Abstract
The aerosol oxidative potential (OP) is considered to better represent the acute health hazards of aerosols than the mass concentration of fine particulate matter (PM2.5). The proposed major contributors to OP are water soluble transition metals and organic compounds, but the relative magnitudes of these compounds to the total OP are not yet fully understood. In this study, as the first step toward the numerical prediction of OP, the cumulative OP (OPtm*) based on the top five key transition metals, namely, Cu, Mn, Fe, V, and Ni, was defined. The solubilities of metals were assumed constant over time and space based on measurements. Then, the feasibility of its prediction was verified by comparing OPtm* values based on simulated metals to that based on observed metals in East Asia. PM2.5 typically consists of primary and secondary species, while OPtm* only represents primary species. This disparity caused differences in the domestic contributions of PM2.5 and OPtm*, especially in large cities in western Japan. The annual mean domestic contributions of PM2.5 were 40%, while those of OPtm* ranged from 50 to 55%. Sector contributions to the OPtm* emissions in Japan were also assessed. The main important sectors were the road brake and iron–steel industry sectors, followed by power plants, road exhaust, and railways.
Subject terms: Environmental impact, Atmospheric chemistry
Introduction
The aerosol oxidative potential (OP), the potential to generate reactive oxygen species (ROS) in cells that induce airway oxidative stress and inflammation, is considered to better represent the health hazards of aerosols than the mass concentration of very fine particulate matter (PM2.5)1–3. Several methods have been proposed to quantify OP4, and among them, the dithiothreitol (DTT) assay5 has been widely applied. The DTT activity is quantified as the consumption rate of the reducing agent, i.e., DTT, in buffer with the extraction of aerosols5. The DTT activity exhibits a stronger association than does PM2.5 with the fractional exhaled nitric oxide, a biomarker of airway inflammation6. The DTT activity has also been found to be more strongly associated with emergency department (ED) visits related to asthma/wheezing and congestive heart failure than the PM2.5 mass concentration7. Population-level analysis of the health effect of the measured ambient DTT level has demonstrated that the 3-d moving average of the DTT activity is highly associated with ED visits for multiple cardiorespiratory outcomes, especially in regard to ischemic heart disease8.
Even though the DTT assay is an in vitro system, the relative contributions of chemical compounds to the total DTT activity are not yet fully understood. Charrier and Anastasio9 indicated that water–soluble transition metals, such as Cu(II), Mn(II), Fe(II), and Fe(III), account for 80% of the DTT activity measured in California, and organics, such as phenanthrenequinone, account for 20% of the measured DTT activity. However, Nishita-Hara et al.10 reported, based on samples measured in Japan, that water-soluble transition metals only explain 37% and 60% of the DTT activities of fine- and coarse-mode particles, respectively. Saffari et al.11 demonstrated that the DTT activity is strongly associated with water-soluble and water-insoluble organics and elemental carbon. In fact, dissolved oxygen causes interfacial catalytic oxidation of DTT in the presence of elemental carbon particles12. Verma et al.13 revealed the importance of humic-like substances (HULIS), such as quinones and secondary organic aerosols, and Yu et al.14 indicated that the interactions between HULIS and transition metals likely contribute to the DTT activity. In addition to catalytic redox reactions of transition metals and quinones, noncatalytic DTT-active organics such as organic hydroperoxides and electron-deficient alkenes have been highlighted15. Thus far, the relative importance of chemical compounds to the total DTT activity is not fully understood, but the importance of the coexistence of metals and organics is widely accepted1–3,9,14,16–18.
To date, several experimental studies have been performed to relate OP, chemical compounds, and health outcomes4–18. In terms of numerical simulations, a model has been proposed to determine the chemical reactions producing ROS in epithelial lining fluids19 and a statistical model, called the land use regression model20,21, to predict the spatial variations in OP. However, none of these studies has derived the spatiotemporal variations in OP via the direct simulation using 3-dimensional numerical modeling. Very recently, Daellenbach et al.22 derived the spatiotemporal variations in OP in Europe by combining the 3-dimensional simulations of organic aerosols and NOx and the statistical relationship between the measured OP and aerosol components in Switzerland and Liechtenstein. Daellenbach et al.22 did not directly predict the redox active aerosol components such as transition metals and quinones but demonstrate that the simulated OP agreed very well with that observed in the measurement sites.
Within the context mentioned above, we developed a 3-dimensional model and emission inventories of the DTT-active transition metals in Asia (TMI-Asia) and Japan (TMI-Japan) and evaluated simulation results based on field measurements in Japan23. Before directly predicting the total OP, as a first step, we defined the cumulative OP based only on transition metals (OPtm*) and assessed its predictability in this study. OPtm* was defined as the summation of the (simulated or observed) DTT-active transition metal concentrations multiplied by the DTT consumption rate per unit mass (obtained by laboratory experiments). This study is the first trial to directly predict the oxidative potential, but it should be noted that the contributions of other components, such as the effects of organics22 and the interactions between organics and metals, have not yet been considered. Many studies focused on OP only of PM2.51–9,11–21 and so another feature of the current study is that we include the OPtm* contributions of coarse-mode particles and Asian dust because lung deposition of fine particulate matter (PM10) may be nonnegligible in some human conditions24, and Asian dust particles containing metals may adversely affect health25. Nishita-Hara et al.10 and Daellenbach et al.22 also considered the coarse-mode particles.
The main objectives of this study are thus summarized as follows: (1) to assess the predictability of OPtm* by numerical simulations, (2) to show the differences of horizontal distributions and the source-receptor relationship between OPtm* and PM2.5, and (3) to identify the major emission sectors for anthropogenic OPtm*.
Results
Temporal variations in OPtm* based on simulations and observations and their comparison
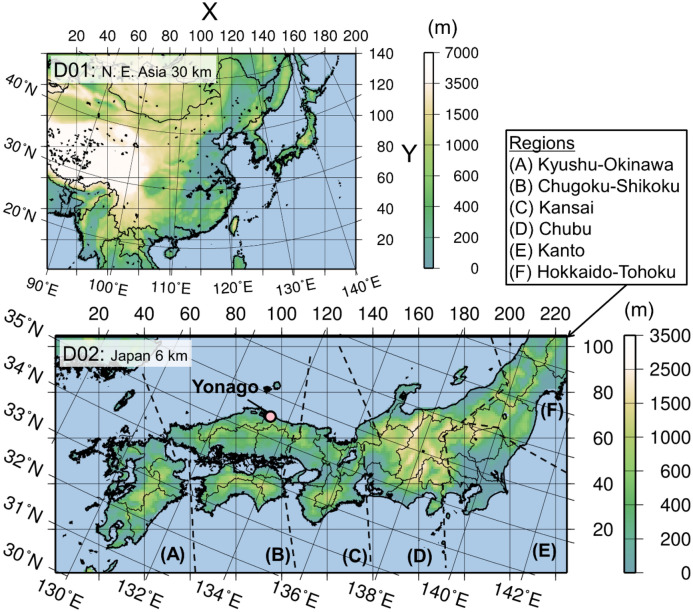
Figure 1 (identical to Fig. 1 of Kajino et al.23) shows the mother domain (D01) and nested domain (D02) for the simulation of the transition metals and cumulative OP (OPtm*) based on the top five key transition metals, namely, Cu, Mn, Fe, V, and Ni, as described in Eq. (2) and Table 2 in Method section. D01 covers East Asia with a horizontal grid resolution of 30 km to simulate the long-range transport from the Asian continent to Japan via synoptic-scale disturbances such as the fronts of cyclones and migrating anticyclones. D02 covers the densely populated and industrial areas of Japan with a complex topography, and a fine grid resolution (i.e., 6 km) is necessary to accurately predict the domestic and transboundary contributions to the surface concentrations of air pollutants.
Figure 1.
Model domains, topography, and regions considered for the analysis. The mother domain (D01) covers East Asia with Δx = 30 km, and the nested domain (D02) covers the central parts of Japan with Δx = 6 km. The observation site (Yonago) and regional names adopted in the analysis are shown. The national and prefecture borders are depicted in D01 and D02, respectively. This figure is identical to Fig. 1 of Kajino et al.23. The map was generated using the Generic Mapping Tools v4.5.7 (https://www.generic-mapping-tools.org).
Table 2.
Values assumed for the parameters of Eq. (2) to estimate OPtm*(CA) and OPtm*(F).
| Unit | ri for OPtm*(CA) | ri for OPtm*(F) | χi | fi |
|---|---|---|---|---|
| μM-DTT min−1 μM−1 | μM-DTT min−1 μM−1 | |||
| Cu(II) | 1.80 | 7.69 | 0.66 | 0.303 |
| Mn(II) | 0.720 | 0.398 | 0.54 | 0.833 |
| Fe(*)a | 0.0361 | 0.616 | 0.23 | 0.909 |
| V(V) | 0.101 | 0 | 0.90 | 0.385 |
| Ni(II) | 0.106 | 4.08 | 0.73 | 0.270 |
aAssuming the equal presence of Fe(II) and Fe(III).
OPtm* based on the simulated transition metals (referred to as the simulated OPtm* hereinafter for simplicity) was compared to OPtm* based on the observed transition metals (hereinafter referred to as the observed OPtm*) of the total suspended particles (TSP) collected at the Yonago site (Fig. 1). As described below, the ri values of Eq. (2) substantially varied among laboratories, as experimental methods such as buffer usage, pH, reaction time, and control volume differed2. To consider the above experimental variability, we derived two different OPtm* values based on ri retrieved from two different experiments, namely, Charrier and Anastasio9 and Fujitani et al.17, which are referred to as OPtm*(CA) and OPtm*(F), respectively.
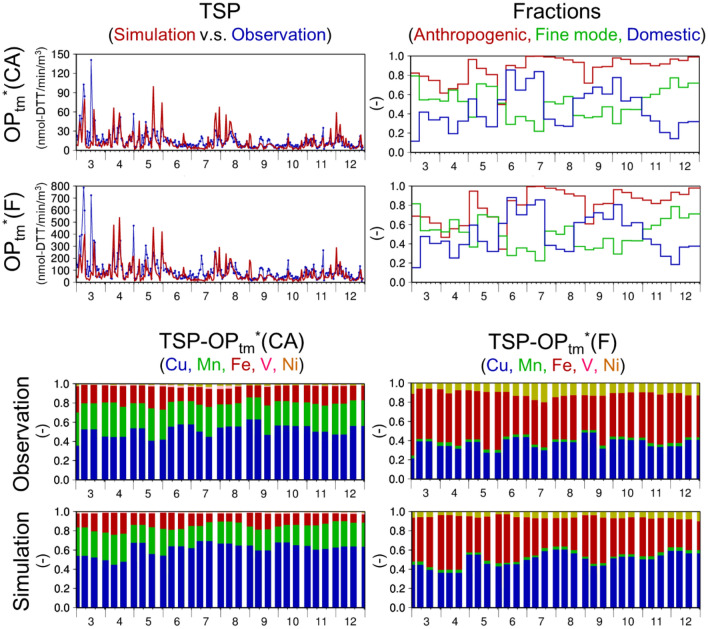
Figure 2 shows a time series of the simulated (PM10) and observed (TSP) OPtm*(CA) and OPtm*(F) at Yonago, as well as the fractions of anthropogenic, fine-mode, and domestic components and the contributions of various elements to the simulated OPtm*. Statistical metrics for the comparison are summarized in Table 1. Note that the OPtm* simulated with Eq. (2) includes correction factor fi based on measurements (the nationwide PM2.5 survey conducted by the Ministry of Environment, Japan (MOEJ); http://www.env.go.jp/air/osen/pm/monitoring.html; last accessed: 6 November 2020), which were independent from the Yonago data.
Figure 2.
Temporal variations in the daily OPtm*(CA) and OPtm*(F) and fractions of anthropogenic vs natural compounds, fine vs coarse particles, domestic vs transboundary contributions, and DTT-active elements. Temporal variations in (upper-left panels) the daily OPtm*(CA) and OPtm*(F) based on (red) the simulated (D01) and (blue) observed transition metals in TSP (nmol-DTT min−1 m−3), (upper-right panels) 10-d mean simulated (D02) fractions of (red) anthropogenic compounds to the total compounds, (green) anthropogenic fine-mode particles to anthropogenic total particles, and (blue) anthropogenic domestic contributions to the total OPtm*(CA) and OPtm*(F), (lower-left panels) relative contributions of each metal to the observed and simulated OPtm*(CA) and (lower-right panels) same as the lower-left panels but for OPtm*(F) at Yonago. Note that the simulated concentration of TSP is equivalent to the simulated PM10 concentration.
Table 1.
Statistical metrics to compare the simulated (D02) and observed daily OPtm* values at Yonago.
| Unit | Na | Observation Medianb | Sim:Obsc | Rd | Fa2e | Fa5f. |
|---|---|---|---|---|---|---|
| – | nmol-DTT min−1 m−3 | – | – | – | – | |
| OPtm*(CA) | 298 | 11.4 | 0.71 | 0.57 | 0.68 | 0.97 |
| OPtm*(F) | 298 | 93.3 | 0.84 | 0.63 | 0.65 | 0.95 |
aNumber of available data.
bMedian of the observation data.
cSimulation to observation median ratio.
dCorrelation coefficient.
eFraction of the simulated values within a factor of two of the observed values.
fFraction of the simulated values within a factor of five of the observed values.
As indicated in Table 1, OPtm* was suitably predicted by the numerical simulations with correction factors based on independent MOEJ nationwide observations, even though OPtm* was primarily contributed by Cu and the discrepancies between the simulated and observed Cu were large (Tables 4 and 5 of Kajino et al.23). Nevertheless, it is not surprising because the approach was analogous to the application of a multimodel ensemble, which generally reduces the uncertainty in each model. The summation of Eq. (2) reduced the uncertainty in the simulation of each metal element. In fact, the normalized root mean square errors (NRMSE; RMSE divided by observation average) for OPtm*(CA) and OPtm*(F) in TSP at Yonago (0.47 and 0.48) were smaller than those for Cu (4.7), Fe (1.0), Mn (0.87), Ni (1.4), and V (1.4). The median values of OPtm*(CA) were almost one order of magnitude smaller than those of OPtm*(F) due to the experimental variations and thus the discussion on the absolute values of OPtm* is not a scope of this study. Therefore, the relative magnitudes in time and space (i.e., the temporal and spatial variations, respectively) are mainly discussed. The simulated relative contributions of metals to OPtm* were consistent with those based on the observations, while those to OPtm*(CA) and OPtm* (F) differed. OPtm*(CA) primarily consisted of Cu, followed by Mn and Fe, which was similar to measurements obtained in California. However, due to the relatively high ri values for Ni and Fe(II) and relatively low ri value for Mn, the major contributors of OPtm*(F) were Fe and Cu, followed by Ni. Although the relative contributions of each metal were different between the two methods, the relative magnitudes of anthropogenic compounds vs. Asian dust, anthropogenic fine-mode vs. coarse-mode particles, and anthropogenic domestic vs. transboundary OPtm* values were similar. Their variations were consistent with those in the metals, as shown in Fig. 6 of Kajino et al.23. Specifically, the contribution of Asian dust was large in spring, and the transboundary contribution was large in colder seasons except from late July to early August, while the fine-mode fractions were inversely correlated with the domestic contributions, which are explained in the next subsection.
Spatial distribution and seasonal variation in OPtm*
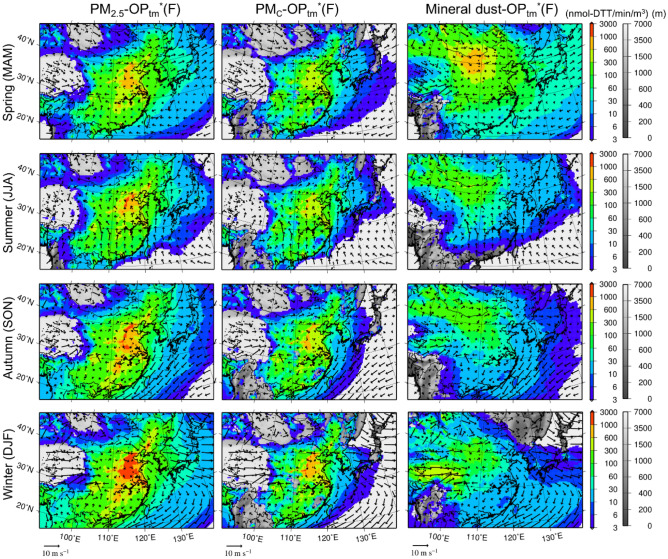
Figure 3 shows the seasonal mean surface air concentrations of the anthropogenic PM2.5-OPtm*(F), anthropogenic coarse-mode PMc-OPtm*(F) (simulated PM10 minus PM2.5), and OPtm*(F) of Asian dust. The simulated OPtm*(CA) is not shown because the horizontal distributions were very similar.
Figure 3.
Seasonal mean surface OPtm*(F). Seasonal mean surface air concentrations of (left to right) anthropogenic PM2.5-OPtm*(F), anthropogenic PMc-OPtm*(F), and OPtm*(F) of Asian dust in (top to bottom) the spring, summer, autumn, and winter of 2013 with the surface wind vectors over D01. The model terrestrial elevations are depicted in grayscale under the various colors. The map was generated using the Generic Mapping Tools v4.5.7 (https://www.generic-mapping-tools.org).
Generally, PM2.5-OPtm*(F) is higher than PMc-OPtm*(F). These anthropogenic surface concentrations were the highest in the winter under stable meteorological conditions. However, due to the presence of surface snow, the emissions of Asian dust were suppressed in the winter. The surface concentrations of Asian dust were the highest in the spring over the Gobi Desert and were almost equivalent to those of PM2.5-OPtm*(F). As shown in Fig. 2, the long-range transport of PM2.5 was more prominent than that of PMc.
The long-range transport of air pollutants from the Asian continent to Japan is influential during the cold seasons such as the spring and winter. A westerly wind prevails in the winter, while northerly and westerly winds prevail in the spring, which results in high concentrations in the respective downwind areas. The long-range transport of Asian dust was influential in the spring. Due to the presence of a Pacific high, the long-range transport was generally insignificant in the summer. The summer of 2013 was an exception. An anticyclone persisted over the southwestern part of the Japanese archipelago from late July to mid-August, which continuously carried pollutants from the Asian continent to Japan via the marginal flow along the northern edge of the anticyclone. The seasonal mean wind pattern exhibited features of the Pacific high, but the high-surface concentration areas extended east off the coast of the continent. In fact, the domestic contribution was small during this period, as shown in Fig. 2.
Domestic contributions of OPtm* and differences from those of PM2.5
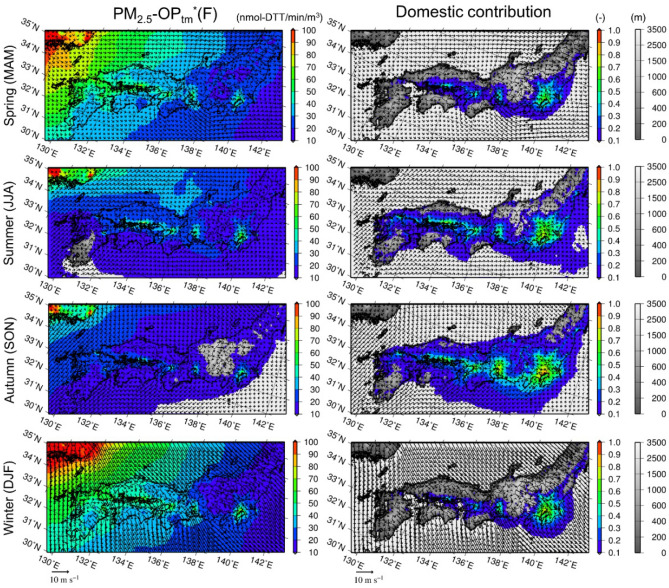
The spatial distribution of the simulated anthropogenic OPtm*(F) in PM2.5 over D02, together with its domestic contributions, is shown in Fig. 4. A contrast between the domestic and transboundary components and their seasonal differences are clearly observed in the figure. Transboundary air pollution dominated in the spring and winter, and there was a clear horizontal gradient in the surface PM2.5-OPtm*(F) concentrations from west to east during that season. However, high surface PM2.5-OPtm*(F) concentrations were found in Kanto, including the Tokyo Metropolitan Area, and the domestic contribution exceeded 50% throughout the year. In addition to the Kanto region, high-concentration areas were observed around large cities, such as Nagoya (in Chubu) and Osaka (in Kansai), where the domestic contributions were as large as those in Kanto (even though the areas were smaller). The domestic contributions were large over the inland seas and their surroundings in the western part of Japan, such as the Seto Inland Sea between Chugoku, Shikoku, and Kyushu and the Bungo Channel between Kyushu and Shikoku. Under the strong influence of the transboundary transport in the spring and winter, the concentrations over the areas were higher than those in the other areas at the same longitudes. The Seto Inland Sea is a major route of vessels in Japan, and thus, large industrial regions are located along the coast, and as a result, the transition metal emissions from ships and industries are high in this region, as shown in Fig. 6.
Figure 4.
Seasonal mean surface anthropogenic PM2.5-OPtm*(F) and its domestic contributions. Seasonal mean (left) surface air concentrations of anthropogenic PM2.5-OPtm*(F) (nmol-DTT min−1 m−3) and (right) its domestic contributions over D02 in (top to bottom) the spring, summer, autumn, and winter of 2013. The model terrestrial elevations are depicted in grayscale under the various colors. The map was generated using the Generic Mapping Tools v4.5.7 (https://www.generic-mapping-tools.org).
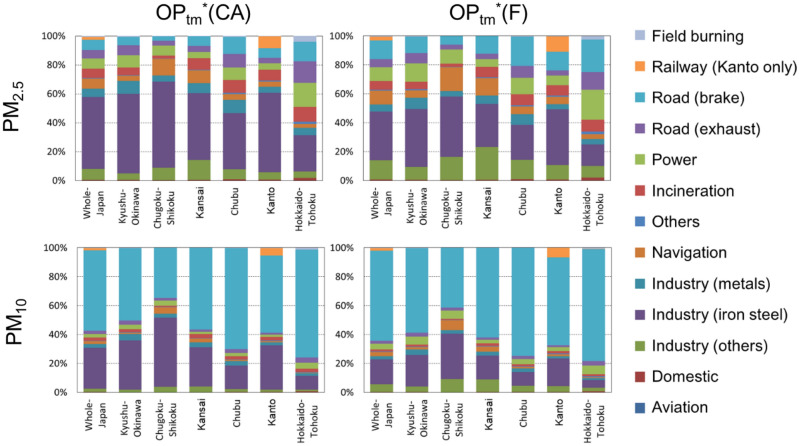
Figure 6.
Relative contributions of the emission sectors to OPtm*. Contributions of each anthropogenic emission sector in TMI-Japan (v1.0) to OPtm*(CA) and OPtm*(F) in regard to PM2.5 and PM10 over all of Japan and the six regions, as shown in Fig. 1. Note that TMI-Japan (v1.0) provides railway emissions only for Kanto.
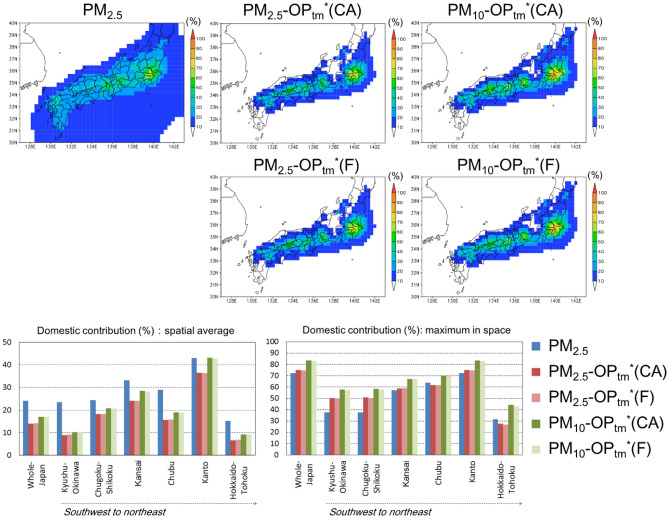
To determine the differences between the health hazard based on OP and the conventional health hazard, i.e., the PM2.5 mass concentration, the domestic contributions of PM2.5 and OPtm* are shown and compared in Fig. 5. The simulation results of PM2.5 were retrieved from a previous study26 using the same bottom-up inventory27 and the same simulation period (2013) with a domain similar to D01 at a different grid resolution (36 km). To quantitatively compare the OPtm* simulation results to the simulated PM2.5 concentrations (using a common inventory and similar resolution) and to estimate the quantities in the regions outside of D02 (such as Hokkaido, all of Tohoku, and Okinawa), the simulation results based on D01 were considered for the comparison.
Figure 5.
Annual mean domestic contributions of PM2.5 and OPtm*(F). (Top five panels) The annual mean domestic contributions of PM2.5 simulated by Kajino26 and anthropogenic OPtm*(CA) and OPtm*(F) in PM2.5 and PM10 simulated in this study. (Bottom two panels) (%) (left) spatially averaged values and (right) maximum values in space of the domestic contributions over all of Japan and the six regions, as shown in Fig. 1. The map was generated using the Grid Analysis and Display System (GrADS) v2.0.2,
available at http://cola.gmu.edu/grads.
The horizontal distributions of the domestic contributions of OPtm*(CA) and OPtm*(F) were very similar. The distributions of the domestic contributions of PM2.5 were much broader than those of the domestic contributions of OPtm*. This result occurred due to the difference in the contributions of the secondary aerosols. OPtm* is composed only of primary aerosols, i.e., metal elements, while PM2.5 is composed of both primary and secondary aerosols. Generally, the relative contributions of secondary aerosols are larger in downwind regions (after long-range transport). Consequently, the domestic contribution of OPtm* is the largest near the source regions, while that of PM2.5 is larger in the downwind regions. As a result, the areal mean values of the PM2.5 domestic contributions are larger than those of PM2.5-OPtm*, but the areal maximum values of PM2.5-OPtm* are as large or even significantly larger than those of PM2.5 especially over the Kyushu-Okinawa and Chugoku-Shikoku regions, where long-range transport is predominant. In these regions, more than 60% of PM2.5 was attributed to transboundary contributions and 40% was attributed to domestic contributions. However, in regard to OP, the domestic contribution was as high as 50%. The domestic contributions of PM10-OPtm* were generally larger than those of PM2.5-PMtm* by up to 5% in terms of the areal average or approximately 10% in terms of the areal maximum. As previously mentioned, this result occurred because the lifetime of PM10 is generally shorter than that of PM2.5. Hence, the relative contributions of long-range transported PM10 are lower than those of PM2.5.
Contributions of the emission sectors to OPtm* in Japan
The relative contributions of each emission sector to OPtm*(CA) and OPtm*(F) of PM2.5 and PM10 are shown in Fig. 6. While large differences occur in the metal contributions to OPtm*(CA) and OPtm*(F), the sector contributions based on these two methods are not very different both in terms of PM2.5 and PM10. However, the sector contributions to PM2.5 and PM10-OPtm* are very different. For example, the road brake sector is the top contributor to PM10-OPtm* but not to PM2.5-OPtm*. It should be noted that the size distribution of the current inventory has not yet been evaluated. In fact, a recent laboratory experiment28 has demonstrated that most brake wear particles occur in the fine mode, i.e., PM2.5. The size apportionment of the emission inventory certainly requires further improvement. The results provided by TMI-Japan are presented below in this section.
The most important sector in regard to PM10-OPtm* was the road brake sector, followed by the iron-steel industry sector. Regarding PM2.5-OPtm*, the other sectors such as other industries (nonmetals), navigation, incineration, power plants, road exhaust, and railways attained almost equal contributions (ranging from only a few percent up to 10–20%). As shown in Fig. 2, the source contributions mainly reflected those of Cu and Fe followed by those of Mn and Ni. The large contribution of the road brake sector to PM10-OPtm* originated from Cu and Fe and that to PM2.5 originated from Fe. The iron-steel industry contribution to OPtm* originated primarily from Fe and Mn, while it originated from Ni and Cu in regard to PM2.5. The contribution of the other industry (nonmetals) sector to PM2.5-OPtm* originated from Ni and Cu, and the contributions of the metal industry sectors other than the iron-steel and incineration sectors originated from Cu and Fe. The power plant sector emitted Fe and Cu, which contributed to PM2.5-OPtm*. The contribution of the road exhaust sector to PM2.5-OPtm*(F) mostly originated from Cu.
The contribution of the navigation sector was the largest in Chugoku-Shikoku, originating from V and Ni. The contribution of the navigation sector was large in regard to PM2.5-OPtm*(F) due to the high ri value for Ni. Vanadium (V) and Ni achieved almost equal contributions to PM2.5-OPtm*(F). The metal emission amounts were the largest in Kanto, the most populated region of Japan, in terms of Cu, Mn, and Fe, while the V and Ni emissions were the largest in Chugoku-Shikoku. As previously described, this result occurred due to the aerosols generated during heavy fuel oil combustion emitted from vessels in the Seto Inland Sea and industrial factories along the coast.
The railway sector contributed approximately 10% to PM2.5-OPtm* in Kanto, which was primarily attributed to Fe (Fe stemming from the railway sector accounted for approximately 15% of the total Fe emission) and Cu. This sector also emitted Mn. Because railway emission data were available only for Kanto, the OPtm* and metal emissions in the other regions could be underestimated at similar fractions.
Discussion
Toward the effective emission control measures
Daellenbach et al.22 and this study indicated that the emission sources for PM2.5 and OP could be very different. There may be a possibility that an emission control measure to reduce PM2.5 surface air concentrations may not necessarily reduce their health risk, if OP is essential to the health hazard of aerosols and if the selected control measure does not reduce OP. For example, reduction of ammonia emission may lead to decrease in surface air concentrations of PM2.5, but not OP because the emission sources of transition metals and quinones are different from those of ammonia. Besides, decrease of ammonia in the air can lead to increase of aerosol acidity and thus solubility of metals, which could enhance OP of aerosols. On the other hand, even though an emission control measure does not significantly decrease the PM2.5 concentration, it may be effective if it decreases OP efficiently.
Certainly, OP is not a sole and perfect health hazard of aerosols, but concerning only PM2.5 may mislead the emission control strategy. In order to seek for the better and effective control measures, OP must be directly simulated by numerical models in addition to PM2.5 and other conventional aerosol components, because numerical models are one of the most powerful tools to quantitatively evaluate the impacts of emission control on the surface air concentrations.
Future research
The current study has the following limitations, which should be resolved in the future. We only simulated the total (water-soluble and water-insoluble) metal concentrations and assumed a constant solubility over time and space. Water solubility of metals depends on their chemical forms and aerosol acidity. As acidity is higher, some of transition metals such as Cu, Mn, and Fe become more water soluble. We should consider specific water solubilities of metals for each emission source and simulate any changes in water solubility of metals due to changes in aerosol acidity occurring during transport29. We only considered metals but organics, especially quinones, are important DTT-active agents9,11–13. The interactions between metals and organics can also enhance DTT consumption and thus should be considered14,19. Metals are primary species, but quinones are secondary and produced in the air. During transport from emission source to downwind regions, the relative contributions of quinones to OP may increase, which can cause differences in dominant emission sectors affecting OP in the two regions. There are species that are not redox active but cause a notable oxidative stress, such as endotoxin30. These species should also be taken into account in the simulation. Finally, epidemiological studies are required to assess the applicability of the new health hazards6–8,31. Further integration of meteorology, chemistry, toxicology, and epidemiology is indispensable in the next study stages.
Methods
Numerical simulations of the transition metals
A transition metal version23 of the Japan Meteorological Agency (JMA) regional-scale meteorology-chemistry model (NHM-Chem32) was adopted in this study. We retrieved simulation results of the considered transition metals from Kajino et al.23, and details are not presented here.
The transition metal version of NHM-Chem employs three aerosol categories: anthropogenic submicron particles (SUB), anthropogenic coarse-mode particles (COR) and mineral dust (MD). The full chemistry version of NHM-Chem fully considers aerosol microphysical processes such as nucleation, condensation, coagulation, and deposition, but the transition metal version only considers deposition processes, such as wet deposition (in-cloud and below-cloud deposition), fog deposition (contact of cloud droplets in the bottom layers of the model to the ground surface33), and dry deposition. Upon emission, the size distributions of the above three aerosol categories are prescribed23, which change during transport only due to advection, turbulent diffusion, and removal processes. A prescribed hygroscopicity is assumed for each category23, which was adopted to obtain cloud condensation nuclei activity used for in-cloud scavenging and fog deposition and hygroscopic growth used for below-cloud scavenging and dry deposition.
As shown in Fig. 1, two model domains were applied to simulate the long-range transport from the Asian continent to Japan and the contributions of local emissions and local transport in Japan. D01 covers East Asian countries with a grid spacing (Δx) of 30 km and 200 × 140 grid cells on the Lambert conformal conic projection to resolve the transport of air pollutants due to synoptic-scale disturbances. D02 covers the Kyushu, Shikoku, and Honshu (only the Chugoku, Kansai, Chubu, and Kanto regions and part of the Tohoku region) islands of Japan with Δx = 6 km and 226 × 106 grid cells on the Lambert conformal conic projection. The horizontal grids of the meteorological and transport parts of NHM-Chem are the same, but the vertical grids differ. There are 38 vertical levels up to 22,055 m above sea level (a.s.l.) for the meteorological simulations and 40 levels up to 18,000 m a.s.l. for the transport simulations. A JRA-55 global analysis34 (1.25° × 1.25°, 6 h) was applied to the initial and boundary conditions of the meteorological simulations in D01. The JMA meso-regional objective analysis (MANAL; 5 km × 5 km, 3 h) was adopted in D02. These analysis data were also used for the spectral nudging of the meteorological simulations.
Kajino et al.23 developed emission inventories of eight DTT-active metals, namely, Cu, Mn, V, Ni, Pb, Fe, Zn, and Cr, in anthropogenic PM2.5 and PM10 in Asia and Japan, referred to as Transition Metal Inventory (TMI)-Asia v1.0 (Δx = 0.25°; monthly, 2000–2008; 9 sectors) and TMI-Japan v1.0 (Δx = 2 km; hourly, weekday/weekend; monthly, 2010; 29 sectors), respectively. Kajino et al.23 also simulated metals originating from Asian dust. TMI-Asia and TMI-Japan were used for the transport simulations over D01 and D02, respectively. Anthropogenic PM2.5 and PM10 emissions were allocated to the SUB and COR categories, and those originating from Asian dust were allocated to the MD category. The simulated metal concentrations of SUB were compared to MOEJ PM2.5 concentration measurements, and the simulated concentrations of COR plus MD were compared to measurements of TSP reported in Kajino et al.23 and this study. A part of mineral dust mass should be included in PM2.5 in reality, but it was neglected in this study.
Surface air concentration measurements of the transition metals
To derive the observed OPtm*, we used the same observation datasets as that reported in Kajino et al.23. The measurement data were collected in Yonago city, Tottori Prefecture, Japan (Fig. 1), from March to December 2013. Aerosols were collected with a TSP sampler (MCAS-03, Murata Keisokuki Service Co. Ltd.) on polytetrafluoroethylene (PTFE) filters (Whatman, PM2.5 Air Monitoring PTFE Membrane Filter, 46.2 mm φ) at a flow rate of 30 L min−1. The sampler was situated on a rooftop terrace of the building of the Faculty of Medicine, Tottori University (35.43°N, 133.33°E), approximately 20 m above ground level. The inorganic elements were analyzed using the fundamental parameter quantification method of energy-dispersive X-ray fluorescence spectrometry (EDXRF-FP), which was developed and evaluated by Okuda et al.35,36.
Definition and derivation of the cumulative oxidative potential based on transition metals (OPtm*)
In this section, we assessed the model predictability of the observed cumulative OP based on the top five DTT-consuming metals in air determined via reagent experiments. It should be noted that this parameter is not the realistic OP in the atmosphere but the idealized OP. The realistic OP can be expressed as follows:
| 1 |
where ri and rj are the specific OP values (DTT consumption rate per unit of mass) of metals i and organic compounds j, respectively, Ci and Cj are the surface air concentrations, and i and j are the metal ions and organic compounds, respectively. The last term is an interaction term between the metal ions and organic compounds14.
However, because water solubility data of the metals and DTT-active organic compounds are not available in the inventory and model, we defined the cumulative OP based only on the total (soluble + insoluble) metals (OPtm*) as follows:
| 2 |
where ri, χconst,i, Ti, and fi are the specific OP, water solubility (assumed constant in time and space), total surface air concentration, and simulation bias correction factor, respectively (i: top five DTT-consuming metals, namely, Cu, Mn, Fe, V, and Ni). The values used in Eq. (2) are listed in Table 2. It is known that the ri values vary among laboratories, as experimental methods such as buffer usage, pH, reaction time, and control volume are different2. To consider the variability in experiments, two different OPtm* values were derived using the ri values of Charrier and Anastasio9 and Fujitani et al.17, referred to as OPtm*(CA) and OPtm*(F), respectively. Charrier and Anastasio9 and Fujitani et al.17 provided fitted ri values by using exponential functions for specific species, but the ri values of all species were fitted with linear functions in this study. Because there was no water solubility information available in the measurements or emission profiles, constant values of χi were assumed and applied, which were obtained from Okuda et al.37. Moreover, fi was equal to 1 for the observed OPtm*, while the inverse of the Sim:Obs ratio obtained from the comparison of the D02 simulations and nationwide PM2.5 measurements of the MOEJ was adopted for fi in regard to the simulated OPtm*. The same fi was applied for PM2.5-OPtm*, PM10-OPtm*, and TSP-OPtm*. Although fi was derived based on simulated and observed PM2.5 without consideration of simulated PM2.5 fraction of mineral dust, it was proved to be reasonable from the comparisons of simulated and observed TSP-OPtm* at Yonago as shown in Table 1. In this paper, OPtm* based on the simulated and observed transition metal concentrations was simply referred to as the simulated OPtm* and observed OPtm*, respectively.
Acknowledgements
The current research was mainly supported by the Environmental Research and Technology Development Fund of the Environmental Restoration and Conservation Agency (ERCA) (JPMEERF20165005 and JPMEERF20165051). It was also supported by the Fundamental Research Budget of MRI (M5 and P5), the Japan Society for the Promotion of Sciences (JSPS) (KAKENHI grant nos. JP19H01155, JP19K19468, JP25870447, JP20H00636), and the Joint Research Program of Arid Land Research Centter, Tottori University (No. 27C2001, 28D20056, 30D2003, and 02C2010). The authors thank Prof. Kazuichi Hayakawa of Kanazawa University for his useful comments on the importance of quinones and Mr. Takuya Kishikawa of the University of Tsukuba for the provided data handling assistance.
Author contributions
M.K. designed the research and conducted the numerical simulations. M.K. wrote the manuscript in collaboration with all coauthors. Y. I. organized the research group, H. H., T. M., and T. F. provided information on the emission inventories, Y. F. provided the experimental data for the oxidative potential determination, and K. O., and T. O. provided the field measurement data of the metals in TSP.
Data availability
The simulated and observed data used for the figures and tables are available at https://mri-2.mri-jma.go.jp/owncloud/s/pfAEWT3Qi3EyGMY (last accessed: 2 November 2020).
Code availability
The NHM-Chem source code is available subject to a license agreement with the JMA. Further information is available at http://www.mri-jma.go.jp/Dep/glb/nhmchem_model/application_en.html (last accessed: 2 November 2020).
Competing interests
The authors declare no competing of interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shiraiwa M, et al. Aerosol health effects from molecular to global scales. Environ. Sci. Technol. 2017;51:13545–13567. doi: 10.1021/acs.est.7b04417. [DOI] [PubMed] [Google Scholar]
- 2.Jiang H, Ahmed CMS, Canchola A, Chen JY, Lin Y-H. Use of dithiothreitol assay to evaluate the oxidative potential of atmospheric aerosols. Atmosphere. 2019;10:571. doi: 10.3390/atmos10100571. [DOI] [Google Scholar]
- 3.Molina C, et al. Airborne aerosols and human health: Leapfrogging from mass concentration to oxidative potential. Atmosphere. 2020;11:917. doi: 10.3390/atmos11090917. [DOI] [Google Scholar]
- 4.Hedayat F, Stevanovic S, Miljevic B, Bottle S, Ristovski ZD. Review-evaluating the molecular assays for measuring the oxidative potential of particulate matter. Chem. Ind. Chem. Eng. Q. 2015;21:201–210. doi: 10.2298/CICEQ140228031H. [DOI] [Google Scholar]
- 5.Kumagai Y, et al. Oxidation of proximal protein sulfhydryls by phenanthraquinone, a component of diesel exhaust particles. Chem. Res. Toxicol. 2015;15:48301–489. doi: 10.1021/tx010099. [DOI] [PubMed] [Google Scholar]
- 6.Delfino RJ, et al. Airway inflammation and oxidative potential of air pollutant particles in a pediatric asthma panel. J. Exp. Sci. Environ. Epidemiol. 2013;23:466–473. doi: 10.1038/jes.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bates JT, et al. Reactive oxygen species generation linked to sources of atmospheric particulate matter and cardiorespiratory effects. Environ. Sci. Technol. 2015;49:13605–13612. doi: 10.1021/acs.est.5b02967. [DOI] [PubMed] [Google Scholar]
- 8.Abrams JY, et al. Associations between ambient fine particulate oxidative potential and cardiorespiratory emergency department visits. Environ. Health Perspect. 2017;125:107008. doi: 10.1289/EHP1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charrier JG, Anastasio C. On dithiothreitol (DTT) as a measure of oxidative potential for ambient particles: evidence for the importance of soluble transition metals. Atmos. Chem. Phys. 2012;12:9321–9333. doi: 10.1054/acp-12-9321-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishita-Hara C, Hirabayashi M, Hara K, Yamazaki A, Hayashi M. Dithiothreitol-measured oxidative potential of size-segregated particulate matter in Fukuoka, Japan: Effects of Asian dust events. GeoHealth. 2019;3:160–173. doi: 10.1029/2019GH000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saffari A, Daher N, Shafer MM, Schauer JJ, Sioutas C. Seasonal and spatial variation in dithiothreitol (DTT) activity of quasi-ultrafine particles in the Los Angeles Basin and its association with chemical species. J. Environ. Sci. Heal. A. 2014;49:441–451. doi: 10.1080/10934529.2014.854677. [DOI] [PubMed] [Google Scholar]
- 12.Sauvain JJ, Rossi MJ. Quantitative aspects of the interfacial catalytic oxidation of dithiothreitol by dissolved oxygen in the presence of carbon nanoparticles. Environ. Sci. Technol. 2016;50:996–1004. doi: 10.1021/acs.est.5b04958. [DOI] [PubMed] [Google Scholar]
- 13.Verma V, et al. Fractionating ambient humic-like substances (HULIS) for their reactive oxygen species activity—Assessing the importance of quinones and atmospheric aging. Atmos. Environ. 2015;120:351–359. doi: 10.1016/j.atmosenv.2015.09.010. [DOI] [Google Scholar]
- 14.Yu H, Wei J, Cheng Y, Subedi K, Verma V. Synergistic and antagonistic interactions among the particulate matter components in generating reactive oxygen species based on the dithiothreitol assay. Environ. Sci. Technol. 2018;52:2261–2270. doi: 10.1021/acs.est.7b04261. [DOI] [PubMed] [Google Scholar]
- 15.Jiang H, et al. Role of functional groups in reaction kinetics of dithiothreitol with secondary organic aerosols. Environ. Pollut. 2020 doi: 10.1016/j.envpol.2020.114402. [DOI] [PubMed] [Google Scholar]
- 16.Saffari A, Daher N, Shafer MM, Schauer JJ, Sioutas C. Global perspective on the oxidative potential of airborne particulate matter: A synthesis of research findings. Environ. Sci. Technol. 2014;48(13):7576–7583. doi: 10.1021/es500937x. [DOI] [PubMed] [Google Scholar]
- 17.Fujitani Y, Furuyama A, Tanabe K, Hirano S. Comparison of oxidative abilities of PM2.5 collected at traffic and residential sites in Japan. Contribution of transition metals and primary and secondary aerosols. Aerosol Air Qual. Res. 2017;17:574–587. doi: 10.4209/aaqr.2016.07.0291. [DOI] [Google Scholar]
- 18.Fang T, Lakey PSJ, Weber RJ, Shiraiwa M. Oxidative potential of particulate matter and generation of reactive oxygen species in epithelial lining fluid. Environ. Sci. Technol. 2019;53(21):12784–12792. doi: 10.1021/acs.est.9b03823. [DOI] [PubMed] [Google Scholar]
- 19.Lakey PSJ, et al. Chemical exposure-response relationship between air pollutants and reactive oxygen species in the human respiratory tract. Sci. Rep. 2016;6:32916. doi: 10.1038/srep32916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang A, et al. Spatial variation and land use regression modeling of the oxidative potential of fine parciels. Environ. Health Perspect. 2015;123:1187–1192. doi: 10.1289/ehp.1408916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jedynska A, et al. Spatial variations and development of land use regression models of oxidative potential in ten European study areas. Atmos. Environ. 2017;150:24–32. doi: 10.1016/j.atmosenv.2016.11.029. [DOI] [Google Scholar]
- 22.Daellenbach KR, et al. Sources of particulate-matter air pollution and its oxidative potential in Europe. Nature. 2014;587:414–419. doi: 10.1038/s41586-020-2902-8. [DOI] [PubMed] [Google Scholar]
- 23.Kajino M, et al. Modeling transition metals in East Asia and Japan and its emission sources. GeoHealth. 2020 doi: 10.1029/2020GH000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.RIVM, Multiple Path Particle Dosimetry Model (MPPD v1.0): A model for human and rat airway particle dosimetry, Bilthoven, The Netherlands. RIVA Report650010030 (2002).
- 25.Hashizume M, et al. Health effects of Asian dust: A systematic review and meta-analysis. Environ. Health Perspect. 2020;128(6):066001. doi: 10.1289/EHP5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kajino, M. et al. Model simulation of atmospheric aerosols, Trans-Boundary Pollution in North-East Asia, Eds. K. Hayakawa, S. Nagao, Y. Inomata, M. Inoue, and A. Matsuki. NOVA Science Publishers. ISBN:978-1-53614-742-2, pp. 147–166 (2018).
- 27.Kurokawa J, et al. Emissions of air pollutants and greenhouse gases over Asian regions during 2000–2008: Regional Emission inventory in ASia (REAS) version 2. Atmos. Chem. Phys. 2013;13:11019–11058. doi: 10.5194/acp-13-11019-2013. [DOI] [Google Scholar]
- 28.Hagino H, Oyama M, Sasaki S. Laboratory testing of airborne brake wear particle emissions using a dynamometer system under urban city driving cycles. Atmos. Environ. 2016;131:269–278. doi: 10.1016/j.atmosenv.2016.02.014. [DOI] [Google Scholar]
- 29.Ito A, Guangxing L, Penner JE. Radiative forcing by light-absorbing aerosols of pyrogenetic iron oxides. Sci. Rep. 2018;8:7347. doi: 10.1038/s41598-018-25756-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rabinovitch N, et al. Importance of the personal endotoxin cloud in school-age children with asthma. J Allergy Clin Immunol. 2005;116:1053–1057. doi: 10.1016/j.jaci.2005.08.045. [DOI] [PubMed] [Google Scholar]
- 31.Onishi K, et al. Predictions of health effects of cross-border atmospheric pollutants using an aerosol forecast model. Environ. Int. 2018;117:48–56. doi: 10.1016/j.envint.2018.04.035. [DOI] [PubMed] [Google Scholar]
- 32.Kajino M, et al. NHM-Chem, the Japan Meteorological Agency’s regional meteorology—Chemistry model: Model evaluations toward the consistent predictions of the chemical, physical, and optical properties of aerosols. J. Meteor. Soc. Japan. 2019;97(2):337–374. doi: 10.2151/jmsj.2019-020. [DOI] [Google Scholar]
- 33.Katata G, et al. Detailed source term estimation of the atmospheric release for the Fukushima Daiichi Nuclear Power Station accident by coupling simulations of an atmospheric dispersion model with an improved deposition scheme and oceanic dispersion model. Atmos. Chem. Phys. 2015;15:1029–1070. doi: 10.5194/acp-15-1029-2015. [DOI] [Google Scholar]
- 34.Kobayashi S, et al. The JRA-55 reanalysis: General specifications and basic characteristics. J. Meteorol. Soc. Jpn. 2015;93:5–48. doi: 10.2151/jmsj.2015-001. [DOI] [Google Scholar]
- 35.Okuda T, et al. Rapid and simple determination of multi-elements in aerosol samples collected on Quartz fiber filters by using EDXRF coupled with fundamental parameter quantification technique. Aerosol Air Qual. Res. 2013;13:1864–1876. doi: 10.4209/aaqr.2012.11.0308. [DOI] [Google Scholar]
- 36.Okuda T, Schauer JJ, Shafer MM. Improved methods for elemental analysis of atmospheric aerosols for evaluating human health impacts of aerosols in East Asia. Atmos. Environ. 2014;97:552–555. doi: 10.1016/j.atmosenv.2014.01.043. [DOI] [Google Scholar]
- 37.Okuda T, Nakao S, Katsuno M, Tanaka S. Source identification of nickel in TSP and PM2.5 in Tokyo, Japan. Atmos. Environ. 2007;41:7642–7648. doi: 10.1016/j.atmosenv.2007.08.050. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The simulated and observed data used for the figures and tables are available at https://mri-2.mri-jma.go.jp/owncloud/s/pfAEWT3Qi3EyGMY (last accessed: 2 November 2020).
The NHM-Chem source code is available subject to a license agreement with the JMA. Further information is available at http://www.mri-jma.go.jp/Dep/glb/nhmchem_model/application_en.html (last accessed: 2 November 2020).