Abstract
Pollen tube (PT) growth as a key step for successful fertilization is essential for angiosperm survival and especially vital for grain yield in cereals. The process of PT growth is regulated by many complex and delicate signaling pathways. Among them, the calcium/calcium-dependent protein kinases (Ca2+/CPKs) signal pathway has become one research focus, as Ca2+ ion is a well-known essential signal molecule for PT growth, which can be instantly sensed and transduced by CPKs to control myriad biological processes. In this review, we summarize the recent progress in understanding the Ca2+/CPKs signal pathway governing PT growth. We also discuss how this pathway regulates PT growth and how reactive oxygen species (ROS) and cyclic nucleotide are integrated by Ca2+ signaling networks.
Keywords: calcium, calcium-dependent protein kinases, pollen tube, plant, signaling
Introduction
The calcium ion (Ca2+), as a central second messenger in plants, coordinates a variety of physiological responses by binding the calcium sensors, which decode the calcium signatures and elicit different cellular responses. In plants, there are four main classes of calcium sensors: calmodulin (CaM) or CaM-like proteins (CMLs), calcineurin B-like proteins (CBLs), CBL interacting protein kinases (CIPKs), and the calcium-dependent protein kinases (CPKs) and their relatives, CDPK-related kinases (CRKs; Harper et al., 2004; Dodd et al., 2010). Among them, CPKs share the unique feature of combining the calcium-binding motifs and protein kinase domain (PKD) on the same peptide. CPKs are implicated in the regulation of plant development, as well as in biotic and abiotic stress signaling. The different tissue- and developmental-stage expressions of the CPKs possess specific functions; for example, AtCPK28 and AtCPK3/4/6/11 have roles in shoot and root development, respectively, and AtCPK6/33 may be involved in the regulation of floral transition (see the review by Yip Delormel and Boudsocq, 2019). Significantly, a number of AtCDPKs are mainly expressed in pollen, indicating their involvement in pollen development and/or pollen tube (PT) growth, which is crucial for sexual reproduction in flowering plants. Successful fertilization begins with pollen grains landing on the stigma and germination of the PT. Upon pollen landing on the stigma, the PT rapidly elongates and penetrates the transmitting tract to deliver the immotile sperm to the ovule for double fertilization (Higashiyama and Yang, 2017). During this process, Ca2+ is well-known to control pollen germination, PT growth, and intercellular communication between PT and female tissue (Ge et al., 2007; Zheng et al., 2019). However, we do not fully understand how these specific Ca2+/CPKs signal pathways regulate PT growth. In this review, we summarize the key findings of the Ca2+/CPKs signaling pathway in PT growth and further address the interrelationship between Ca2+ signaling with other complex signaling networks such as reactive oxygen species (ROS) and cyclic nucleotide.
Composition and Construction of Pollen Tube
The PT is a tubular structure that germinates from the aperture in pollen. In angiosperms, the cell wall of the PT usually comprises two layers: the outer fibrillar layer that is mainly composed of pectin, hemicellulose, and cellulose, and the inner layer of callose (Taylor and Hepler, 1997). The tip of the PT comprises a single pectin layer, which is the most elastic region and the expansion point of PT growth. Some studies indicated that inhibition of cellulose biosynthesis can affect the morphology and structural integrity of Petunia and Lily PTs (Anderson et al., 2002), while pectin that is synthesized in the Golgi apparatus and then secreted into the cell wall by exocytosis can strengthen the mechanical strength and ductility of the PT (Li et al., 1994; Hasegawa et al., 1998). Interestingly, callose is only deposited on the inner layer of the cell wall of the PT, except for the tip, and it also has a role in the correct recognition of pollen and stigma (Lush and Clarke, 1997; Dearnaley et al., 1999; Kuboyama and Takeda, 2000). Further, some glycoproteins are deposited in the PT, i.e., arabinogalactan proteins (AGPs) and lipid transfer protein 5 (LTP5; Cheung et al., 1995; Chae et al., 2009).
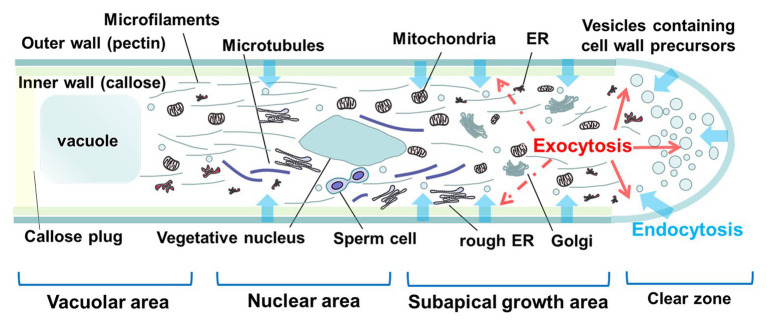
The structure of the PT can be divided into four different zones according to Franklin’s description (Figure 1; Franklin-Tong, 1999). At the extreme tip of the PT, a “clear zone” is filled with secretory vesicles that package many cell wall components, which will then be incorporated into the apical dome of the PT tip for elongation; behind the “clear zone” is a subapical growth area, which contains most of the cytoplasm and organelles such as mitochondria, Golgi complexes, endoplasmic reticulum (ER), and cytoskeletal components. At the bottom of the germinated PT are the vacuolar area and the cell nuclear area, which contain the vegetative nucleus and generative (or sperm) cell. The formation of vacuoles maintains tube turgor and pushes the cytoplasm to the apex of the PT. To restrict the cytoplasm to the apical region of the growing tube, a series of callose plugs are formed at regular intervals behind the tip. Although the composition and construction of the PT are well understood, the mechanics of its elongation are unclear. In addition to the conventional hydrodynamic model, ion dynamics and the cell wall model (Zonia and Munnik, 2011; Liu and Hussey, 2014), a new Hechtian model of PT tip growth has been put forward (Lamport et al., 2017). Briefly, the new model proposes that a viscoplastic pectic cell wall is mechanically coupled to the plasma membrane by Hechtian adhesion, which transmits wall strain to the plasma membrane and thus regulates Ca2+ and other ion fluxes that regulate the exocytosis of wall precursors. Moreover, the “kiss-and-run” mode of exocytosis/endocytosis at the PT apex and “durotropic” movement, namely exhibiting increased movement speed in stiffer materials, have also been proposed and challenge some previous standpoints about PT elongation (see the review by Adhikari et al., 2020). In any case, all the models mentioned above involve Ca2+ as a key signal.
Figure 1.
Diagrammatic representation of the structure of the tip region of a pollen tube (PT). The tip of a PT can be classically divided into four zones from the tip to the base: clear zone, subapical growth area, nuclear area, and vacuolar area. The bulk of exocytosis occurs in the apical region as shown with the solid red arrows, while certain exocytic vesicles may also be secreted to the subapical growth area, as shown with the dotted arrows. Endocytosis can occur in the apical and subapical growth areas and nuclear area as shown with the blue arrows (ER is endoplasmic reticulum).
The Role of Ca2+ in Pollen Tube Growth
It is well established that a tip-focused calcium gradient is essential for pollen germination and PT growth (Holdaway-Clarke et al., 2003; Iwano et al., 2009; Michard et al., 2009; Steinhorst and Kudla, 2013). The elevation of the Ca2+ gradient is correlated with pulsed tube growth. Some studies indicated that Ca2+ can directly affect turgor formation during PT growth by affecting the formation of the vacuole (Li et al., 2017). Additionally, asymmetric Ca2+ accumulation within the tip is associated with reorientation of growth in that direction (Malho and Trewavas, 1996). In most studies, [Ca2+]cyt oscillations correlate with oscillations of PT growth speed (Holdaway-Clarke et al., 1997). Channels that account for oscillatory Ca2+ influx across the plasmalemma mainly include the stretch-activated Ca2+ channels (SACs), the cyclic nucleotide-gated channels (CNGCs; Frietsch et al., 2007), and the glutamate receptor-related channels (GLRs; see the review by Hepler et al., 2012). SACs locate at the extreme apex of the PT and open in response to deformation of the plasma membrane caused by PT growth (Dutta and Robinson, 2004). In Arabidopsis, two SACs, namely MCA1 and MCA2, have been identified in the root; however, it remains unknown if related proteins function in PTs (Nakagawa et al., 2007). In addition to the first identified and molecularly characterized CNGC18, there are five additional CNGCs (namely CNGC7, 8, 9, 10, and 16) that are potentially relevant for pollen ion fluxes (Tunc-Ozdemir et al., 2013; Gao et al., 2016). A recent study indicates that CNGC18/8/7 together with calmodulin 2 (CaM2) constitutes a molecular switch that either opens or closes the calcium channel, depending on [Ca2+]cyt levels during PT growth (Pan et al., 2019). Subsequently, a breakthrough study uncovered that MILDEW RESISTANCE LOCUS-O (MLO) proteins can regulate PT guidance in response to ovular signals by recruiting the CNGC18 to the plasma membrane in order to modify Ca2+ gradients in the growing PT (Meng et al., 2020). Among all known CNGCs, only AtCNGC18 and OsCNGC13 are reported to be highly expressed in the pistils and to act as a novel maternal sporophytic factor required for PT guidance (Gao et al., 2016; Xu et al., 2017). By pharmacology, loss-of-function, and heterologous complementary approaches, some studies indicate that GLRs facilitate Ca2+ influx, modulating the apical [Ca2+]cyt gradient and consequently the impact on PT growth (Qi et al., 2006; Michard et al., 2011; Vincill et al., 2012). Interestingly, AtGLRs are inactive when expressed alone in Xenopus oocytes, implying that GLRs may be subject to a plant-specific activation mechanism by CPKs (Roy et al., 2008; Alfieri et al., 2020). A recent study revealed that CORNICHON HOMOLOG (CNIH) proteins are essential for sorting, trafficking, and localizing AtGLRs (Wudick et al., 2018). More importantly, the result of coexpressing AtCNIH4 or AtCNIH1/4 with a PT expressed AtGLR3.3 or AtGLR3.2 in COS-7 cells further confirms that CNIH proteins can enhance AtGLR channel activity, and with binding specificity (Wudick et al., 2018). In ovules, 1-aminocyclopropane-1-carboxylic acid (ACC), a precursor of ethylene synthesis, stimulates GLR-dependent Ca2+ elevation, which in turn promotes LURE1 secretion and PT attraction (Mou et al., 2020). And whether ACC can also act as the most potent elicitor of GLR-mediated Ca2+ elevations in PT requires further study. In addition to these plasma membrane located Ca2+ channels, some internal Ca2+ channels located at a vacuole or endoplasmic reticula, such as ACA2/7/8/9/10 and ECA1, are responsible for fine-tuning the Ca2+ gradient by sequestration of the ion (Harper et al., 1998; Hwang et al., 2000; Iwano et al., 2009; Lucca and León, 2011; Michard et al., 2017; Li et al., 2018a). In addition to being a signal molecule, the Ca2+ ion is also required for cross-linking cell wall components. At the extreme apex of a growing PT, methyl-pectin is secreted as the main new cell wall material, which forms rather loose ionic bonds with Ca2+, resulting in reduced cell wall rigidity. As soon as [Ca2+]cyt increases, the pectin methylesterase is transported to the apex by exocytosis, resulting in de-methoxylation of methyl-pectin and cross-linking with free Ca2+, which increases cell wall rigidity (Bosch and Hepler, 2005). In the process of cell wall remodeling, a self-regulatory network modulating oscillatory growth cycles of an elongating PT also integrates changes in the concentration of [Ca2+]cyt, apical exocytosis of methyl-pectin and pectin methyl esterase (PME), and regulation of SACs, as well as the contribution of F-actin and ROP1 signaling (see the review by Steinhorst and Kudla, 2013).
The Structure and Functions of Ca2+ Signal Decoder CPKs
The Ca2+ signal can be decoded and relayed by a series of phosphorylation cascades mainly regulated by four families of protein kinases (Harper et al., 2004). Among them, CPKs can be directly activated by Ca2+ and phosphorylate downstream effectors to regulate myriad biological processes. The representative structure of CPKs harbors a variable N-terminal domain (VNTD) followed by a PKD and an auto-inhibitory junction domain (JD) that is linked to the C-terminal calmodulin-like domain (CaMLD) with EF-hand Ca2+-binding sites (Harper et al., 1991; Cheng et al., 2002; Hrabak et al., 2003). The VNTD is important not only for membrane localization when modified by palmitoylation and myristoylation at the cysteine and glycine residues, respectively; it is also essential for specific interaction with targets (Stael et al., 2011; Boudsocq and Sheen, 2013). The JD serves as a pseudosubstrate that blocks the kinase active center in the absence of Ca2+ and releases autoinhibition upon Ca2+ binding to EF-hands within the CaMLD domain (Figure 2; Harmon et al., 1994; Liese and Romeis, 2013). CPKs have been identified throughout the plant kingdom and constitute a large multigene family in various plant species, i.e., 34 CPKs identified in Arabidopsis thaliana, 29 CPKs identified in Oryza sativa, and 32 CPKs identified in Zea mays (Cheng et al., 2002; Asano et al., 2005; Khalid et al., 2019). The CPK superfamily members have been implicated in many biological processes, such as development, metabolism, and biotic and abiotic stress responses (reviewed in Klimecka and Muszynska, 2007; Asano et al., 2012). Given the huge number of CPKs with specific functions in different cells or tissue, one important question is how the Ca2+/CPKs signal pathway regulates pollen germination and PT growth.
Figure 2.
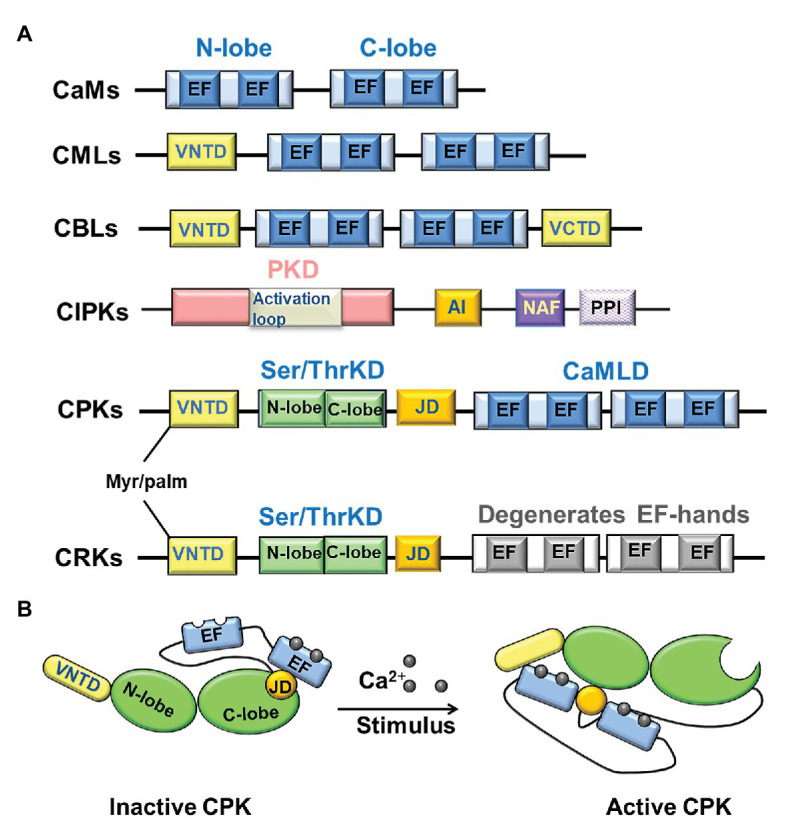
The structures of four calcium sensors and the activation mechanism of calcium-dependent protein kinases (CPKs) by calcium-binding. (A) Domain organization of the four main classes of calcium ion (Ca2+) sensors in plants. (B) Activation mechanism of CPKs by calcium-binding. When Ca2+ concentration is low, the C-lobe is loaded with Ca2+ and interacts with the junction domain (JD), forming an inactive conformation, which blocks the kinase domain access to the substrate. While Ca2+ concentration is elevating, the N-lobe binds Ca2+, resulting in an active conformation, which drives the auto-inhibitory JD out of the active site. EF: EF-hand usually occurs in pairs, N-lobe having a lower calcium affinity than the C-lobe; PKD: protein kinase domain; NAF: asparagine-alanine-phenylalanine domain mediating interaction with the calcineurin B-like proteins (CBLs); PPI: protein-phosphatase interaction domain mediating the interactions of CIPK with 2C-type protein phosphatases (PP2Cs); VNTD: variable N-terminal domain, where the myristoylation (myr) and palmitoylation (palm) occur; VCTD: variable C-terminal domain; JD/AI: an auto-inhibitory junction domain; CaMLD: a C-terminal CaM-like domain classically with 4 EF-hands Ca2+-binding motifs.
The Role of CPKs in Pollen Tube Growth
Calcium-dependent protein kinases, as the vital components in Ca2+ signaling pathways, have been implicated in many aspects of plant life including development and abiotic and biotic stress responses (Simeunovic et al., 2016). The first CPK found to be involved in pollen germination and PT growth was in maize, as the inhibition of this pollen-specific CPK (ZmCPK20) impaired both the pollen germination and growth (Estruch et al., 1994; Moutinho et al., 1998). Further analysis of the expression patterns of ZmCPKs using the maize gene expression atlas revealed that about 12 ZmCPKs were predominantly accumulated in the anther (Stelpflug et al., 2016; Li et al., 2018a). Some proteome studies also found that many CPKs accumulated in maize pollen and many phosphorylate specific substrates upon PT germination and growth. The crucial role of the maize CDPK in PT growth is further substantiated by the function study of ZmCPK32 (Li et al., 2018b). In contrast to most CPKs’ positive regulation of PT growth, ZmCPK32 as a pollen-specific CPK was demonstrated to negatively regulate the PT growth, as a transient expression of ZmCPK32 in tobacco via microparticle bombardment suppressed both the PT germination and growth (Li et al., 2018a). In Petunia inflate, PiCPK1 and PiCPK2 were highly expressed in PT and had distinct functions. The PiCPK2 is involved in PT extension by mediating peroxisome function in conjunction with a small CDPK-interacting protein 1 (PiSCP1; Guo et al., 2013), while the PiCPK1 is likely a key regulator of growth polarity by regulating Ca2+ homeostasis (Yoon et al., 2006). Moreover, five of the 34 CDPK isoforms in the Arabidopsis are highly expressed in pollen, including AtCPK14, 16, 17, 24, and 34 (Harper et al., 2004). Among them, the genetic evidence indicated that AtCPK17 and AtCPK34 are essential for PT growth in response to a Ca2+ signal in the apical dome (Figure 3; Myers et al., 2009). How AtCPK17 and AtCPK34 influence the PT polarized tip growth remains poorly understood, and whether AtCPK17/34 has a regulatory function in the rho-GTPase of plants (ROP) pathway awaits further confirmation (Yang and Fu, 2007; Zhou et al., 2009). Two pollen-specific aquaporins, AtNIP4;1 and AtNIP4;2, were identified, which can be phosphorylated by AtCPK34 in vitro and have a role in pollen germination and PT growth (Di Giorgio et al., 2016). AtCPK2, AtCPK20, and AtCPK6 were shown to promote PT growth by activating the anion channel SLAH3 and ALMT12/13/14 at the pollen tip (Gutermuth et al., 2013, 2018). K+ influx into PT is also essential for PT growth. Further studies showed that AtCPK11 and AtCPK24 (the closest homolog of ZmCPK32) negatively affect PT elongation by mediating the Ca2+-dependent inhibition of the inwardly rectifying K+ channels (Zhao et al., 2013). In O. sativa, OsCPK21 plays an essential role in pollengenesis, possibly via indirectly regulating the transcription of MIKC*-type MADS box proteins (Wen et al., 2019). Moreover, OsCPK25/26 can phosphorylate the predominantly pollen-expressed OIP30 (a RuvB-like DNA helicase 2) and likely affect pollen development by transcriptional control of gene expression (Wang et al., 2011).
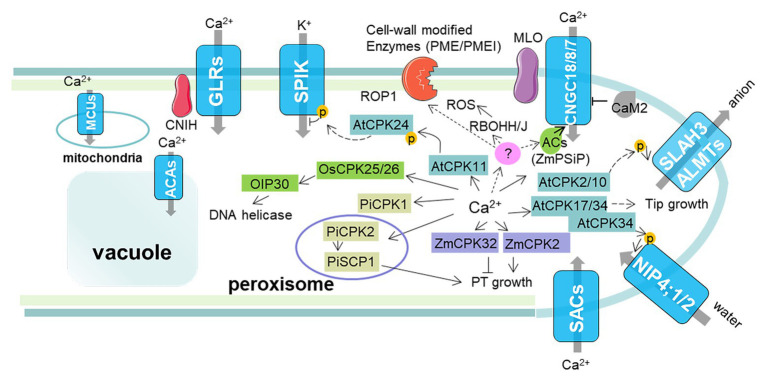
Figure 3.
The proposed Ca2+/CPK signaling regulating PT growth. On the plasmalemma of the PT, calcium entry mainly occurs through three different channels: the stretch-activated Ca2+ channels (SACs), the cyclic nucleotide gated channels (CNGCs), and the glutamate receptor-related channels (GLRs). CNGCs become activated by the binding of adenosine 3', 5'-cyclic monophosphate (cAMP), which are produced by adenylate cyclases (ACs) and inhibited by calmodulin (CaM) binding. Moreover, MLO5/9 can recruit the CNGC18 with asymmetric distribution and result in a change in PT growth direction. The SACs are located at the extreme apex of the tube in response to the deformation of the plasma membrane caused by growth. GLRs as a ligand Ca2+ gated channel are transported, targeted, and activated by CORNICHON HOMOLOG (CNIH) proteins. Some other Ca2+ channels located on the organelle membrane are also involved in fine-tuning of the cytoplasmic Ca2+ concentration and affecting the PT growth, such as mitochondrial calcium uniporters (MCUs) and Ca2+-ATPases (ACAs). Ca2+ signals are perceived by CPKs that decode the information presented in specific Ca2+ signatures and regulate PT growth. In Arabidopsis, the Ca2+/AtCPK11 signal pathway phosphorylates AtCPK24, which will further phosphorylate the K+ influx channel SPIK, resulting in the inhibition of PT elongation. The Ca2+/AtCPK2/10 signal pathway phosphorylates the anion channel SLAH3 and some ALMTs to export anion at the PT tip. Ca2+/AtCPK17/34 can promote pollen tip growth and tropism. The Ca2+/AtCPK34 signal pathway can phosphorylate pollen-specific aquaporins NIP4;1 and NIP4;2 to ensure pollen germination and PT growth. In maize, Ca2+/ZmCPK20 positively regulates PT growth, while Ca2+/ZmCPK32 negatively regulates PT growth. In petunias, PiCPK2 can interact with the small CDPK-interacting protein 1 PiSCP1 to affect PT growth, presumably by mediating peroxisome function, while PiCPK1, which is localized in the plasma membrane, can regulate the polarity of PT growth. In rice, Ca2+/OsCPK25/26 can phosphorylate DNA helicase OIP30 in mature pollen. Moreover, the Ca2+/CPK signal may also integrate and coordinate with other signaling systems, such as ROP1 signaling, reactive oxygen species (ROS), and cAMP.
How Ca2+/CPKs Regulates the Pollen Tube Growth
Although mounting evidence indicates that the Ca2+/CPKs signal pathway has a role in PT growth, how it regulates PT growth is still unclear. The key to unlocking the underlying mechanisms depends on the identification of downstream signal pathway targets. Simeunovic et al. (2016) have comprehensively summarized the identified CPKs targets in plants, while only a small number of CPKs targets have been identified in pollen, mainly including some ion channels (or aquaporin) such as AtSPIK, AtSLAH3, AtACA8, and AtNIP4;1/2 (see Table 1). The activity of PT specific shaker pollen inward K+ channel (SPIK) was inhibited by AtCPK24, which is phosphorylated and activated by AtCPK11 (Figure 3; Zhao et al., 2013). Disruption of SPIK will reduce K+ influx and impair pollen germination and PT growth (Mouline et al., 2002). Moreover, the Ca2+/CPKs signal pathway to control PT growth via anion channel (AtSLAH3 and ALMT12/13/14) activation is confirmed by reverse genetics and electrophysiology (Figure 3; Gutermuth et al., 2018). The tip-focused Ca2+ gradient is essential for PT growth, which requires Ca2+ channel distributions in PT. The cyclic nucleotide-gated channel 18 (CNGC18) is functionally validated for Ca2+ influx across the plasma membrane of PT. Some research reveals a potential feed-forward mechanism in which CPK32 activates CNGC18, further promoting calcium entry during the elevation phase of Ca2+ oscillations in the polar growth of PTs (Zhou et al., 2014). And whether the activities of other CNGCs are directly and indirectly influenced by Ca2+/CPKs requires further investigation. AtACA8, a Ca2+-ATPases to extrude Ca2+ to the apoplast, is confirmed to be phosphorylated by AtCPK16 in vitro (Giacometti et al., 2012). All these results suggest that the Ca2+/CPKs signal pathway may regulate PT growth by maintaining the appropriate intracellular ion concentrations at the apex via fine-tuned diversified ion channels. Besides these, the Ca2+/CPKs signal pathway may crosstalk with other signal molecules such as ROS, which are generated by respiratory burst oxidase homolog (Rboh) NADPH oxidases and also involved in PT growth. In Arabidopsis, RBOHH and RBOHJ were revealed to not only slow down PT growth but also maintain PT integrity when regulated by the RALF-BUPS/ANX complex (Boisson-Dernier et al., 2013). The direct regulation of RBOHD activity by Ca2+/CPKs has been reported in Arabidopsis and Potato (Kobayashi et al., 2007; Liu and He, 2016). However, whether there are some specific CPKs in pollen that are responsive to phosphorylate Rboh remains unknown so far. Moreover, cell wall-modifying enzymes are crucial for PT growth. Some studies show that PME and PME inhibitor (PMEI) modulate the rapid growth of the PT (Röckel et al., 2008). It will be interesting to explore these enzymes, which are potential downstream targets of the Ca2+/CPKs signal pathway. We also summarized a model to illustrate the Ca2+/CPKs signal pathway regulating PT growth.
Table 1.
Overview of the identified CPKs in pollen.
| Namea | Gene IDb | Locationc | Targetsd | Physiological relevancee | Referencesf |
|---|---|---|---|---|---|
| Arabidopsis thaliana | |||||
| AtCPK2 | AT3G10660 | ER, MB | AtRBOHD/F (PM), AtSLAH3 (PM), ALMT12/13/14 (PM) | Reduced ROS production in cpk1,2 double mutants; reduced anion currents and fluxes are reduced in cpk2,20 double mutants | Lu and Hrabak, 2002; Harper et al., 2004; Gao et al., 2013; Gutermuth et al., 2013, 2018 |
| AtCPK14 | AT2G41860 | MB | - | - | Harper et al., 2004 |
| AtCPK16 | AT2G17890 | PM | ACA8 (PM), AtDi19-2 (N, C) | - | Curran et al., 2011; Giacometti et al., 2012 |
| AtCPK17 | AT5G12180 | PM | - | Reduced pollen transmission efficiency in cpk17/34 double mutants | Myers et al., 2009 |
| AtCPK20 | AT2G38910 | S, MB | AtSLAH3 (PM), ALMT12/13/14 (PM) | Anion currents and fluxes are reduced in cpk2,20 double mutants | Gutermuth et al., 2013, 2018 |
| AtCPK24 | AT2G31500 | PM, N | SPIK,14-3-3 | Impairing the Ca2+-dependent inhibition of K+ in currents and PT elongation | Zhao et al., 2013; Swatek et al., 2014 |
| AtCPK26 | AT4G38230 | N, C | - | - | Harper et al., 2004 |
| AtCPK34 | AT5G19360 | PM | NIP4;1/2 | Fewer seeds per silique and reduced pollen germination and PT length in nip4;1/2 mutant | Di Giorgio et al., 2016 |
| Zea mays | |||||
| ZmCPK20 | GRMZM2G365815 | - | - | - | Estruch et al., 1994 |
| ZmCPK32 | GRMZM2G332660 | PM | - | Inhibition of PT growth by transient expression of ZmCPK32 in tobacco pollen | Li et al., 2018a |
| Petunia inflate | |||||
| PiCPK1 | DQ147913 | PM | PiSCP1 | Loss of growth polarity Inhibited pollen germination and tube growth | Yoon et al., 2006; Guo et al., 2013 |
| PiCPK2 | DQ147912 | P | PiSCP1 | Inhibition of PT extension but did not affect growth polarity or germination rates | Guo et al., 2013 |
| Oryza sativa | |||||
| OsCPK25 | Os11g04170 | - | - | - | Wang et al., 2011; Tang and Page, 2013 |
| OsCPK26 | Os12g03970 | - | - | - | Wang et al., 2011; Tang and Page, 2013 |
Short name.
Gene identifier according to TAIR (A. thaliana CDPKs) or GeneBank (other species).
Subcellular localization published in the literature: S, soluble; MB, membranes; N, nucleus; C, cytoplasm; P, peroxisomes; PM, plasma membrane; ER, endoplasmic reticulum.
Lists of published CPK target genes with their published subcellular localization in parentheses.
Physiological relevance is defined by phenotypes of knockdown or overexpressing lines, when available, or other physiological traits.
Corresponding references.
Crosstalk with Other Signaling Networks in Pollen Tube Growth
Certainly, proper growth of the PT depends on an elaborate mechanism, which not only needs the central Ca2+/CPK signal but also needs integration and coordination with other molecules and signaling systems, such as ROP1 signaling, inositol-polyphosphates (IP3/6) and numerous pistil factors (γ-aminobutyric acid, long-chain base phosphates, and polyamines; Wu et al., 2014; Yu et al., 2014; Aloisi et al., 2017; Domingos et al., 2019). Additionally, some evidence reveals a link between [Ca2+]cyt and pHcyt plays a role in PT growth (Behera et al., 2018; Mangano et al., 2018). Further, we will emphasize some interconnections and convergence points of Ca2+ signaling with ROS and adenosine 3',5'-cyclic monophosphate (cAMP). ROS generated by NADPH oxidases (NOXs) that are shown to be involved in various processes in PT growth, including germination, polarized, and ovule-targeted growth, and PT burst during fertilization (see review by Wudick and Feijo, 2014). Some direct evidence indicates that binding of Ca2+ will activate some NOXs activities, such as RbohH and RbohJ (Potocky et al., 2012; Kaya et al., 2014). This activation mechanism probably occurs synergistically with phosphorylation of NOXs, although phosphorylation seems to be a prerequisite for Ca2+-mediated NOX activation (Kimura et al., 2012). Based on these findings, a positive feedback model for Ca2+/ROS signaling in PT growth is raised, in which Ca2+-induced NOXs activity leads to ROS mediated activation of some Ca2+ channels, which in turn causes an increase in the cytosolic Ca2+ level (Breygina et al., 2016; Makavitskaya et al., 2018). An interesting recent finding is that LINC-complex mediated VN proximity to the PT tip is required for both responses to exogenous ROS and internal nuclear Ca2+ fluctuations (Moser et al., 2020). Cyclic nucleotides (cNMPs), such as cAMP and guanosine 3',5'-cyclic monophosphate (cGMP), as the activators of CNGCs are undoubtedly involved in PT growth (Duszyn et al., 2019). Presently, information is limited because there are only seven experimentally confirmed adenylate cyclases (ACs) in higher plants, which limits the knowledge about how cAMPs were synthesized and how they regulate the CNGCs during PT growth (Yang et al., 2020). Among them, ZmPSiP is preferentially expressed in PT and catalyzes the production of cAMP, which is responsible for PT growth and reorientation (Moutinho et al., 2001). As for cGMP, it is noteworthy that nitric oxide can activate guanylyl cyclase and possibly activate CNGCs through increases in cGMP levels, leading to an influx of extracellular Ca2+ and actin filament organization during cell wall construction in Pinus bungeana PTs (Wang et al., 2009; Marondedze et al., 2017). Therefore, capturing a more complete picture of the Ca2+/CPK signaling in PT growth requires an exhaustive investigation of the other integrated molecular and signaling systems.
Concluding Remarks and Future Perspectives
Although substantial progress has been made in the past decades, the mechanism of the Ca2+/CPKs signal pathway for regulating PT growth is still fragmented. Only a few relatively complete signal transduction chains are reported. Besides the regulation of the cell wall properties and ion concentrations, the related researches about the Ca2+/CPKs signal pathway involved in other processes such as endo- and exo-cytosis and cytoskeletal regulation fine-tuning of Ca2+ concentration in organelles (vacuole, dictyosome, and mitochondria) need exhaustive investigation (Steinhorst et al., 2015; Selles et al., 2018; Flores-Herrera et al., 2019; Guo and Yang, 2020). Moreover, identification of the unknown targets of CPKs (particularly for nuclear targets such as TFs) and depiction of the elaborate internetwork of the Ca2+/CPKs pathway with other signal pathways will lead to important insights into the mechanisms of PT growth. The progress of experimental techniques such as various omics techniques, Y2H screens, CRISPR/Cas gene editing, and RNAi by directly adding the siRNAs into the PT culture medium (Suwinska et al., 2017), various molecular probes (Mravec et al., 2017), microfluidics and microrobotics (Burri et al., 2020), and computational methods (Damineli et al., 2017) will provide new opportunities and boost our understanding of the Ca2+/CPKs signal pathway in PT growth.
Author Contributions
HY, CY, SY, and YZ wrote the manuscript. FY, NC, XL, YL, and XH revised and critically evaluated the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This review was supported by grants from the Key R&D and Promotion Projects of Henan Province (China; grant no.192102110004), the Youth Program of National Natural Science Foundation of China (China; grant no.31800260), and the Key Laboratory of Plant Molecular Physiology, Chinese Academy of Sciences fund.
References
- Adhikari P. B., Liu X., Kasahara R. D. (2020). Mechanics of pollen tube elongation: a perspective. Front. Plant Sci. 11:589712. 10.3389/fpls.2020.589712, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfieri A., Doccula F. G., Pederzoli R., Grenzi M., Bonza M. C., Luoni L., et al. (2020). The structural bases for agonist diversity in an Arabidopsis thaliana glutamate receptor-like channel. Proc. Natl. Acad. Sci. U. S. A. 117, 752–760. 10.1073/pnas.1905142117, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloisi I., Cai G., Faleri C., Navazio L., Serafini-Fracassini D., Del Duca S. (2017). Spermine regulates pollen tube growth by modulating Ca2+-dependent actin organization and cell wall structure. Front. Plant Sci. 8:1701. 10.3389/fpls.2017.01701, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. R., Barnes W. S., Bedinger P. (2002). 2,6-Dichlorobenzonitrile, a cellulose biosynthesis inhibitor, affects morphology and structural integrity of petunia and lily pollen tubes. J. Plant Physiol. 159, 61–67. 10.1078/0176-1617-00651 [DOI] [Google Scholar]
- Asano T., Hayashi N., Kikuchi S., Ohsugi R. (2012). CDPK-mediated abiotic stress signaling. Plant Signal. Behav. 7, 817–821. 10.4161/psb.20351, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano T., Tanaka N., Yang G., Hayashi N., Komatsu S. (2005). Genome-wide identification of the rice calcium-dependent protein kinase and its closely related kinase gene families: comprehensive analysis of the CDPKs gene family in rice. Plant Cell Physiol. 46, 356–366. 10.1093/pcp/pci035, PMID: [DOI] [PubMed] [Google Scholar]
- Behera S., Xu Z., Luoni L., Bonza M. C., Doccula F. G., de Michelis M. I., et al. (2018). Cellular Ca2+ signals generate defined ph signatures in plants. Plant Cell 30, 2704–2719. 10.1105/tpc.18.00655, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisson-Dernier A., Lituiev D. S., Nestorova A., Franck C. M., Thirugnanarajah S., Grossniklaus U. (2013). ANXUR receptor-like kinases coordinate cell wall integrity with growth at the pollen tube tip via NADPH oxidases. PLoS Biol. 11:e1001719. 10.1371/journal.pbio.1001719, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch M., Hepler P. K. (2005). Pectin methylesterases and pectin dynamics in pollen tubes. Plant Cell 17, 3219–3226. 10.1105/tpc.105.037473, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudsocq M., Sheen J. (2013). CDPKs in immune and stress signaling. Trends Plant Sci. 18, 30–40. 10.1016/j.tplants.2012.08.008, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breygina M. A., Abramochkin D. V., Maksimov N. M., Yermakov I. P. (2016). Hydrogen peroxide affects ion channels in lily pollen grain protoplasts. Plant Biol. 18, 761–767. 10.1111/plb.12470, PMID: [DOI] [PubMed] [Google Scholar]
- Burri J. T., Munglani G., Nelson B. J., Grossniklaus U., Vogler H. (2020). Quantification of mechanical forces and physiological processes involved in pollen tube growth using microfluidics and microrobotics. Methods Mol. Biol. 2160, 275–292. 10.1007/978-1-0716-0672-8_20, PMID: [DOI] [PubMed] [Google Scholar]
- Chae K., Kieslich C. A., Morikis D., Kim S. C., Lord E. M. (2009). A gain-of-function mutation of Arabidopsis lipid transfer protein 5 disturbs pollen tube tip growth and fertilization. Plant Cell 21, 3902–3914. 10.1105/tpc.109.070854, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S. H., Willmann M. R., Chen H. C., Sheen J. (2002). Calcium signaling through protein kinases. The Arabidopsis calcium-dependent protein kinase gene family. Plant Physiol. 129, 469–485. 10.1104/pp.005645, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A. Y., Wang H., Wu H. -m. (1995). A floral transmitting tissue-specific glycoprotein attracts pollen tubes and stimulates their growth. Cell 82, 383–393. 10.1016/0092-8674(95)90427-1, PMID: [DOI] [PubMed] [Google Scholar]
- Curran A., Chang I. F., Chang C. L., Garg S., Miguel R. M., Barron Y. D., et al. (2011). Calcium-dependent protein kinases from Arabidopsis show substrate specificity differences in an analysis of 103 substrates. Front. Plant Sci. 2:36. 10.3389/fpls.2011.00036, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damineli D. S. C., Portes M. T., Feijo J. A. (2017). Oscillatory signatures underlie growth regimes in Arabidopsis pollen tubes: computational methods to estimate tip location, periodicity, and synchronization in growing cells. J. Exp. Bot. 68, 3267–3281. 10.1093/jxb/erx032, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearnaley J. D. W., Clark K. M., Heath I. B., Lew R. R., Goring D. R. (1999). Neither compatible nor self-incompatible pollinations of Brassica napus involve reorganization of the papillar cytoskeleton. New Phytol. 141, 199–207. 10.1046/j.1469-8137.1999.00334.x [DOI] [PubMed] [Google Scholar]
- Di Giorgio J. A., Bienert G. P., Ayub N. D., Yaneff A., Barberini M. L., Mecchia M. A., et al. (2016). Pollen-specific aquaporins NIP4;1 and NIP4;2 are required for pollen development and pollination in Arabidopsis thaliana. Plant Cell 28, 1053–1077. 10.1105/tpc.15.00776, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd A. N., Kudla J., Sanders D. (2010). The language of calcium signaling. Annu. Rev. Plant Biol. 61, 593–620. 10.1146/annurev-arplant-070109-104628, PMID: [DOI] [PubMed] [Google Scholar]
- Domingos P., Dias P. N., Tavares B., Portes M. T., Wudick M. M., Konrad K. R., et al. (2019). Molecular and electrophysiological characterization of anion transport in Arabidopsis thaliana pollen reveals regulatory roles for pH, Ca2+ and GABA. New Phytol. 223, 1353–1371. 10.1111/nph.15863, PMID: [DOI] [PubMed] [Google Scholar]
- Duszyn M., Swiezawska B., Szmidt-Jaworska A., Jaworski K. (2019). Cyclic nucleotide gated channels (CNGCs) in plant signalling-current knowledge and perspectives. J. Plant Physiol. 241:153035. 10.1016/j.jplph.2019.153035, PMID: [DOI] [PubMed] [Google Scholar]
- Dutta R., Robinson K. R. (2004). Identification and characterization of stretch-activated ion channels in pollen protoplasts. Plant Physiol. 135, 1398–1406. 10.1104/pp.104.041483, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estruch J. J., Kadwell S., Merlin E., Crossland L. (1994). Cloning and characterization of a maize pollen-specific calcium dependent calmodulin independent protein kinase. Proc. Natl. Acad. Sci. U. S. A. 91, 8837–8841. 10.1073/pnas.91.19.8837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Herrera C., Preciado-Linares G., Gonzalez-Vizueth I., Corona de la Pena N., Gutierrez-Aguilar M. (2019). In situ assessment of mitochondrial calcium transport in tobacco pollen tubes. Protoplasma 256, 503–509. 10.1007/s00709-018-1316-z, PMID: [DOI] [PubMed] [Google Scholar]
- Franklin-Tong V. E. (1999). Signaling and the modulation of pollen tube growth. Plant Cell 11, 727–738. 10.1105/tpc.11.4.727, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frietsch S., Wang Y. F., Sladek C., Poulsen L. R., Romanowsky S. M., Schroeder J. I., et al. (2007). A cyclic nucleotide-gated channel is essential for polarized tip growth of pollen. Proc. Natl. Acad. Sci. U. S. A. 104, 14531–14536. 10.1073/pnas.0701781104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Chen X., Lin W., Chen S., Lu D., Niu Y., et al. (2013). Bifurcation of Arabidopsis NLR immune signaling via Ca2+-dependent protein kinases. PLoS Pathog. 9:e1003127. 10.1371/journal.ppat.1003127, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q. F., Gu L. L., Wang H. Q., Fei C. F., Fang X., Hussain J., et al. (2016). Cyclic nucleotide-gated channel 18 is an essential Ca2+ channel in pollen tube tips for pollen tube guidance to ovules in Arabidopsis. Proc. Natl. Acad. Sci. U. S. A. 113, 3096–3101. 10.1073/pnas.1524629113, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge L. L., Tian H. Q., Russell S. D. (2007). Calcium function and distribution during fertilization in angiosperms. Am. J. Bot. 94, 1046–1060. 10.3732/ajb.94.6.1046, PMID: [DOI] [PubMed] [Google Scholar]
- Giacometti S., Marrano C. A., Bonza M. C., Luoni L., Limonta M., De Michelis M. I. (2012). Phosphorylation of serine residues in the N-terminus modulates the activity of ACA8, a plasma membrane Ca2+-ATPase of Arabidopsis thaliana. J. Exp. Bot. 63, 1215–1224. 10.1093/jxb/err346, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J., Yang Z. (2020). Exocytosis and endocytosis: coordinating and fine-tuning the polar tip growth domain in pollen tubes. J. Exp. Bot. 71, 2428–2438. 10.1093/jxb/eraa134, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F., Yoon G. M., McCubbin A. G. (2013). PiSCP1 and PiCDPK2 localize to peroxisomes and are involved in pollen tube growth in Petunia inflata. Plan. Theory 2, 72–86. 10.3390/plants2010072, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutermuth T., Herbell S., Lassig R., Brosche M., Romeis T., Feijo J. A., et al. (2018). Tip-localized Ca2+-permeable channels control pollen tube growth via kinase-dependent R- and S-type anion channel regulation. New Phytol. 218, 1089–1105. 10.1111/nph.15067, PMID: [DOI] [PubMed] [Google Scholar]
- Gutermuth T., Lassig R., Portes M. T., Maierhofer T., Romeis T., Borst J. W., et al. (2013). Pollen tube growth regulation by free anions depends on the interaction between the anion channel SLAH3 and calcium-dependent protein kinases CPK2 and CPK20. Plant Cell 25, 4525–4543. 10.1105/tpc.113.118463, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon A. C., Yoo B. C., McCaffery C. (1994). Pseudosubstrate inhibition of CDPK, a protein kinase with a calmodulin-like domain. Biochemistry 33, 7278–7287. 10.1021/bi00189a032, PMID: [DOI] [PubMed] [Google Scholar]
- Harper J. F., Breton G., Harmon A. (2004). Decoding Ca2+ signals through plant protein kinases. Annu. Rev. Plant Biol. 55, 263–288. 10.1146/annurev.arplant.55.031903.141627, PMID: [DOI] [PubMed] [Google Scholar]
- Harper J. F., Hong B., Hwang I., Guo H. Q., Stoddard R., Huang J. F., et al. (1998). A novel calmodulin-regulated Ca2+-ATPase (ACA2) from Arabidopsis with an N-terminal autoinhibitory domain. J. Biol. Chem. 273, 1099–1106. 10.1074/jbc.273.2.1099, PMID: [DOI] [PubMed] [Google Scholar]
- Harper J. F., Sussman M. R., Schaller G. E., Putnam-Evans C., Charbonneau H., Harmon A. C. (1991). A calcium-dependent protein kinase with a regulatory domain similar to calmodulin. Science 252, 951–954. 10.1126/science.1852075, PMID: [DOI] [PubMed] [Google Scholar]
- Hasegawa Y., Nakamura S., Kakizoe S., Sato M., Nakamura N. (1998). Immunocytochemical and chemical analyses of Golgi vesicles isolated from the germinated pollen of Camellia japonica. J. Plant Res. 111, 421–429. 10.1007/BF02507807 [DOI] [Google Scholar]
- Hepler P. K., Kunkel J. G., Rounds C. M., Winship L. J. (2012). Calcium entry into pollen tubes. Trends Plant Sci. 17, 32–38. 10.1016/j.tplants.2011.10.007, PMID: [DOI] [PubMed] [Google Scholar]
- Higashiyama T., Yang W. C. (2017). Gametophytic pollen tube guidance: attractant peptides, gametic controls, and receptors. Plant Physiol. 173, 112–121. 10.1104/pp.16.01571, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdaway-Clarke T. L., Feijo J. A., Hackett G. R., Kunkel J. G., Hepler P. K. (1997). Pollen tube growth and the intracellular cytosolic calcium gradient oscillate in phase while extracellular calcium influx is delayed. Plant Cell 9, 1999–2010. 10.2307/3870560, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdaway-Clarke T. L., Weddle N. M., Kim S., Robi A., Parris C., Kunkel J. G., et al. (2003). Effect of extracellular calcium, pH and borate on growth oscillations in Lilium formosanum pollen tubes. J. Exp. Bot. 54, 65–72. 10.1093/jxb/erg004, PMID: [DOI] [PubMed] [Google Scholar]
- Hrabak E. M., Chan C. W., Gribskov M., Harper J. F., Choi J. H., Halford N., et al. (2003). The Arabidopsis CDPK-SnRK superfamily of protein kinases. Plant Physiol. 132, 666–680. 10.1104/pp.102.011999, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I., Sze H., Harper J. F. (2000). A calcium-dependent protein kinase can inhibit a calmodulin-stimulated Ca2+ pump (ACA2) located in the endoplasmic reticulum of Arabidopsis. Proc. Natl. Acad. Sci. U. S. A. 97, 6224–6229. 10.1073/pnas.97.11.6224, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwano M., Entani T., Shiba H., Kakita M., Nagai T., Mizuno H., et al. (2009). Fine-tuning of the cytoplasmic Ca2+ concentration is essential for pollen tube growth. Plant Physiol. 150, 1322–1334. 10.1104/pp.109.139329, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya H., Nakajima R., Iwano M., Kanaoka M. M., Kimura S., Takeda S., et al. (2014). Ca2+-activated reactive oxygen species production by Arabidopsis RbohH and RbohJ is essential for proper pollen tube tip growth. Plant Cell 26, 1069–1080. 10.1105/tpc.113.120642, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalid M. H. B., Raza M. A., Yu H. Q., Khan I., Sun F. A., Feng L. Y., et al. (2019). Expression, subcellular localization, and interactions of CPK family genes in maize. Int. J. Mol. Sci. 20:6173. 10.3390/ijms20246173, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S., Kaya H., Kawarazaki T., Hiraoka G., Senzaki E., Michikawa M., et al. (2012). Protein phosphorylation is a prerequisite for the Ca2+-dependent activation of Arabidopsis NADPH oxidases and may function as a trigger for the positive feedback regulation of Ca2+ and reactive oxygen species. Biochim. Biophys. Acta 1823, 398–405. 10.1016/j.bbamcr.2011.09.011 [DOI] [PubMed] [Google Scholar]
- Klimecka M., Muszynska G. (2007). Structure and functions of plant calcium-dependent protein kinases. Acta Biochim. Pol. 54, 219–233. 10.18388/abp.2007_3242, PMID: [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Ohura I., Kawakita K., Yokota N., Fujiwara M., Shimamoto K., et al. (2007). Calcium-dependent protein kinases regulate the production of reactive oxygen species by potato NADPH oxidase. Plant Cell 19, 1065–1080. 10.1105/tpc.106.048884, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuboyama T., Takeda G. (2000). Genomic factors responsible for abnormal morphology of pollen tubes in the interspecific cross Nicotiana tabacum × N. rustica. Sex. Plant Reprod. 12, 333–337. 10.1007/s004970000027 [DOI] [Google Scholar]
- Lamport D. T. A., Tan L., Held M., Kieliszewski M. J. (2017). Pollen tube growth and guidance: Occam’s razor sharpened on a molecular arabinogalactan glycoprotein rosetta stone. New Phytol. 217, 491–500. 10.1111/nph.14845 [DOI] [PubMed] [Google Scholar]
- Li Y. Q., Chen F., Linskens H. F., Cresti M. (1994). Distribution of unesterified and esterified pectins in cell walls of pollen tubes of flowering plants. Sex. Plant Reprod. 7, 145–152. 10.1007/BF00228487 [DOI] [Google Scholar]
- Li Y., Guo J., Yang Z., Yang D. -L. (2018a). Plasma membrane-localized calcium pumps and copines coordinately regulate pollen germination and fertility in Arabidopsis. Int. J. Mol. Sci. 19:1774. 10.3390/ijms19061774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D. X., Hu H. Y., Li G., Ru Z. G., Tian H. Q. (2017). Calcium controls the formation of vacuoles from mitochondria to regulate microspore development in wheat. Plant Reprod. 30, 131–139. 10.1007/s00497-017-0309-y, PMID: [DOI] [PubMed] [Google Scholar]
- Li J., Li Y., Deng Y., Chen P., Feng F., Chen W., et al. (2018b). A calcium-dependent protein kinase, ZmCPK32, specifically expressed in maize pollen to regulate pollen tube growth. PLoS One 13:e0195787. 10.1371/journal.pone.0195787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liese A., Romeis T. (2013). Biochemical regulation of in vivo function of plant calcium-dependent protein kinases (CDPK). Biochim. Biophys. Acta 1833, 1582–1589. 10.1016/j.bbamcr.2012.10.024 [DOI] [PubMed] [Google Scholar]
- Liu Y., He C. (2016). Regulation of plant reactive oxygen species (ROS) in stress responses: learning from AtRBOHD. Plant Cell Rep. 35, 995–1007. 10.1007/s00299-016-1950-x, PMID: [DOI] [PubMed] [Google Scholar]
- Liu J., Hussey P. J. (2014). Dissecting the regulation of pollen tube growth by modeling the interplay of hydrodynamics, cell wall and ion dynamics. Front. Plant Sci. 5:392. 10.3389/fpls.2014.00392, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S. X., Hrabak E. M. (2002). An Arabidopsis calcium-dependent protein kinase is associated with the endoplasmic reticulum. Plant Physiol. 128, 1008–1021. 10.1104/pp.010770, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucca N., León G. (2011). Arabidopsis ACA7, encoding a putative auto-regulated Ca2+-ATPase, is required for normal pollen development. Plant Cell Rep. 31, 651–659. 10.1007/s00299-011-1182-z [DOI] [PubMed] [Google Scholar]
- Lush W. M., Clarke A. E. (1997). Observations of pollen tube growth in Nicotiana alata and their implications for the mechanism of self-incompatibility. Sex. Plant Reprod. 10, 27–35. 10.1007/s004970050064 [DOI] [Google Scholar]
- Makavitskaya M., Svistunenko D., Navaselsky I., Hryvusevich P., Mackievic V., Rabadanova C., et al. (2018). Novel roles of ascorbate in plants: induction of cytosolic Ca2+ signals and efflux from cells via anion channels. J. Exp. Bot. 69, 3477–3489. 10.1093/jxb/ery056, PMID: [DOI] [PubMed] [Google Scholar]
- Malho R., Trewavas A. J. (1996). Localized apical increases of cytosolic free calcium control pollen tube orientation. Plant Cell 8, 1935–1949. 10.2307/3870403, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangano S., Martinez Pacheco J., Marino-Buslje C., Estevez J. M. (2018). How does pH fit in with oscillating polar growth? Trends Plant Sci. 23, 479–489. 10.1016/j.tplants.2018.02.008, PMID: [DOI] [PubMed] [Google Scholar]
- Marondedze C., Wong A., Thomas L., Irving H., Gehring C. (2017). Cyclic nucleotide monophosphates in plants and plant signaling. Handb. Exp. Pharmacol. 238, 87–103. 10.1007/164_2015_35, PMID: [DOI] [PubMed] [Google Scholar]
- Meng J. G., Liang L., Jia P. F., Wang Y. C., Li H. J., Yang W. C. (2020). Integration of ovular signals and exocytosis of a Ca2+ channel by MLOs in pollen tube guidance. Nat. Plants 6, 143–153. 10.1038/s41477-020-0599-1, PMID: [DOI] [PubMed] [Google Scholar]
- Michard E., Alves F., Feijo J. A. (2009). The role of ion fluxes in polarized cell growth and morphogenesis: the pollen tube as an experimental paradigm. Int. J. Dev. Biol. 53, 1609–1622. 10.1387/ijdb.072296em, PMID: [DOI] [PubMed] [Google Scholar]
- Michard E., Lima P. T., Borges F., Silva A. C., Portes M. T., Carvalho J. E., et al. (2011). Glutamate receptor-like genes form Ca2+ channels in pollen tubes and are regulated by pistil D-serine. Science 332, 434–437. 10.1126/science.1201101, PMID: [DOI] [PubMed] [Google Scholar]
- Michard E., Simon A. A., Tavares B., Wudick M. M., Feijo J. A. (2017). Signaling with ions: the keystone for apical cell growth and morphogenesis in pollen tubes. Plant Physiol. 173, 91–111. 10.1104/pp.16.01561, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser M., Kirkpatrick A., Groves N. R., Meier I. (2020). LINC-complex mediated positioning of the vegetative nucleus is involved in calcium and ROS signaling in Arabidopsis pollen tubes. Nucleus 11, 149–163. 10.1080/19491034.2020.1783783, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou W., Kao Y. T., Michard E., Simon A. A., Li D., Wudick M. M., et al. (2020). Ethylene-independent signaling by the ethylene precursor ACC in Arabidopsis ovular pollen tube attraction. Nat. Commun. 11:4082. 10.1038/s41467-020-17819-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouline K., Very A. A., Gaymard F., Boucherez J., Pilot G., Devic M., et al. (2002). Pollen tube development and competitive ability are impaired by disruption of a shaker K+ channel in Arabidopsis. Genes Dev. 16, 339–350. 10.1101/gad.213902, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutinho A., Hussey P. J., Trewavas A. J., Malho R. (2001). cAMP acts as a second messenger in pollen tube growth and reorientation. Proc. Natl. Acad. Sci. U. S. A. 98, 10481–10486. 10.1073/pnas.171104598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutinho A., Trewavas A. J., Malho R. (1998). Relocation of a Ca2+-dependent protein kinase activity during pollen tube reorientation. Plant Cell 10, 1499–1510. 10.1105/tpc.10.9.1499, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mravec J., Kracun S. K., Rydahl M. G., Westereng B., Pontiggia D., De Lorenzo G., et al. (2017). An oligogalacturonide-derived molecular probe demonstrates the dynamics of calcium-mediated pectin complexation in cell walls of tip-growing structures. Plant J. 91, 534–546. 10.1111/tpj.13574, PMID: [DOI] [PubMed] [Google Scholar]
- Myers C., Romanowsky S. M., Barron Y. D., Garg S., Azuse C. L., Curran A., et al. (2009). Calcium-dependent protein kinases regulate polarized tip growth in pollen tubes. Plant J. 59, 528–539. 10.1111/j.1365-313X.2009.03894.x, PMID: [DOI] [PubMed] [Google Scholar]
- Nakagawa Y., Katagiri T., Shinozaki K., Qi Z., Tatsumi H., Furuichi T., et al. (2007). Arabidopsis plasma membrane protein crucial for Ca2+ influx and touch sensing in roots. Proc. Natl. Acad. Sci. U. S. A. 104, 3639–3644. 10.1073/pnas.0607703104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Chai X., Gao Q., Zhou L., Zhang S., Li L., et al. (2019). Dynamic interactions of plant cngc subunits and calmodulins drive oscillatory Ca2+ channel activities. Dev. Cell 48, 710.e715–725.e715. 10.1016/j.devcel.2018.12.025 [DOI] [PubMed] [Google Scholar]
- Potocky M., Pejchar P., Gutkowska M., Jimenez-Quesada M. J., Potocka A., Alche Jde D., et al. (2012). NADPH oxidase activity in pollen tubes is affected by calcium ions, signaling phospholipids and Rac/Rop GTPases. J. Plant Physiol. 169, 1654–1663. 10.1016/j.jplph.2012.05.014, PMID: [DOI] [PubMed] [Google Scholar]
- Qi Z., Stephens N. R., Spalding E. P. (2006). Calcium entry mediated by GLR3.3, an Arabidopsis glutamate receptor with a broad agonist profile. Plant Physiol. 142, 963–971. 10.1104/pp.106.088989, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röckel N., Wolf S., Kost B., Rausch T., Greiner S. (2008). Elaborate spatial patterning of cell-wall PME and PMEI at the pollen tube tip involves PMEI endocytosis, and reflects the distribution of esterified and de-esterified pectins. Plant J. 53, 133–143. 10.1111/j.1365-313X.2007.03325.x, PMID: [DOI] [PubMed] [Google Scholar]
- Roy S. J., Gilliham M., Berger B., Essah P. A., Cheffings C., Miller A. J., et al. (2008). Investigating glutamate receptor-like gene co-expression in Arabidopsis thaliana. Plant Cell Environ. 31, 861–871. 10.1111/j.1365-3040.2008.01801.x, PMID: [DOI] [PubMed] [Google Scholar]
- Selles B., Michaud C., Xiong T. C., Leblanc O., Ingouff M. (2018). Arabidopsis pollen tube germination and growth depend on the mitochondrial calcium uniporter complex. New Phytol. 219, 58–65. 10.1111/nph.15189, PMID: [DOI] [PubMed] [Google Scholar]
- Simeunovic A., Mair A., Wurzinger B., Teige M. (2016). Know where your clients are: subcellular localization and targets of calcium-dependent protein kinases. J. Exp. Bot. 67, 3855–3872. 10.1093/jxb/erw157, PMID: [DOI] [PubMed] [Google Scholar]
- Stael S., Bayer R. G., Mehlmer N., Teige M. (2011). Protein N-acylation overrides differing targeting signals. FEBS Lett. 585, 517–522. 10.1016/j.febslet.2011.01.001, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhorst L., Kudla J. (2013). Calcium—a central regulator of pollen germination and tube growth. Biochim. Biophys. Acta 1833, 1573–1581. 10.1016/j.bbamcr.2012.10.009 [DOI] [PubMed] [Google Scholar]
- Steinhorst L., Mahs A., Ischebeck T., Zhang C., Zhang X., Arendt S., et al. (2015). Vacuolar CBL-CIPK12 Ca2+-sensor-kinase complexes are required for polarized pollen tube growth. Curr. Biol. 25, 1475–1482. 10.1016/j.cub.2015.03.053, PMID: [DOI] [PubMed] [Google Scholar]
- Stelpflug S. C., Sekhon R. S., Vaillancourt B., Hirsch C. N., Buell C. R., de Leon N., et al. (2016). An expanded maize gene expression atlas based on RNA sequencing and its use to explore root development. Plant Genome 9, 1–16. 10.3835/plantgenome2015.04.0025, PMID: [DOI] [PubMed] [Google Scholar]
- Suwinska A., Wasag P., Zakrzewski P., Lenartowska M., Lenartowski R. (2017). Calreticulin is required for calcium homeostasis and proper pollen tube tip growth in Petunia. Planta 245, 909–926. 10.1007/s00425-017-2649-0, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swatek K. N., Wilson R. S., Ahsan N., Tritz R. L., Thelen J. J. (2014). Multisite phosphorylation of 14-3-3 proteins by calcium-dependent protein kinases. Biochem. J. 459, 15–25. 10.1042/BJ20130035, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W., Page M. (2013). Transcription factor AtbZIP60 regulates expression of Ca2+-dependent protein kinase genes in transgenic cells. Mol. Biol. Rep. 40, 2723–2732. 10.1007/s11033-012-2362-9, PMID: [DOI] [PubMed] [Google Scholar]
- Taylor L. P., Hepler P. K. (1997). Pollen germination and tube growth. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 461–491. 10.1146/annurev.arplant.48.1.461, PMID: [DOI] [PubMed] [Google Scholar]
- Tunc-Ozdemir M., Tang C., Ishka M. R., Brown E., Groves N. R., Myers C. T., et al. (2013). A cyclic nucleotide-gated channel (CNGC16) in pollen is critical for stress tolerance in pollen reproductive development. Plant Physiol. 161, 1010–1020. 10.1104/pp.112.206888, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincill E. D., Bieck A. M., Spalding E. P. (2012). Ca2+ conduction by an amino acid-gated ion channel related to glutamate receptors. Plant Physiol. 159, 40–46. 10.1104/pp.112.197509, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. W., Chen W. C., Lin L. J., Lee C. T., Tseng T. H., Leu W. M. (2011). OIP30, a RuvB-like DNA helicase 2, is a potential substrate for the pollen-predominant OsCPK25/26 in rice. Plant Cell Physiol. 52, 1641–1656. 10.1093/pcp/pcr094, PMID: [DOI] [PubMed] [Google Scholar]
- Wang Y., Chen T., Zhang C., Hao H., Liu P., Zheng M., et al. (2009). Nitric oxide modulates the influx of extracellular Ca2+ and actin filament organization during cell wall construction in Pinus bungeana pollen tubes. New Phytol. 182, 851–862. 10.1111/j.1469-8137.2009.02820.x, PMID: [DOI] [PubMed] [Google Scholar]
- Wen K., Chen Y., Zhou X., Chang S., Feng H., Zhang J., et al. (2019). OsCPK21 is required for pollen late-stage development in rice. J. Plant Physiol. 240:153000. 10.1016/j.jplph.2019.153000, PMID: [DOI] [PubMed] [Google Scholar]
- Wu J., Qin X., Tao S., Jiang X., Liang Y. K., Zhang S. (2014). Long-chain base phosphates modulate pollen tube growth via channel-mediated influx of calcium. Plant J. 79, 507–516. 10.1111/tpj.12576, PMID: [DOI] [PubMed] [Google Scholar]
- Wudick M. M., Feijo J. A. (2014). At the intersection: merging Ca2+ and ROS signaling pathways in pollen. Mol. Plant 7, 1595–1597. 10.1093/mp/ssu096, PMID: [DOI] [PubMed] [Google Scholar]
- Wudick M. M., Portes M. T., Michard E., Rosas-Santiago P., Lizzio M. A., Nunes C. O., et al. (2018). CORNICHON sorting and regulation of GLR channels underlie pollen tube Ca2+ homeostasis. Science 360, 533–536. 10.1126/science.aar6464, PMID: [DOI] [PubMed] [Google Scholar]
- Xu Y., Yang J., Wang Y., Wang J., Yu Y., Long Y., et al. (2017). OsCNGC13 promotes seed-setting rate by facilitating pollen tube growth in stylar tissues. PLoS Genet. 13:e1006906. 10.1371/journal.pgen.1006906, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Fu Y. (2007). ROP/RAC GTPase signaling. Curr. Opin. Plant Biol. 10, 490–494. 10.1016/j.pbi.2007.07.005, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Zhao Y., Chen N., Liu Y., Yang S., Du H., et al. (2020). A new adenylyl cyclase, putative disease resistance RPP13-like protein 3, participates in abscisic acid-mediated heat stress resistance in maize. J. Exp. Bot. 72, 283–301. 10.1093/jxb/eraa431, PMID: [DOI] [PubMed] [Google Scholar]
- Yip Delormel T., Boudsocq M. (2019). Properties and functions of calcium-dependent protein kinases and their relatives in Arabidopsis thaliana. New Phytol. 224, 585–604. 10.1111/nph.16088, PMID: [DOI] [PubMed] [Google Scholar]
- Yoon G. M., Dowd P. E., Gilroy S., McCubbin A. G. (2006). Calcium-dependent protein kinase isoforms in Petunia have distinct functions in pollen tube growth, including regulating polarity. Plant Cell 18, 867–878. 10.1105/tpc.105.037135, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G. H., Zou J., Feng J., Peng X. B., Wu J. Y., Wu Y. L., et al. (2014). Exogenous gamma-aminobutyric acid (GABA) affects pollen tube growth via modulating putative Ca2+-permeable membrane channels and is coupled to negative regulation on glutamate decarboxylase. J. Exp. Bot. 65, 3235–3248. 10.1093/jxb/eru171, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L. N., Shen L. K., Zhang W. Z., Zhang W., Wang Y., Wu W. H. (2013). Ca2+-dependent protein kinase11 and 24 modulate the activity of the inward rectifying K+ channels in Arabidopsis pollen tubes. Plant Cell 25, 649–661. 10.1105/tpc.112.103184, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng R. H., Su S., Xiao H., Tian H. Q. (2019). Calcium: a critical factor in pollen germination and tube elongation. Int. J. Mol. Sci. 20:420. 10.3390/ijms20020420, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Fu Y., Yang Z. (2009). A genome-wide functional characterization of Arabidopsis regulatory calcium sensors in pollen tubes. J. Integr. Plant Biol. 51, 751–761. 10.1111/j.1744-7909.2009.00847.x, PMID: [DOI] [PubMed] [Google Scholar]
- Zhou L., Lan W., Jiang Y., Fang W., Luan S. (2014). A calcium-dependent protein kinase interacts with and activates a calcium channel to regulate pollen tube growth. Mol. Plant 7, 369–376. 10.1093/mp/sst125, PMID: [DOI] [PubMed] [Google Scholar]
- Zonia L., Munnik T. (2011). Understanding pollen tube growth: the hydrodynamic model versus the cell wall model. Trends Plant Sci. 16, 347–352. 10.1016/j.tplants.2011.03.009, PMID: [DOI] [PubMed] [Google Scholar]