Abstract
Numerous post-Streptococcal syndromes (PSS) have been described in the literature.
The role of antibiotic therapy in the management of PSS is best established with acute rheumatic fever.
We present a patient with streptococcus-associated medium vessel vasculitis with multiple flares despite immunosuppressive therapy that achieved a sustained remission with long term oral penicillin V 250 mg twice daily.
Keywords: Vasculitis, Streptococcus, Antibiotic treatment
Case presentation
A 63-year-old man was referred to the rheumatology department following several months of episodic fevers, rigors, cognitive dysfunction and asthenia.
One month prior to the onset of these symptoms, new heamatospermia led to the diagnosis of a Gleason 7 grade prostate cancer. He had curative robotic prostatectomy with no adjuvant therapy.
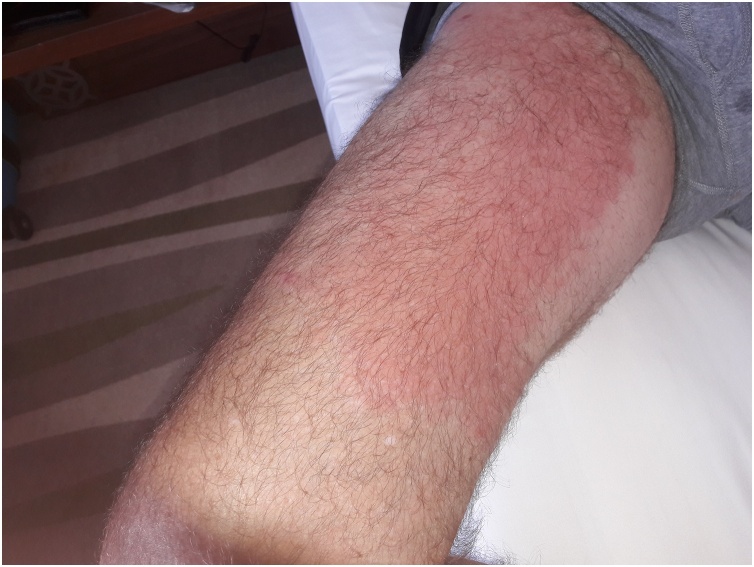
The patient reported paroxysmal episodes of fevers, night sweats and "fuzzy-headedness". Each episode lasted approximately two days, with asymptomatic intervals of three to four weeks. During the most recent episode, a transient rash appeared - the first time on his inner right thigh (Fig. 1) that his primary care provider diagnosed as herpes zoster and treated with oral acyclovir. The rash resolved within 2–3 days.
Fig. 1.
First right thigh vasculitic rash.
Two months later, he was admitted for recurrence of the rash on the same right thigh with systemic symptoms. C-reactive protein (CRP) was 129 mg/L at this time. Patient was discharged on Acyclovir. Two days later, he developed right knee monoarthritis which limited his mobility. He was again evaluated in the emergency department. Laboratory results included CRP of 23 mg/L, elevated anti-streptolysin O titre (ASOT) of 800 IU/L, negative blood cultures, negative respiratory viral throat screening panel, negative HIV 1 and 2 antigen/antibody test, and negative IgM antibody for Varicella Zoster, cytomegalovirus, Epstein-Barr and parvovirus. The knee aspirate performed twice during this admission, showed moderate number of neutrophil polymorphs, synovial matrix and occasional foamy macrophages. It was negative for bacterial organisms, herpes simplex virus PCR and crystal deposition. Three days later, he had a significant improvement and was discharged home.
Ten days later, he was seen in ambulatory follow up in the Rheumatology department. The patient was white with no known Mediterranean or Asian ancestry. He had no antecedent history of polyarthralgia, sicca symptoms or orogenital ulcerations. During his paroxysmal episodes he denied dysuria, respiratory or abdominal symptoms. Examination revealed mild residual erythema on the right thigh and a subtle effusion of the right knee. There was no lymphadenopathy. Chest and abdominal examinations were normal. Foot pulses were present. No microangiopathic phenomena or nail changes were identified. Laboratory evaluation revealed mildly raised inflammatory markers (CRP 6 mg/L and ESR 24 mm/h), mild neutrophilia (11.2 × 109/L), lymphopenia (0.4 × 109/L) and a uric acid level of 0.41 mmol/L. The remaining biochemistry results, serum protein electrophoresis, autoimmune screening tests and other various general pathology investigations were normal (Table 1).
Table 1.
General pathology tests.
| Test | Result | Ref Range & Units |
|---|---|---|
| Urea & Electrolytes | ||
| Serum sodium | 140 | 136−145 mmol/L |
| Serum potassium | 4.1 | 3.5−5.1 mmol/L |
| Serum urea | 5.8 | 2.8−8.1 mmol/L |
| Serum creatinine | 83 | 62−106 umol/L |
| eGFR (MDRD) per 1.73 sq m | >60 | 60−90 ml/min |
| Liver function tests | ||
| Serum albumin | 40 | 35−52 g/L |
| Serum total protein | 71 | 66−87 g/L |
| Serum globulin | 30 | 18−36 g/L |
| Serum alkaline phosphatase | 70 | 40−129 iu/L |
| Serum total bilirubin | 6 | 0−21 umol/L |
| Serum ALT | 15 | 0−41 iu/L |
| Bone profile (serum) | ||
| Serum calcium | 2.30 | 2.2−2.55 mmol/L |
| Serum adjusted calcium | 2.30 | 2.2−2.55 mmol/L |
| Serum phosphate | 0.71 | 0.87−1.45 mmol/L |
| Serum ferritin | 507 | 30−400 Ug/L |
| Prolactin serum | 276 | 86−324 miu/L |
| Serum cortisol | 129 | nmol/L |
| Serum LDH | 249 | 240−480 iu/L |
| Clotting screening | ||
| INR | 1.0 | 0.8−1.2 |
| APTT ratio | 1.0 | 0.8−1.2 |
| Fibrinogen | 7.8 | 1.8−4.5 g/L |
| Serum Creatinine Kinase (CK) | 35 | 39−308 iu/L |
| Thyroid function tests | ||
| Serum free thyroxine | 13.1 | 12−22 pmol/L |
| Serum TSH | 3.26 | 0.27−4.2 mu/L |
| Serum ACE (angiotensin converting enzyme) | 27 | 8−52 u/L |
| PSA (Serum prostate – specific Ag) | 0.34 | 0−41 ug/L |
| Serum protein electrophoresis | ||
| Serum immunoglobulin G | 8.9 | 7−16 g/L |
| Serum immunoglobulin A | 2.0 | 0.7−4 g/L |
| Serum immunoglobulin M | 0.9 | 0.4−2.3 g/L |
| Serum total protein | 65 | 66−87 g/L |
| Comment for SPE | Paraprotein band not detected | |
| Serum light chains | ||
| Serum Kappa light chains | 14 | 3−19 mg/L |
| Serum Lambda light chains | 9 | 6−29 mg/L |
| Serum Kappa: Lambda ratio | 1.56 | 0.26−1.65 |
| CD 4 + 8 | ||
| CD4 | 37 | 28−57 % |
| CD4 (abs) | 570 | 200−900 10^6/L |
| CD8 | 16 | 10−39 % |
| CD8 (abs) | 248 | 200−900 10^6/L |
| B&T CELL COUNT | ||
| T cell (abs) | 1233 | 700−2100 10^6/L |
| B cell | 7 | 6−19 % |
| B cell (abs) | 123 | 100−500 10^6/L |
| NK cell | 18 | 7−31 % |
| NK cell (abs) | 295 | 90−600 10^6/L |
| T cell | 74 | 55−83 % |
| Urine electrophoresis | ||
| Urine protein electrophoresis | No evidence of abnormal light chains | |
| Urine protein concentration | <40.0 | 0−150 mg/L |
| HLA B27 | Negative | |
| Autoimmune screening | ||
| p ANCA | negative | |
| c ANCA | negative | |
| Serum C3 | 1.58 | 0.81−1.57 g/L |
| Serum C4 | 0.36 | 0.13−0.39 g/L |
| Anti –nuclear antibody (ANA) | negative | negative |
| Extractable nuclear antigen autoantibodies (ENA) | negative | negative |
| Rheumatoid factor (RF) | 12 | 0−14 iu/mL |
| Anti –CCP antibodies (anti-citrullinated proten antibodies) | 1.1 | 0−7 u/mol |
| Infection screen | ||
| B. Burgdorferi IgM | Negative | |
| B. Burgdorferi Ig G | Negative | |
| Tropheryma Whippley PCR serology | Negative | |
| Throat swab culture | Negative | |
| Hepatitis B surface antigen | Negative | |
| Hepatitis C antibody | Negative | |
| Hepatitis A IgM | Negative | |
| Pneumococcal Ig G | 34.78 mg/L | 30 mg/L |
| Enteric pathogens (faeces) | ||
| Salmonella species DNA | Not detected | |
| Shigella species DNA | Not detected | |
| Campylobacter species DNA | Not detected | |
| VTEC DNA | Not detected | |
| Microscopy (Concentrate) | Pathogenic parasites not seen | |
| C. parvuum/C. hominis DNA | Not detected | |
| Giardia Lamblia DNA | Not detected | |
A plain chest film revealed no focal or interstitial changes. ECG and transthoracic echocardiogram were normal.
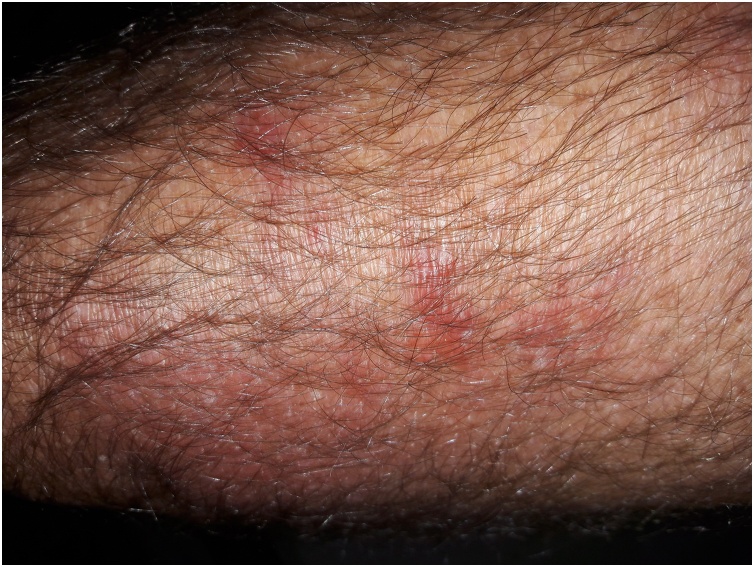
A presumed diagnosis of reactive arthritis secondary to an unidentified infective agent was made. The patient was treated with Naproxen 500 mg twice daily for a week. Owing to the recent prostatectomy, genitourinary infection was considered as a potential precipitant. During follow up eight 8 weeks later, the patient reported that his knee pain, swelling erythema had resolved fully, but he continued to have intermittent rigors, with the most recent episode two days prior. He again had an erythematous rash of the anterior right thigh. On this occasion, the rash was mildly tender and was not associated with lymphadenopathy (Fig. 2). Erythrocyte Sedimentation Rate (ESR) was mildly elevated 24 mm/hr, CRP was 123 mg/L and ferritin of 507 ug/L. Microscopic hematuria and proteinuria were present on urinalysis. Urine protein/creatinine ratio was normal and there were no casts on microscopy.
Fig. 2.
Recurrent right thigh vasculitic rash.
Multiple differential diagnoses were considered including Adult-onset Stills' disease, although the rash was not characteristic and the lymphopaenia, with only a very modest ferritin rise, would be uncommon. A periodic fever syndrome was excluded as subsequent molecular genetic testing was negative and indeed the majority of these syndromes arise before the third decade. A paraneoplastic phenomenon secondary to prostate cancer was considered but his prostate specific antigen (PSA) was in normal range and oncology follow ups confirm that the cancer was in remission. Reactive phenomena related to an unclear infective agent remained the main differential diagnosis.
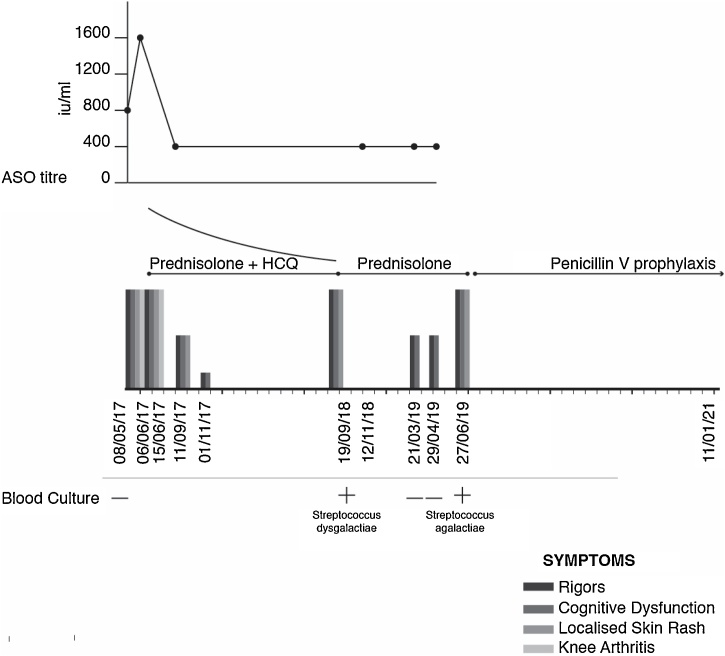
ASOT was 1600 IU/L (Fig. 3) on this occasion. The increasing ASOT suggested that the patient has been infected with Group A streptococcus in the preceding months. However, the chronology of ASOT levels did not initially appear to correlate with symptoms, nor was there evidence of active streptococcal infection. As such, antimicrobial therapy was not initiated. Group A streptococcus would be an unlikely urinary pathogen, as it is not normally associated with urinary tract infections or urogenital surgery. Further infectious disease investigations were normal (Table 1).
Fig. 3.
Clinical course corroborated with ASOT levels and blood cultures.
A punch biopsy from the thigh skin rash revealed an acute medium vessel vasculitis with some fat necrosis. A diagnosis of cutaneous polyarteritis nodosa (cPAN) was suspected. CT-angiogram was normal, the patient was normotensive, had no painful skin nodules, neuropathy, or orchitis and was hepatitis B negative, suggesting systemic PAN would be unlikely.
A repeat skin biopsy during a further episode a month later revealed marked neutrophilic infiltration of small artery walls, however no fibrinoid necrosis was present. Given the vasculitic nature of the skin rash, the patient was treated with oral prednisolone 30 mg daily for two weeks followed by a tapering regime within the following months. This lead to a significant improvement. However, when tapered below 5 mg prednisolone a day the systemic symptoms and rash recurred. Remission of symptoms was eventually achieved and maintained with 10 mg daily of prednisolone.
Hydroxychloroquine (HCQ) was added at 200 mg twice a day as a steroid-sparing agent. He required 7.5 mg prednisolone every other day to maintain control of his disease.
One year later, while still taking HCQ and prednisolone, the patient was admitted with a flare of symptoms. CRP was 72 mg/L and ASOT was 400 IU/mL. Group C streptococcus (S. dysgalactiae) was identified in a blood culture. He received 8 hourly intravenous Co-amoxiclav 1.2 g for two days followed by 6 hourly oral Clindamicin 450 mg for 10 days with resolution of all symptoms. HCQ was discontinued due to lack of significant benefit and the patient was advised to reduce the prednisolone by 1 mg every month.
Six months later he was re-admitted with similar symptoms. Further blood cultures were negative. Positron Emission Tomography with FDG tracer (FDG PETCT) revealed increased uptake in the colon, however targeted biopsies were normal. No uptake was identified on the heart valves and repeat transthoracic echocardiography was not suggestive of infective endocarditis.
Several months later, another flare lead to hospitalization and blood cultures on this occasion revealed Group B streptococcus (S. agalactiae). He was treated again with 8 hourly intravenous Co-amoxiclav 1.2 g for one day, followed by 8 hourly oral Amoxicillin 1 g for 10 days, resulting in rapid resolution of symptoms.
A diagnosis of streptococcus-associated medium vessel vasculitis was made.
The patient was discharged with oral phenoxymethylpenicillin 250 mg twice a day as prophylactic treatment and no further immunosuppressive therapies were given. There have been no further flares to date following prophylactic antibiotic therapy, with the patient reporting full symptom remission for the last 18 months (Fig.3).
Discussion
Several years, were required to reach optimal management in this case of relapsing medium size vessel vasculitis manifesting with periodic skin involvement and systemic symptoms. A suspected relationship with infection was considered from the early phase of his illness, however it was difficult to demonstrate an association. Following the first flare, Group A streptococcus infection was suspected, but the organism was not recovered on culture and throat swab. In the majority of post-streptococcal syndromes there is often a preceding infection that appears within one to 3 weeks. Only in some occult post streptococcal syndromes (e.g. movement and neuropsychiatric disorders) has delayed symptom onset been demonstrated [1].
Following identification of two other streptococci (S. agalactiae and dysgalactiae) during subsequent admissions with continued elevated ASOT, suspicion increased for an infective precipitant. It was particularly notable that during repeated admissions empiric antibiotic therapy would achieve a prompt resolution of symptoms. With penicillin antibiotic prophylaxis alone, the patient achieved remission without the need for ongoing immunosuppressive therapy.
The role of antibiotic therapy in the management of post-Streptococcal syndromes (PSS) is best established with acute rheumatic fever. The role of penicillin prophylaxis has been suggested in paediatric cases of streptococcus associated cPAN [[2], [3], [4], [5], [6], [7]]. The same prophylactic treatment was also suggested when a few adult streptococcus related cPAN have been reported but those cases have had the first vasculitis attack during childhood [8,9]. Our case is different because the patient developed streptococcus related medium vessel vasculitis in adult life.
With respect to the length of the penicillin prophylactic treatment duration for streptococcus related recurrent cPAN there isn’t a consensus approach. Some authors recommend a similar approach to the rheumatic fever [2]. At the time of their publication some reported good results with penicillin prophylaxis up to 2 years and as long as up 25 years [6,7]. Others recommended a lifelong penicillin prophylaxis [8].
For our patient we plan to continue with penicilline V 250 mg twice daily as prophylaxis for a total of two years. Following this we will instigate active surveillance for any flares. In case of flares, depending on their intensity (moderate or severe) we may prolong the prophylactic period up to 5 years or indeed lifelong if required.
This case is also unique by virtue of relapsing vasculitis being associated with different types of streptococcus (S agalactiae and dysgalactiae) infection without an identifiable source. Acquired or genetically-determined immunodeficiency was clearly a possibility to consider. However, the patient was found to have a normal serum protein electrophoresis and immunoglobulin levels. B and T lymphocyte subset analysis, complement levels, and Pneumococcal IgG titre were all normal, as was his spleen size and structure.
The clinical picture was not compatible with inherited complement deficiency as our patient had no recurrent infections in childhood. Inherited C1q deficiency, for example, is typically associated with severe SLE presenting also in childhood, not with adult onset vasculitis [10].
Leucocyte adhesion deficiency is typically associated with recurrent pyogenic infection, but also presents in infancy or childhood.
However, it is known that the type of capsular polysaccharide (CPS) found on Group B streptococci and also S. suis, notably the presence of terminal sialic acid, has in itself an immunosuppressive effect, particularly on dendritic cell function [11]. This may be relevant to the pathophysiology of both the recurrent infections, and the associated inflammatory disease observed in this case.
Learning points/take home messages
-
•
For relapsing medium vessel vasculitis of uncertain aetiology, thorough investigations for associated Streptococcal infections are warranted.
-
•
The diagnosis of Streptococcus-Associated Medium Vessel Vasculitis can be challenging. The exact temporal relationship between the inciting infection and the vasculitis onset is unclear but we suspect it can be variable (days, weeks or months).
-
•
Antibiotic prophylaxis should be considered in relapsing post-Streptococcal vasculitis, particularly if immunosuppressive treatment alone is unsuccessful.
Author statement
All authors have no conflict of interest to declare.
RS drafted the case report. KT, IO, VH and CT prepared the literature search and discussion. KAD had significant contribution in discussion. All authors reviewed and contributed towards the final manuscript.
Declaration of Competing Interest
No conflicts of interest are declared by any authors.
Acknowledgements
The authors are grateful to Igor Brbre, for evidence search: Recurrent streptococcus and vasculitis (29th January, 2021). Brighton, UK: Brighton and Sussex Library and Knowledge Service’’
The authors would also like to acknowledge Dr. Fang En Sin, Dr. Daniel Agranoff and Dr. Catherine Sargent for their efforts in the clinical management of this case.
The authors also thank BSUH (Brighton and Sussex University Hospital) Rheumatology CHARITABLE FUND that contributed towards paying part of the publication fee.
References
- 1.Gimzal Aylan, Topcuoglu Volkan, Yanki Yazgan M. Acute rheumatic fever, Sudenham’s chorea and psychopathology. Turk Psikiayatri Derg. 2002;13(2):137–141. Summer. [PubMed] [Google Scholar]
- 2.Faria de Paula Isabela H., Ramalho Carlos Eduardo, Almeida Fabiola D., Alves Andressa G., Santos Maria Carolina, Sacchetti Silvana B. Association between paediatric cutaneous polyarteritis nodosa and streptococcal infection-a single tertiary centre experience. Paediatr Rheumatol. 2017;15(Suppl 1):37. [Google Scholar]
- 3.Kumar A., Gupta A., Singhal M., Gupta A., Nada R., Minz R.W. A single centre experience of childhood polyarteritis nodosa from north India. Ann Rheum Dis. 2016;75:764. [Google Scholar]
- 4.Fathalla Basil M., Miller Laurie, Brady Stephen, Schaller Jane G. Cutaneous polyarteritis nodosa in children. J Am Acad Dermatol. 2005;53:724–728. doi: 10.1016/j.jaad.2005.03.065. [DOI] [PubMed] [Google Scholar]
- 5.Falcini F. Vascular and connective tissue diseases in the paediatric world. Lupus. 2004;13:77–84. doi: 10.1191/0961203304lu517rr. [DOI] [PubMed] [Google Scholar]
- 6.Falcini F., Lionetti P., Simoni G., Resti M., Cimaz R. Severe abdominal involvement as the initial manifestation of cutaneous polyarteritis nodosa in a young girl. Clin Exp Rheumatol. 2001;19:349–351. [PubMed] [Google Scholar]
- 7.Tonnelier Jeane-Marie, Ansart Severine, Tilly-Gentric Armelle, Pennec Yvon-Louis. Juvenile relapsing periarteritis nodosa and streptococcal infection. Joint Bone Spine. 2000;67:346–348. [PubMed] [Google Scholar]
- 8.Till S.H., Amos R.S. Long-term follow-up of juvenile-onset (cutaneous polyarteritis nodosa associated with streptococcal infection. Br J Rheumatol. 1997;36:909–911. doi: 10.1093/rheumatology/36.8.909. [DOI] [PubMed] [Google Scholar]
- 9.Anthony Albornoz M., Benedetto Anthony V., Korman Michael, McFall Susan, Tourtellotte Charles D., Myers Allen R. Relapsing cutaneous polyarteritis nodosa associated with streptococcal infections. Int J Dermatol. 1998;37:664–666. doi: 10.1046/j.1365-4362.1998.00493.x. [DOI] [PubMed] [Google Scholar]
- 10.Bowness P., Davies K.A., Norsworthy P.J., Athanassiou P., Taylor-Wiedeman J., Borysiewicz L.K. Hereditary C1q deficiency and systemic lupus erythematosus. QJM. 1994;87(August (8)):455–464. [PubMed] [Google Scholar]
- 11.Calzas Cynthia, Goyette-Desjardins Guillaume, Lemire Paul, Gagnon Fleur, Lachance Claude, Calsteren Marie-rose Van. Group B Streptococcus and Streptococcus suis capsular polysaccharides induce chemokine production by dendritic cells via toll-like receptor 2- and MyD88-dependent and -Independent pathways. Infect Immun. 2013;81(August (9)):3106–3118. doi: 10.1128/IAI.00113-13. [DOI] [PMC free article] [PubMed] [Google Scholar]