (2016). miR-223 enhances the sensitivity of non-small cell lung cancer cells to erlotinib by targeting the insulin-like growth factor-1 receptor. Int. J. Mol. Med. 38 183–191. 10.3892/ijmm.2016.2588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F., Tang Q., Zheng X. H., Wu J., Huang H., Zhang H., et al. (2018). Inactivation of Stat3 and crosstalk of miRNA155-5p and FOXO3a contribute to the induction of IGFBP1 expression by beta-elemene in human lung cancer. Exp. Mol. Med. 50:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F., Wang X., Zheng W., Zhao J. (2019). Long noncoding RNA HOXA-AS2 promotes cell migration and invasion via upregulating IGF-2 in non-small cell lung cancer as an oncogene. Eur. Rev. Med. Pharmacol. Sci. 23 4793–4799. [DOI] [PubMed] [Google Scholar]
- Zheng F., Zhang Z., Flamini V., Jiang W. G., Cui Y. (2017). The axis of CXCR4/SDF-1 plays a role in colon cancer cell adhesion through regulation of the AKT and IGF1R signalling pathways. Anticancer Res. 37 4361–4369. [DOI] [PubMed] [Google Scholar]
- Zhong Z., Li F., Li Y., Qin S., Wen C., Fu Y., et al. (2018). Inhibition of microRNA-19b promotes ovarian granulosa cell proliferation by targeting IGF-1 in polycystic ovary syndrome. Mol. Med. Rep. 17 4889–4898. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhou F., Nie L., Feng D., Guo S., Luo R. (2017). MicroRNA-379 acts as a tumor suppressor in non-small cell lung cancer by targeting the IGF1R-mediated AKT and ERK pathways. Oncol. Rep. 38 1857–1866. 10.3892/or.2017.5835 [DOI] [PubMed] [Google Scholar]
- Zhou H., Rao J., Lin J., Yin B., Sheng H., Lin F., et al. (2011). The insulin-like growth factor-I receptor kinase inhibitor NVP-ADW742 sensitizes medulloblastoma to the effects of chemotherapy. Oncol. Rep. 25 1565–1571. [DOI] [PubMed] [Google Scholar]
- Zhou N., Sun Z., Li N., Ge Y., Zhou J., Han Q., et al. (2018). miR197 promotes the invasion and migration of colorectal cancer by targeting insulinlike growth factorbinding protein 3. Oncol. Rep. 40 2710–2721. [DOI] [PubMed] [Google Scholar]
- Zhou Q. (2015). BMS-536924, an ATP-competitive IGF-1R/IR inhibitor, decreases viability and migration of temozolomide-resistant glioma cells in vitro and suppresses tumor growth in vivo. OncoTargets Ther. 8:689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Chen N., Xu H., Zhou X., Wang J., Fang X., et al. (2020). Regulation of Hippo-YAP signaling by insulin-like growth factor-1 receptor in the tumorigenesis of diffuse large B-cell lymphoma. J. Hematol. Oncol. 13 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Shen F., Ma P., Hui H., Pei S., Chen M., et al. (2015). GSK1838705A, an IGF-1R inhibitor, inhibits glioma cell proliferation and suppresses tumor growth in vivo. Mol. Med. Rep. 12 5641–5646. 10.3892/mmr.2015.4129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Zhao X., Li X., Ping G., Pei S., Chen M., et al. (2016). PQ401, an IGF-1R inhibitor, induces apoptosis and inhibits growth, proliferation and migration of glioma cells. J. Chemother. 28 44–49. 10.1179/1973947815y.0000000026 [DOI] [PubMed] [Google Scholar]
- Zhou Y., Li S., Li J., Wang D., Li Q. (2017). Effect of microRNA-135a on cell proliferation, migration, invasion, apoptosis and tumor angiogenesis through the IGF-1/PI3K/Akt signaling pathway in non-small cell lung cancer. Cell Physiol. Biochem. 42 1431–1446. 10.1159/000479207 [DOI] [PubMed] [Google Scholar]
- Zhu H., Xue C., Yao M., Wang H., Zhang P., Qian T., et al. (2018). miR-129 controls axonal regeneration via regulating insulin-like growth factor-1 in peripheral nerve injury. Cell Death Dis. 9:720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W., Lei J., Bai X., Wang R., Ye Y., Bao J. (2018). MicroRNA-503 regulates hypoxia-induced cardiomyocytes apoptosis through PI3K/Akt pathway by targeting IGF-1R. Biochem. Biophys. Res. Commun. 506 1026–1031. 10.1016/j.bbrc.2018.10.160 [DOI] [PubMed] [Google Scholar]
- Zhuang S. T., Cai Y. J., Liu H. P., Qin Y., Wen J. F. (2020). LncRNA NEAT1/miR-185-5p/IGF2 axis regulates the invasion and migration of colon cancer. Mol. Genet. Genom. Med. 8:e1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
IGF-associated miRNAs in cancers (NNTs: nearby normal tissues).
Role of different drug inhibitors in suppressing the IGF-1R activity and attenuating tumorigenesis as well as drug resistance in various human cancer cells and promoting response to treatment.
Epigenetic regulation of different genes associated with the IGF signaling pathway in human cancers.
Data Availability Statement
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.