Abstract
OBJECTIVE
Higher resting heart rate (rHR) and lower heart rate variability (HRV) are associated with increased risk of cardiovascular disease (CVD) and all-cause mortality in people with and without diabetes. It is unknown whether temporal changes in rHR and HRV may contribute to this risk. We investigated associations between 5-year changes in rHR and HRV and risk of future CVD and death, taking into account participants’ baseline glycemic state.
RESEARCH DESIGN AND METHODS
In this prospective, population-based cohort study we investigated 4,611 CVD-free civil servants (mean [SD] age, 60 [5.9] years; 70% men). We measured rHR and/or six indices of HRV. Associations of 5-year change in 5-min rHR and HRV with fatal and nonfatal CVD and all-cause mortality or the composite of the two were assessed, with adjustments made for relevant confounders. Effect modification by glycemic state was tested.
RESULTS
At baseline, 63% of participants were normoglycemic, 29% had prediabetes, and 8% had diabetes. During a median (interquartile range) follow-up of 11.9 (11.4; 12.3) years, 298 participants (6.5%) experienced a CVD event and 279 (6.1%) died of non–CVD-related causes. We found no association between 5-year changes in rHR and HRV and future events. Only baseline rHR was associated with all-cause mortality. A 10 bpm–higher baseline HR level was associated with an 11.4% higher rate of all-cause mortality (95% CI 1.0–22.9%; P = 0.032). Glycemic state did not modify associations.
CONCLUSIONS
Changes in rHR and HRV and possibly also baseline values of these measures are not associated with future CVD or death in people with or without dysglycemia.
Introduction
Cardiovascular disease (CDV) is the leading cause of death globally of patients with and without dysglycemia globally (1). Thus, identifying persons at risk for CVD prior to development of overt disease, by use of risk markers, is essential to prevent later complications. In large, longitudinal, population-based cohort studies, single baseline assessments of high resting heart rate (rHR) and low heart rate variability (HRV), characterizing cardiac autonomic function, have been established as markers of increased risk of future CVD and death in people without diabetes (2–5) and patients with diabetes (6–9). High rHR and low HRV are also associated with preclinical pathological disease states, such as subclinical inflammation (10), dysglycemia (11,12), and vascular damage (13), all of which contribute to increased risk of future CVD and death. Individuals with prediabetes (14) and diabetes (15,16) are particularly prone to adverse levels of rHR and HRV. This can be a sign of diabetic autonomic neuropathy, a prevalent complication to prediabetes and diabetes.
Measures of rHR and HRV are easily obtainable by short-term noninvasive measures derived from electrocardiograms (ECGs); thus, they are obvious candidates for risk stratification. Temporal changes in rHR and HRV could be associated with an additional risk of CVD and death, over and above the risk conveyed by baseline rHR and HRV levels. If this were the case, sequential measures could prove to be useful monitoring tools in patient care, because rHR and HRV assessments could be obtained regularly at many patient consultations with low cost and little effort. To date, however, to our knowledge, associations between temporal changes in rHR and HRV and the risk of CVD and death have not been investigated in observational epidemiological settings of individuals without CVD.
To address this limitation, we investigated the prospective associations of measures of 5-year changes in rHR and HRV with incident CVD and death in normoglycemic individuals and those with dysglycemia, such as prediabetes and diabetes, in the large, population-based Whitehall II cohort study with repeated measurements of rHR and six indices of HRV.
Research Design and Methods
Access to data from the Whitehall study is possible by contacting the Whitehall team directly (whitehall2@ucl.ac.uk).
Study Participants
The Whitehall II study is an occupational cohort of 10,308 British civil servants (6,896 men and 3,412 women aged 35–55 years) of mainly White race who have been followed with clinical examinations every 5 years since 1985 (17).
Both rHR and HRV were measured at phase 5 (1997–1999), phase 7 (2002–2004), and phase 9 (2007–2009) of the study. Although rHR was assessed for all participants, HRV measurement involved only a subcohort of Whitehall participants. We used data from phases 5, 7, and 9 to assess 5-year change in rHR and HRV, with phase 7 being the baseline for this study. Changes were assessed for participants with data from phases 5 and 7. If participants also had additional data at phase 9, then changes between phases 7 and 9 also were included in the analyses.
Of the 6,967 study participants at phase 7, we excluded 1,111 (15.9%) with events of study outcomes such as previous CVD and stroke. Another 1,245 (17.9%) were excluded who had missing phase 5 data on rHR or HRV, leaving 4,611 participants for analyses. Although 5-year changes in rHR were available for all 4,611 study participants, only 1,675 (36.3%) had information on 5-year changes in HRV.
The study was reviewed and approved by the U.K. National Health Service Health Research Authority London-Harrow Ethics Committee, and written informed consent was obtained from each participant at each examination phase. The study was conducted according to the principles of the Helsinki Declaration.
Measurements and Definitions
The rHR and HRV indices were derived from 5-min resting 12-lead ECG recordings obtained subsequent to 5-min of rest in the supine position at phases 5, 7, and 9 of the study. Recordings were filtered through an automated algorithm, allowing the analyses of suitable normal-to-normal sinus rhythm R–R intervals without the presence of arrhythmias, ectopic beats, and branch blocks (normal-to-normal [N–N] intervals).
The following six indices of HRV were analyzed: 1) the SD of all N–N intervals; 2) the root mean square of the sum of the squares of differences between consecutive N–N intervals as measures of the time domain; 3) low-frequency power (i.e., in the 0.04–0.15 Hz frequency band); 4) high-frequency power (in the 0.15–0.4 Hz frequency band); 5) total power (in the ≤0.4 Hz frequency band) as measures of the frequency domain, using a Blackman-Tukey algorithm; and 6) the calculated ratio between low-frequency and high-frequency power. Root mean square of the sum of the squares of differences between consecutive normal-to-normal intervals and high-frequency power outcomes are associated mainly with parasympathetic modulations, whereas the remaining measures characterize mixed sympathetic and parasympathetic influences.
The rHR was obtained from either the aforementioned 5-min rHR measures or, if such measures were not assessable, rHR was obtained from standard 10-s 12-lead ECG recordings by the Burdick Eclipse 850 ECG recorder.
Plasma glucose, serum insulin, and serum lipid levels, as well as HbA1c and systolic and diastolic blood pressure at phases 5, 7, and 9 were measured as described previously (18). Information on smoking habit (never, ex-smoker, or current), physical activity (hours per week of mild, moderate, and vigorous physical activity), and medication use also was collected, using self-administered questionnaires, at phases 5, 7, and 9 (19).
Glycemic status was determined by measuring HbA1c using the following American Diabetes Association criteria (20): normal glycemia (<5.6%; 39 mmol/mol), prediabetes (5.6–6.4%; 39–47 mmol/mol), or diabetes (≥6.5%; 48 mmol/mol). Diabetes cases were also diagnosed outside the study by the treating physician.
Outcome Ascertainment
The primary outcomes were fatal and nonfatal CVD, total number of deaths, and a composite end point of CVD or death. The participants’ unique National Health Service (NHS) identification numbers were linked to the NHS Hospital Episode Statistics database (21). Incidence of CVD was assessed over the follow-up period from 2002 to 2004 to the end of follow-up (30 June 2015) and included fatal and nonfatal coronary heart disease (defined by ICD-9 codes 410–414 or ICD-10 codes I20–25) and stroke. Nonfatal myocardial infarction was determined using data from questionnaires, study ECGs, hospital acute ECGs, cardiac enzyme levels, and physician records (22). In the definition of stroke, cases identified by self-report only were excluded. Stroke included first subarachnoid hemorrhage, cerebral infarction, intracerebral hemorrhage, not-specified stroke (ICD-10 codes I60–I64), and transient cerebral ischemic attacks (ICD-10 code G45). Cases of stroke were ascertained from participants’ general practitioners, by information extracted from hospital medical records, or from the NHS Hospital Episode Statistics database. Ascertainment of cardiovascular events in the Whitehall II study has recently been validated (23). All-cause mortality was assessed from 2002 to 2004 to end of follow-up by flagging participants in the NHS Central Registry, which provided information on the cause and date of death.
Statistical Analysis
Incidence rates for CVD, all-cause mortality, or the composite of the two were estimated in separate models using Poisson regression analysis with the corresponding log-risk time as offset. For each participant, the follow-up period was split into 1-year age bands to account for the nonconstant effect of age over time on CVD risk and death (24) Exposure was 5-year changes in HR or HRV measures between phases 5 and 7.
Analyses were adjusted for age, sex, race/ethnicity, and baseline rHR or HRV measures (model 1), and additional adjustments were performed for glycemic state (normoglycemia, prediabetes, diabetes); BMI; physical activity; smoking; systolic blood pressure; levels of total cholesterol, LDL cholesterol, and triglycerides; and use of tricyclic antidepressants, diuretics, and β-blockers in a subsequent model (model 2). In model 2, we further tested for a modifying effect of baseline glycemic state on the outcome-exposure associations using the following interaction term: glycemic state multiplied by rHR or HRV. If significant, associations were calculated for the glycemic states separately in both models 1 and 2. For all HRV exposures, the models were additionally adjusted for the simultaneously measured HR at the current and previous phase.
For the subset of individuals with rHR or HRV measurements at phase 9 (86.6% for HR and 80.8% for HRV), we updated the covariates accordingly, treating all covariates as time varying. For example, a participant with prediabetes at phase 7 in whom diabetes developed between phases 7 and 9 first contributed with risk time in the prediabetes group and subsequently to the diabetes group.
To allow direct comparisons of incidence rate ratios between the exposure variables, we further calculated standardized regression coefficients for the subset of the population for whom rHR and all six HRV indices were available at the same time points (ie, the subset with autonomic function assessed). In a subsidiary analysis, we repeated the analyses with baseline levels of rHR or HRV measurements as exposure (i.e., removing the covariate for the 5-year changes from the models).
To avoid exclusion of patients with missing values, which may lead to biased results (25), the multivariate imputations by chained equations method (26) with missing-at-random assumptions (n = 50 imputations), and including a number of auxiliary data on the participants not used in the analyses, was used to impute missing data on the covariates at each phase. Exposure and outcome variables were not imputed. Estimates of parameters of interest were averaged across the imputation copies according to Rubin rules (27).
Subset analyses on determinants and future events were performed for patients not receiving β-blocker treatment. Statistical analyses were performed in R, version 3.6.1 (The R Foundation for Statistical Computing) and SAS, version 9.4 (SAS Institute, Cary, NC).
Data and Resource Availability
Whitehall II data, protocols, and other metadata are available to bona fide researchers for research purposes. Please refer to the Whitehall II data sharing policy at https://www.ucl.ac.uk/whitehallII/data-sharing.
Results
The median (interquartile range) follow-up time was 11.9s (11.4; 12.3) years for death and 11.9 (11.3; 12.2) years for CVD and the composite outcome, respectively. In the study population, rHR measures were available for analysis from 4,611, 4,611, and 4,029 individuals at phases 5, 7, and 9, respectively. A total of 4,611 participants had rHR measures at both phases 5 and 7 and 3,991 of these individuals had additional rHR measures at phase 9. In total, 8,602 pairs of measures of rHR at ∼5 years apart were used for analyses of change.
In the study population, HRV measures were collected from 2,381, 3,069, and 3,495 individuals at phases 5, 7, and 9, respectively. A total of 1,675 participants had HRV measures at both phases 5 and 7 and 1,353 of these individuals had additional HRV measures at phase 9. In total, 3,028 pairs of measures of HRV at ∼5 years apart were used for analyses of 5-year HRV change.
All measures of HRV diminished during the study period, as described previously (28). Changes during the study are presented in Supplementary Fig. 1. At baseline (phase 7), the study population consisted mainly of men (3,235 [70.2%] men vs 1,376 [29.8%] women); the mean (SD) age was 60.5 (5.9) years and the mean (SD) rHR was 68.0 (11.4) bpm. A majority of participants were normoglycemic, and glycemic state of participants was distributed as follows (interquartile range reported in parentheses): 63.3% (61.9; 64.7) were normoglycemic, 29.1% (27.8; 30.5) had prediabetes, 1.8% (1.4; 2.2) had diabetes detected on screening, and 5.8% (5.1; 6.5) had known diabetes. Detailed characteristics of the study population at phase 7 are listed in Table 1.
Table 1.
Baseline (phase 7) characteristics of the study participants by sex
| n (% available)* | Total | Men | Women | |
|---|---|---|---|---|
| No. of participants | 4,611 | 3,235 | 1,376 | |
| White race (%) | 4,611 (100) | 93.0 (92.2–93.7) | 95.0 (94.2–95.7) | 88.2 (86.4–89.9) |
| Age (years) | 4,611 (100) | 60.5 (5.9) | 60.3 (5.8) | 60.7 (5.9) |
| Height (cm) | 4,610 (100) | 171.3 (9.1) | 175.4 (6.6) | 161.5 (6.5) |
| BMI (kg/m2) | 4,599 (100) | 26.4 (4.2) | 26.3 (3.7) | 26.7 (5.1) |
| Current smoker (%) | 4,580 (99) | 7.4 (6.7–8.2) | 6.9 (6.0–7.8) | 8.8 (7.3–10.4) |
| Moderate to vigorous exercise (h/week) | 4,538 (98) | 11.5 (4.3; 20.0) | 12.8 (5.0; 21.5) | 8.5 (2.5; 16.3) |
| Alcohol intake (units/week) | 4,541 (98) | 8.0 (3.0; 17.0) | 10.0 (4.0; 20.0) | 4.0 (0.0; 10.0) |
| Glycemic state | ||||
| Normoglycemia (%) | 4,525 (98) | 63.3 (61.9–64.7) | 63.8 (62.1–65.4) | 62.3 (59.7–64.9) |
| Prediabetes (%) | 4,525 (98) | 29.1 (27.8–30.5) | 29.4 (27.8–31) | 28.5 (26.1–31) |
| Screening-detected diabetes (%) | 4,525 (98) | 1.8 (1.4–2.2) | 1.6 (1.2–2.1) | 2.2 (1.5–3.2) |
| Known diabetes (%) | 4,525 (98) | 5.8 (5.1–6.5) | 5.3 (4.5–6.1) | 6.9 (5.6–8.4) |
| Family history of diabetes (%) | 4,545 (99) | 10.3 (9.4–11.2) | 9.2 (8.2–10.3) | 13.0 (11.2–14.9) |
| Medication | ||||
| Antihypertensive treatment (%) | 4,592 (100) | 18.4 (17.3–19.5) | 17.8 (16.5–19.1) | 19.8 (17.7–22.0) |
| Lipid lowering treatment (%) | 4,592 (100) | 6.9 (6.1–7.6) | 6.5 (5.7–7.4) | 7.7 (6.4–9.3) |
| Tricyclic antidepressants (%) | 4,611 (100) | 2.6 (2.1–3.1) | 2.1 (1.6–2.7) | 3.7 (2.8–4.8) |
| Diuretics (%) | 4,592 (100) | 6.9 (6.2–7.7) | 5.9 (5.1–6.8) | 9.2 (7.7–10.9) |
| β-blockers (%) | 4,592 (100) | 6.8 (6.0–7.5) | 6.6 (5.8–7.5) | 7.1 (5.8–8.6) |
| Blood measurements | ||||
| Total cholesterol (mmol/L) | 4,574 (99) | 5.8 (1.0) | 5.7 (1.0) | 6.0 (1.0) |
| HDL cholesterol (mmol/L) | 4,574 (99) | 1.6 (0.5) | 1.5 (0.4) | 1.9 (0.5) |
| LDL cholesterol (mmol/L) | 4,526 (98) | 3.6 (0.9) | 3.6 (0.9) | 3.6 (1.0) |
| Triglycerides (mmol/L) | 4,574 (99) | 1.4 (0.9) | 1.4 (1.0) | 1.2 (0.7) |
| Systolic blood pressure (mmHg) | 4,608 (100) | 127.2 (16.3) | 127.9 (15.6) | 125.6 (17.8) |
| Diastolic blood pressure (mmHg) | 4,608 (100) | 74.2 (10.3) | 74.7 (10.2) | 72.8 (10.4) |
| Fasting plasma glucose (mmol/L) | 4,567 (99) | 5.4 (1.1) | 5.5 (1.1) | 5.2 (1.0) |
| 2-h plasma glucose (mmol/L) | 3,852 (84) | 6.5 (2.0) | 6.5 (2.1) | 6.5 (1.9) |
| HbA1c (%) | 4,519 (98) | 5.7 (0.6) | 5.7 (0.6) | 5.7 (0.7) |
| HbA1c (mmol/mol) | 4,519 (98) | 38.4 (6.7) | 38.3 (6.4) | 38.7 (7.3) |
| HR indices | ||||
| HR from ECG (bpm) | 4,611 (100) | 68.0 (11.4) | 67.7 (11.7) | 68.9 (10.6) |
| SDNN (ms) | 3,069 (67) | 33.9 (25.5; 44.6) | 33.8 (25.3; 44.7) | 34.1 (26.1; 44.5) |
| RMSSD (ms) | 3,069 (67) | 20.5 (13.6; 30.1) | 19.7 (13.2; 28.7) | 21.8 (14.6; 32.7) |
| LF power (ms2) | 3,069 (67) | 286.9 (158.8; 528.7) | 301.1 (165.0; 554.2) | 264.7 (151.6; 474.4) |
| HF power (ms2) | 3,069 (67) | 115.0 (55.5; 232.6) | 105.9 (50.5; 213.7) | 146.4 (66.3; 295.0) |
| LF/HF ratio | 3,069 (67) | 2.59 (1.58; 4.09) | 2.91 (1.81; 4.51) | 1.92 (1.14; 3.03) |
| Total power (ms2) | 3,069 (67) | 1,011 (573; 1,742) | 1,010 (571; 1,778) | 1,012 (586; 1,678) |
Data are reported as mean (SD), median (25th; 75th percentiles), or proportion (95% CI). HF, high-frequency; LF, low-frequency; RMSSD, root mean square of the sum of the squares of differences between consecutive N–N R–R intervals; SDNN, standard deviation of N–N R–R intervals.
n (%) refers to the number of participants (percentage of total cohort) with data on the respective variable prior to multiple imputation.
During follow-up, 298 (6.5%) of the study participants had a CVD event (n = 47 fatal; n = 251 nonfatal). An additional 279 (6.1%) died of non–CVD-related causes, resulting in a total of 577 (12.5%) events for the composite outcome (Table 2).
Table 2.
Number of CVD events, all-cause mortality, or the composite of the two by glycemic state in study population prior to imputation
| CVD | All-cause mortality | Composite outcome | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Normal glycemia | 161 | 5.6 | 184 | 6.4 | 321 | 11.2 |
| Prediabetes | 90 | 6.8 | 102 | 9.7 | 181 | 13.7 |
| Diabetes | 38 | 11.1 | 33 | 8.1 | 61 | 17.8 |
| Unclassifiable | 9 | 10.5 | 7 | 8.1 | 14 | 16.3 |
| Total population | 298 | 6.5 | 319 | 6.9 | 577 | 12.5 |
n = number of events; % = percentage of group with an event.
There was no modifying effect of glycemic state on any of the associations between 5-year change in rHR or HRV measures and the rate of an event (P ≥ 0.051). Hence, the interaction term was removed from the model.
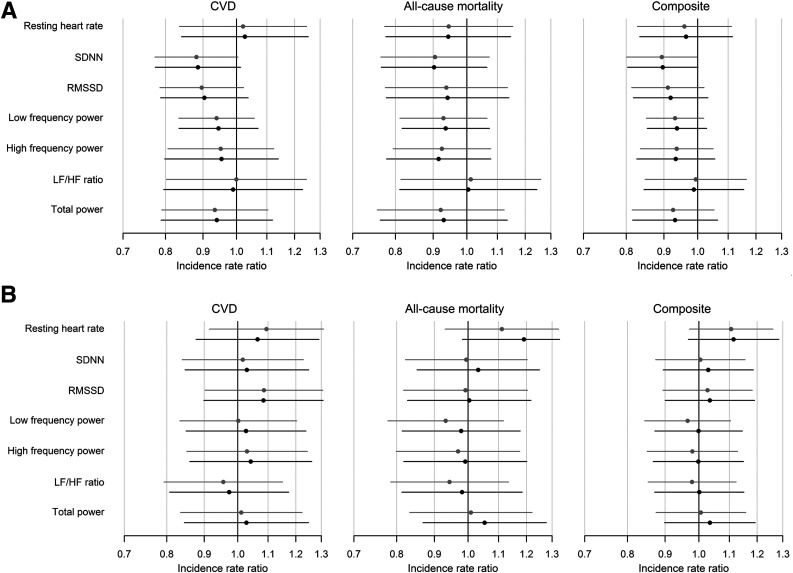
We found no statistically significant association of 5-year changes in rHR or HRV with risk of first CVD event, all-cause mortality, or the composite (see Table 3, Fig. 1, and Supplementary Table 1 for estimates of an SD in change in determinants). The range of rate ratios per 10-unit change in rHR or HRV measures was from 0.96 to 1.10, indicating small or neutral effect size. One association (between 5-year change in SD of all N–N intervals and rate of the composite event) reached statistical significance (P = 0.046), suggesting a clinically not important negative association, which was expected, given the number of tests performed. Subset analyses on patients not receiving β-blocker treatment (Supplementary Table 3) showed no difference in results.
Table 3.
Rate ratios with 95% CI of 10-unit difference in 5-year changes in HR or HRV on the incidence of CVD, all-cause mortality, or the composite of the two
| CVD | All-cause mortality | Composite | |||||
|---|---|---|---|---|---|---|---|
| 5-years change in: | Model | RR | P* | RR | P* | RR | P* |
| rHR (10 bpm) | 1 | 1.08 (0.95–1.23) | 0.258 | 1.01 (0.90–1.14) | 0.855 | 1.03 (0.93–1.12) | 0.602 |
| 2 | 1.10 (0.96–1.25) | 0.166 | 1.01 (0.90–1.14) | 0.864 | 1.03 (0.94–1.13) | 0.518 | |
| SDNN (10 ms) | 1 | 0.96 (0.92–1.00) | 0.058 | 0.97 (0.92–1.02) | 0.250 | 0.97 (0.93–1.00) | 0.046 |
| 2 | 0.96 (0.93–1.00) | 0.076 | 0.97 (0.92–1.02) | 0.224 | 0.97 (0.94–1.00) | 0.053 | |
| RMSSD (10 ms) | 1 | 0.98 (0.95–1.00) | 0.103 | 0.99 (0.95–1.03) | 0.511 | 0.98 (0.96–1.00) | 0.106 |
| 2 | 0.98 (0.95–1.01) | 0.150 | 0.99 (0.95–1.03) | 0.538 | 0.98 (0.96–1.01) | 0.155 | |
| LF power (10 ms2) | 1 | 1.00 (1.00–1.00) | 0.301 | 1.00 (1.00–1.00) | 0.294 | 1.00 (1.00–1.00) | 0.123 |
| 2 | 1.00 (1.00–1.00) | 0.371 | 1.00 (1.00–1.00) | 0.342 | 1.00 (1.00–1.00) | 0.172 | |
| HF power (10 ms2) | 1 | 1.00 (1.00–1.00) | 0.559 | 1.00 (1.00–1.00) | 0.316 | 1.00 (1.00–1.00) | 0.259 |
| 2 | 1.00 (1.00–1.00) | 0.604 | 1.00 (1.00–1.00) | 0.289 | 1.00 (1.00–1.00) | 0.272 | |
| LF/HF ratio (10 units) | 1 | 1.00 (0.37–2.66) | 0.997 | 1.06 (0.40–2.80) | 0.913 | 0.97 (0.48–1.97) | 0.941 |
| 2 | 0.95 (0.36–2.52) | 0.924 | 1.02 (0.39–2.65) | 0.968 | 0.95 (0.47–1.90) | 0.882 | |
| Total power (10 ms2) | 1 | 1.00 (1.00–1.00) | 0.424 | 1.00 (1.00–1.00) | 0.420 | 1.00 (1.00–1.00) | 0.240 |
| 2 | 1.00 (1.00–1.00) | 0.487 | 1.00 (1.00–1.00) | 0.475 | 1.00 (1.00–1.00) | 0.298 | |
HF, high frequency; LF, low frequency; RMSSD, root mean square of the sum of the squares of differences between consecutive N–N R–R intervals; RR, rate ratio; SDNN, standard deviation of N–N R–R intervals.
P value for the test of the rate ratio being equal to unity (i.e., no effect).
Figure 1.
Association of 5-year change in HR and HRV (A) or higher baseline values of the measures (B) with incidence of events. Association of (A) 1 SD difference in 5-year change in rHR and HRV or of (B) 1 population SD higher baseline value in HR or in the log of HRV indices at baseline with incidence of a fatal or nonfatal CVD, all-cause mortality, or the composite of the two. Data shown for rHR are only for participants with simultaneous HRV measures. Analyses were adjusted for age, sex, race/ethnicity, and, for (A), also baseline HR/HRV (model 1: gray lines). HRV indices were additionally adjusted for the simultaneously measured HR. Additional adjustments were made for glycemic state; BMI; physical activity; smoking; systolic blood pressure; levels of total cholesterol, LDL cholesterol, and triglycerides; and use of tricyclic antidepressants, diuretics, and β-blockers (model 2: black lines). The x-axis is logarithmic. HF, high frequency; LF, low frequency; RMSSD, root mean square of the sum of the squares of differences between consecutive N–N intervals; SDNN, standard deviation of all normal-to-normal R-R intervals.
In the subsidiary analyses of baseline levels of rHR and HRV, we found similar results. There was no modifying effect of glycemic state on any of the associations (P ≥ 0.298), and there was no association between baseline levels of rHR or HRV and risk of first CVD event, all-cause mortality, or the composite end point (Fig. 1 and Supplementary Table 2). The range of rate ratios per 10-unit–higher baseline rHR or HRV measures was from 0.91 to 1.11 (most at ∼1.00), indicating small or neutral effect size. Again, one association (between baseline rHR and rate all-cause mortality) with the expected direction reached statistical significance (P = 0.040) with a rate ratio of 1.11 (95% CI 1.00–1.22).
Conclusions
In this large, population-based, longitudinal study of people without CVD, 5-year changes in rHR indices or HRV were not associated with future CVD events in a median follow-up of 12 years. The null finding was observed irrespective of participants’ glycemic state at the beginning of follow-up. In subsidiary analyses, high baseline rHR was associated with increased mortality risk, but no other associations of baseline levels of HRV with subsequent events were found in this cohort.
To our knowledge, this is the first study to assess temporal changes in rHR and HRV in relation CVD and death in people without CVD. In a post hoc analysis of the double-blind, randomized trials Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial and Telmisartan Randomized Assessment Study in ACE Intolerant Subjects With Cardiovascular Disease (6) with a 56-month follow-up, the associations of changes in rHR with cardiovascular events were studied in patients with coronary, peripheral, or cerebrovascular disease, or diabetes with end-organ damage. Participants were assigned to receive 10 mg of ramipril daily, 80 mg of telmisartan daily, or their combination, or either 80 mg of telmisartan daily or placebo telmisartan daily, or their combination. Both higher baseline rHR and greater in-trial increases of rHR were associated with an increased CVD incidence with no threshold value for rHR (6). These observations may suggest that temporal changes in rHR may be a valid risk marker only in patients at a high risk of CVD.
Except for greater mortality risk in participants with high baseline rHR, baseline levels of rHR and HRV were not associated with the end points of our study. In previous investigations, researchers have reported partly inconsistent findings. In the U.S. Framingham (3,29) and Atherosclerosis Risk in Communities (ARIC) studies (2), for example, high rHR and low HRV were linked to increased risk of future CVD and all-cause mortality. Similar findings for rHR were seen in the FINRISK cohort with regard to CVD (4). The baseline clinical markers of our study population were collected more recently (2002–2004) than the clinical markers from the Framingham (1948–1971), ARIC (1987–1989), and FINRISK (1982–1997) studies, reflecting a period of more aggressive preventive medication treatments, which may explain weaker associations in the Whitehall II study. However, the level of HRV (measured during 1989–1990) was not associated with death in participants with and without diabetes in the Monitoring of Trends and Determinants in Cardiovascular Disease/Cooperative Health Research in the Region of Augsburg (MONICA/KORA) study (30).
Among patients with diabetes in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) (31), Action to Control Cardiovascular Risk in Diabetes (ACCORD) (8), and Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) (9) trials, baseline measures of rHR and HRV were linked to future events. The baseline clinical markers were collected in 1993–2006, 2001–2005, and 2001–2013, respectively, and thus recommendations for CVD prevention were similar as for the Whitehall population. These were interventional trials and assessments were done on either in-trial associations or post-trial follow-up. Hence, results cannot be regarded as observational and may be confounded by trial participation. On the other hand, a causal relationship cannot be ruled out either. It has been hypothesized that increased rHR and sympathetic overactivation, as seen in prediabetes and diabetes, can contribute to vascular disease through mechanisms such as the promotion of atherosclerosis, increasing oxygen demand of the heart, and the promotion of cardiac arrythmias (32–34). These mechanisms may play a role in the development of vascular disease in prediabetes and diabetes. However, a causal relationship has yet to be established. Notably, interventional trials targeted at a reduction of HR in patients with existing CVD has only shown an improvement in heart failure outcomes (35) but not in vascular disease (36). There is not a tangible explanation of why the data from this study do not exhibit any differences in association in different glycemic states. It could be speculated that autonomic dysfunction characterized as high rHR and low HRV could be signs of different pathogenic mechanisms in these states. Patients with normoglycemia are not exposed to high blood glucose levels and, therefore, autonomic dysfunction in this group may be a sign of other conditions than in individuals with dysglycemia. However, the lack of differences between glycemic groups may be just a sign of the possible lack of predictive value of rHR and HRV in general.
The lack of associations in our study could be attributed to recent changes in the distribution of risk factors of CVD in younger birth cohorts. Such cohort effects have been described in the Whitehall II cohort study, showing that younger birth cohorts have a higher BMI compared with older cohorts but with lower levels of total cholesterol (37). This may indicate a change in associations between CVD risk markers (e.g., that higher BMI is not as strongly associated with cholesterol levels as previously) and, therefore, may not be as strong a risk marker for CVD when preventive treatment with, for example,, cholesterol-lowering medication is implemented. It is possible that rHR and HRV may have lost their association with later events due to preventive medicine.
Strengths and Weaknesses
Our study benefits from its large sample size, the comprehensive measurements of outcomes and exposures assessed simultaneously, and an extensive adjustment for confounders. Importantly, the prospective design with repeated data allowed us to assess associations of changes in rHR and HRV with major future events. End points were adjudicated by health care professionals ensuring valid diagnoses.
The study population was predominantly of European descent and based on people employed as civil servants, so the results may not be generalizable to other ethnic groups, unemployed people, those employed in the private sector or who do manual labor. In addition, the cohort seems to have a high level of physical activity and a low prevalence of smokers compared with national surveys performed in the U.K. (38). Therefore, the study findings may not be fully generalizable to the general population. In addition, the present cohort consists of a relatively small subset of participants with diabetes at baseline (n = 343) and, therefore, interpretation of results for patients with diabetes should be done with caution. The HRV measures were short-term measures of 5 min. Such measures may be prone to some issues with reproducibility, which may affect out findings. It is possible that longer measuring periods might have revealed an association with end point (e.g., the circadian rhythm of HRV is associated with future events [39]). In addition, more robust measures for estimating autonomic dysfunction, such as cardiovascular autonomic reflex tests, may prove to have associations in contemporary cohorts with future CVD, as show previously (40).
For cerebral vascular disease, transient ischemic attack was accepted as a valid diagnosis for cerebral CVD. Transient ischemic attack may be a less reliable diagnosis for cerebral ischemia because it is a transient deficit that may not exhibit measurable paraclinical signs that can validate the diagnosis.
Conclusion
In summary, our study findings suggest changes in rHR and 5-min HRV may not be useful risk markers for the development of the first CVD event or all-cause mortality in people with and without dysglycemia. Also, baseline measures of theses markers may not be risk markers of future events in a contemporary, general, CVD-free population.
Article Information
Acknowledgments. The authors thank all the participants in the Whitehall II Study, as well as all Whitehall II research scientists, study and data managers, and clinical and administrative staff who make the study possible.
Funding. The U.K. Medical Research Council (grants K013351 and R024227), British Heart Foundation, and the U.S. National Institutes of Health (grants R01HL36310 and R01AG013196) have supported collection of data in the Whitehall II study. D.R.W. is supported by the Danish Diabetes Academy, which is funded by an unrestricted grant from the Novo Nordisk Foundation. M.K. had research grants from the U.K. Medical Research Council (grants K013351 and R024227), the U.S. National Institute on Aging (grant R01AG056477), NordForsk, the Academy of Finland (grant 311492), and Helsinki Institute of Life Science during the conduct of the study. M.M. has a New Horizons grant from British Heart Foundation (grant NH/16/2/32499).
The funders of the study had no role in study design, data collection, analysis, interpretation, or writing of the report.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. C.S.H., M.E.J., D.R.W., E.J.B., A.G.T., M.K., and D.V. contributed to the study concept and design. M.M., D.R.W., E.J.B., A.G.T., and M.K. contributed the data. C.S.H. and D.V. planned the statistical analysis and drafted the manuscript. D.V. conducted the statistical analysis. All authors contributed to, critically revised, and approved the final version of the manuscript. C.S.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented at the European Association for the Study of Diabetes 2019 annual meeting, Barcelona, Spain, 16–20 September 2019.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.13526351.
References
- 1.Gregg EW, Cheng YJ, Srinivasan M, et al. Trends in cause-specific mortality among adults with and without diagnosed diabetes in the USA: an epidemiological analysis of linked national survey and vital statistics data. Lancet 2018;391:2430–2440 [DOI] [PubMed] [Google Scholar]
- 2.Dekker JM, Crow RS, Folsom AR, et al. Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes: the ARIC Study. Atherosclerosis Risk in Communities. Circulation 2000;102:1239–1244 [DOI] [PubMed] [Google Scholar]
- 3.Tsuji H, Larson MG, Venditti FJ Jr., et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation 1996;94:2850–2855 [DOI] [PubMed] [Google Scholar]
- 4.Cooney MT, Vartiainen E, Laatikainen T, Juolevi A, Dudina A, Graham IM. Elevated resting heart rate is an independent risk factor for cardiovascular disease in healthy men and women [published correction appears in Am Heart J 2010;160:208]. Am Heart J 2010;159:612–619.e3 [DOI] [PubMed] [Google Scholar]
- 5.Ho JE, Larson MG, Ghorbani A, et al. Long-term cardiovascular risks associated with an elevated heart rate: the Framingham Heart Study. J Am Heart Assoc 2014;3:e000668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lonn EM, Rambihar S, Gao P, et al. Heart rate is associated with increased risk of major cardiovascular events, cardiovascular and all-cause death in patients with stable chronic cardiovascular disease: an analysis of ONTARGET/TRANSCEND. Clin Res Cardiol 2014;103:149–159 [DOI] [PubMed] [Google Scholar]
- 7.Pop-Busui R, Braffett BH, Zinman B, et al.; DCCT/EDIC Research Group . Cardiovascular autonomic neuropathy and cardiovascular outcomes in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes Care 2017;40:94–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pop-Busui R, Evans GW, Gerstein HC, et al.; Action to Control Cardiovascular Risk in Diabetes Study Group . Effects of cardiac autonomic dysfunction on mortality risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care 2010;33:1578–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hillis GS, Woodward M, Rodgers A, et al. Resting heart rate and the risk of death and cardiovascular complications in patients with type 2 diabetes mellitus. Diabetologia 2012;55:1283–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aeschbacher S, Schoen T, Dörig L, et al. Heart rate, heart rate variability and inflammatory biomarkers among young and healthy adults. Ann Med 2017;49:32–41 [DOI] [PubMed] [Google Scholar]
- 11.Hillebrand S, Swenne CA, Gast KB, et al. The role of insulin resistance in the association between body fat and autonomic function. Nutr Metab Cardiovasc Dis 2015;25:93–99 [DOI] [PubMed] [Google Scholar]
- 12.Saito I, Hitsumoto S, Maruyama K, et al. Heart rate variability, insulin resistance, and insulin sensitivity in Japanese adults: the Toon Health Study. J Epidemiol 2015;25:583–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubin J, Blaha MJ, Budoff MJ, et al. The relationship between resting heart rate and incidence and progression of coronary artery calcification: the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 2012;220:194–200 [DOI] [PubMed] [Google Scholar]
- 14.Papanas N, Vinik AI, Ziegler D. Neuropathy in prediabetes: does the clock start ticking early? Nat Rev Endocrinol 2011;7:682–690 [DOI] [PubMed] [Google Scholar]
- 15.Carnethon MR, Golden SH, Folsom AR, Haskell W, Liao D. Prospective investigation of autonomic nervous system function and the development of type 2 diabetes: the Atherosclerosis Risk in Communities study, 1987-1998. Circulation 2003;107:2190–2195 [DOI] [PubMed] [Google Scholar]
- 16.Wulsin LR, Horn PS, Perry JL, Massaro JM, D’Agostino RB. Autonomic imbalance as a predictor of metabolic risks, cardiovascular disease, diabetes, and mortality. J Clin Endocrinol Metab 2015;100:2443–2448 [DOI] [PubMed] [Google Scholar]
- 17.Hansen CS, Færch K, Jørgensen ME, et al. Heart rate, autonomic function, and future changes in glucose metabolism in individuals without diabetes: the Whitehall II cohort study. Diabetes Care 2019;42:867–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tabák AG, Jokela M, Akbaraly TN, Brunner EJ, Kivimäki M, Witte DR. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Lancet 2009;373:2215–2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stringhini S, Batty GD, Bovet P, et al. Association of lifecourse socioeconomic status with chronic inflammation and type 2 diabetes risk: the Whitehall II prospective cohort study. PLoS Med 2013;10:e1001479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Diabetes Association . 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018;41(Suppl. 1):S13–S27 [DOI] [PubMed] [Google Scholar]
- 21.Hinnouho GM, Czernichow S, Dugravot A, et al. Metabolically healthy obesity and the risk of cardiovascular disease and type 2 diabetes: the Whitehall II cohort study. Eur Heart J 2015;36:551–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marmot M, Brunner E. Cohort profile: the Whitehall II study. Int J Epidemiol 2005;34:251–256 [DOI] [PubMed] [Google Scholar]
- 23.Kivimäki M, Batty GD, Singh-Manoux A, Britton A, Brunner EJ, Shipley MJ. Validity of cardiovascular disease event ascertainment using linkage to UK hospital records. Epidemiology 2017;28:735–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carstensen B. Age-period-cohort models for the Lexis diagram. Stat Med 2007;26:3018–3045 [DOI] [PubMed] [Google Scholar]
- 25.Janssen KJ, Donders AR, Harrell FE Jr., et al. Missing covariate data in medical research: to impute is better than to ignore. J Clin Epidemiol 2010;63:721–727 [DOI] [PubMed] [Google Scholar]
- 26.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res 2007;16:219–242 [DOI] [PubMed] [Google Scholar]
- 27.Marshall A, Altman DG, Holder RL, Royston P. Combining estimates of interest in prognostic modelling studies after multiple imputation: current practice and guidelines. BMC Med Res Methodol 2009;9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jandackova VK, Scholes S, Britton A, Steptoe A. Are changes in heart rate variability in middle-aged and older people normative or caused by pathological conditions? Findings from a large population-based longitudinal cohort study. J Am Heart Assoc 2016;5:e002365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kannel WB, Kannel C, Paffenbarger RS Jr., Cupples LA. Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J 1987;113:1489–1494 [DOI] [PubMed] [Google Scholar]
- 30.Ziegler D, Zentai CP, Perz S, et al.; KORA Study Group . Prediction of mortality using measures of cardiac autonomic dysfunction in the diabetic and nondiabetic population: the MONICA/KORA Augsburg Cohort Study. Diabetes Care 2008;31:556–561 [DOI] [PubMed] [Google Scholar]
- 31.Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group . Risk factors for cardiovascular disease in type 1 diabetes. Diabetes 2016;65:1370–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beere PA, Glagov S, Zarins CK. Retarding effect of lowered heart rate on coronary atherosclerosis. Science 1984;226:180–182 [DOI] [PubMed] [Google Scholar]
- 33.Custodis F, Schirmer SH, Baumhäkel M, Heusch G, Böhm M, Laufs U. Vascular pathophysiology in response to increased heart rate. J Am Coll Cardiol 2010;56:1973–1983 [DOI] [PubMed] [Google Scholar]
- 34.Palatini P, Casiglia E, Julius S, Pessina AC. High heart rate: a risk factor for cardiovascular death in elderly men. Arch Intern Med 1999;159:585–592 [DOI] [PubMed] [Google Scholar]
- 35.Swedberg K, Komajda M, Böhm M, et al.; SHIFT Investigators . Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study [published correction appears in Lancet 2010;376:1988]. Lancet 2010;376:875–885 [DOI] [PubMed] [Google Scholar]
- 36.Fox K, Ford I, Steg PG, Tendera M, Robertson M, Ferrari R; BEAUTIFUL Investigators . Heart rate as a prognostic risk factor in patients with coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a subgroup analysis of a randomised controlled trial. Lancet 2008;372:817–821 [DOI] [PubMed] [Google Scholar]
- 37.Hulmán A, Tabák AG, Nyári TA, et al. Effect of secular trends on age-related trajectories of cardiovascular risk factors: the Whitehall II longitudinal study 1985-2009. Int J Epidemiol 2014;43:866–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.NHS Digital . Health Survey for England 2018. Accessed 26 January 2021. Available from http://healthsurvey.hscic.gov.uk/support-guidance/public-health/health-survey-for-england-2018.aspx
- 39.Malik M, Farrell T, Camm AJ. Circadian rhythm of heart rate variability after acute myocardial infarction and its influence on the prognostic value of heart rate variability. Am J Cardiol 1990;66:1049–1054 [DOI] [PubMed] [Google Scholar]
- 40.Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care 2003;26:1553–1579 [DOI] [PubMed] [Google Scholar]