Abstract
Background
In metastatic castration-resistant prostate cancer (mCRPC), assessing treatment response and bone lesions with technetium-99m is limited by image resolution and subjectivity. We evaluated bone scan lesion area (BSLA), a quantitative imaging assessment of response in patients with mCRPC receiving radium-223 alone or in combination with androgen receptor pathway inhibitors (abiraterone/prednisone or enzalutamide).
Patients and methods
This randomized, non-comparative phase IIa three-arm trial (NCT02034552) evaluated technetium-99m-based BSLA response rate (RR), safety, radiologic progression-free survival (rPFS), and time to first symptomatic skeletal event (SSE) in men with mCRPC and bone metastases receiving radium-223 with/without abiraterone/prednisone or enzalutamide. The primary endpoint was week 24 BSLA RR.
Results
Overall, 63 patients received treatment (abiraterone/prednisone combination, n = 22; enzalutamide combination, n = 22; radium-223 monotherapy, n = 19). Median treatment duration (first to last dose of any study treatment) was 12 months (abiraterone/prednisone combination), 10 months (enzalutamide combination), and 3 months (radium-223 monotherapy). Week 24 BSLA RR was 58% [80% confidence interval (CI) 41% to 74%; one-sided P < 0.0001; 11/19 patients] with abiraterone/prednisone combination, 50% (32% to 68%; one-sided P < 0.0001; 8/16 patients) with enzalutamide combination, and 22% (10% to 40%; one-sided P = 0.0109; 4/18 patients) with radium-223 monotherapy. Median rPFS was not evaluable for combination arms and 4 months (80% CI 4 to 12) for monotherapy. SSEs were reported in 32% of patients; median time to first SSE was not estimable. Fatigue and back pain were the most commonly reported treatment-emergent adverse events (TEAEs); more patients receiving combination therapy than monotherapy had TEAEs. Fractures were reported in 18% receiving abiraterone/prednisone, 32% receiving enzalutamide, and 11% receiving radium-223 monotherapy. Fracture rates were lower in patients taking bone health agents versus not taking bone health agents at baseline.
Conclusions
Technetium-99m imaging BSLA may offer objective, quantifiable assessment of isotope uptake changes, and potentially treatment response, in patients with mCRPC and bone metastases treated with radium-223 alone or in combination with abiraterone/prednisone or enzalutamide. In this largely treatment-naive population, BSLA RR was numerically lower with radium-223 monotherapy versus combination therapy, indicating a limited role as first-line treatment. Use of radium-223 should follow evidence-based treatment guidelines and the licensed indication.
Key words: metastatic castration-resistant prostate cancer, radium-223, abiraterone, enzalutamide, bone scan lesion area, technetium-99m
Highlights
-
•
Radium-223 is a targeted alpha therapy for bone-dominant metastatic castration-resistant prostate cancer.
-
•
Assessing bone lesions and treatment response with technetium-99m is limited by image resolution and subjectivity.
-
•
We used computer-aided detection (CAD) of bone scan lesion area (BSLA) to assess radium-223 ± abiraterone/enzalutamide.
-
•
On preliminary assessment, CAD-based BSLA demonstrated positive response to radium-223 ± abiraterone/enzalutamide therapy.
-
•
Use of radium-223 should follow evidence-based treatment guidelines and the licensed indication.
Introduction
Multiple life-prolonging therapies are approved for metastatic castration-resistant prostate cancer (mCRPC), including androgen receptor pathway inhibitors (ARPIs; abiraterone/prednisone and enzalutamide), chemotherapy (docetaxel, cabazitaxel), immunotherapy (sipuleucel-T), and targeted alpha therapy (radium-223 dichloride). Abiraterone (a CYP17 inhibitor), enzalutamide (a second-generation androgen receptor inhibitor1), and radium-223 (a targeted alpha therapy that selectively binds to areas of increased bone turnover in bone metastases2) have all demonstrated overall survival (OS) benefit in patients with mCRPC in phase III clinical trials.3, 4, 5, 6, 7, 8, 9, 10 The different mechanisms of action of these agents and limited overlapping toxicity provided a rationale for their use in combination.1 However, additional clarity about such combination therapies is now required in light of recent negative findings for abiraterone/prednisone plus radium-223 combination therapy compared with abiraterone/prednisone alone in the ERA 223 trial in men with chemotherapy-naive, asymptomatic, or mildly symptomatic mCRPC.11
Bone metastases occur in approximately 90% of men with mCRPC,3,10 but there are challenges with staging and determining treatment response.12 The conventional approach with technetium-99m uses visual assessment of images to monitor metastases based on the number of bone lesions over time. However, limited image resolution and the subjectivity of visual assessment precludes reliable quantitative assessment of lesion size. These issues of specificity and sensitivity mean that conventional bone scan imaging is too ‘blurry’ for clear visual discernment and resolution of distinct lesions.12,13 Consequently, currently there is no reliable means to evaluate changes in bone lesion burden in mCRPC. Accordingly, Response Evaluation Criteria in Solid Tumors (RECIST) 1.114 and Prostate Cancer Clinical Trials Working Group 3 (PCWG3) criteria15 recommend limited use of imaging endpoints in evaluating bone lesions compared with soft-tissue lesions. Both RECIST 1.1 and PCWG3 use bone lesions as non-target lesions (assessed qualitatively); thus, both criteria permit assessment of complete response (CR) and progressive disease (PD), but cannot distinguish partial response (PR) from stable disease (SD). This severely limits the use of bone lesion response as an efficacy endpoint. Therefore, when determining treatment efficacy in patients with mCRPC and bone metastases, quantifiable time-to-event-based efficacy outcomes, including radiologic progression-free survival (rPFS) or time to symptomatic skeletal events (SSEs), may be more reliable than qualitative response categories (CR, PD, or neither). Accordingly, the PCWG3 suggests using alternative endpoints to response, even in early-phase clinical trials of new agents for the treatment of mCRPC.15
Recently, bone scan lesion area (BSLA), determined using an automated computer-aided detection (CAD) system, has been used to quantify whole-body bone scintigraphic images and act as a biomarker for bone tumor burden.16 This automated system was reported to have a sensitivity of 94% and specificity of 89% for tumor pixels on bone scans, compared with ∼77% sensitivity and 84% to 96% specificity of manual interpretation.16 In a subsequent validation study in men with mCRPC and bone metastases receiving either abiraterone/prednisone or placebo, BSLA <200 cm2 at baseline was prognostic for delayed progression and predictive of prolonged OS in treated patients. Furthermore, patients with PD, defined as a 30% increase in BSLA from baseline to week 12 of treatment, had a significantly shorter OS than patients without progression.13
This phase IIa three-arm study evaluated bone scan response rate (RR) using the above-mentioned CAD system to measure technetium-99m bone scan BSLA, safety, and other outcomes in patients with mCRPC following treatment with radium-223 alone or in combination with either abiraterone/prednisone or enzalutamide. The study also explored the clinical value of different imaging modalities [diffusion-weighted magnetic resonance imaging (DW-MRI) and sodium fluoride positron emission tomography/computed tomography (Na18F PET/CT)] for evaluating treatment response in bone lesions. Detailed rationales for investigating BSLA, DW-MRI, and Na18F PET/CT in this setting are provided in the Supplementary Materials, available at https://doi.org/10.1016/j.esmoop.2021.100082. This study was initiated and patients completed radium-223 treatments before the results of the ERA 223 trial of abiraterone/prednisone plus radium-223 were available and before publication of the CAD BSLA validation study.
Patients and methods
Study design and conduct
The study was a randomized, non-comparative, open-label, multicenter, phase IIa trial (NCT02034552). The study protocol and all subsequent amendments were approved by an independent ethics committee or institutional review board at each participating site (19 sites in 15 states and the District of Columbia within the United States). The study was conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice guidelines of the International Conference on Harmonization. All patients provided written informed consent before participating in the study.
Patients
Patients aged ≥18 years were eligible for inclusion if they had histologically or cytologically confirmed progressive CRPC and two or more bone metastases detected by whole-body bone imaging, but no known visceral metastases. Key exclusion criteria included history of or known visceral metastases, treatment with >1 chemotherapy agent for prostate cancer, or previous abiraterone/prednisone, enzalutamide, radium-223, or systemic radiotherapy. The definition of castration-resistant disease and a full list of inclusion/exclusion criteria are provided in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100082.
Study design and treatment regimens
Following screening, eligible patients were randomized in a 1 : 1 : 1 ratio to one of three treatment arms: radium-223 plus abiraterone acetate and prednisone, radium-223 plus enzalutamide, or radium-223 monotherapy. Radium-223 55 kBq/kg was administered intravenously once every 4 weeks for six cycles. Patients in the combination arms also received either oral abiraterone acetate (1000 mg once daily) and oral prednisone/prednisolone (5 mg twice daily) or oral enzalutamide (160 mg once daily). Abiraterone/prednisone or enzalutamide were administered concurrently with radium-223. Patients could continue abiraterone/prednisone or enzalutamide for up to 2 years after the last radium-223 dose if investigators determined that patients would receive clinical benefit.
During treatment, all concomitant medications, including those for prostate cancer, but excluding medication for procedures and imaging, were to be recorded. Hormonal therapy (e.g. luteinizing hormone-releasing hormone agonists or antagonists, anti-androgens) was permitted in any treatment arm. In the radium-223 arm only, other prostate cancer therapies (e.g. diethylstilbestrol, estradiol, ketoconazole, dexamethasone, hydrocortisone, prednisone) could be used according to routine clinical practice, at the discretion of the investigator, with the exception of concomitant abiraterone/prednisone or enzalutamide. Differential permissibility was considered ethically necessary, given the lack of expected efficacy of radium-223 in soft tissue disease, notwithstanding its potential for bias.
The primary endpoint for each treatment arm was BSLA RR at week 24 calculated from each patient's digitized technetium-99m bone scan and based on a series of intermediate calculations. Bone scans were evaluated by central review using CAD software (MedQIA, LLC, Los Angeles, CA) to evaluate each patient's digitized whole-body technetium-99m bone scan at each time point (see Supplementary Materials, available at https://doi.org/10.1016/j.esmoop.2021.100082 for details). The reviewer identified bone pixels and determined the BSLA (in cm2), defined as the sum of the pixel areas identified as bone lesions from the set of whole-body technetium-99m bone imaging pixels, and disease status (bone lesion or not bone lesion) for each pixel. For each treatment arm, response was classified as a decrease in BSLA of ≥30% from baseline at week 24.
Secondary endpoints included rPFS (non-bone or bone progression, whichever occurred first), time to radiologic non-bone (soft-tissue) progression (modified RECIST 1.1), time to radiologic bone progression (adapted PCWG2 guidelines),17 SSE-free survival (SSE-FS), time to first SSE, OS, and safety. An SSE was defined as any of the following: use of external-beam radiotherapy (EBRT) to relieve skeletal pain, new symptomatic pathologic bone fractures (vertebral or nonvertebral), spinal cord compression, or tumor-related orthopedic surgical intervention. Bone fractures and bone-associated events were assessed both as SSEs and as adverse events (AEs). Use of bone health agents (BHAs) was assessed at baseline and during treatment and follow-up. Follow-up safety assessments occurred every 12 weeks from the end of treatment and for up to 2 years from the last radium-223 dose.
The study treatment period was from treatment initiation up to 30 days after the last dose of study treatment. Patients entered an active follow-up period of up to 2 years after the last radium-223 dose for efficacy, imaging, and select safety information. After the active follow-up period, patients entered a long-term follow-up period for up to 7 years from the last radium-223 dose. Following a protocol amendment, patients were transitioned into a separate long-term safety follow-up study for the remainder of their long-term follow-up.
All AEs (severity, seriousness, and duration) and laboratory values were reported during the study treatment period. Any AEs arising during this period were classified as treatment-emergent AEs (TEAEs). All AEs related to study medication and all serious AEs (SAEs) were also reported during active follow-up. Select safety data were collected throughout the study, including long-term follow-up data, regardless of the investigator's causality assessment. These data included bone fractures and bone-associated events, e.g. osteoporosis (reported as AEs or SAEs); all occurrences of leukemia, myelodysplastic syndrome, aplastic anemia, and primary bone cancer, or any other new primary malignancy (reported as SAEs); and survival.
All AEs were coded according to the Medical Dictionary for Regulatory Activities (MedDRA) v.21.0, graded according to the Common Terminology Criteria for Adverse Events (CTCAE) v.4.0, and assessed by the investigator for their relationship to treatment.
Clinical and imaging-related exploratory endpoints are described in the Supplementary Materials, available at https://doi.org/10.1016/j.esmoop.2021.100082.
Patients were assessed at screening and at weeks 8, 16, and 24 using a whole-body technetium-99m bone scan and MRI/CT imaging of the chest, abdomen, and pelvis. Assessment continued every 12 weeks or until confirmed radiologic progression (bone and/or soft tissue). Patients were also assessed for SSEs at each clinic visit. Exploratory imaging with DW-MRI and Na18F PET/CT was conducted at screening, at weeks 8, 16, and 24, and then every 12 weeks at selected sites. Results from each imaging method underwent separate central review.
Statistical analysis
Patient baseline characteristics for the intention-to-treat (ITT) population (all randomized patients) were reported by treatment arm using descriptive statistics. The efficacy population comprised a modified ITT population (mITT; patients who received at least one dose of study drug). The primary imaging analysis population included all patients in the mITT population with evaluable baseline technetium-99m bone scans. The safety population comprised all patients who received at least one radium-223 dose.
The primary endpoint analysis in each treatment arm was based on formal non-comparative hypothesis tests for BSLA RR at week 24, without adjustment for multiplicity. The hypothesis tested in each arm was H0: BSLA RR ≤5% versus HA: BSLA RR >5%, using an exact single-arm binomial test with a one-sided alpha of 0.10. Twenty evaluable participants were required to obtain 90% power to detect a bone scan RR of >5% at week 24 when the true RR was 30%. Bone scan RR at week 24 was estimated with exact binomial confidence intervals (CIs). There were no statistical comparisons between treatment arms.
Efficacy endpoints and AEs were reported using summary statistics. Time-to-event variables (rPFS, SSE-FS, OS, time to first SSE, time to radiologic bone progression) were summarized by treatment arm using the Kaplan–Meier method, with median values and Brookmeyer–Crowley CIs. Week 24 rates for time-to-event efficacy variables were based on a Kaplan–Meier analysis. Frequencies and percentages were tabulated for categorical variables.
Results
Patients and study regimen
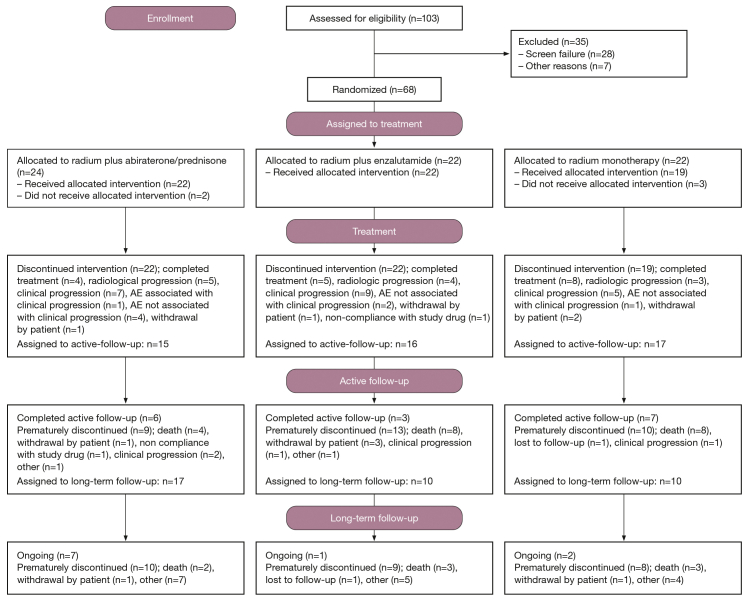
From March 2014 to May 2018, 68 patients were entered into the randomized study and 63 received treatment. Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2021.100082, shows the efficacy, imaging, and safety populations in each treatment arm. At baseline, overall, the median time since diagnosis of bone metastasis was 16 months, and 44% of patients had 6 to 20 metastases (Table 1). Patients received a median of six radium-223 injections in each combination arm and four in the radium-223 monotherapy arm. Median duration of treatment (from first to last dose of any study treatment) was 12 months with the abiraterone/prednisone combination, 10 months with the enzalutamide combination, and 3 months with radium-223 monotherapy. The median duration of the active follow-up period (up to 2 years after the last radium-223 dose) was 7 months for both combination regimens and 19 months for radium-223 monotherapy. This was expected, as patients in the radium-223 arm received a maximum of six doses of radium-223, whereas patients in the combination arms could receive abiraterone/prednisone or enzalutamide for up to 2 years after the last radium-223 dose until progression.
Table 1.
Baseline patient characteristics (ITT population)
| Radium-223 monotherapy (n = 22) | Radium-223 + abiraterone/prednisone (n = 24) | Radium-223 + enzalutamide (n = 22) | Total (N = 68) | |
|---|---|---|---|---|
| Median age, years | 72 | 68 | 73 | 71 |
| ECOG PS, n (%) | ||||
| 0 | 10 (45) | 13 (54) | 11 (50) | 34 (50) |
| 1 | 9 (41) | 9 (38) | 11 (50) | 29 (43) |
| Median total ALP, U/l | 96 | 101 | 98 | 99 |
| Median PSA, μg/l | 31 | 17 | 19 | 19 |
| Median time since PC diagnosis, months | 25 | 52 | 48 | 46 |
| Median time since first cancer progression, months | 15 | 32 | 20 | 21 |
| Median time since bone metastasis initial diagnosis, months | 10 | 15 | 22 | 16 |
| Extent of disease, n (%) | ||||
| <6 metastases | 9 (41) | 6 (25) | 6 (27) | 21 (31) |
| 6-20 metastases | 7 (32) | 11 (46) | 12 (55) | 30 (44) |
| >20 lesions | 3 (14) | 5 (21) | 4 (18) | 12 (18) |
| Superscan | 1 (5) | 0 | 0 | 1 (1) |
| Median baseline BSLA, mm2 | 4315 | 7479 | 7516 | 7266 |
| Prior systemic anticancer therapies, n (%)a | ||||
| Sipuleucel-T | 5 (23) | 6 (25) | 3 (14) | 14 (21) |
| Docetaxel | 4 (18) | 3 (13) | 5 (23) | 12 (18) |
| Prior BHA use, n (%) | 8 (42)b | 7 (32)c | 8 (36) | 23 (37)d |
| Denosumab | 7 (37)b | 6 (27)c | 7 (32) | 20 (32)d |
| Zoledronic acid | 1 (5)b | 1 (5)c | 1 (5) | 3 (5)d |
ALP, alkaline phosphatase; BHA, bone health agent; BSLA, bone scan lesion area; ECOG PS, Eastern Cooperative Oncology Group performance status; ITT, intention-to-treat; PC, prostate cancer; PSA, prostate-specific antigen.
>15% of patients overall.
n = 19.
n = 22.
n = 63.
Most patients (85%) received ≥1 concomitant systemic anticancer therapy during the treatment period (abiraterone/prednisone arm: 83%, enzalutamide arm: 95%, and radium-223 arm: 77%), and 81% received ≥1 concomitant hormonal therapy (83%, 86%, and 73%, respectively). The majority of patients received androgen-deprivation therapies such as leuprorelin/leuprorelin acetate (75% of patients), degarelix/degarelix acetate (4%), and goserelin (4%). Few patients received first-generation androgen receptor inhibitors, such as bicalutamide (3%), and other prostate cancer therapies were reported in <3% of patients each. During follow-up, 88% of patients received ≥1 systemic anticancer therapy (83%, 96%, and 86%, respectively), and 87% received ≥1 hormonal therapy (83%, 91%, and 86%, respectively).
Efficacy
Primary endpoint
The BSLA RR at week 24 based on technetium-99m bone scans in the imaging analysis population was 58% (80% CI 41% to 74%; one-sided P < 0.0001; 11/19 patients) for radium-223 plus abiraterone/prednisone, 50% (80% CI 32% to 68%; one-sided P < 0.0001; 8/16 patients) for radium-223 plus enzalutamide, and 22% (80% CI 10% to 40%; one-sided P = 0.0109; 4/18 patients) for radium-223 monotherapy (Table 2).
Table 2.
Primary and secondary efficacy endpoints (mITT population)
| Radium-223 monotherapy (n = 19) | Radium-223 + abiraterone/prednisone (n = 22) | Radium-223 + enzalutamide (n = 22) | |
|---|---|---|---|
| BSLA RR at week 24, % (80% CI),a P value |
22 (10-40); P = 0.0109b | 58 (41-74); P < 0.0001b | 50 (32-68); P < 0.0001b |
| Median rPFS, months (80% CI)c | 4 (4-12) | NE (19-NE) | NE (10-NE) |
| Median time to radiologic disease (non-bone) progression, months (80% CI)c | 5 (4-NE) | NE (NE-NE) | NE (NE-NE) |
| Median time to radiologic bone progression, months (80% CI)c | 12 (4-12) | NE (NE-NE) | NE (NE-NE) |
| Patients with an SSE, n (%) | 6 (32) | 7 (32) | 7 (32) |
| Median SSE-FS, months (80% CI) | 12 (10-25) | NE (17-NE) | 20 (12-28) |
| Median time to first SSE, months (80% CI) | NE (13-NE) | NE (17-NE) | NE (20-NE) |
| Median OS, months (80% CI) | 36 (21-41) | 38 (36-NE) | 30 (27-NE) |
BSLA, bone scan lesion area; CI, confidence interval; mITT, modified intention-to-treat; NE, not estimable; OS, overall survival; rPFS, radiologic progression-free survival; RR, response rate; SSE, symptomatic skeletal event; SSE-FS, SSE-free survival.
Imaging population: radium-223 + abiraterone/prednisone, n = 19; radium-223 + enzalutamide, n = 16; radium-223 monotherapy, n = 18.
Test of the null hypothesis of BSLA RR ≤5% at week 24 using an exact single-arm binomial test in each treatment group with one-sided alpha = 0.10.
Central review.
Secondary endpoints
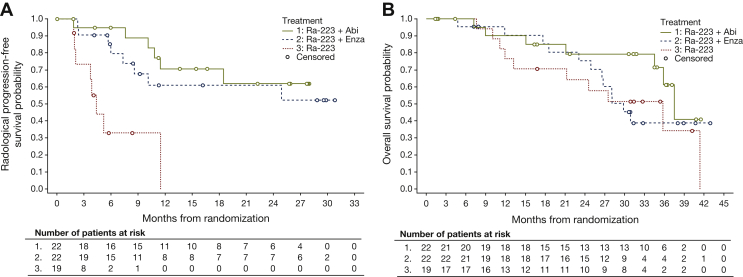
Median rPFS, time to radiologic non-bone progression, time to radiologic bone progression, and time to first SSE are shown in Table 2 and Figure 1A. In each arm, 32% of patients had an SSE. Median SSE-FS was also not estimable for the radium-223 plus abiraterone/prednisone arm (Table 2). The 24-month SSE-FS rates were 66% (80% CI 50% to 79%), 47% (80% CI 33% to 61%), and 35% (80% CI 20% to 50%) for radium-223 plus abiraterone/prednisone, radium-223 plus enzalutamide, and radium-223 monotherapy, respectively.
Figure 1.
Kaplan–Meier curves of (A) radiologic progression-free survival by central review and (B) overall survival (mITT population). Abi, abiraterone/prednisone; Enza, enzalutamide; mITT, modified intention-to-treat; Ra-223, radium-223.
Median OS was 38 months (80% CI 36 to not estimable) for radium-223 plus abiraterone/prednisone, 30 months (80% CI 27 to not estimable) for radium-223 plus enzalutamide, and 36 months (80% CI 21 to 41) for radium-223 monotherapy (Table 2); 24-month OS rates were ≥75% for each combination treatment and 64% for radium-223 monotherapy (Figure 1B).
Exploratory endpoints
Clinical exploratory endpoints
The alkaline phosphatase (ALP) RR at the end of radium-223 treatment was 36% (80% CI 26% to 59%) for radium-223 plus abiraterone/prednisone, 50% (80% CI 41% to 74%) for radium-223 plus enzalutamide, and 42% (80% CI 30% to 65%) for radium-223 monotherapy. Median percentage change in ALP from baseline to the end of radium-223 treatment was −25% for radium-223 plus abiraterone/prednisone, −31% for radium-223 plus enzalutamide, and −21% for radium-223 monotherapy. Median time to confirmed ALP progression on study was 10 months (80% CI 8 to 13) for radium-223 plus abiraterone/prednisone, 9 months (80% CI 8 to 16) for radium-223 plus enzalutamide, and not estimable for radium-223 monotherapy.
The prostate-specific antigen (PSA) RR at the end of radium-223 treatment was 64% (80% CI 60% to 90%) for each combination arm and 21% (80% CI 11% to 4%) for radium-223 monotherapy. Median percentage change in PSA from baseline was −84% for radium-223 plus abiraterone/prednisone, −93% for radium-223 plus enzalutamide, and 50% for radium-223 monotherapy. Median time to confirmed PSA progression was 26 months (80% CI 19 to not estimable) for radium-223 plus abiraterone/prednisone, 10 months (80% CI 9 to 25) for radium-223 plus enzalutamide, and 4 months (80% CI 3 to not estimable) for radium-223 monotherapy.
Technetium-99-based imaging exploratory endpoints
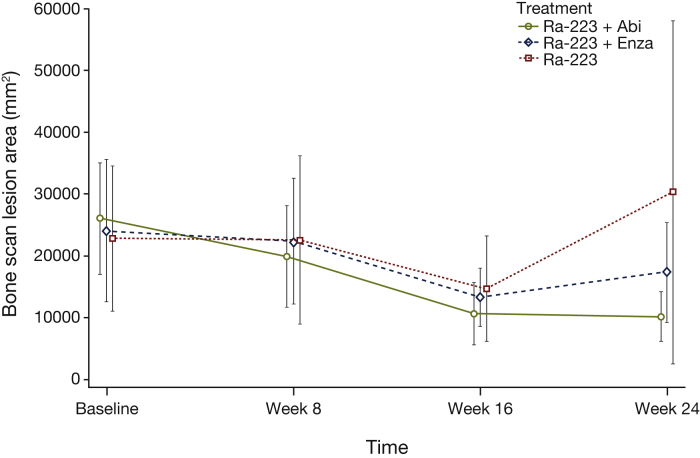
Mean BSLA at week 24 was numerically lower than at baseline for the combination treatments and higher than baseline for radium-223 monotherapy; CIs were overlapping (Figure 2). Based on BSLA best overall response rate (BORR) during the study using technetium-99m bone imaging, 13/19 patients (68%) had a response with radium-223 plus abiraterone/prednisone, 14/16 patients (88%) with radium-223 plus enzalutamide, and 5/18 patients (28%) with radium-223 monotherapy. These values are higher than the BSLA RR at week 24 because responses in some patients occurred later than week 24.
Figure 2.
Bone scan lesion area over time based on technetium-99m imaging (imaging analysis population). Abi, abiraterone/prednisone; Enza, enzalutamide; Ra-223, radium-223.
At week 24, >30% of patients across the three treatment arms had quantitative involvement in all four axial regions (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2021.100082).
Additional exploratory radiologic methods for imaging bone metastases (DW-MRI and Na18F PET/CT) are reported in the Supplementary Information, available at https://doi.org/10.1016/j.esmoop.2021.100082.
Safety
Adverse events
All patients, except one in the radium-223 monotherapy arm, experienced at least one TEAE during the study (Table 3). Generally, the percentage of patients with TEAEs was higher with combination treatment than with radium-223 monotherapy. Overall, a greater percentage of patients receiving combination treatment [12 patients (55%) with abiraterone/prednisone and 15 patients (68%) with enzalutamide] experienced Grade 3-4 TEAEs compared with those receiving radium-223 monotherapy [seven patients (37%)]. One patient in the enzalutamide arm died from Grade 5 general physical deterioration considered unrelated to study treatment. Treatment-related TEAEs were reported in 43 patients (68%) overall: 17 (77%), 18 (82%), and 8 (42%) in the radium-223 plus abiraterone/prednisone, radium-223 plus enzalutamide, and radium-223 monotherapy arms, respectively. The most commonly reported any-grade treatment-related TEAEs overall were fatigue (n = 17; 27%), diarrhea (n = 14, 22%), and nausea (n = 8, 13%). The only treatment-related serious TEAE was nausea in one patient receiving radium-223 plus abiraterone/prednisone.
Table 3.
Treatment-emergent adverse events (any grade) occurring in ≥20% of patients in any treatment arm (safety population)
| Adverse event (MedDRA preferred term), n (%) | Radium-223 monotherapy (n = 19) | Radium-223 + abiraterone/prednisone (n = 22) | Radium-223 + enzalutamide (n = 22) | Total (N = 63) |
|---|---|---|---|---|
| Any TEAE | 18 (95) | 22 (100) | 22 (100) | 62 (98) |
| Fatigue | 6 (32) | 8 (36) | 9 (41) | 23 (37) |
| Back pain | 5 (26) | 8 (36) | 9 (41) | 22 (35) |
| Diarrhea | 4 (21) | 8 (36) | 9 (41) | 21 (33) |
| Nausea | 2 (11) | 9 (41) | 6 (27) | 17 (27) |
| Arthralgia | 3 (16) | 5 (23) | 6 (27) | 14 (22) |
| Decreased appetite | 3 (16) | 3 (14) | 5 (23) | 11 (17) |
| Hypertension | 3 (16) | 2 (9) | 6 (27) | 11 (17) |
| Constipation | 1 (5) | 6 (27) | 3 (14) | 10 (16) |
| Dizziness | 1 (5) | 3 (14) | 6 (27) | 10 (16) |
| Hot flush | 1 (5) | 3 (14) | 5 (23) | 9 (14) |
| Vomiting | 2 (11) | 6 (27) | 1 (5) | 9 (14) |
| Headache | 1 (5) | 5 (23) | 1 (5) | 7 (11) |
| Upper respiratory tract infection | 0 | 5 (23) | 2 (9) | 7 (11) |
MedDRA, Medical Dictionary for Regulatory Activities (v.21.0); TEAE, treatment-emergent adverse event.
Drug-related AEs during active follow-up were reported in six patients (10%) overall: one patient receiving radium-223 plus abiraterone/prednisone (pathological fracture), three patients receiving radium-223 plus enzalutamide (pathological fracture, arthralgia, and acute promyelocytic leukemia), and two patients receiving radium-223 monotherapy (constipation and atypical femur fracture). Post-treatment drug-related SAEs occurred in one patient receiving radium-223 plus enzalutamide (acute promyelocytic leukemia) and one patient receiving radium-223 monotherapy (atypical femur fracture). In addition to the one patient in the enzalutamide combination arm who died during the study treatment period, 20 patients died during active 2-year follow-up, and 8 died during long-term follow-up. Most deaths during follow-up were related to prostate cancer (n = 26); none was considered to be study drug related.
Fractures/time to first fracture
The incidence of fractures during study treatment or active follow-up and median time to first fracture (censoring for death or loss to follow-up) are shown in Table 4. Fracture rates were generally lower in patients taking BHAs at baseline than in patients not taking BHAs at baseline (Table 4).
Table 4.
Number of fractures and time to first fracture during and after treatment (safety population)
| All patients in the safety population | Radium-223 monotherapy (n = 19) | Radium-223 + abiraterone/prednisone (n = 22) | Radium-223 + enzalutamide (n = 22) |
|---|---|---|---|
| Number of patients with ≥1 fracture, n (%) | 2 (11) | 4 (18) | 7 (32) |
| Median time to first fracture, months (80% CI) | 18 (6-18) | NE (17-NE) | 35 (24-35) |
| Event-free rate at 2 years, % (95% CI) | 0 | 72 (39-89) | 67 (38-85) |
| Patients without baseline BHA use, n | 11 | 15 | 14 |
| Patients with ≥1 fracture, n (%) | 1 (9) | 4 (27) | 5 (36) |
| Median time to first fracture, months (80% CI) | 18 (NE-NE) | NE (8-NE) | 35 (8-35) |
| Event-free rate at 2 years, % (95% CI) | 0 | 60 (23-84) | 58 (17-85) |
| Patients with baseline BHA use, n | 8 | 7 | 8 |
| Patients with ≥1 fracture, n (%) | 1 (13) | 0 | 2 (25) |
| Median time to first fracture, months (80% CI) | NE (6-NE) | NE (NE-NE) | NE (2-NE) |
| Event-free rate at 2 years, % (95% CI) | NE (NE) | 100 (100-100) | 75 (31-93) |
BHA, bone health agent; CI, confidence interval; NE, not estimable.
Discussion
This non-comparative trial was conducted to evaluate BSLA RR using technetium-99m bone scans in patients with mCRPC and bone metastases treated with radium-223 as monotherapy or in combination with either abiraterone/prednisone or enzalutamide. Patients in the combination arms generally had a longer median duration of treatment and a greater median number of radium-223 injections than patients in the monotherapy arm. The primary endpoint of a BSLA RR >5% at week 24 was met in each treatment arm and BSLA RRs were numerically greater in each combination arm than in the radium-223 monotherapy arm. The BSLA BORR during the study was consistent with the BSLA RR at week 24. Median rPFS and median times to radiologic non-bone and bone progression were not estimable in either combination arm (due to insufficient follow-up time) but were reached in the monotherapy arm. As patients generally stopped follow-up for radiologic progression at the first progression event, estimates of the components of radiologic progression (bone and non-bone) may be biased. A similar proportion of patients in each treatment arm experienced SSEs, but median SSE-FS was not estimable or longer with the combinations than with radium-223 monotherapy, suggesting that SSEs occurred later with combination therapy than with monotherapy.
The toxicity profile of each drug was consistent with previously reported clinical experience.3, 4, 5, 6, 7 Furthermore, although this study was non-comparative and low patient numbers preclude between-arm conclusions regarding survival and disease progression, the study had rigorous follow-up for drug-related and SSE-related events and no new safety signals for the combination regimens were reported for up to 2 years following the last study treatment.
Proportionally more patients had fractures with combination treatment than with radium-223 monotherapy. However, for all three treatment arms combined, the proportion of patients who experienced fractures was lower in patients receiving BHAs at baseline than in patients not receiving BHAs at baseline, although sample sizes and numbers of events were small. Time to first fracture results by treatment arm were inconclusive. Although current treatment guidelines recommend a BHA, such as denosumab or zoledronic acid, for all patients with mCRPC for the prevention of skeletal-related events,18,19 at the time the study was conducted (2014-2018), only 37% of patients were receiving BHAs at baseline, and BHA use during the study was not mandatory. Recent findings from the PEACE III trial of enzalutamide alone or in combination with radium-223 in patients with mCRPC showed that the increased risk of fracture with this combination is almost eliminated with mandatory use of BHAs,20 suggesting that mandatory BHA use in the current study may have reduced fracture rates for combination regimens. In the ERA 223 trial, which was not completed at the time of initiation of the current study, an increased fracture rate was reported with abiraterone/prednisone plus radium-223 (compared with abiraterone/prednisone plus placebo), yet only 39% of patients in the abiraterone/prednisone plus radium-233 group received concomitant BHAs. Moreover, in the ERA 223 combination therapy group, the fracture rate was lower in patients taking BHAs rather than not taking BHAs (15% versus 37%, respectively).11
Nevertheless, our results should be interpreted with caution as patients were followed for different durations in each treatment arm. The combination arms, which allowed abiraterone/prednisone or enzalutamide therapy for up to 2 years, had substantially longer treatment periods than the monotherapy arm, where treatment was generally limited to 24 weeks. In addition, discrepancies were evident in the proportions of patients who had received prior docetaxel therapy: 18% of patients in the radium-223 monotherapy group; 13% in the abiraterone/prednisone group; and 23% in the enzalutamide group. These differences may have contributed to intergroup differences in the primary outcome. However, such subgroup differences are normal and consistent with chance in a small randomized study. Consequently, the effect of these differences cannot be evaluated, given the small sample size. Future real-world evidence studies of sequential ARPI-radium-223 therapy in clinical practice would be useful to determine the impact of prior taxane therapy on BSLA measurements and rPFS in men with progressive CRPC and bone metastases. In addition, it is possible that the differential permissibility of concomitant therapy in the radium-223 monotherapy group compared with the other treatment groups may have resulted in bias.
This study also explored the feasibility of CAD-derived BSLA based on technetium-99m bone imaging to assess treatment response.16 A whole-body bone scan is the standard imaging assessment for patients with prostate cancer.12 However, visual assessment of lesion numbers based on technetium-99 imaging is not a reliable method to quantify changes in total bone lesion burden, as lack of image resolution and subjectivity in visual evaluation yield variability in scan interpretation.16 Consequently, standard bone scans are inadequate for measuring bone lesions and existing technology limitations require RECIST to classify bone metastases as non-measurable disease.14 Similarly, PCWG3 guidelines focused on new lesion detection rather than changes to existing lesions.17 In the current study, BSLA measurements derived using an automated CAD system provided a feasible, quantitative, and objective method to measure changes in isotope uptake in patients with mCRPC. Although additional evaluation of its reliability is required, this method may be able to differentiate between PR and SD, thereby permitting a response assessment. A meaningful comparison between technetium-99m and DW-MRI and Na18F PET/CT was not possible because the number of patient scans was too low.
Recently, the prognostic value of an automated bone scan index (aBSI) derived using artificial intelligence has been increasingly reported,21, 22, 23, 24 although for patients treated with radium-223, the aBSI requires validation in prospective studies.22 The aBSI primarily has been evaluated as a prognostic biomarker, whereas the BSLA is a quantitative imaging biomarker being evaluated primarily for treatment response assessment in prostate cancer.13 There has been clinical interest in the use of baseline aBSI and changes in aBSI during treatment as possible predictors of OS and time to symptom progression.21, 22, 23, 24 A recent analysis of 721 evaluable men with mCRPC enrolled in a phase III trial of tasquinimod reported that baseline aBSI was an independent prognostic imaging marker of survival in mCRPC,25 and a recent meta-analysis of aBSI in 567 patients with mCRPC from nine studies suggested that high baseline aBSI and a high change in aBSI during treatment were associated with poor survival.26 However, this technique had not been validated at the time our study was designed, and prospective studies of systemic therapies are needed to clearly define the utility of changes in aBSI over time as a primary endpoint in clinical trials and as a prognostic biomarker in routine clinical practice.22,24
Limitations of our study include its small sample size, lack of correlative studies with survival, the open-label design, differential concomitant therapy, and the small number of patients evaluable to explore the utility of an automated CAD system to measure BSLA and the use of DW-MRI and Na18F PET/CT to assess treatment response. The primary endpoint of BSLA RR was based on an experimental method, which could be considered a limitation, although conventional endpoints were also measured. Differences in median durations of treatment between arms make it difficult to draw conclusions on AE frequency between arms. Therefore, interpretation of the results of this phase IIa trial should consider the limited sample size and the use of BSLA as an exploratory method that was not confirmed by larger randomized studies at the time our study was conducted. Subsequent to the conduct of this study, a clinical validation was published of baseline BSLA and 12-week disease control calculated by the CAD BSLA method as a surrogate biomarker for OS in 198 men with mCRPC.13 However, a limitation of this validation study is that the two BSLA-based endpoints validated are different from the primary endpoint in our study, 24-week BSLA response.
In conclusion, in our study in patients with mCRPC and bone metastases treated with radium-223 alone or in combination with abiraterone/prednisone or enzalutamide, the primary endpoint of BSLA RR >5% was met in each treatment arm. In the combination arms, rPFS and time to radiologic progression were not estimable, and a lower bone fracture incidence was observed in patients who were receiving BHAs at baseline than in patients who were not receiving BHAs at baseline. Although patients receiving combination treatment experienced more drug-related TEAEs than patients receiving radium-223 monotherapy, the safety profile of each drug was consistent with previous experience and there were no new safety signals. However, no preferred treatment combination or sequencing can be recommended based on the results of this trial. The use of radium-223 should follow current evidence-based treatment guidelines.18,19 Our findings suggest that technetium-99m imaging BSLA, using a CAD system, may be an objective and quantifiable means of assessing isotope uptake changes in this patient population, and represents a potential method for assessing treatment response, improving assessment of technetium-99m bone scans, complementing other imaging modalities, and improving scan results in countries where more expensive imaging is unavailable. Although this study was too small to reliably correlate CAD-based technetium-99m imaging BSLA with clinical outcomes, a validation of this approach was subsequently conducted, although not with the 24-week BSLA response endpoint, which was the primary endpoint in our study. Future studies should examine its utility further in this setting.
Acknowledgements
Writing support for the preparation of this article was provided by Yvonne E. Yarker, PhD, of OPEN Health Communications (London, UK) with financial support from Bayer HealthCare.
Funding
This work was supported by Bayer HealthCare Pharmaceuticals Inc., Whippany, NJ, USA (no grant number).
Disclosure
DPP has received honoraria from Advanced Accelerator Applications, Amgen, Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Clovis Oncology, Eli Lilly, Exelixis, Incyte, Janssen, Pfizer, Pharmacyclics, Roche, Seattle Genetics, and UroGen; and has received research grants/funding from Advanced Accelerator Applications, Astellas, AstraZeneca, Bayer, Bristol-Myers Squibb, Clovis Oncology, Eli Lilly, Endocyte, Genentech, Innocrin, MedImmune, Merck, Novartis, Pfizer, Progenics, Roche, Sanofi Aventis, and Seattle Genetics. UNV has received honoraria, has acted as an advisor/consultant, and has received research grants/funding from Bayer, Sanofi Inc., Exelixis, Bristol-Myers Squibb, Pfizer, and EMD Serono Inc. CSH has acted as an advisor/consultant for Aptevo, Asana, Astellas, AstraZeneca, Bayer, Blue Earth Diagnostics, Churchill Pharma, Clovis Oncology, Dendreon, Endocyte, Ferring, Hinova Pharma, Janssen, Myriad Genetics, Orion Corporation, and Pfizer; has received research grants/funding from Aptevo, Bayer, Aragon Pharma, Astellas, AstraZeneca, Dendreon, Genentech, Hoffman-La Roche, Medivation, Sanofi, and Pfizer; and has received travel, accommodation, and expenses from Bayer, Blue Earth Diagnostics, Clovis Oncology, Ferring, Genentech, Hinova, Janssen, Myriad Genetics, Orion Corporation, and Pfizer. CA has acted as a speaker bureau/expert testimony for Sanofi. NAD has acted as a speaker bureau/expert testimony for Astellas/Pfizer and Janssen. BAM has received honoraria, travel, and accommodation expenses, and acted as an advisor/consultant for Astellas, Amgen, Bayer, Janssen, and Pfizer; and has received grants/funding from Astellas, Bayer, Janssen, and Pfizer. DIQ has received research grants/funding from Seattle Genetics, MSD, and Novartis; has acted as an advisor/consultant for Astellas, AstraZeneca, Bayer, BMS, Dendreon, Exelixis, Roche, Janssen, MSD, Novartis, Pfizer, and Sanofi; and has received travel, accommodation, and expenses from Pfizer, MSD, AstraZeneca, BMS, and Roche. OS reports grand and/or fees from Advanced Accelerator Applications, Astellas, AstraZeneca, Bayer, Bellicum, Blue Earth Diagnostics, Inc., Bristol-Myers Squibb, Celgene, Constellation, Dendreon, EMD Serono, Innocrin, Invitae, Johnson & Johnson, Merck, Myovant, Pfizer, Sanofi, and SOTIO. VJW, JS, and LT are employees of Bayer HealthCare Pharmaceuticals. All other authors have declared no conflicts of interest.
Supplementary data
Supplementary Figure S1.
Supplementary Figure S2.
References
- 1.Lorente D., Fizazi K., Sweeney C., de Bono J.S. Optimal treatment sequence for metastatic castration-resistant prostate cancer. Eur Urol Focus. 2016;2:488–498. doi: 10.1016/j.euf.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Bruland O.S., Nilsson S., Fisher D.R., Larsen R.H. High-linear energy transfer irradiation targeted to skeletal metastases by the alpha-emitter 223Ra: adjuvant or alternative to conventional modalities? Clin Cancer Res. 2006;12:6250s–6257s. doi: 10.1158/1078-0432.CCR-06-0841. [DOI] [PubMed] [Google Scholar]
- 3.de Bono J.S., Logothetis C.J., Molina A. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scher H.I., Fizazi K., Saad F. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 5.Ryan C.J., Smith M.R., de Bono J.S. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beer T.M., Armstrong A.J., Rathkopf D.E. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–433. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parker C., Nilsson S., Heinrich D. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 8.Kantoff P.W., Higano C.S., Shore N.D. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 9.de Bono J.S., Oudard S., Ozguroglu M. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 10.Tannock I.F., de Wit R., Berry W.R. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 11.Smith M., Parker C., Saad F. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:408–419. doi: 10.1016/S1470-2045(18)30860-X. [DOI] [PubMed] [Google Scholar]
- 12.Cook G.J., Azad G., Padhani A.R. Bone imaging in prostate cancer: the evolving roles of nuclear medicine and radiology. Clin Transl Imaging. 2016;4:439–447. doi: 10.1007/s40336-016-0196-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown M.S., Kim G.H.J., Chu G.H. Quantitative bone scan lesion area as an early surrogate outcome measure indicative of overall survival in metastatic prostate cancer. J Med Imaging (Bellingham) 2018;5:011017. doi: 10.1117/1.JMI.5.1.011017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisenhauer E.A., Therasse P., Bogaerts J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Scher H.I., Morris M.J., Stadler W.M. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol. 2016;34:1402–1418. doi: 10.1200/JCO.2015.64.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown M.S., Chu G.H., Kim H.J. Computer-aided quantitative bone scan assessment of prostate cancer treatment response. Nucl Med Commun. 2012;33:384–394. doi: 10.1097/MNM.0b013e3283503ebf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scher H.I., Halabi S., Tannock I. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Comprehensive Cancer Network® Prostate cancer version 2.2021 – February 17, 2021. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf Available at: Accessed March 11, 2021.
- 19.Mottet N, Cornford P, van den Bergh RCN, et al. EAU - EANM - ESTRO - ESUR - SIOG Guidelines on Prostate Cancer 2020. Available at: https://uroweb.org/wp-content/uploads/EAU-EANM-ESTRO-ESUR-SIOG-Guidelines-on-Prostate-Cancer-2020v4.pdf. Accessed March 11, 2021.
- 20.Tombal B.F., Loriot Y., Saad F. Decreased fracture rate by mandating bone-protecting agents in the EORTC 1333/PEACE III trial comparing enzalutamide and Ra223 versus enzalutamide alone: an interim safety analysis. J Clin Oncol. 2019;37(suppl 15):5007. [Google Scholar]
- 21.Ali A., Hoyle A.P., Parker C.C. The automated bone scan index as a predictor of response to prostate radiotherapy in men with newly diagnosed metastatic prostate cancer: an exploratory analysis of STAMPEDE's “M1|RT Comparison”. Eur Urol Oncol. 2020;3:412–419. doi: 10.1016/j.euo.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anand A., Tragardh E., Edenbrandt L. Assessing radiographic response to (223)Ra with an automated bone scan index in metastatic castration-resistant prostate cancer patients. J Nucl Med. 2020;61:671–675. doi: 10.2967/jnumed.119.231100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reza M., Wirth M., Tammela T. Automated bone scan index as an imaging biomarker to predict overall survival in the Zometa European Study/SPCG11. Eur Urol Oncol. 2019;4:49–55. doi: 10.1016/j.euo.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Mota J.M., Armstrong A.J., Larson S.M. Measuring the unmeasurable: automated bone scan index as a quantitative endpoint in prostate cancer clinical trials. Prostate Cancer Prostatic Dis. 2019;22:522–530. doi: 10.1038/s41391-019-0151-4. [DOI] [PubMed] [Google Scholar]
- 25.Armstrong A.J., Anand A., Edenbrandt L. Phase 3 assessment of the automated bone scan index as a prognostic imaging biomarker of overall survival in men with metastatic castration-resistant prostate cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2018;4:944–951. doi: 10.1001/jamaoncol.2018.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song H., Jin S., Xiang P. Prognostic value of the bone scan index in patients with metastatic castration-resistant prostate cancer: a systematic review and meta-analysis. BMC Cancer. 2020;20:238. doi: 10.1186/s12885-020-06739-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.