Abstract
Background
In locations where the alveolar bone height is low, such as at the maxillary molars, implant placement can be difficult, or even impossible, without procedures aimed at generating new bone, such as sinus lifts. Various types of bone graft materials are used after a sinus lift. In our study, a three-dimensional image analysis using a volume analyzer was performed to measure and compare the volume of demineralized bovine bone mineral (Bio-Oss®) and carbonate apatite (Cytrans®) after a sinus lift, as well as the amount of bone graft material resorption. Patient data were collected from cone-beam computed tomography images taken before, immediately following, and 6 months after the sinus lift. Using these images, both the volume and amount of resorption of each bone graft material were measured using a three-dimensional image analysis system.
Results
The amount of bone resorption in the Bio-Oss®-treated group was 25.2%, whereas that of the Cytrans®-treated group was 14.2%. A significant difference was found between the two groups (P < 0.001).
Conclusions
Our findings indicate that the volume of bone resorption was smaller in the Cytrans®-treated group than in the Bio-Oss®-treated group, suggesting that Cytrans® is more promising for successful implant treatments requiring a sinus lift.
Keywords: CBCT, Cone-beam computed tomography, Maxillary sinus augmentation, Dental implant, Sinus lift, Bio-Oss®, Cytrans®
Background
Dental implant therapy has advanced significantly over the last decade. Currently, implants are available in various sizes and surface textures to provide patients with improved implant therapy options [1–3]. However, at sites in the upper molar region, the alveolar bone height is less than that at other sites, and the effects of alveolar bone resorption after tooth extractions should also be considered. Therefore, implant placement may be difficult, or impossible, without bone augmentation procedures, such as a sinus lift [4–6]. While the sinus lift procedure is an established treatment option [7–9], the most suitable choice of bone-filling material is unclear, and novel bone-filling materials are currently under development [10–12]. In this study, we evaluate the utility of demineralized bovine bone mineral (DBBM), commercially known as Bio-Oss®, and carbonate apatite (CO3Ap), commercially known as Cytrans®, as bone-filling materials in a sinus lift. We conducted a comparative study by measuring the volume and the amount of resorption of each material using a three-dimensional image analysis system.
Methods
Patients
This study was conducted on patients who elected to receive dental implant therapy requiring a sinus lift between January 2019 and May 2020 (12 individuals, mean age 58.2 years). The sinus lift sites examined in this study consisted of a total of 14, with 8 sites included in the DBBM-treated (Bio-Oss®) group and 6 sites in the CO3Ap-treated (Cytrans®) group.
These procedures were carried out once the study parameters were explained to the patients, and they gave their consent. This study was approved by the Kanagawa Dental University Ethics Committee (approval number 697). The following were the inclusion criteria for the subjects: age of at least 20 years old, free of systemic diseases, non-smokers, with bone height of less than 3 mm, and with no thickening of the sinus mucosa.
Before performing the sinus lift, patients whose teeth still remained were treated for periodontal disease; if there was decay in the teeth adjacent to the defect, the decay was treated, and if there was a root canal lesion, root canal therapy was performed to reduce the risk of surgery.
Surgical procedure
All patients were instructed to take an oral dose (1 g) of amoxicillin hydrate (Sawacillin Capsules®, LTL Pharma, Tokyo, Japan) 1 h before surgery. Infiltration anesthesia was administered (Lidocaine/Adrenaline bitartrate®, Showa Yakuhin Kako Co., Ltd., Tokyo, Japan), a gingival incision was made, and avulsion was performed up to the sidewall of the maxillary sinus. The Schneiderian membrane was elevated using the lateral window technique, and bone-filling was performed using either DBBM (Bio-Oss®, Geistlich, Wolhusen, Switzerland) or CO3Ap (Cytrans®, GC, Tokyo, Japan).
Finally, the fenestration site was covered with a collagen membrane (Bio-Gide®, Geistlich, Wolhusen, Switzerland) and sutured. All surgical procedures were performed in two stages. The stitches were removed 2 weeks after surgery.
All surgeries were performed by the same doctor, a teaching associate in the Department of Implantology at our university hospital. The transplant material was also infiltrated with saline solution.
Cytrans (size, M; particle size, 0.6–1.0 mm) and Bio-Oss (size, L; particle size, 1–2 mm) were the grafting materials used.
Volume evaluation method
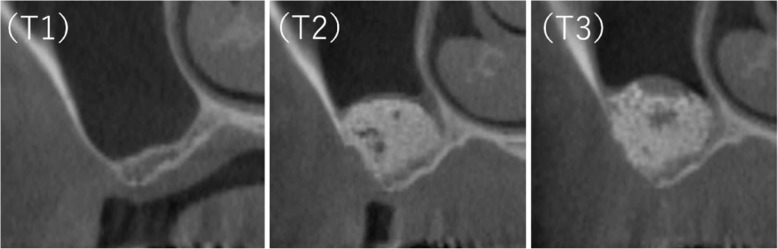
Evaluations were conducted using patient data obtained from cone beam computed tomography (CBCT) images (3DX®, Morita, Tokyo, Japan) collected before (T1), immediately following the sinus lift surgery (T2), and 6 months later (T3) (Fig. 1).
Fig. 1.
CBCT images of the maxillary sinus: (T1) before surgery, (T2) immediately after surgery, and (T3) 6 months after surgery
The collected images were superimposed using a three-dimensional image analysis system volume analyzer (SYNAPSE VINCENT®, FUJIFILM, Tokyo, Japan), and the volume of bone-filling material was measured. Volumes were quantified according to the following method: Using the fusion function of the three-dimensional (3D) visualization software SYNAPSE VINCENT®, the volume immediately after surgery was measured by overlaying T1 and T2 images, and the volume at 6 months after surgery was measured by overlaying T1 and T3 images. The amount of resorption was calculated by subtracting the volume on T3 images from that on T2 images.
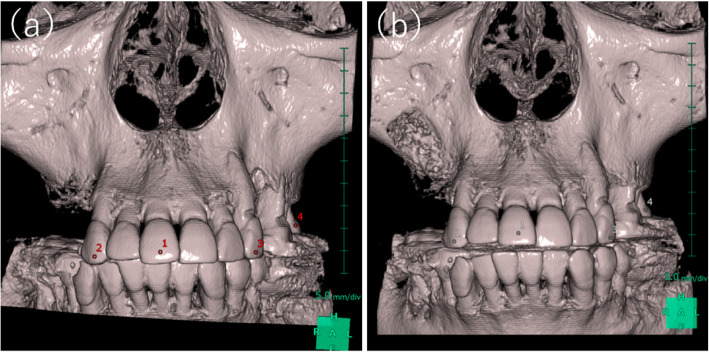
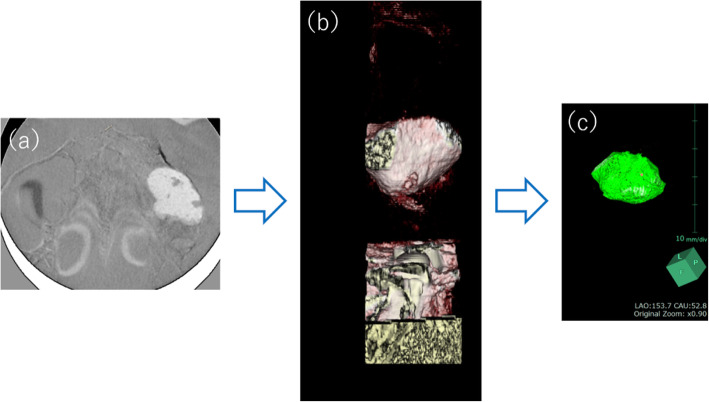
Specifically, the overlay of images involved the import of the two images into SYNAPSE VINCENT®, followed by a “manual image alignment” (Fig. 2) and an “automatic image alignment” using maxillary arch anatomical landmarks as references (Fig. 3). Finally, the overlay of processed images was produced. Next, an “image reconstruction” step was performed, followed by the removal of excess information by trimming the resulting 3D data, and “overall measurement” was selected among the measurement methods available to obtain the volume of the bone-filling material (Fig. 4).
Fig. 2.
“Manual alignment” was performed using residual teeth as reference points. a Preoperative CBCT data. b Postoperative CBCT data
Fig. 3.
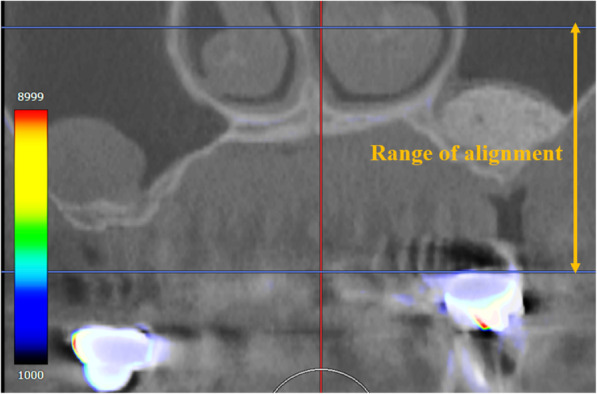
“Automatic alignment” was performed using maxillary arch anatomical landmarks as reference points
Fig. 4.
The overlaid images were trimmed, and the volume was measured using “overall measurements.” Image obtained after a “image reconstruction,” b trimming on the vertical axis, and c trimming of unnecessary data (information)
Statistical methods
The comparison of the amount of resorption in the context of each bone-filling material was performed using the Student’s t-test (P < 0.05).
Results
In the Bio-Oss®-treated group, bone resorption was 25.2%, whereas in the Cytrans®-treated group, bone resorption was 14.2%. There was a significant difference in bone resorption between the two bone-filling materials (P = 0.001) (Fig. 5) (Table 1). In addition, there was no observed increase in bone mass.
Fig. 5.
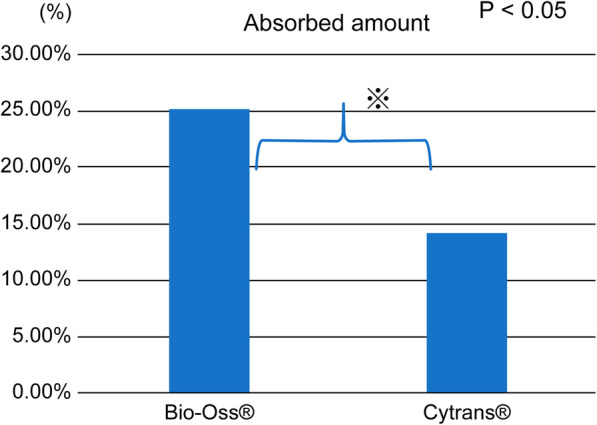
Amount of resorption of Bio-Oss® and Cytrans®
Table 1.
Clinical data on the volume of bone-filling material in procedures using Bio-Oss® and Cytrans®
| Treatment group | Patient no. | Age | Gender | Deficit condition (FDI) | T2 (ml) | T3 (ml) | Absorbed amount (%) |
|---|---|---|---|---|---|---|---|
| Bio-Oss® (n = 8) | 1 | 65 | F | Edentulous | 3.762 | 2.538 | 32.50 |
| 2 | 59 | M | 23–27 | 1.583 | 1.167 | 26.30 | |
| 3 | 53 | M | 26, 27 | 2.271 | 1.772 | 22.00 | |
| 4 | 59 | F | 14–17 | 4.880 | 3.836 | 21.40 | |
| 5 | 21 | F | 14–16 | 1.428 | 0.916 | 35.90 | |
| 6 | 70 | F | 26, 27 | 2.163 | 1.622 | 25.00 | |
| 7 | 71 | F | Edentulous | 2.436 | 1.973 | 19.00 | |
| 8 | 71 | F | Edentulous | 3.198 | 2.581 | 19.30 | |
| Cytrans® (n = 6) | 1 | 67 | F | 14–17 | 2.547 | 2.371 | 6.90 |
| 2 | 41 | F | 16, 17 | 2.108 | 1.791 | 15.00 | |
| 3 | 59 | M | 25–27 | 1.506 | 1.222 | 18.90 | |
| 4 | 67 | M | Edentulous | 3.763 | 3.375 | 10.30 | |
| 5 | 67 | M | Edentulous | 2.392 | 2.150 | 10.10 | |
| 6 | 67 | F | 24–27 | 2.780 | 2.386 | 14.20 |
Postoperatively, most patients developed swelling on the second day, followed by slight internal bleeding on the cheek in some cases. The internal bleeding disappeared in about 2 weeks.
All patients showed a good postoperative prognosis and were able to reach 6 months postoperatively without any problems.
Discussion
Several reports have examined the changes that occur in bone replacement materials after a sinus lift. Our findings suggest that the amount of resorption after surgery could be reduced by using Cytrans® as the bone-filling material.
Various methods have been used to measure the changes in the volume of bone-filling materials after a sinus lift. In a study conducted on 27 participants, Kim et al. used preoperative and postoperative panoramic X-ray images to measure and compare the distance of implants placed simultaneously at three points: mesial, central, and distal. They reported that in all cases, bone resorption occurred over time [13]. Although the avoidance of CBCT imaging may be useful in the evaluation of ongoing follow-up treatment because of the reduced exposure of patients to radiation [14, 15], a complete picture of the anatomical morphology is difficult to obtain using 2D images alone compared with panoramic x-rays and CBCT data [16–18].
Gorla et al. quantified the volume of bone-filling material after a sinus lift using CBCT by measuring the cross-sectional area and height at randomly selected locations [19]; however, in recent years, three-dimensional measurements using CBCT have been made possible. Previously, Kwon et al. conducted sinus lifts using DBBM (Bio-Oss®) and measured the changes in bone-filling volume using a method similar to ours and reported that the volume was fully resorbed in 2 to 26 weeks after surgery [20]. In a similar study using DBBM (Bio-Oss®), Younes et al. reported that the volume of the graft was 1418.26 mm3 2 weeks after surgery, 1201.21 mm3 3 months after surgery, and 1130.13 mm3 2 years after surgery, and that the stability of the bone-filling materials was 79.7% [21].
Previous studies have reported favorable DBBM (Bio-Oss®) bone resorption values after a sinus lift, including 19.4% according to Guo et al., 9.39 ± 3.01% according to Gultekin et al., and 26% according to Kirmeier et al. [22–24]. Thus, sinus lifts using DBBM (Bio-Oss®) as the bone-filling material typically yield positive results. It is often debated whether DBBM (Bio-Oss®) should be mixed with autologous bone during bone transplantation. In a systematic review of sinus lift procedures, Rickert et al. concluded that artificial bone substitutes should be mixed with autologous bone to promote bone formation [25]. A systematic review by Aludden et al. revealed similar results [26]. Furthermore, Hatano et al. previously reported positive results using graft materials made of a mixture of Bio-Oss® and autologous bone at a ratio of 2:1 [27]. However, in experiments conducted by Kim et al., no difference in new bone formation was observed, regardless of whether the graft material was composed of Bio-Oss® alone or made of Bio-Oss® mixed with 25% of autologous bone [28]. A systematic review published by Jensen et al. described analogous results [29]. Similarly, Starch-Jensen et al. reported that the long-term prognosis after a sinus lift was favorable, regardless of the filling material used [30]. Mixing DBBM (Bio-Oss®) with an autologous bone is believed to promote bone formation; however, the optimal method to collect sufficient amounts of autologous bone remains unclear.
Clinical reports using Cytrans® are few; however, Kudoh et al. used this material to perform sinus lifts in humans with good results [31]. Ishikawa et al. and Mano et al. compared the osteogenic potential of three different types of synthetic bones (Neobone®, Cytrans®, and Cerasorb®) in dogs and reported that the degree of bone replacement was the largest using Cytrans® [32, 33]. Additionally, Fujisawa et al. reported that the porosity and carbonate content of Cytrans® were 25.4 ± 0.6% and 12.1 ± 0.6%, respectively, whereas those of Bio-Oss® were 57.0 ± 0.5% and 5.6 ± 0.1%, respectively.
The low crystallinity, porous structure, and high porosity of Bio-Oss® make it desirable for the replacement of osteoconductive bone. In contrast, Cytrans, with a higher carbonate content, led to a faster rate of bone formation and a higher amount of new bone formation. However, the justification of these differences in bone formation is yet unknown [34]. Of note, Spence et al. reported that a high carbonate content can increase osteoclastogenesis in the context of carbonate-substituted hydroxyapatite [35]; increased osteoclastogenesis leads to the activation of osteoblasts. Therefore, we hypothesize that the potential increased osteoclastogenesis in the context of carbonate-rich Cytrans leads to an increase in the rate of bone formation.
In this study, the amount of resorption in the Cytrans® group was low. As reported above, Cytrans® has a high carbonate content and a high rate of replacement in new bone, which may have resulted in less postoperative resorption.
Chan et al. classified the maxillary sinus floor as follows: narrow, average, and wide, and reported that the narrow floor makes the lateral approach technique more difficult [36]. Cho et al. reported that the angular difference between the medial and lateral walls of the maxillary sinus makes surgery difficult and increases the likelihood of the perforation of the Schneider’s membrane [37]. The presence of a septum in the maxillary sinus is also thought to be one of the factors that makes surgery difficult [38]. However, in this surgery, there was no septum and no perforation of the Schneider membrane; thus, the volume was not affected.
The limitation of this study is that although we could visually confirm the change of the graft material from sparse to dense on the 3D visualization software, it was impossible to evaluate the density based on the CT values because of the CBCT imaging. Pauwels et al. compared CBCT with multidetector CT and reported that CBCT produces a large variability in gray values due to its limited field size, relatively large amount of scattered radiation, and limitations of currently applied reconstruction algorithms. Of note, they reported that CBCT should not be used to assess bone quality and density [39]. In addition, it was not possible to calculate the bone resorption and survival rate from the time of superstructure attachment; this represents an area of focus for future research.
Conclusion
Our study findings revealed that the amount of volume resorption was smaller when Cytrans® was used as a bone-filling material than when Bio-Oss® was used. This study provides a potential framework for improved dental implant treatment outcomes in the future.
Acknowledgements
Not applicable.
Abbreviations
- 3D
Three-dimensional
- CBCT
Cone beam computed tomography
- DBBM
Demineralized bovine bone mineral
- CO3Ap
Carbonate apatite
Authors’ contributions
H.K. and K.N. conceived the study; K.F., K.N., and R.K. performed the experiments; Y.O., K.W., H.S., HJ.K., and M.O. analyzed the data; H.K. and K.F. contributed new methods or models; K.N. wrote the manuscript. All authors have read and approved the final manuscript.
Funding
This study received no external funding.
Availability of data and materials
The datasets obtained and analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Kanagawa Dental University Ethics Committee (approval number 697). Written informed consent was obtained from all patients.
Consent for publication
Not applicable
Competing interests
Koudai Nagata, Kei Fuchigami, Ryoji Kitami, Yurie Okuhama, Kana Wakamori, Hirokazu Sumitomo, Hyunjin Kim, Manabu Okubo, and Hiromasa Kawana declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Smeets R, Stadlinger B, Schwarz F, Beck-Broichsitter B, Jung O. Impact of dental implant surface modifications on osseointegration. Biomed Res Int. 2016;2016:6285620. doi: 10.1155/2016/6285620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Makowiecki A, Hadzik J, Błaszczyszyn A, Gedrange T, Dominiak M. An evaluation of superhydrophilic surfaces of dental implants - a systematic review and meta-analysis. BMC Oral Health. 2019;19:79. doi: 10.1186/s12903-019-0767-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papaspyridakos P, De Souza A, Vazouras K, Gholami H, Pagni S, Weber HP. Survival rates of short dental implants (≤6 mm) compared with implants longer than 6 mm in posterior jaw areas: a meta-analysis. Clin Oral Implants Res. 2018;29(Suppl 16):8–20. doi: 10.1111/clr.13289. [DOI] [PubMed] [Google Scholar]
- 4.Choi YJ, Kim YH, Han SS, Jung UW, Lee C, Lee A, et al. Alveolar bone height according to the anatomical relationship between the maxillary molar and sinus. J Periodontal Implant Sci. 2020;50:38–47. doi: 10.5051/jpis.2020.50.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walter C, Dagassan-Berndt DC, Kühl S, Weiger R, Lang NP, Zitzmann NU. Is furcation involvement in maxillary molars a predictor for subsequent bone augmentation prior to implant placement? A pilot study. Clin Oral Implants Res. 2014;25:1352–1358. doi: 10.1111/clr.12275. [DOI] [PubMed] [Google Scholar]
- 6.Tian XM, Qian L, Xin XZ, Wei B, Gong Y. An analysis of the proximity of maxillary posterior teeth to the maxillary sinus using cone-beam computed tomography. J Endod. 2016;42:371–377. doi: 10.1016/j.joen.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 7.Bornstein MM, Chappuis V, von Arx T, Buser D. Performance of dental implants after staged sinus floor elevation procedures: 5-year results of a prospective study in partially edentulous patients. Clin Oral Implants Res. 2008;19:1034–1043. doi: 10.1111/j.1600-0501.2008.01573.x. [DOI] [PubMed] [Google Scholar]
- 8.Boyne PJ, James RA. Grafting of the maxillary sinus floor with autogenous marrow and bone. J Oral Surg. 1980;38:613–616. [PubMed] [Google Scholar]
- 9.Brugger OE, Bornstein MM, Kuchler U, Janner SF, Chappuis V, Buser D. Implant therapy in a surgical specialty clinic: an analysis of patients, indications, surgical procedures, risk factors, and early failures. Int J Oral Maxillofac Implants. 2015;30:151–160. doi: 10.11607/jomi.3769. [DOI] [PubMed] [Google Scholar]
- 10.Liu R, Yan M, Chen S, Huang W, Wu D, Chen J. Effectiveness of platelet-rich fibrin as an adjunctive material to bone graft in maxillary sinus augmentation: a meta-analysis of randomized controlled trails. Biomed Res Int. 2019;17:7267062. doi: 10.1155/2019/7267062.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silva LD, de Lima VN, Faverani LP, de Mendonça MR, Okamoto R, Pellizzer EP. Maxillary sinus lift surgery-with or without graft material? A systematic review. Int J Oral Maxillofac Surg. 2016;45:1570–1576. doi: 10.1016/j.ijom.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 12.Pérez-Martínez S, Martorell-Calatayud L, Peñarrocha-Oltra D, García-Mira B, Peñarrocha-Diago M. Indirect sinus lift without bone graft material: systematic review and meta-analysis. J Clin Exp Dent. 2015;7:e316–e319. doi: 10.4317/jced.51716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim DH, Ko MJ, Lee JH, Jeong SN. A radiographic evaluation of graft height changes after maxillary sinus augmentation. J Periodontal Implant Sci. 2018;48:174–181. doi: 10.5051/jpis.2018.48.3.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin HS, Nam KC, Park H, Choi HU, Kim HY, Park CS. Effective doses from panoramic radiography and CBCT (cone beam CT) using dose area product (DAP) in dentistry. Dentomaxillofac Radiol. 2014;43:20130439. doi: 10.1259/dmfr.20130439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Signorelli L, Patcas R, Peltomäki T, Schätzle M. Radiation dose of cone-beam computed tomography compared to conventional radiographs in orthodontics. J Orofac Orthop. 2016;77:9–15. doi: 10.1007/s00056-015-0002-4. [DOI] [PubMed] [Google Scholar]
- 16.Malina-Altzinger J, Damerau G, Grätz KW, Stadlinger PD. Evaluation of the maxillary sinus in panoramic radiography—a comparative study. Int J Implant Dent. 2015;1:17. doi: 10.1186/s40729-015-0015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu KS, Choi DY, Lee WJ, Kim HJ, Jung UW, Kim S. Reliability of two different presurgical preparation methods for implant dentistry based on panoramic radiography and cone-beam computed tomography in cadavers. J Periodontal Implant Sci. 2012;42:39–44. doi: 10.5051/jpis.2012.42.2.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reddy MS, Mayfield-Donahoo T, Vanderven FJ, Jeffcoat MK. A comparison of the diagnostic advantages of panoramic radiography and computed tomography scanning for placement of root form dental implants. Clin Oral Implants Res. 1994;5:229–238. doi: 10.1034/j.1600-0501.1994.050406.x. [DOI] [PubMed] [Google Scholar]
- 19.Gorla LF, Spin-Neto R, Boos FB, RdS P, Garcia-Junior IR, Hochuli-Vieira E. Use of autogenous bone and beta-tricalcium phosphate in maxillary sinus lifting: a prospective, randomized, volumetric computed tomography study. Int J Oral Maxillofac Surg. 2015;44:1486–1491. doi: 10.1016/j.ijom.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Kwon JJ, Hwang J, Kim YD, Shin SH, Cho BH, Lee JY. Automatic three-dimensional analysis of bone volume and quality change after maxillary sinus augmentation. Clin Implant Dent Relat Res. 2019;21:1148–1155. doi: 10.1111/cid.12853. [DOI] [PubMed] [Google Scholar]
- 21.Younes F, Cosyn J, De Bruyckere T, Cleymaet R, Eghbali A. A 2-year prospective case series on volumetric changes, PROMs, and clinical outcomes following sinus floor elevation using deproteinized bovine bone mineral as filling material. Clin Implant Dent Relat Res. 2019;21:301–309. doi: 10.1111/cid.12730. [DOI] [PubMed] [Google Scholar]
- 22.Guo XH, Jiang Q, Ruan H, Luo Y, Yu YC. Evaluation of three-dimensional changes after sinus floor augmentation with DBBM. Shanghai Kou Qiang Yi Xue. 2013;22:448–452. [PubMed] [Google Scholar]
- 23.Gultekin BA, Borahan O, Sirali A, Karabuda ZC, Mijiritsky E. Three-dimensional assessment of volumetric changes in sinuses augmented with two different bone substitutes. Biomed Res Int. 2016;2016:4085079. doi: 10.1155/2016/4085079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirmeier R, Payer M, Wehrschuetz M, Jakse N, Platzer S, Lorenzoni M. Evaluation of three-dimensional changes after sinus floor augmentation with different grafting materials. Clin Oral Implants Res. 2008;19:366–372. doi: 10.1111/j.1600-0501.2007.01487.x. [DOI] [PubMed] [Google Scholar]
- 25.Rickert D, Slater JJRH, Meijer HJA, Vissink A, Raghoebar GM. Maxillary sinus lift with solely autogenous bone compared to a combination of autogenous bone and growth factors or (solely) bone substitutes. A systematic review. Int J Oral Maxillofac Surg. 2012;41:160–167. doi: 10.1016/j.ijom.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Aludden HC, Mordenfeld A, Hallman M, Dahlin C, Jensen T. Lateral ridge augmentation with Bio-Oss alone or Bio-Oss mixed with particulate autogenous bone graft: a systematic review. Int J Oral Maxillofac Surg. 2017;46:1030–1038. doi: 10.1016/j.ijom.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Hatano N, Shimizu Y, Ooya K. A clinical long-term radiographic evaluation of graft height changes after maxillary sinus floor augmentation with a 2:1 autogenous bone/xenograft mixture and simultaneous placement of dental implants. Clin Oral Implants Res. 2004;15:339–345. doi: 10.1111/j.1600-0501.2004.00996.x. [DOI] [PubMed] [Google Scholar]
- 28.Kim YJ, Saiki CET, Silva K, Massuda CKM, de Souza Faloni AP, Braz-Silva PH, et al. Bone formation in grafts with Bio-Oss and autogenous bone at different proportions in rabbit calvaria. Int J Dent. 2020;2494128:2020. doi: 10.1155/2020/2494128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jensen T, Schou S, Stavropoulos A, Terheyden H, Holmstrup P. Maxillary sinus floor augmentation with Bio-Oss or Bio-Oss mixed with autogenous bone as graft: a systematic review. Clin Oral Implants Res. 2012;23:263–273. doi: 10.1111/j.1600-0501.2011.02168.x. [DOI] [PubMed] [Google Scholar]
- 30.Starch-Jensen T, Aludden H, Hallman M, Dahlin C, Christensen AE, Mordenfeld A. A systematic review and meta-analysis of long-term studies (five or more years) assessing maxillary sinus floor augmentation. Int J Oral Maxillofac Surg. 2018;47:103–116. doi: 10.1016/j.ijom.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Kudoh K, Fukuda N, Kasugai S, Tachikawa N, Koyano K, Matsushita Y, et al. Maxillary sinus floor augmentation using low-crystalline carbonate apatite granules with simultaneous implant installation: first-in-human clinical trial. J Oral Maxillofac Surg. 2019;77:985.e1–985.e11. doi: 10.1016/j.joms.2018.11.026. [DOI] [PubMed] [Google Scholar]
- 32.Ishikawa K, Miyamoto Y, Tsuchiya A, Hayashi K, Tsuru K, Ohe G. Physical and histological comparison of hydroxyapatite, carbonate apatite, and β-tricalcium phosphate bone substitutes. Materials (Basel) 2018;11:1993. doi: 10.3390/ma11101993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mano T, Akita K, Fukuda N, Kamada K, Kurio N, Ishikawa K, et al. Histological comparison of three apatitic bone substitutes with different carbonate contents in alveolar bone defects in a beagle mandible with simultaneous implant installation. J Biomed Mater Res B Appl Biomater. 2020;108:1450–1459. doi: 10.1002/jbm.b.34492. [DOI] [PubMed] [Google Scholar]
- 34.Fujisawa K, Akita K, Fukuda N, Kamada K, Kudoh T, Ohe G, et al. Compositional and histological comparison of carbonate apatite fabricated by dissolution-precipitation reaction and Bio-Oss®. J Mater Sci Mater Med. 2018;29:121. doi: 10.1007/s10856-018-6129-2. [DOI] [PubMed] [Google Scholar]
- 35.Spence G, Patel N, Brooks R, Bonfield W, Rushton N. Osteoclastogenesis on hydroxyapatite ceramics: the effect of carbonate substitution. J Biomed Mater Res A. 2010;92(4):1292–300. [DOI] [PubMed]
- 36.Chan HL, Suarez F, Monje A, Benavides E, Wang HL. Evaluation of maxillary sinus width on cone-beam computed tomography for sinus augmentation and new sinus classification based on sinus width. Clin Oral Implants Res. 2014;25:647–52. [DOI] [PubMed]
- 37.Cho SC, Wallace SS, Froum SJ, Tarnow DP. Influence of anatomy on Schneiderian membrane perforations during sinus elevation surgery: three-dimensional analysis. Pract Proced Aesthet Dent. 2001;13:160–163. [PubMed] [Google Scholar]
- 38.Al-Moraissi E, Elsharkawy A, Abotaleb B, Alkebsi K, Al-Motwakel H. Does intraoperative perforation of Schneiderian membrane during sinus lift surgery causes an increased the risk of implants failure?: A systematic review and meta regression analysis. Clin Implant Dent Relat Res. 2018;20:882–889. doi: 10.1111/cid.12660. [DOI] [PubMed] [Google Scholar]
- 39.Pauwels R, Jacobs R, Singer SR, Mupparapu M. CBCT-based bone quality assessment: are Hounsfield units applicable? Dentomaxillofac Radiol. 2015;44(1):20140238. doi: 10.1259/dmfr.20140238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets obtained and analyzed during the current study are available from the corresponding author upon reasonable request.