Abstract
Background
Chronic kidney disease is associated with chronic inflammation and progressive loss of peripheral muscle strength and the ability to exercise, and these changes are highly pronounced in patients receiving hemodialysis (HD). We evaluated hand grip strength (HGS) and leg muscle strength (LMS) in patients receiving HD and attempted to identify factors associated with muscle strength.
Methods
We screened HGS (opposite the fistula side) and LMS (both sides) in HD patients at a single center (n = 112) by using digital hand and leg dynamometers (T.K.K. 5401 and 5710e/5715, Takei Scientific Instruments Co. Ltd., Niigata, Japan).
Results
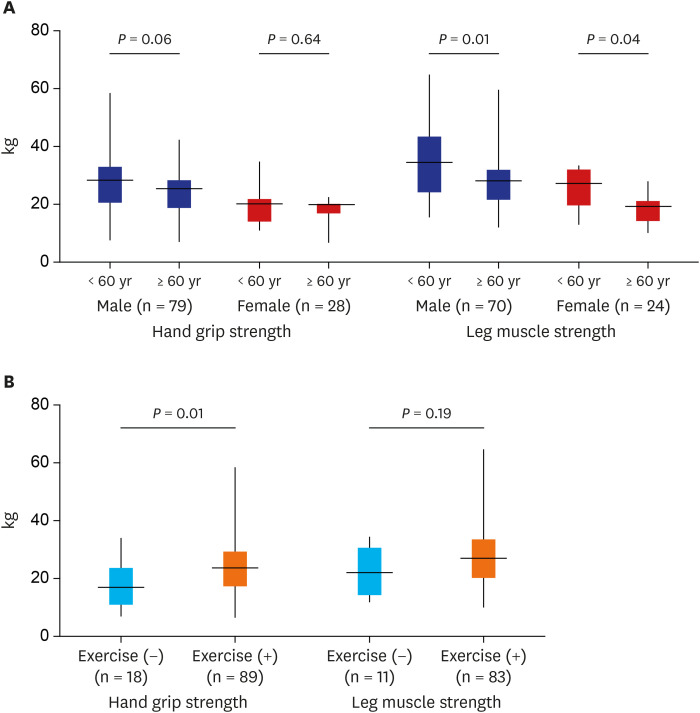
The mean age of patients was 62.6 years, and 73.2% of the patients were male. Diabetes was the cause of kidney failure in 50% of the patients, and the median HD vintage was 34 months. A total of 77.7% of patients reported that they participated in regular home-based exercise, and 29.5% of patients regularly participated in hospital-based resistance exercise. HGS and LMS showed good correlation (r = 0.715, P < 0.001). HGS (25.1 vs. 17.0 kg) and LMS (30.1 vs. 20.4 kg) were greater in males (P < 0.001 and P < 0.001, respectively) than in females. Older patients (≥ 60 years) showed less LMS than younger patients in both males and females (P = 0.012 and P = 0.037, respectively), but HGS did not differ according to age. Patients performing regular home- or hospital-based exercise showed higher HGS than those who did not exercise (24.2 vs. 18.6 kg, P = 0.011), but LMS was not significantly different (29.3 vs. 23.6 kg, P = 0.185). Multiple linear regression analysis proved that male sex, younger age, and any type of exercise were factors associated with improved HGS and LMS. Groups of older age (≥ 60 years), male sex, and shorter duration of HD (< median) benefitted more from exercise.
Conclusion
Sex, age, and exercise were the most important determinants of muscle strength in HD patients. We need to encourage patients to engage in regular home or group exercise from the beginning of dialysis and introduce new feasible forms of exercise for HD patients.
Keywords: Hand Grip Strength, Hemodialysis, Leg Muscle Strength
Graphical Abstract
INTRODUCTION
Protein-energy wasting (PEW) was defined by the International Society of Renal Nutrition and Metabolism (ISRNM) in 2008 and is characterized by the loss of body protein mass and fuel reserves in chronic kidney disease (CKD) or acute kidney injury patients.1 PEW also indicates metabolic and nutritional derangements in chronic disease states.2 Sarcopenia is characterized by loss of muscle mass and strength, a decreased quality of life (QOL) with age, morbidities, and immobility and is associated with PEW in CKD and end stage renal disease (ESRD) patients.3 Sarcopenia is also associated with functional decline, increased risk of falling, and increased mortality in CKD and ESRD patients as well as in older adults.2,4
Aging is associated with sarcopenia and an increased prevalence of CKD (the mean age ± standard deviation [SD] of dialysis patients in Korea was 62.3 ± 13.4 years in 2018), which accelerates normal physiological muscle wasting.5 It is well known that CKD is associated with ‘accelerated aging.’6,7 CKD-associated catabolic alterations can explain the profound features of sarcopenia in CKD patients.5 Muscle wasting is severe and occurs early in CKD patients, and sarcopenia is commonly observed in ESRD patients.8,9 CKD is associated with chronic low-grade inflammation leading to progressive weight loss, muscle weakness, and the loss of the ability to exercise. These changes are highly pronounced in dialysis patients due to fatigue and inactivity.10,11,12,13,14 Inflammatory cytokines and inactivity-mediated destruction of protein homeostasis result in the catabolic destruction of structural and functional proteins, resulting in skeletal muscle wasting.15
The prevalence of PEW increases as renal function decreases, and its prevalence range from 28% to 54% in ESRD patients receiving maintenance dialysis.16 Sarcopenia prevalence is also negatively correlated with declining renal function.9 Its prevalence in dialysis patients was about 50% two decades ago.17 In addition, the prevalence of frailty, defined as weakness, a slow gait, exhaustion, low tolerance for physical activity, unintentional weight loss, and muscle mass < 10% of that of age- and sex-matched population controls, can reach 30% in incident hemodialysis (HD) patients. This may be due to profound deconditioning, increased vulnerability, and characteristics related to old age as well as altered nutritional status in dialysis patients.18
Reduced exercise capacity and functional decline are important because of their impact on QOL and morbidity.19 Structural and functional changes in the cardiovascular system and skeletal muscles, which determine exercise capacity and VO2max were less than 75% of the predicted values in CKD patients.20 Approximately 71% of HD patients had a decrease in quadriceps force that was less than 2 SDs from the average of normal controls.5
Hand grip strength (HGS) measurement is widely used as a functional test of overall strength as well as an indicator of general health and nutritional status.21,22 HGS has also been studied in dialysis patients; it has been associated with a high prevalence of sarcopenia and malnutrition and strongly associated with mortality.23,24 A meta-analysis proved the global association of HGS with survival in dialysis patients, and a Japanese study also suggested a link between decreased functional status and increased mortality risk.25,26
In this study, we cross-sectionally evaluated the leg muscle strength (LMS) as well as HGS of HD patients and attempted to identify risk factors for the loss of muscle strength.
METHODS
Study design
This study was a cross-sectional study conducted at a single HD center. We screened HGS (opposite the fistula side) and LMS (both sides) (n = 112) by using digital hand and leg dynamometers (T.K.K. 5401 and 5710e/5715, Takei Scientific Instruments Co. Ltd., Niigata, Japan).
A digital hand dynamometer (T.K.K. 5401) was used to measure the sitting position HGS (opposite the fistula side). Participants were seated, with the shoulder along the body and no rotation, 90 degrees elbow flexion, and neutral flexion. Participants were encouraged to grasp strongly two times at intervals of 1 to 2 minutes, and greater HGS was used in the analysis. A digital leg dynamometer (T.K.K. 5710e/5715) was used to measure the LMS of the knee joint extension muscles (both sides) as directed in the manufacturer's instructions. Participants were seated, with 90 degrees of knee flexion, and measurements were repeated two times at intervals of 1 to 2 minutes, and the greatest LMS was used in the analysis.
We collected data on demographics (age, sex); anthropometrics (height, dry weight); medical histories, including diabetes, hypertension, coronary artery disease, cerebro-cardiovascular disease, peripheral artery occlusive disease/interventions, malignancies, and chronic liver diseases; cause of ESRD, and HD vintage. We also collected biochemical data, including Kt/V, hemoglobin, blood urea nitrogen (BUN)/serum creatinine, calcium/phosphorus, total CO2, highly sensitive C-reactive protein (hs-CRP), intact parathyroid hormone (PTH), and iron/total iron-binding capacity (TIBC).
In addition, we evaluated the exercise status. We interviewed on the regular home-based exercise performed at least 3 times a week. The type, duration, and strength of exercise were variable. Therefore, we only considered whether the regular exercise would be performed. There was no interval between the interview on exercise status and muscle strength measurement. We also considered participation in the hospital-based resistance exercise during the dialysis. We had implemented hospital-based resistance exercise using a latex band during the dialysis in September 2018. All the HD patients in our center have been encouraged to participate in the hospital-based exercise program and we followed the patient's decision whether to exercise or not. Therefore, the duration of hospital-based exercise before muscle strength measurement was variable due to the diversity in the dialysis initiation and the agreement to the exercise. But, all the patients in the analysis performed hospital-based resistance exercise more than 3 months. Patients in the exercise program performed hospital-based resistance exercise after the initiation of dialysis on one's own volition and an assigned physician assistant helped patients in terms of numbers of repetition, strength, and duration. Nephrologists, physician assistants, and nurses in the HD center had a check-up meeting once a month and gathered opinions whether to change the exercise prescription or not. However, final decision was wholly dependent on patients.
Ethics statement
The study was approved by the Institutional Review Boards of the National Medical Center, Seoul, Korea (NMC-2006-021). Written informed consents were acquired from participants.
Statistical analysis
Continuous variables are expressed as mean ± SD (for variables with normal distributions) or medians (25th–75th percentiles) (for variables with nonnormal distributions), and categorical variables are expressed as number (%). The baseline characteristics, biochemical results and muscle strength results were compared using the χ2 test, Student's t-test, Mann-Whitney U test, or ANOVA/Kruskal-Wallis tests, as appropriate. Multiple regression analysis was used to identify significant factors related to muscle strength by using variables with P values below 0.05. All statistical analyses were performed with IBM SPSS Statistics, Version 20.0 (IBM Corp., Chicago, IL, USA). P values were 2-sided and were considered significant at P < 0.05.
RESULTS
Baseline characteristics
A total of 112 patients receiving maintenance HD participated. The baseline characteristics of total participants and the comparison according to the exercise status are summarized in Table 1. The mean age was 62.6 years, and 73.2% of the patients were male. Diabetes was the cause of kidney failure in 50% of the patients, and the median HD vintage was 34 months. A total of 78.6% of patients reported that they performed regular home-based exercise, and 29.5% of patients regularly participated in hospital-based intradialytic resistance exercise. 26.8% of patients performed both home- and hospital-based exercise while 18.8% of patients did not exercise at all. Median HGS opposite the fistula side and LMS (greater value between both sides) were 21.8 and 26.9 kg, respectively. HD vintage, Kt/V, serum level of creatinine, albumin, calcium, hs-CRP, and iron showed statistical difference among exercise status groups.
Table 1. Baseline characteristics of participants.
| Variables | Total (n = 112) | No exercise (n = 21) | Home- or hospital-based exercise (n = 61) | Both home- and hospital-based exercise (n = 30) | P value | |
|---|---|---|---|---|---|---|
| Age, yr | 62.6 ± 12.7 | 62.5 ± 13.2 | 61.3 ± 12.3 | 65.4 ± 13.1 | 0.346 | |
| Sex (male) | 82 (73.2) | 18 (85.7) | 42 (68.9) | 22 (73.3) | 0.375 | |
| Cause of ESRD | 0.780 | |||||
| Diabetic nephropathy | 56 (50.0) | 12 (57.1) | 30 (49.2) | 14 (46.7) | ||
| Hypertensive nephrosclerosis | 10 (8.9) | 0 (0) | 7 (11.7) | 3 (10.0) | ||
| Glomerulonephritis | 6 (5.4) | 2 (9.5) | 4 (6.7) | 0 (0.0) | ||
| Polycystic kidney disease | 3 (2.7) | 0 (0.0) | 2 (3.3) | 1 (3.3) | ||
| Others | 13 (11.6) | 3 (14.3) | 6 (10.0) | 4 (13.3) | ||
| Unknown | 24 (21.4) | 4 (19.0) | 12 (20.0) | 8 (26.7) | ||
| Past medical history | ||||||
| Diabetes | 72 (64.3) | 17 (81.0) | 38 (62.3) | 17 (56.7) | ||
| Hypertension | 109 (97.3) | 21 (100.0) | 60 (98.4) | 28 (93.3) | ||
| Cerebro-cardiovascular disease | 42 (37.5) | 8 (38.1) | 21 (34.4) | 13 (43.3) | ||
| Peripheral artery obstructive disease | 10 (8.9) | 4 (18.9) | 5 (8.2) | 1 (27.0) | ||
| Malignancy | 9 (8.0) | 2 (9.5) | 4 (6.6) | 3 (10.0) | ||
| Chronic liver disease | 9 (8.0) | 1 (4.8) | 4 (6.6) | 4 (13.3) | ||
| No exercise | 21 (18.8) | 21 (100.0) | ||||
| Regular home-based exercise | 88 (78.6) | 58 (95.1) | ||||
| Hospital-based intradialytic resistance exercise | 33 (29.5) | 3 (4.9) | ||||
| Both home- and hospital-based exercise | 30 (26.8) | 30 (100.0) | ||||
| Dry weight, kg | 61.6 ± 10.6 | 60.1 ± 8.8 | 61.7 ± 12.1 | 62.6 ± 8.6 | 0.721 | |
| BMI, kg/m2 | 22.7 ± 3.3 | 21.8 ± 3.1 | 22.6 ± 3.4 | 23.4 ± 3.2 | 0.251 | |
| HD vintage, mon | 34 (11–62) | 25 (8–62) | 42 (18–74) | 21 (7–41) | 0.030 | |
| Kt/V | 1.43 ± 0.19 | 1.34 ± 0.15 | 1.47 ± 0.21 | 1.42 ± 0.17 | 0.025 | |
| Hemoglobin, g/dL | 10.5 (9.9–11.1) | 9.9 (9.3–10.9) | 10.4 (10.0–11.2) | 10.6 (10.2–11.3) | 0.068 | |
| Blood urea nitrogen, mg/dL | 59 (48–77) | 42 (41–72) | 63 (49–80) | 58 (50–75) | 0.251 | |
| Serum creatinine, mg/dL | 9.27 ± 2.52 | 8.07 ± 2.66 | 9.71 ± 2.62 | 9.22 ± 1.92 | 0.035 | |
| Serum albumin, g/dL | 4.0 (3.8–4.3) | 3.6 (3.2–3.9) | 4.1 (3.9–4.3) | 4.0 (3.9–4.2) | < 0.001 | |
| Serum calcium, mg/dL | 8.8 ± 0.6 | 8.4 ± 0.6 | 8.9 ± 0.5 | 8.8 ± 0.6 | 0.007 | |
| Serum phosphorus, mg/dL | 4.6 ± 1.3 | 4.2 ± 1.5 | 4.8 ± 1.2 | 4.4 ± 1.1 | 0.103 | |
| Total CO2, meq/L | 19.6 ± 3.7 | 19.4 ± 3.3 | 19.6 ± 3.6 | 20.0 ± 4.0 | 0.808 | |
| hs-CRP, mg/dL | 0.14 (0.05–0.48) | 0.41 (0.18–1.51) | 0.09 (0.04–0.30) | 0.08 (0.03–0.56) | 0.001 | |
| Intact PTH, pg/mL | 146.1 (77.8–238.7) | 114.9 (60.4–166.1) | 149.2 (70.9–269.2) | 148.7 (96.2–264.8) | 0.331 | |
| Iron, μg/dL | 63 (47–79) | 59 (51–84) | 67 (50–79) | 60 (44–90) | 0.692 | |
| TIBC, μg/dL | 229 (187–263) | 179 (166–229) | 238 (196–270) | 234 (189–266) | 0.004 | |
| Transferrin saturation, % | 28.9 (21.0–38.8) | 36.2 (21.7–42.0) | 27.3 (22.7–36.0) | 27.2 (17.5–40.9) | 0.326 | |
| Hand grip strength, (opposite the fistula side, kg) | 21.8 (17.0–29.2) | 18.4 (11.4–23.9) | 24.7 (18.5–30.3) | 21.6 (16.6–25.3) | 0.013 | |
| Male | 25.1 (19.7–30.4) | 18.9 (13.1–24.9) | 28.5 (24.0–32.1) | 23.9 (19.7–28.1) | 0.001 | |
| Female | 17.0 (14.5–20.5) | 11.1 (11.1–11.1) | 18.4 (16.6–20.6) | 15.8 (13.9–21.0) | 0.210 | |
| Leg muscle strength (better side, kg) | 26.9 (20.3–33.5) | 24.5 (14.5–30.8) | 30.4 (20.5–36.8) | 24.0 (19.4–30.4) | 0.107 | |
| Male | 30.1 (23.0–37.2) | 24.5 (14.5–30.8) | 33.1 (24.9–43.2) | 27.8 (22.4–31.3) | 0.005 | |
| Female | 20.4 (15.8–25.0) | None | 20.3 (15.1–25.8) | 21.8 (17.8–25.5) | 0.849 | |
Continuous variables are expressed as the mean ± SD (normal distribution) or median (25–75 percentile) (non-normal distribution), and categorical variables are expressed as number (%).
BMI = body mass index, ESRD = end stage renal disease, HD = hemodialysis, hs-CRP = highly sensitive C-reactive protein, TIBC = total iron-binding capacity.
HGS and LMS
Table 2 compares HGS and LMS between the general population (Korea National Health and Nutrition Examination VI-3 survey27 and Takei Scientific Instruments Co. Ltd. presented) and HD patients. The HGS of HD patients was approximately 25%–50% less than that of the general population in almost all age groups in both males and females. The LMS of female and male HD patients started to decrease after the age of 60 (female) and 70 (male), and it was approximately 30%–40% less than that of the general population.
Table 2. Comparison of mean hand grip and leg muscle strength between general population and hemodialysis patients.
| Age | Hand grip strength, kg | Leg muscle strength, kg | ||||||
|---|---|---|---|---|---|---|---|---|
| General population | Hemodialysis patients | General population | Hemodialysis patients | |||||
| Malea | Femalea | Male | Female | Maleb | Femaleb | Male | Female | |
| 20–29 | 41.3 (329) | 24.4 (339) | 35.1 (7) | 21.8 (6) | ||||
| 30–39 | 44.4 (298) | 26.2 (416) | 22.3 (2) | 23.4 (4) | 33.6 (23) | 22.7 (28) | 64.7 (1) | 24.8 (4) |
| 40–49 | 43.7 (367) | 28.6 (464) | 31.8 (8) | 16.6 (3) | 32.4 (24) | 23.2 (19) | 33.4 (8) | 23.8 (3) |
| 50–59 | 40.6 (423) | 24.7 (548) | 26.4 (22) | 16.2 (4) | 32.0 (26) | 22.5 (20) | 34.7 (21) | 24.1 (3) |
| 60–69 | 37.8 (330) | 23.3 (405) | 27.3 (21) | 17.2 (9) | 32.7 (46) | 25.9 (75) | 32.7 (18) | 18.5 (7) |
| 70–79 | 31.2 (204) | 20.2 (252) | 20.1 (19) | 17.2 (7) | 36.2 (81) | 26.1 (91) | 25.1 (16) | 20.2 (6) |
| ≥ 80 | 26.9 (56) | 16.7 (191) | 23.5 (7) | 9.4 (1) | 36.2 (10) | 19.7 (6) | 22.2 (6) | 13.0 (1) |
Data are presented as mean (number).
aSource: Each value means the average of age-specific hand grip strength using data from the Korea National Health and Nutrition Examination Survey (KNHANES) VI-3 (2015) survey. bSource: Takei Scientific Instruments Co. Ltd., Niigata, Japan presented. ‘Participants from Department of physical education and department of public health, Gifu University, Japan.’
HGS and LMS showed good correlation (r = 0.715, P < 0.001). HGS (25.1 vs. 17.0 kg) and LMS (30.1 vs. 20.4 kg) were greater in males (P < 0.001 and P < 0.001, respectively) than females. Older patients (≥ 60 years) had less LMS than younger patients in both sex (P = 0.012 and P = 0.037, respectively), but HGS did not differ by age (P = 0.060 and P = 0.640, respectively) (Fig. 1A). Patients performing regular home- or hospital-based exercise had greater HGS than those performing no exercise (24.2 vs. 18.6 kg, P = 0.011), but LMS was not significantly different between the groups (29.3 vs. 23.6 kg, P = 0.185) (Fig. 1B). Serum albumin (r = 0.30, P = 0.002 and r = 0.27, P = 0.007, respectively) and creatinine (r = 0.35, P = 0.001 and r = 0.35, P = 0.001, respectively) were positively correlated with HGS and LMS, and hs-CRP was negatively correlated only with HGS (r = −0.23, P = 0.020). Body mass index (BMI) was positively correlated with LMS (r = 0.24, P = 0.020), but HGS was not influenced by BMI (r = 0.16, P = 0.094). The presence of diabetes, hypertension, cerebro-cardiovascular disease, peripheral artery obstructive disease, malignancy, and chronic liver disease did not affect LMS. However, HGS was lower in patients with cerebro-cardiovascular disease than in those without cerebro-cardiovascular disease (20.6 vs. 24.8 kg, P = 0.018). There were no differences in HGS and LMS according to HD vintage tertile (P = 0.059-and 0.929, respectively).
Fig. 1. Hand grip and leg muscle strength according to age and sex (A) and the home- or hospital-based exercise (B). Whiskers go from minimum to maximum values with 1st and 3rd quartile box points. The horizontal dotted line represents the median level.
Factors related with muscle strength
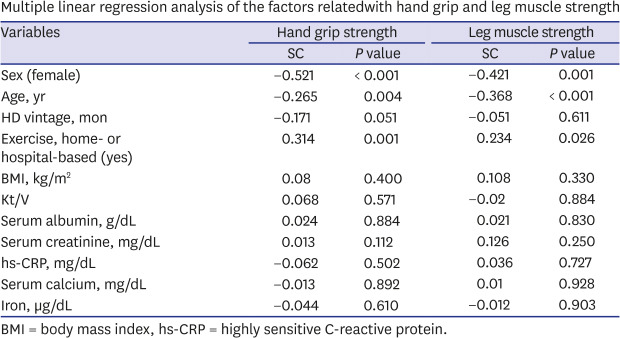
Multiple linear regression analysis considering sex, age, BMI, exercise, and biochemical markers proved that male sex, younger age, and any type of exercise were factors associated with greater HGS and LMS. And groups of older age (≥ 60 years), male sex, and shorter duration of HD (< median [34 months]) benefitted more from exercise than their corresponding counterparts (Table 3). The association of muscle strength with male sex, younger age, and any type of exercise were maintained even after we selected patients who checked both HGS and LMS (n = 93) (Supplementary Table 1).
Table 3. Multiple linear regression analysis of the factors related with hand grip and leg muscle strength.
| Variables | Total | Age < 60 | Age ≥ 60 | Male | Female | HD vintage < 34 | HD vintage ≥ 34 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SC | P value | SC | P value | SC | P value | SC | P value | SC | P value | SC | P value | SC | P value | ||
| Hand grip strength | |||||||||||||||
| Sex (female) | −0.521 | < 0.001 | −0.365 | 0.132 | −0.545 | < 0.001 | −0.38 | 0.029 | −0.68 | < 0.001 | |||||
| Age, yr | −0.265 | 0.004 | −0.245 | 0.037 | −0.459 | 0.101 | −0.327 | 0.021 | 0.012 | 0.933 | |||||
| HD vintage, mon | −0.171 | 0.051 | −0.163 | 0.398 | −0.222 | 0.054 | −0.211 | 0.054 | 0.166 | 0.484 | |||||
| Exercise, home- or hospital-based (yes) | 0.314 | 0.001 | 0.22 | 0.233 | 0.362 | 0.010 | 0.354 | 0.004 | −0.148 | 0.667 | 0.446 | 0.004 | 0.081 | 0.600 | |
| BMI, kg/m2 | 0.08 | 0.400 | −0.01 | 0.957 | 0.14 | 0.259 | 0.05 | 0.670 | 0.523 | 0.068 | 0.037 | 0.790 | 0.135 | 0.340 | |
| Kt/V | 0.068 | 0.571 | −0.123 | 0.646 | 0.142 | 0.341 | 0.088 | 0.495 | −0.431 | 0.194 | −0.025 | 0.879 | 0.322 | 0.077 | |
| Serum albumin, g/dL | 0.024 | 0.884 | 0.207 | 0.465 | −0.004 | 0.973 | −0.004 | 0.967 | 0.057 | 0.864 | 0.01 | 0.938 | 0.184 | 0.247 | |
| Serum creatinine, mg/dL | 0.013 | 0.112 | 0.162 | 0.438 | 0.84 | 0.128 | 0.217 | 0.071 | 0.11 | 0.637 | 0.147 | 0.310 | 0.169 | 0.244 | |
| hs-CRP, mg/dL | −0.062 | 0.502 | −0.047 | 0.821 | −0.065 | 0.619 | −0.038 | 0.745 | −0.693 | 0.035 | −0.012 | 0.932 | −0.02 | 0.889 | |
| Serum calcium, mg/dL | −0.013 | 0.892 | 0.116 | 0.587 | −0.173 | 0.191 | −0.003 | 0.981 | −0.416 | 0.106 | −0.069 | 0.662 | 0.011 | 0.941 | |
| Iron, μg/dL | −0.044 | 0.610 | −0.105 | 0.523 | −0.043 | 0.966 | −0.006 | 0.954 | −0.288 | 0.164 | 0.08 | 0.562 | −0.024 | 0.851 | |
| Leg muscle strength | |||||||||||||||
| Sex (female) | −0.421 | 0.001 | −0.135 | 0.585 | −0.478 | 0.007 | −0.356 | 0.050 | −0.542 | 0.008 | |||||
| Age, yr | −0.368 | < 0.001 | −0.384 | 0.003 | −0.488 | 0.164 | −0.5 | 0.001 | −0.13 | 0.833 | |||||
| HD vintage (months) | −0.051 | 0.611 | 0.046 | 0.826 | −0.093 | 0.504 | −0.051 | 0.670 | 0.261 | 0.425 | |||||
| Exercise, home- or hospital-based (yes) | 0.234 | 0.026 | 0.212 | 0.268 | 0.318 | 0.048 | 0.273 | 0.031 | 0.299 | 0.059 | 0.157 | 0.320 | |||
| BMI, kg/m2 | 0.108 | 0.330 | 0.242 | 0.241 | 0.013 | 0.934 | 0.087 | 0.505 | 0.288 | 0.450 | 0.203 | 0.174 | −0.093 | 0.607 | |
| Kt/V | −0.02 | 0.884 | −0.327 | 0.225 | 0.091 | 0.629 | −0.031 | 0.831 | −0.39 | 0.517 | 0.142 | 0.420 | −0.118 | 0.585 | |
| Serum albumin, g/dL | 0.021 | 0.830 | 0.389 | 0.203 | −0.07 | 0.615 | 0.018 | 0.879 | 0.165 | 0.712 | 0.06 | 0.668 | 0.262 | 0.201 | |
| Serum creatinine, mg/dL | 0.126 | 0.250 | 0.067 | 0.770 | 0.123 | 0.430 | 0.186 | 0.159 | −0.183 | 0.500 | 0.165 | 0.293 | 0.045 | 0.789 | |
| hs-CRP, mg/dL | 0.036 | 0.727 | 0.068 | 0.750 | 0.124 | 0.416 | 0.095 | 0.457 | −0.309 | 0.323 | 0.115 | 0.446 | 0.045 | 0.792 | |
| Serum calcium, mg/dL | 0.01 | 0.928 | −0.233 | 0.313 | 0.089 | 0.566 | 0.016 | 0.908 | −0.191 | 0.616 | −0.139 | 0.380 | 0.07 | 0.689 | |
| Iron, μg/dL | −0.012 | 0.903 | −0.141 | 0.424 | 0.193 | 0.178 | 0.071 | 0.565 | −0.443 | 0.092 | 0.005 | 0.974 | −0.003 | 0.984 | |
Units of age and HD vintage are years and months, respectively.
BMI = body mass index, hs-CRP = highly sensitive C-reactive protein, SC = standardized coefficient.
DISCUSSION
This study showed a good correlation between HGS and LMS, and both HGS and LMS were greater in men than in women. Age had greater impact on LMS than HGS. HGS and LMS in patients who performed regular home- or hospital-based exercise were greater than those in patients who did not exercise, although the difference was significant for only HGS. Serum albumin and creatinine were positively correlated with both HGS and LMS. CRP showed a negative correlation with HGS while BMI showed a positive correlation with LMS. Sex, age, and exercise were the most important determinants of both HGS and LMS in HD patients. And groups of older age, male sex, and shorter duration of HD benefitted more from exercise.
Muscle strength (HGS and LMS) in HD patients, except LMS in male HD patients, was 25%–50% less than that of general population in almost all age groups. Structural and functional decline to the cardiovascular system and skeletal muscle occur in CKD and ESRD patients, and this contributes to an exercise capacity that is 40%–50% less than that of normal people.20 Muscle strength is not the sole factor determining physical function, but the results of this study may offer a way to explain the decrease in physical function in CKD and ESRD patients.
Prevention of PEW and sarcopenia are important clinical issues in CKD and ESRD patients. Insufficient food intake due to loss of appetite and diet restrictions, especially protein restriction for CKD patients with progression of renal dysfunction, contributes to PEW, and an increased catabolic state, including persistent inflammation, metabolic acidosis, hormonal imbalance, and physical inactivity, leads to excessive muscle wasting.2,18,28,29 Therefore, serum bicarbonate normalization, serum uric acid and phosphorus control, and exercise as well as nutritional support are receiving attention as ways to treat these patients.2 ATP-dependent ubiquitin-proteasome (UPS) proteolysis is the major cause of increased skeletal muscle degradation in CKD. Inflammation and metabolic acidosis play a major role in activating the UPS.5
Inflammation markers, including interleukin (IL)-6 and CRP, were negatively correlated with muscle mass in dialysis patients.30 NFκB and insulin/insulin growth factor (IGF)-1 are well-known to be involved in protein catabolism.5 Hyperphosphatemia also induced systemic inflammation and malnutrition independent of renal function in animal models.31 In this study, CRP was negatively correlated with HGS, which is concordant with previous reports, although serum phosphorus levels were not associated with muscle strength.
Although total CO2 did not show any correlation with muscle strength in this study, metabolic acidosis has a prominent role in PEW and sarcopenia. Metabolic acidosis can induce glucocorticoid secretion and insulin resistance and increase branched chain amino acid (BCAA) oxidation in skeletal muscles, which is associated with decreased muscle protein synthesis.32,33,34 In addition, sodium bicarbonate supplementation in CKD patients slowed the decline in renal function, increased dietary protein intake, and reduced normalized protein nitrogen appearance, which reflects decreased protein breakdown associated with increased lean body mass.35 Bicarbonate supplementation in pre-dialysis CKD patients also increased total and appendicular lean muscle mass and reduced plasma myostatin level which is a surrogate of muscle degradation in addition to the improvement in lower extremity muscle strength.36,37
Serum albumin and creatinine were positively correlated with both HGS and LMS in this study. Malnutrition is considered as a nontraditional cardiovascular risk factor in dialysis patients. Hypoalbuminemia is one of the most potent risk factors for mortality in HD patients.38
The 2012 Kidney Disease Improving Global Outcomes (KDIGO) guideline recommends a protein intake of 0.8–1.3 g/kg/day in CKD patients with progressive renal dysfunction.38 The typical dietary protein intake recommendation for ESRD patients is 1.2 g/kg/day.39 However, some experts recommended a dietary protein intake of more than 1.8 g/kg/day in dialysis patients because they do not have to prevent CKD progression and are in a hypercatabolic state due to dialysis itself.40 Protein restriction in CKD patients should be regulated considering the development of PEW, which can lead to poor clinical outcomes. The obesity paradox and reverse epidemiological trends in CKD and ESRD patients should also be considered. Weight loss of more than 10% was 50% less common in obese than in nonobese CKD patients.41 Significant weight loss occurred at a relatively early stage of CKD and was associated with an increased risk of mortality after dialysis.42
BMI, possibly implies nutritional state and muscle mass, was positively correlated with LMS in this study, which is concordant with previous reports. And serum creatinine was positively correlated with both HGS and LMS in this study. Serum creatinine can be a good surrogate marker of muscle mass in dialysis patients with no or minimal residual renal function, and a study analyzing 2 Korean HD cohorts and matched US Caucasian and African American cohorts reported better survival in patients with higher BMI and serum creatinine than in patients with lower BMI and serum creatinine.43,44,45 A new simplified PEW scoring system that includes serum creatinine/body surface area successfully predicted survival in HD patients and could potentially replace the muscle wasting category in the diagnosis of PEW.46
Any type of exercise (regular home-based or hospital-based resistance exercise) showed a beneficial effect on muscle strength in this study. One anabolic-based strategy for the treatment of PEW is exercise in addition to nutritional support, anabolic steroids, and growth hormones. The reduction in protein intake reduces protein catabolism as well as protein synthesis; therefore, nitrogen balance is not disturbed. Net protein catabolism occurs when protein and energy intake are inadequately low. However, resistance training during protein restriction counteracts impaired muscle cell metabolism.47 Experts report that resistance training together with protein or amino acid supplementation can have beneficial effects on muscle mass and/or muscle function. Exercise improves muscle size, the protein synthesis rate, neuromuscular function, insulin sensitivity, and inflammation associated with increased insulin-stimulated glucose and amino acid transport.4,48 Regular exercise also showed positive effects on nutritional markers such as serum albumin, prealbumin, and energy intake regardless of type, intensity, length, or supervision.47 These results may be related to the alleviation of depression or improvement in appetite due to exercise.49 In addition, intradialytic exercise (IDE) combined with oral or parenteral nutrition enhanced amino acid uptake and protein content in the muscles of HD patients, indicating an improved anabolic effect of nutritional supplementation.50
Cardiovascular exercise, such as cycle training, was well tolerated during HD and improved oxygen uptake, inflammation, arterial compliance, muscle strength, physical activity, and psychosocial functioning.51,52,53 Exercise induced vascular adaptation and improved endothelial function, which are associated with improved exercise capacity.54 Strengthening exercise was associated with skeletal muscle hypertrophy and increased muscle strength in CKD and ESRD patients.55 It also reduced inflammation and improved PEW by increasing muscle IGF-1 and decreasing myostatin in HD patients.56 In addition, IDE improved the efficiency of dialysis (Kt/V) and solute clearance, including serum phosphorus; physical function; arterial stiffness; QOL; depression; and the hospitalization rate.57,58 IDE also increased blood pressure in patients with intradialytic hypotension, which has been associated with decreased cardiac output and increased mortality.59,60 IDE can be a convenient option to ensure compliance and reduce uncomfortable dialysis-related symptoms such as restless legs and cramping.61
Physical inactivity is an important clinical problem among CKD and dialysis patients.47 Providing exercise therapy to patients who are unable to perform conventional dynamic training due to position limitations, hemodynamic instability during dialysis, low motivation, or fatigue is a major challenge.20 HD patients, especially elderly patients, reported low levels of physical activity, which is associated with poor physical function and frailty. HD itself contributes to physical inactivity.62 The Kidney Disease Outcome Quality Initiative (K/DOQI) and European guidelines recommend that nephrologists counsel and encourage dialysis patients to increase physical activity.63,64 Physicians caring for CKD and ESRD patients should encourage patients to engage in physical activity such as aerobic, resistant, coordination, and flexibility exercises.62
Patient groups of older age, male sex, and shorter duration of HD benefitted more from exercise than their corresponding counterparts in this study. This may imply that patients with reduced habitual physical activity (older age) and a considerable amount of muscle mass (male sex and shorter duration of HD) can benefit from exercise. We need to encourage patients to exercise from the beginning of HD, even from pre-dialysis CKD stages.
This study has several limitations, including the absence of body composition data, inaccurate measurement of home-based exercise (i.e., exercise was self-reported and the information on the type, duration, and strength of exercise was incomplete), the lack of information on dietary and nutritional status, and systemic shortcomings due to the cross-sectional study design. However, we obtained general features of HGS and LMS in HD patients in this study.
In conclusion, sex, age, and exercise were the most important determinants of muscle strength in HD patients. We need to encourage patients to do regular home or group exercise from the beginning of dialysis and introduce and implement new feasible forms of exercise for HD patients.
Footnotes
Disclosures: The authors have no conflicts of interest to disclose.
- Conceptualization: Cha RH.
- Data curation: Cha RH, Lee GS, Yoo JY, Rhee OB, Jeon YD.
- Formal analysis: Cha RH.
- Writing - original draft: Cha RH.
- Writing - review & editing: Cha RH.
SUPPLEMENTARY MATERIAL
Multiple linear regression analysis of the factors related with muscle strength in patients who checked both hand grip and leg muscle strength
References
- 1.Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73(4):391–398. doi: 10.1038/sj.ki.5002585. [DOI] [PubMed] [Google Scholar]
- 2.Hanna RM, Ghobry L, Wassef O, Rhee CM, Kalantar-Zadeh K. A practical approach to nutrition, protein-energy wasting, sarcopenia, and cachexia in patients with chronic kidney disease. Blood Purif. 2020;49(1-2):202–211. doi: 10.1159/000504240. [DOI] [PubMed] [Google Scholar]
- 3.Moon SJ, Kim TH, Yoon SY, Chung JH, Hwang HJ. Relationship between stage of chronic kidney disease and sarcopenia in Korean aged 40 years and older using the Korea National Health and Nutrition Examination Surveys (KNHANES IV-2, 3, and V-1, 2), 2008–2011. PLoS One. 2015;10(6):e0130740. doi: 10.1371/journal.pone.0130740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goisser S, Kemmler W, Porzel S, Volkert D, Sieber CC, Bollheimer LC, et al. Sarcopenic obesity and complex interventions with nutrition and exercise in community-dwelling older persons--a narrative review. Clin Interv Aging. 2015;10:1267–1282. doi: 10.2147/CIA.S82454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fahal IH. Uraemic sarcopenia: aetiology and implications. Nephrol Dial Transplant. 2014;29(9):1655–1665. doi: 10.1093/ndt/gft070. [DOI] [PubMed] [Google Scholar]
- 6.Stenvinkel P, Larsson TE. Chronic kidney disease: a clinical model of premature aging. Am J Kidney Dis. 2013;62(2):339–351. doi: 10.1053/j.ajkd.2012.11.051. [DOI] [PubMed] [Google Scholar]
- 7.Anand S, Johansen KL, Kurella Tamura M. Aging and chronic kidney disease: the impact on physical function and cognition. J Gerontol A Biol Sci Med Sci. 2014;69(3):315–322. doi: 10.1093/gerona/glt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Domański M, Ciechanowski K. Sarcopenia: a major challenge in elderly patients with end-stage renal disease. J Aging Res. 2012;2012:754739. doi: 10.1155/2012/754739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foley RN, Wang C, Ishani A, Collins AJ, Murray AM. Kidney function and sarcopenia in the United States general population: NHANES III. Am J Nephrol. 2007;27(3):279–286. doi: 10.1159/000101827. [DOI] [PubMed] [Google Scholar]
- 10.Fried LF, Boudreau R, Lee JS, Chertow G, Kurella-Tamura M, Shlipak MG, et al. Kidney function as a predictor of loss of lean mass in older adults: health, aging and body composition study. J Am Geriatr Soc. 2007;55(10):1578–1584. doi: 10.1111/j.1532-5415.2007.01398.x. [DOI] [PubMed] [Google Scholar]
- 11.Workeneh BT, Mitch WE. Review of muscle wasting associated with chronic kidney disease. Am J Clin Nutr. 2010;91(4):1128S–32S. doi: 10.3945/ajcn.2010.28608B. [DOI] [PubMed] [Google Scholar]
- 12.Wang XH, Mitch WE. Muscle wasting from kidney failure-a model for catabolic conditions. Int J Biochem Cell Biol. 2013;45(10):2230–2238. doi: 10.1016/j.biocel.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stenvinkel P, Carrero JJ, von Walden F, Ikizler TA, Nader GA. Muscle wasting in end-stage renal disease promulgates premature death: established, emerging and potential novel treatment strategies. Nephrol Dial Transplant. 2016;31(7):1070–1077. doi: 10.1093/ndt/gfv122. [DOI] [PubMed] [Google Scholar]
- 14.Jhamb M, Weisbord SD, Steel JL, Unruh M. Fatigue in patients receiving maintenance dialysis: a review of definitions, measures, and contributing factors. Am J Kidney Dis. 2008;52(2):353–365. doi: 10.1053/j.ajkd.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lenk K, Schuler G, Adams V. Skeletal muscle wasting in cachexia and sarcopenia: molecular pathophysiology and impact of exercise training. J Cachexia Sarcopenia Muscle. 2010;1(1):9–21. doi: 10.1007/s13539-010-0007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koppe L, Fouque D, Kalantar-Zadeh K. Kidney cachexia or protein-energy wasting in chronic kidney disease: facts and numbers. J Cachexia Sarcopenia Muscle. 2019;10(3):479–484. doi: 10.1002/jcsm.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clyne N. Physical working capacity in uremic patients. Scand J Urol Nephrol. 1996;30(4):247–252. doi: 10.3109/00365599609182300. [DOI] [PubMed] [Google Scholar]
- 18.Obi Y, Qader H, Kovesdy CP, Kalantar-Zadeh K. Latest consensus and update on protein-energy wasting in chronic kidney disease. Curr Opin Clin Nutr Metab Care. 2015;18(3):254–262. doi: 10.1097/MCO.0000000000000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rogan A, McCarthy K, McGregor G, Hamborg T, Evans G, Hewins S, et al. Quality of life measures predict cardiovascular health and physical performance in chronic renal failure patients. PLoS One. 2017;12(9):e0183926. doi: 10.1371/journal.pone.0183926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGregor G, Ennis S, Powell R, Hamborg T, Raymond NT, Owen W, et al. Feasibility and effects of intra-dialytic low-frequency electrical muscle stimulation and cycle training: a pilot randomized controlled trial. PLoS One. 2018;13(7):e0200354. doi: 10.1371/journal.pone.0200354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bohannon RW. Hand-grip dynamometry predicts future outcomes in aging adults. J Geriatr Phys Ther. 2008;31(1):3–10. doi: 10.1519/00139143-200831010-00002. [DOI] [PubMed] [Google Scholar]
- 22.Norman K, Stobäus N, Gonzalez MC, Schulzke JD, Pirlich M. Hand grip strength: outcome predictor and marker of nutritional status. Clin Nutr. 2011;30(2):135–142. doi: 10.1016/j.clnu.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 23.Kittiskulnam P, Chertow GM, Carrero JJ, Delgado C, Kaysen GA, Johansen KL. Sarcopenia and its individual criteria are associated, in part, with mortality among patients on hemodialysis. Kidney Int. 2017;92(1):238–247. doi: 10.1016/j.kint.2017.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vogt BP, Borges MC, Goés CR, Caramori JC. Handgrip strength is an independent predictor of all-cause mortality in maintenance dialysis patients. Clin Nutr. 2016;35(6):1429–1433. doi: 10.1016/j.clnu.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 25.Hwang SH, Lee DH, Min J, Jeon JY. Handgrip Strength as a Predictor of all-cause mortality in patients with chronic kidney disease undergoing dialysis: a meta-analysis of prospective cohort studies. J Ren Nutr. 2019;29(6):471–479. doi: 10.1053/j.jrn.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Matsuzawa R, Kamitani T, Roshanravan B, Fukuma S, Joki N, Fukagawa M. Decline in the functional status and mortality in patients on hemodialysis: results from the Japan Dialysis Outcome and Practice Patterns Study. J Ren Nutr. 2019;29(6):504–510. doi: 10.1053/j.jrn.2018.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoo JI, Choi H, Ha YC. Mean hand grip strength and cut-off value for sarcopenia in Korean adults using KNHANES VI. J Korean Med Sci. 2017;32(5):868–872. doi: 10.3346/jkms.2017.32.5.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hyun YY, Lee KB, Han SH, Kim YH, Kim YS, Lee SW, et al. Nutritional status in adults with predialysis chronic kidney disease: KNOW-CKD Study. J Korean Med Sci. 2017;32(2):257–263. doi: 10.3346/jkms.2017.32.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carrero JJ, Stenvinkel P, Cuppari L, Ikizler TA, Kalantar-Zadeh K, Kaysen G, et al. Etiology of the protein-energy wasting syndrome in chronic kidney disease: a consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM) J Ren Nutr. 2013;23(2):77–90. doi: 10.1053/j.jrn.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 30.Kaizu Y, Ohkawa S, Odamaki M, Ikegaya N, Hibi I, Miyaji K, et al. Association between inflammatory mediators and muscle mass in long-term hemodialysis patients. Am J Kidney Dis. 2003;42(2):295–302. doi: 10.1016/s0272-6386(03)00654-1. [DOI] [PubMed] [Google Scholar]
- 31.Yamada S, Tokumoto M, Tatsumoto N, Taniguchi M, Noguchi H, Nakano T, et al. Phosphate overload directly induces systemic inflammation and malnutrition as well as vascular calcification in uremia. Am J Physiol Renal Physiol. 2014;306(12):F1418–28. doi: 10.1152/ajprenal.00633.2013. [DOI] [PubMed] [Google Scholar]
- 32.Kalantar-Zadeh K, Mehrotra R, Fouque D, Kopple JD. Metabolic acidosis and malnutrition-inflammation complex syndrome in chronic renal failure. Semin Dial. 2004;17(6):455–465. doi: 10.1111/j.0894-0959.2004.17606.x. [DOI] [PubMed] [Google Scholar]
- 33.Kalantar-Zadeh K, Fouque D. Nutritional management of chronic kidney disease. N Engl J Med. 2017;377(18):1765–1776. doi: 10.1056/NEJMra1700312. [DOI] [PubMed] [Google Scholar]
- 34.Bailey JL, Zheng B, Hu Z, Price SR, Mitch WE. Chronic kidney disease causes defects in signaling through the insulin receptor substrate/phosphatidylinositol 3-kinase/Akt pathway: implications for muscle atrophy. J Am Soc Nephrol. 2006;17(5):1388–1394. doi: 10.1681/ASN.2004100842. [DOI] [PubMed] [Google Scholar]
- 35.de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol. 2009;20(9):2075–2084. doi: 10.1681/ASN.2008111205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kittiskulnam P, Srijaruneruang S, Chulakadabba A, Thokanit NS, Praditpornsilpa K, Tungsanga K, et al. Impact of serum bicarbonate levels on muscle mass and kidney function in pre-dialysis chronic kidney disease patients. Am J Nephrol. 2020;51(1):24–34. doi: 10.1159/000504557. [DOI] [PubMed] [Google Scholar]
- 37.Abramowitz MK, Melamed ML, Bauer C, Raff AC, Hostetter TH. Effects of oral sodium bicarbonate in patients with CKD. Clin J Am Soc Nephrol. 2013;8(5):714–720. doi: 10.2215/CJN.08340812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toyoda K, Kuragano T, Kawada H, Taniguchi T, Nakanishi T. Effect of progression in malnutrition and inflammatory conditions on adverse events and mortality in patients on maintenance hemodialysis. Blood Purif. 2019;47(Suppl 2):3–11. doi: 10.1159/000496629. [DOI] [PubMed] [Google Scholar]
- 39.KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Chapter 3: management of progression and complications of CKD. Kidney Int Suppl. 2013;3(1):73–90. doi: 10.1038/kisup.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft AJ, Morley JE, et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc. 2013;14(8):542–559. doi: 10.1016/j.jamda.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 41.Ziolkowski SL, Long J, Baker JF, Chertow GM, Leonard MB. Chronic kidney disease and the adiposity paradox: valid or confounded? J Ren Nutr. 2019;29(6):521–528. doi: 10.1053/j.jrn.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ku E, Kopple JD, Johansen KL, McCulloch CE, Go AS, Xie D, et al. Longitudinal weight change during CKD progression and its association with subsequent mortality. Am J Kidney Dis. 2018;71(5):657–665. doi: 10.1053/j.ajkd.2017.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel SS, Molnar MZ, Tayek JA, Ix JH, Noori N, Benner D, et al. Serum creatinine as a marker of muscle mass in chronic kidney disease: results of a cross-sectional study and review of literature. J Cachexia Sarcopenia Muscle. 2013;4(1):19–29. doi: 10.1007/s13539-012-0079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Canaud B, Granger Vallée A, Molinari N, Chenine L, Leray-Moragues H, Rodriguez A, et al. Creatinine index as a surrogate of lean body mass derived from urea Kt/V, pre-dialysis serum levels and anthropometric characteristics of haemodialysis patients. PLoS One. 2014;9(3):e93286. doi: 10.1371/journal.pone.0093286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park J, Jin DC, Molnar MZ, Dukkipati R, Kim YL, Jing J, et al. Mortality predictability of body size and muscle mass surrogates in Asian vs white and African American hemodialysis patients. Mayo Clin Proc. 2013;88(5):479–486. doi: 10.1016/j.mayocp.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moreau-Gaudry X, Jean G, Genet L, Lataillade D, Legrand E, Kuentz F, et al. A simple protein-energy wasting score predicts survival in maintenance hemodialysis patients. J Ren Nutr. 2014;24(6):395–400. doi: 10.1053/j.jrn.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 47.Cupisti A, D'Alessandro C, Fumagalli G, Vigo V, Meola M, Cianchi C, et al. Nutrition and physical activity in CKD patients. Kidney Blood Press Res. 2014;39(2-3):107–113. doi: 10.1159/000355784. [DOI] [PubMed] [Google Scholar]
- 48.Jung HW, Kim SW, Kim IY, Lim JY, Park HS, Song W, et al. Protein intake recommendation for Korean older adults to prevent sarcopenia: Expert consensus by the Korean Geriatric Society and the Korean Nutrition Society. Ann Geriatr Med Res. 2018;22(4):167–175. doi: 10.4235/agmr.18.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frey S, Mir AR, Lucas M. Visceral protein status and caloric intake in exercising versus nonexercising individuals with end-stage renal disease. J Ren Nutr. 1999;9(2):71–77. doi: 10.1016/s1051-2276(99)90003-1. [DOI] [PubMed] [Google Scholar]
- 50.Majchrzak KM, Pupim LB, Flakoll PJ, Ikizler TA. Resistance exercise augments the acute anabolic effects of intradialytic oral nutritional supplementation. Nephrol Dial Transplant. 2008;23(4):1362–1369. doi: 10.1093/ndt/gfm773. [DOI] [PubMed] [Google Scholar]
- 51.Heiwe S, Jacobson SH. Exercise training in adults with CKD: a systematic review and meta-analysis. Am J Kidney Dis. 2014;64(3):383–393. doi: 10.1053/j.ajkd.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 52.Johansen KL, Painter P. Exercise in individuals with CKD. Am J Kidney Dis. 2012;59(1):126–134. doi: 10.1053/j.ajkd.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koh KP, Fassett RG, Sharman JE, Coombes JS, Williams AD. Effect of intradialytic versus home-based aerobic exercise training on physical function and vascular parameters in hemodialysis patients: a randomized pilot study. Am J Kidney Dis. 2010;55(1):88–99. doi: 10.1053/j.ajkd.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 54.Ashor AW, Lara J, Siervo M, Celis-Morales C, Oggioni C, Jakovljevic DG, et al. Exercise modalities and endothelial function: a systematic review and dose-response meta-analysis of randomized controlled trials. Sports Med. 2015;45(2):279–296. doi: 10.1007/s40279-014-0272-9. [DOI] [PubMed] [Google Scholar]
- 55.Kirkman DL, Mullins P, Junglee NA, Kumwenda M, Jibani MM, Macdonald JH. Anabolic exercise in haemodialysis patients: a randomised controlled pilot study. J Cachexia Sarcopenia Muscle. 2014;5(3):199–207. doi: 10.1007/s13539-014-0140-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moraes C, Marinho SM, da Nobrega AC, de Oliveira Bessa B, Jacobson LV, Stockler-Pinto MB, et al. Resistance exercise: a strategy to attenuate inflammation and protein-energy wasting in hemodialysis patients? Int Urol Nephrol. 2014;46(8):1655–1662. doi: 10.1007/s11255-014-0712-3. [DOI] [PubMed] [Google Scholar]
- 57.Salhab N, Karavetian M, Kooman J, Fiaccadori E, El Khoury CF. Effects of intradialytic aerobic exercise on hemodialysis patients: a systematic review and meta-analysis. J Nephrol. 2019;32(4):549–566. doi: 10.1007/s40620-018-00565-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rhee SY, Song JK, Hong SC, Choi JW, Jeon HJ, Shin DH, et al. Intradialytic exercise improves physical function and reduces intradialytic hypotension and depression in hemodialysis patients. Korean J Intern Med. 2019;34(3):588–598. doi: 10.3904/kjim.2017.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fotbolcu H, Duman D, Ecder SA, Oduncu V, Cevik C, Tigen K, et al. Attenuated cardiovascular response to sympathetic system activation during exercise in patients with dialysis-induced hypotension. Am J Nephrol. 2011;33(6):491–498. doi: 10.1159/000327829. [DOI] [PubMed] [Google Scholar]
- 60.Flythe JE, Xue H, Lynch KE, Curhan GC, Brunelli SM. Association of mortality risk with various definitions of intradialytic hypotension. J Am Soc Nephrol. 2015;26(3):724–734. doi: 10.1681/ASN.2014020222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kirkman DL, Edwards DG, Lennon-Edwards S. Exercise as an Adjunct therapy in chronic kidney disease. Renal Nutr Forum. 2014;33(4):1–8. [PMC free article] [PubMed] [Google Scholar]
- 62.Johansen KL, Chertow GM, Ng AV, Mulligan K, Carey S, Schoenfeld PY, et al. Physical activity levels in patients on hemodialysis and healthy sedentary controls. Kidney Int. 2000;57(6):2564–2570. doi: 10.1046/j.1523-1755.2000.00116.x. [DOI] [PubMed] [Google Scholar]
- 63.K/DOQI Workgroup. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45(4) Suppl 3:S1–S153. [PubMed] [Google Scholar]
- 64.Fouque D, Vennegoor M, ter Wee P, Wanner C, Basci A, Canaud B, et al. EBPG guideline on nutrition. Nephrol Dial Transplant. 2007;22(Suppl 2):ii45–ii87. doi: 10.1093/ndt/gfm020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multiple linear regression analysis of the factors related with muscle strength in patients who checked both hand grip and leg muscle strength