Abstract
Modelling human colon cancer has long been the ambition of researchers and oncologists with the aim to better replicate disease progression and treatment response. Advances in our understanding of genetics, stem cell biology, tumour microenvironment and immunology have prepared the groundwork for recent major advances. In the last two years the field has seen the progression of: using patient derived organoids (alone and in co-culture) as predictors of treatment response; molecular stratification of tumours that predict outcome and treatment response; mouse models of metastatic disease; and transplant models that can be used to de-risk clinical trials. We will discuss these advances in this review.
Current Opinion in Genetics and Development 2021, 66:50–56
This review comes from a themed issue on Cancer genomics
Edited by David J. Adams, Marcin Imielinski and C. Daniela Robles-Espinoza
For a complete overview see the Issue and the Editorial
Available online 7th January 2021
https://doi.org/10.1016/j.gde.2020.12.003
0959-437X/© 2021 The Authors. Published by Elsevier Ltd. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
Introduction
Colorectal cancer (CRC) with ∼800 000 deaths per year globally is still one of the most common cancers in men and women [1]. This heavy public health burden is predicted to increase if no improvement in early detection and effective interventions for late stage CRC are discovered. Currently routine colonoscopic imaging followed by surgical resection of primary tumours and oligo-metastases are the first intervention option for CRC patients [2]. Depending on stage, combination treatments of cytotoxic chemotherapies like 5-fluoruracil (5-FU), oxaliplatin or irinotecan show some benefits, which can be increased in combination with vascular endothelial growth factor receptor (VEGFR) or epidermal growth factor receptor (EGFR) treatments [3,4]. While the use of EGFR inhibitor depends on the mutation status of KRAS and BRAF [5], VEGFR inhibitors are routinely applied for stage IV patients [6]. The decisions for systemic treatments are based on routine diagnostics such as histopathological characteristics of tumours, the status of lymph-nodes and metastasis (TNM). This grading system doesn’t account for additional factors that impact on response and patients’ outcome. For example, BRAF mutations are associated with right-sided colon cancers with specific molecular features and poor-prognosis [7,8] however the clinical treatment decision is independent of these recognised characteristics. In addition, the therapies above are the same anti-proliferative combination treatments that have been used since the 1990’s, drug design and development has not kept pace with our understanding of the biology of CRC. One attempt to embrace the wide spectrum of inter-patient heterogeneity, and importantly take different molecular features into account, is the definition of molecular subtypes [9, 10, 11].
Here we discuss the most recent advances in the field of CRC research and highlight technical improvements which may lead to discovery of novel treatment concepts. Focusing particularly on the fast progressing field of organoids, the molecular characterisation of CRC, and the importance of next-generation mouse models, which aim to de-risk the translation of pre-clinical results into the clinics by improving human relevant features (Figure 1).
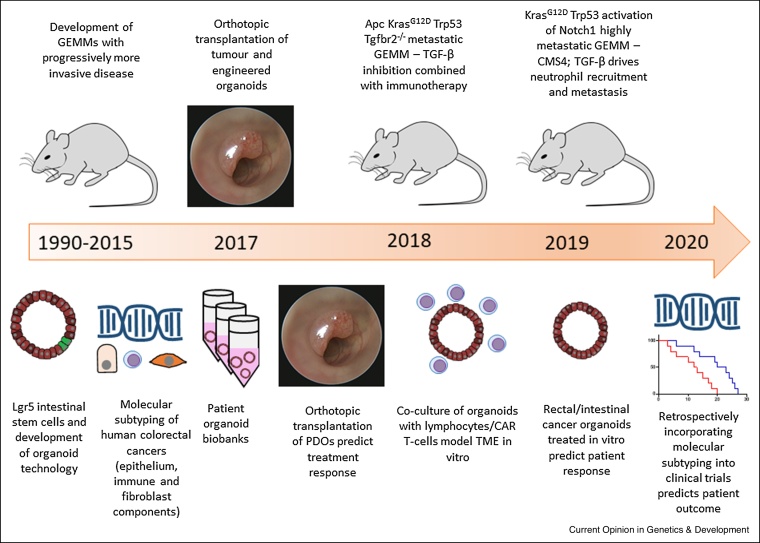
Figure 1.
Timeline of recent advances in mouse models of CRC in parallel with molecular, technological and clinical scientific advances. Decades of research provided the field with GEMMs that permitted investigation of driver mutations in CRC, however, the models had long latency and were minimally metastatic. Concomitant discovery of intestinal stem cell markers precipitated the organoid revolution, which could be subsequently engineered using CRISPR, and provided means to store patient samples in biobanks. Molecular subtyping of CRC biopsies revealed patient stratification relating to epithelium, immune and fibroblast components. Advancements in transplantation techniques enabled orthotopic grafting of mouse or patient tumour-derived/engineered organoids, importantly PDOs were shown to be able to predict patient treatment response. 2018 and 2019 saw breakthroughs in the development of increasingly metastatic GEMMs with the KPN mouse exhibiting 100% metastasis and correlating with CMS4, the most aggressive CRC subtype. TGF-β and immunotherapy treatments demonstrated utility in reducing metastatic burden in these models. The same period of time saw advancement of in vitro organoid models, with both co-culture with immune cells and in vitro therapeutic treatment of organoids alone demonstrating patient responsiveness. More recently, the retrospective incorporation of molecular subtyping into clinical trial outcomes is predictive of patient outcome, establishing the importance of patient stratification. Modified from Figure 1 in Jackstadt and Sansom [37]. It is used under license CC BY 4.0. https://creativecommons.org/licenses/by/4.0/.
Modelling CRC in vitro - a guide for treatments
Classical 2D adherent cell lines served for many years as valuable tools to discover key functional aspects of CRC biology; however, these cell lines show reduced complexity compared to patient tumours. Significant improvement followed identification of the intestinal stem cell (ISC) marker Lgr5 [12] and the subsequent opportunity to culture self-organising mouse intestinal organoids derived and arising from this stem cell population. The generation of mouse and human organoids from virtually every organ followed shortly [13]. Sequential genetic alterations using CRISPR/CAS9 to model acquisition of CRC driver mutations in human organoids confirmed the concept proposed by Vogelstein in 1990 [14,15]. Besides the opportunity to culture healthy tissues, tumour derived organoids were proven to retain morphological, genetic and transcriptional features of the initial founder tumour [16,17]. Importantly, cultures of CRC patient-derived organoids (PDOs) were used as avatars to predict patient response ex vivo [18, 19, 20, 21]. Interestingly the generation of a liver metastases PDO biobank revealed a modest inter-patient heterogeneity in pharmacological response and may serve to identify specific treatment responder versus non-responder transcriptional profiles [22]. Strikingly, treatment response following orthotopic transplantation of liver metastasis or rectal tumour PDOs into immune-deficient mice correlated with patient response [23••,24]. These elegant studies provide the proof-of-concept that tumour response and resistance can be predicted in ‘window-of-opportunity’ trials that can help guide clinical decision making. Some financial, logistical and technical limitations still present obstacles that need to be overcome before precision medicine of organoids and drug-discovery can be applied routinely in the clinic [25].
One substantial limitation of organoid cultures is the lack of tumour microenvironment (TME) components and other physiological parameters. The remarkable results for checkpoint inhibition in immunogenic microsatellite instable (MSI) colon cancer [26] demonstrates the potential power of microenvironmental epithelial cell-extrinsic influence over cancer cell fate and highlights the necessity of immune competent model systems when testing cancer therapies. To circumvent this limitation, co-culture systems of organoids with mesenchymal cells and lymphocytes have been developed [27,28]. Neal et al. modulated air-liquid interphases and demonstrated that lymphocytes in culture treated with PD1 or PDL1 inhibitors generate a cytotoxic response to cancer cells [27]. In co-culture systems of chimeric antigen receptor (CAR)-engineered lymphocytes with PDOs, specific cancer cell killing was demonstrated and proposed as a personalised in vitro testing platform [29]. These studies demonstrate that a holistic approach is a suitable way to investigate cancer cell-immune cell interactions with relevance for cancer therapies. However, the culture conditions in which these cells are propagated can never truly replicate the nuanced tissue intercompartmental signalling ecosystem within a tumour, and this should be considered when using these systems.
Stratified medicine and tailored treatments
It is a critical challenge to distinguish responders from non-responders, or identify stage III patients with increased likelihood of recurrence. In an ideal scenario this discrimination would take place prior to treatment, consequently our understanding of the underlying molecular features that drive resistance are crucial to this endeavour. One strategy is to classify patients, and identify inter-patient heterogeneity, with the use of transcriptional profiles of whole tumours, including epithelial cancer cell and stromal components. Various studies identified 3–6 subtypes with distinct molecular features and pathway activation (reviewed in Ref. [30]). An international consortium set out to clarify these classifications and ultimately defined four comprehensive consensus molecular subtypes (CMSs) with different molecular features [9]. CMS1 is characterized by mutations in BRAF and microsatellite instability/DNA mismatch repair deficiency (MSI/dMMR). These tumours display high cytotoxic lymphocyte infiltration response to checkpoint inhibition, thus called the ‘immune’ subtype. CMS2 the ‘canonical’ subtype shows high WNT signalling activation, epithelial clusters and high levels of chromosomal instability (CIN). CMS3 differs slightly and shows increased KRAS mutations accompanied by association to metabolic changes and is accordingly the ‘metabolic’ subtype. CMS4, the ‘mesenchymal’ subtype, is dominated by stromal content, fibroblasts, monocytic and lymphocytic lineages. Molecularly, this subtype shows increased activation of TGF-β signalling, epithelial-mesenchymal transition (EMT), and predicts worst survival. Together, these classifications demonstrate heterogeneity between patients and highlight the opportunity to treat patients according to their subtype. A number of clinical trials retrospectively incorporating the CMSs show specific response [30,31,32•] demonstrating the importance of patient stratification and the value of implementing CMS in clinical practice. However, a number of confounding factors can influence the accuracy of CMS calling. Particularly, the composition of the stromal compartment is of increased importance in CMS1/4 tumours and contributes strongly to CMS identification. To this end, bioinformatics tools were developed to query the stromal composition in detail [33]. While this was useful to decipher the stromal content of the tumour, CMS2/3 tumours comprise of much more epithelium. To capture the signature of epithelial cells, which are influenced by the environment and account for intra-tumoral heterogeneity, the CRC cell intrinsic subtypes (CRIS) were developed [10,11]. Importantly, CRIS are less dependent on regional variation within a tumour, thus subtypes are classified much more robustly [34]. However, a faster and less costly way to identify CMS is needed to increase the use in clinical diagnosis. The use of image based CMS (imCMS), where machine learning algorithms are capable of automated detection of CMS specific morphological features from H&E slide alone, is a promising application with potential for clinical implication [35]. In addition, CMS calling has been optimized at protein level by immunohistochemistry for CDX2, FRMD6, HTR2B, pan-cytokeratin, ZEB1 and microsatellite status [36].
In vivo models of intestinal cancer: emerging pre-clinical tools
As we have covered in a previous review [37], the suite of mouse models available for pre-clinical research were lacking a number of essential features relevant to human disease. In this section, we will focus on the recent improvements of genetically engineered mouse models (GEMMs) and the improved transplantation of organoids to recapitulate human colon cancer progression.
It is beyond doubt that GEMMs are the most appropriate tools to recapitulate the complexity of the tumour ecosystem [38]. Spontaneous tumours that develop in a fully immune-competent environment create systemic inflammation which substantially contributes to tumour progression [39]. They are also subject to the same evolutionary bottlenecks of transformation, invasion and metastasis as human tumours and undergo a natural selection during expansion. However, a persistent issue of these models was the low penetrance of metastasis to distant organs. If mice developed spontaneous metastasis the rate was below 25% with a latency which made pre-clinical investigations nearly impossible [37]. To this end, we developed a model of CMS4 intestinal cancer, driven by KrasG12D mutation, which is highly penetrant to the liver (>80%) with a strong desmoplastic reaction in primary tumours and metastasis [40••]. In combination with Trp53 mutation, activation of Notch1 signalling rewired the TME towards an immunosuppressive neutrophil rich environment driven by TGF-β mediated neutrophil attraction. In line with these findings, Germann et al. reported the importance of a neutrophil rich TME for the activation of latent TGF-β ligands by neutrophil secreted metalloproteinases [41]. Besides the generation of an immunosuppressive TME, expression of activated epithelial Notch1 (Rosa26N1icd) drives poor prognosis subtypes CMS4 and CRIS-B; however, Notch1 activation in the context of Apc mutations (Apcfl/+) didn’t trigger a subtype switch and tumours remained non-metastatic CMS2/3 tumours [40••]. Further evidence for the role of Notch signalling in metastatic CRC came from a recent publication by Varga et al. whom observed increased Notch3 activation in a GEMM with mutations in Trp53 and constitutive activation of AKT [42]. The mice developed metastatic CRC (∼80% lymph nodes affected) that resembled CMS4 when treated with the carcinogen azoxymethane (AOM) and therapeutic treatment with a NOTCH3 antibody reduced primary and metastatic burden [42].
Classical CRC is thought to progress from adenoma to adenocarcinoma with initiating mutations in the APC tumour suppressor and subsequent mutations in KRAS, TP53, SMAD4. When mutations along the classical route of progression to colon cancer were combined in mice, they develop spontaneous metastatic tumours albeit with a penetrance of maximal 40% (often lower) [43, 44, 45]. Orthotopic transplantation of organoids derived from such tumours, either into the colon or the liver, has proven to be a rapid and reliable tool to investigate multiple aspects of advanced CRC [42,43,45, 46, 47, 48, 49]. For example, these novel models were used to demonstrate the effect of CXCR2 and TGF-β inhibition on metastasis [40••], with increased efficacy when combined with checkpoint inhibition [43,50]. Furthermore, the role of Lgr5 positive and negative cancer stem cells were explored using a transplant model with the surprising finding that only liver metastases, and not the primary tumour, show a remarkable sensitivity to Lgr5+ cell depletion when diphtheria toxin receptor mediated killing was utilised [49,51•]. Moreover, engineered organoids monitored by intra-vital imaging uncovered that most disseminated cells when they leave the primary tumour and seed to the liver are Lgr5 negative [51•]. During expansion to macro-metastasis these cells de-differentiate and regain Lgr5 expression. In addition, primary tumours showed recurrence post depletion [49,52], indicating a fundamental impact of the TME on stem cell plasticity. Future studies should address mechanisms, which control this plasticity and test the therapeutic potential.
Importantly, these GEMMs and organoid models were proven to recapitulate characteristics of human cancers and could be stratified into different CMS [40••,43,44]. It is interesting to note that the field is lacking an epithelial CMS2/3 GEMM that metastasises spontaneously with high penetrance. Half of stage IV CRC patients comprise of CMS2/3 and half of liver metastases are CMS2, thus CMS2 appears able to drive metastatic progression [53,54]. Alternatively, it may be an issue of intra-tumour heterogeneity compromising accurate subtyping and it is possible that CMS2/3 tumours simply contain a CMS4 region that drives the aggressive disease [55]. As previously discussed, CRIS analysis could be used as an alternative to CMS to more robustly classify the epithelium in tumours. Another possibility is the temporary acquisition of more aggressive features, CMS2 CRC has been demonstrated to subtype switch from CMS2 in bulk human CRC biopsies to CMS4 in cells budding from the invasive margin, suggestive of a mechanism as to how these subtypes could become metastatic[56]. As observed with the Lgr5 stem cell plasticity described above it may be that upon seeding to the distant site the established metastases revert to the CMS of the primary tumour rather than persist as CMS4. Obtaining multiple tumour biopsies from different regions and treating patients according to the most aggressive pathology contained within may improve outcomes.
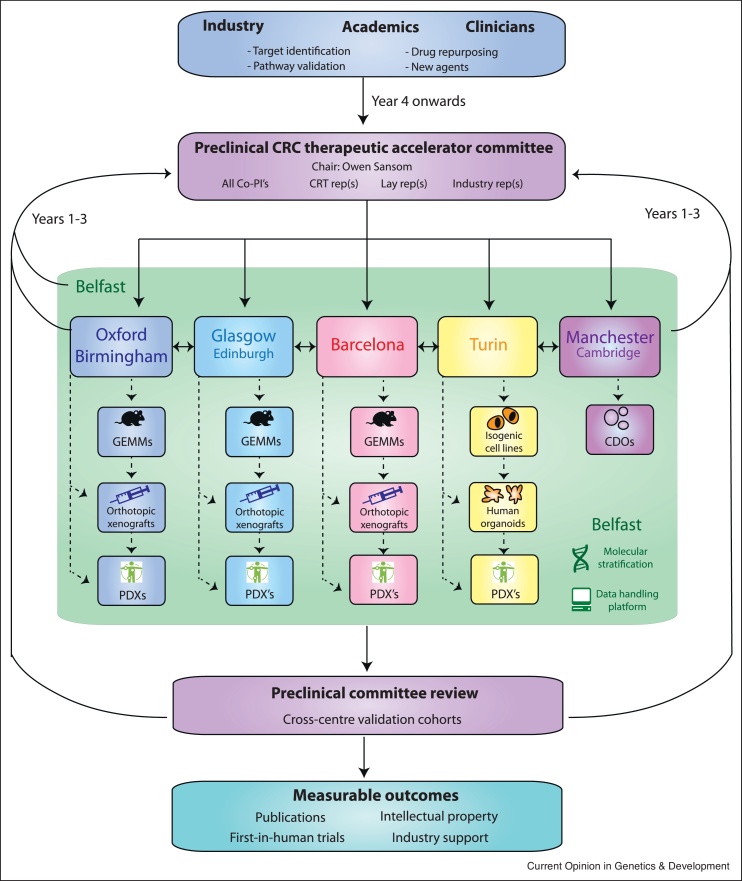
It will be an important task for the future to generate a comprehensive cross-comparison of mouse models and human datasets to allow for precise subtyping of mouse models. To this end, we have initiated a Cancer Research UK funded consortium (ACRCelerate: Colorectal Cancer Stratified Medicine Network) which will characterise a suite of state-of-the-art mouse models that recapitulate human disease to generate reliable and robust pre-clinical data and de-risk the failure of promising drug candidates in clinical trials (Figure 2).
Figure 2.
ACRCelerate: Colorectal Cancer Stratified Medicine Network Platform design and governance. Project proposals arising from industry partners, academics or clinicians will be submitted to a preclinical therapeutic committee for discussion. Execution of the project will be allocated to the various hubs based on centre expertise and interest before cross centre validation is undertaken. We also seek to develop complementary circulating tumour cell-derived organoid (CDO) technology. This pipeline will contribute to the advancement of translational research in CRC by bringing together CMS relevant GEMMs, orthotopic transplant models, PDOs and xenografts to couple preclinical information with clinical science.
Conclusions
Overall, the in vitro and in vivo landscape of CRC mouse models in recent years has improved substantially; however, a number of key features are not appropriately represented in the currently available models. PDO cultures have demonstrated their usefulness in correlating with patient outcome and predicting patient responsiveness to therapy. The ongoing development of co-culture techniques with TME components is essential to more closely replicate patient disease, and ultimately treatment response on a time-scale that could direct patient treatment in real-time. As discussed above, subtyping patient disease and mouse models has been proven as a valuable concept, and although mouse tumours show a narrower phenotype compared to the wide spectrum of human disease, this feature should be used to identify the most representative models of specific patient groups. As the number of mouse models expands it may be possible to take a primary approach to subtype mouse tumours themselves, before relating back to human subtypes. This may allow functional assessment of the impact of key driver genes. With the recent advantages in single cell techniques, tumour heterogeneity can be analysed with a much higher resolution. Initial comparative studies in lung cancer and CRC highlight a striking similarity between human and mouse tumours [57•,58]. Single cell technologies will allow for an even better cross-comparison of human and mouse tumours, to identify targetable modules in various cell lineages. Additionally, spontaneous GEMMs which precisely generate tumours in human relevant parts of the colon are needed. These models, driven by genetic alterations detected in humans should enable the community to investigate therapeutic options for example specifically in right-sided and rectal cancer under fully immune-competent conditions.
Conflict of interest statement
SJL has received funding from UCB and Redx Pharma. OJS has received funding from AstraZeneca, Redx Pharma, BMS and Novartis.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
CRediT authorship contribution statement
Tamsin RM Lannagan: Writing - original draft. Rene Jackstadt: Writing - original draft. Simon J Leedham: Writing - review & editing. Owen J Sansom: Writing - review & editing.
Acknowledgements
We apologise to those whose work was omitted due to space restrictions. TRML is funded by CRUK Accelerator Programme Award (ACRCelerate Grant No. A26825), RJ is funded by the Dietmar Hopp Foundation, SJL is funded by Wellcome Trust Senior Clinical Research Fellowship (206314/Z/17/Z), OJS is funded by Cancer Research UK (Core Grant No. A21139).
References
- 1.Siegel R.L. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145–164. doi: 10.3322/caac.21601. [DOI] [PubMed] [Google Scholar]
- 2.Hafez N., Gettinger S. Oligometastatic disease and local therapies: a medical oncology perspective. Cancer J. 2020;26:144–148. doi: 10.1097/PPO.0000000000000439. [DOI] [PubMed] [Google Scholar]
- 3.Douillard J.Y. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol. 2014;25:1346–1355. doi: 10.1093/annonc/mdu141. [DOI] [PubMed] [Google Scholar]
- 4.Saltz L.B. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 5.Lievre A., Laurent-Puig P. Genetics: predictive value of KRAS mutations in chemoresistant CRC. Nat Rev Clin Oncol. 2009;6:306–307. doi: 10.1038/nrclinonc.2009.69. [DOI] [PubMed] [Google Scholar]
- 6.Punt C.J., Koopman M., Vermeulen L. From tumour heterogeneity to advances in precision treatment of colorectal cancer. Nat Rev Clin Oncol. 2017;14:235–246. doi: 10.1038/nrclinonc.2016.171. [DOI] [PubMed] [Google Scholar]
- 7.Yaeger R. Clinical sequencing defines the genomic landscape of metastatic colorectal cancer. Cancer Cell. 2018;33:125–136.e3. doi: 10.1016/j.ccell.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee G.H. Is right-sided colon cancer different to left-sided colorectal cancer? - A systematic review. Eur J Surg Oncol. 2015;41:300–308. doi: 10.1016/j.ejso.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Guinney J. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350–1356. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunne P.D. Cancer-cell intrinsic gene expression signatures overcome intratumoural heterogeneity bias in colorectal cancer patient classification. Nat Commun. 2017;8 doi: 10.1038/ncomms15657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isella C. Selective analysis of cancer-cell intrinsic transcriptional traits defines novel clinically relevant subtypes of colorectal cancer. Nat Commun. 2017;8 doi: 10.1038/ncomms15107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barker N. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 13.Schutgens F., Clevers H. Human organoids: tools for understanding biology and treating diseases. Annu Rev Pathol. 2020;15:211–234. doi: 10.1146/annurev-pathmechdis-012419-032611. [DOI] [PubMed] [Google Scholar]
- 14.Fearon E.R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 15.Kawasaki K. Chromosome engineering of human colon-derived organoids to develop a model of traditional serrated adenoma. Gastroenterology. 2020;158:638–651.e8. doi: 10.1053/j.gastro.2019.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Fujii M. A colorectal tumor organoid library demonstrates progressive loss of niche factor requirements during tumorigenesis. Cell Stem Cell. 2016;18:827–838. doi: 10.1016/j.stem.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 17.van de Wetering M. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. 2015;161:933–945. doi: 10.1016/j.cell.2015.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clevers H.C. Organoids: avatars for personalized medicine. Keio J Med. 2019;68:95. doi: 10.2302/kjm.68-006-ABST. [DOI] [PubMed] [Google Scholar]
- 19.Ooft S.N. Patient-derived organoids can predict response to chemotherapy in metastatic colorectal cancer patients. Sci Transl Med. 2019;11 doi: 10.1126/scitranslmed.aay2574. [DOI] [PubMed] [Google Scholar]
- 20.Yao Y. Patient-derived organoids predict chemoradiation responses of locally advanced rectal cancer. Cell Stem Cell. 2020;26:17–26.e6. doi: 10.1016/j.stem.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Narasimhan V. Medium-throughput drug screening of patient-derived organoids from colorectal peritoneal metastases to direct personalized therapy. Clin Cancer Res. 2020;26:3662–3670. doi: 10.1158/1078-0432.CCR-20-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruun J. Patient-derived organoids from multiple colorectal cancer liver metastases reveal moderate intra-patient pharmacotranscriptomic heterogeneity. Clin Cancer Res. 2020 doi: 10.1158/1078-0432.CCR-19-3637. [DOI] [PubMed] [Google Scholar]
- 23••.Ganesh K. A rectal cancer organoid platform to study individual responses to chemoradiation. Nat Med. 2019;25:1607–1614. doi: 10.1038/s41591-019-0584-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; Rectal cancer has been particularly underrepresented in mouse models of colorectal cancer. Ganesh et al. (2019) established a biobank of 65 human rectal cancer organoid lines that maintained the molecular and histopathological features of the original cancer. They demonstrated that in vitro responses of these lines to chemotherapy correlated with patient clinical outcome, validating the preclinical potential of using PDOs. Further they engrafted these lines into the rectums of immune-compromised mice and treated with chemotherapy, again tumour growth correlated with patient response. This PDX model also metastasised to the lung and liver as it does in patients. This paper demonstrates the utility of organoid and in vivo transplant models and it will be an exciting next step to move into an immune-competent setting.
- 24.Vlachogiannis G. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science. 2018;359:920–926. doi: 10.1126/science.aao2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hedayat S., Valeri N. Patient-derived organoids: promises, hurdles and potential clinical applications. Clin Oncol (R Coll Radiol) 2020;32:213–216. doi: 10.1016/j.clon.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 26.Ganesh K. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol. 2019;16:361–375. doi: 10.1038/s41575-019-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neal J.T. Organoid modeling of the tumor immune microenvironment. Cell. 2018;175:1972–1988.e16. doi: 10.1016/j.cell.2018.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dijkstra K.K. Generation of tumor-reactive T cells by co-culture of peripheral blood lymphocytes and tumor organoids. Cell. 2018;174:1586–1598.e12. doi: 10.1016/j.cell.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schnalzger T.E. 3D model for CAR-mediated cytotoxicity using patient-derived colorectal cancer organoids. EMBO J. 2019;38 doi: 10.15252/embj.2018100928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buikhuisen J.Y., Torang A., Medema J.P. Exploring and modelling colon cancer inter-tumour heterogeneity: opportunities and challenges. Oncogenesis. 2020;9:66. doi: 10.1038/s41389-020-00250-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stintzing S. Consensus molecular subgroups (CMS) of colorectal cancer (CRC) and first-line efficacy of FOLFIRI plus cetuximab or bevacizumab in the FIRE3 (AIO KRK-0306) trial. Ann Oncol. 2019;30:1796–1803. doi: 10.1093/annonc/mdz387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32•.Lenz H.J. Impact of consensus molecular subtype on survival in patients with metastatic colorectal cancer: results from CALGB/SWOG 80405 (Alliance) J Clin Oncol. 2019;37:1876–1885. doi: 10.1200/JCO.18.02258. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lenz et al. (2019) sought to investigate if CMS classification of 581 patients with metastatic CRC (mCRC) enrolled in a phase III clinical trial (CALB/SWOG 80405) would provide predictive and/or prognostic information. The trial was designed to compare the overall survival (OS) and progression-free survival (PFS) of patients treated with standard-of-care chemotherapy alongside cetuximab or bevacizumab. It was found that CMS was significantly prognostic for OS and PFS, a novel finding for mCRC, as well as predictive of treatment response, with CMS1 patients benefitting from bevacizumab but not cetuximab and CMS2 patients benefitting from cetuximab over bevacizumab. Strengthening the argument for including CMS as part of clinical decision making.
- 33.Becht E. Immune and stromal classification of colorectal cancer is associated with molecular subtypes and relevant for precision immunotherapy. Clin Cancer Res. 2016;22:4057–4066. doi: 10.1158/1078-0432.CCR-15-2879. [DOI] [PubMed] [Google Scholar]
- 34.Alderdice M. Prospective patient stratification into robust cancer-cell intrinsic subtypes from colorectal cancer biopsies. J Pathol. 2018;245:19–28. doi: 10.1002/path.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sirinukunwattana K. Image-based consensus molecular subtype (imCMS) classification of colorectal cancer using deep learning. Gut. 2020 doi: 10.1136/gutjnl-2019-319866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ten Hoorn S. Classification of colorectal cancer in molecular subtypes by immunohistochemistry. Methods Mol Biol. 2018;1765:179–191. doi: 10.1007/978-1-4939-7765-9_11. [DOI] [PubMed] [Google Scholar]
- 37.Jackstadt R., Sansom O.J. Mouse models of intestinal cancer. J Pathol. 2016;238:141–151. doi: 10.1002/path.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kersten K. Genetically engineered mouse models in oncology research and cancer medicine. EMBO Mol Med. 2017;9:137–153. doi: 10.15252/emmm.201606857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garner H., de Visser K.E. Immune crosstalk in cancer progression and metastatic spread: a complex conversation. Nat Rev Immunol. 2020 doi: 10.1038/s41577-019-0271-z. [DOI] [PubMed] [Google Scholar]
- 40••.Jackstadt R. Epithelial NOTCH signaling rewires the tumor microenvironment of colorectal cancer to drive poor-prognosis subtypes and metastasis. Cancer Cell. 2019;36:319–336.e7. doi: 10.1016/j.ccell.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; A highly penetrant metastatic GEMM of CRC has long been sought after. In 2019, Jackstadt et al. presented the KPN mouse, which along with activation of epithelial Notch1, an activating mutation in Kras and loss of Trp53 drove a form of CRC with serrated features. The model exhibited intestinal adenocarcinoma and 100% metastasis, including to lymph node, liver and lung. Characterisation of the model revealed it to be akin to CMS4/CRIS-B, the poorest prognosis CRC subtypes, and that Notch1 activation was driving Tgf-β signalling, which was in turn recruiting neutrophils which drove metastasis. Blocking Tgf- β signalling or targeting neutrophils reduced metastasis, demonstrating the therapeutic potential of targeting neutrophils in mCRC.
- 41.Germann M. Neutrophils suppress tumor-infiltrating T cells in colon cancer via matrix metalloproteinase-mediated activation of TGFbeta. EMBO Mol Med. 2020;12 doi: 10.15252/emmm.201910681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Varga J. AKT-dependent NOTCH3 activation drives tumor progression in a model of mesenchymal colorectal cancer. J Exp Med. 2020;217 doi: 10.1084/jem.20191515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tauriello D.V.F. TGFbeta drives immune evasion in genetically reconstituted colon cancer metastasis. Nature. 2018;554:538–543. doi: 10.1038/nature25492. [DOI] [PubMed] [Google Scholar]
- 44.Boutin A.T. Oncogenic Kras drives invasion and maintains metastases in colorectal cancer. Genes Dev. 2017;31:370–382. doi: 10.1101/gad.293449.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sakai E. Combined mutation of Apc, Kras, and Tgfbr2 effectively drives metastasis of intestinal cancer. Cancer Res. 2018;78:1334–1346. doi: 10.1158/0008-5472.CAN-17-3303. [DOI] [PubMed] [Google Scholar]
- 46.Fumagalli A. Genetic dissection of colorectal cancer progression by orthotopic transplantation of engineered cancer organoids. Proc Natl Acad Sci U S A. 2017;114:E2357–E2364. doi: 10.1073/pnas.1701219114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Rourke K.P. Transplantation of engineered organoids enables rapid generation of metastatic mouse models of colorectal cancer. Nat Biotechnol. 2017;35:577–582. doi: 10.1038/nbt.3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roper J. In vivo genome editing and organoid transplantation models of colorectal cancer and metastasis. Nat Biotechnol. 2017;35:569–576. doi: 10.1038/nbt.3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Sousa e Melo F. A distinct role for Lgr5(+) stem cells in primary and metastatic colon cancer. Nature. 2017;543:676–680. doi: 10.1038/nature21713. [DOI] [PubMed] [Google Scholar]
- 50.Liao W. KRAS-IRF2 axis drives immune suppression and immune therapy resistance in colorectal cancer. Cancer Cell. 2019;35:559–572.e7. doi: 10.1016/j.ccell.2019.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51•.Fumagalli A. Plasticity of Lgr5-negative cancer cells drives metastasis in colorectal cancer. Cell Stem Cell. 2020;26:569–578.e7. doi: 10.1016/j.stem.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]; The ability to target cancer stem cells (CSCs) is of great interest to the CRC field as a potential means to eradicate cancer and prevent the spread of disseminating cells that seed metastases. However, this elegant study by Fumagalli et al. (2020) using multiphoton microscopy in an in vivo mouse model demonstrated that the classic intestinal stem cell marker, Lgr5, was actually absent from CRC cells that resulted in metastases. These Lgr5− cells exhibited plasticity in that once they seeded in a distant organ, such as the liver, they would eventually give rise to Lgr5+ CSCs. These data suggest that it is not sufficient to target CSCs as a means of treating CRC as cells that would not be considered to be CSCs maintain an intrinsic hierarchy that can be reactivated.
- 52.Shimokawa M. Visualization and targeting of LGR5(+) human colon cancer stem cells. Nature. 2017;545:187–192. doi: 10.1038/nature22081. [DOI] [PubMed] [Google Scholar]
- 53.Coebergh van den Braak R.R.J. Interconnectivity between molecular subtypes and tumor stage in colorectal cancer. BMC Cancer. 2020;20:850. doi: 10.1186/s12885-020-07316-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fontana E. Context matters-consensus molecular subtypes of colorectal cancer as biomarkers for clinical trials. Ann Oncol. 2019;30:520–527. doi: 10.1093/annonc/mdz052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dunne P.D. Challenging the cancer molecular stratification dogma: intratumoral heterogeneity undermines consensus molecular subtypes and potential diagnostic value in colorectal cancer. Clin Cancer Res. 2016;22:4095–4104. doi: 10.1158/1078-0432.CCR-16-0032. [DOI] [PubMed] [Google Scholar]
- 56.De Smedt L. Expression profiling of budding cells in colorectal cancer reveals an EMT-like phenotype and molecular subtype switching. Br J Cancer. 2017;116:58–65. doi: 10.1038/bjc.2016.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57•.Zhang L. Single-cell analyses inform mechanisms of myeloid-targeted therapies in colon cancer. Cell. 2020;181:442–459.e29. doi: 10.1016/j.cell.2020.03.048. [DOI] [PubMed] [Google Scholar]; The application of single-cell RNA sequencing (scRNA-seq) has facilitated the characterisation of cell heterogeneity in tumours. Zhang et al. (2020) examined myeloid lineages in biopsies from CRC patient samples and found distinct populations of tumour-associated macrophages (TAMs) and dendritic cells (DCs). scRNA-seq of mouse tumour models revealed comparable populations present facilitating mechanistic interrogation. This study supports the rationale for clinical testing of immunotherapies that target myeloid populations as well as emphasising the value of mouse preclinical models in the investigation of human disease.
- 58.Zilionis R. Single-cell transcriptomics of human and mouse lung cancers reveals conserved myeloid populations across individuals and species. Immunity. 2019;50:1317–1334.e10. doi: 10.1016/j.immuni.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]