Abstract
OBJECTIVE
The use of remote real-time continuous glucose monitoring (CGM) in the hospital has rapidly emerged to preserve personal protective equipment and reduce potential exposures during coronavirus disease 2019 (COVID-19).
RESEARCH DESIGN AND METHODS
We linked a hybrid CGM and point-of-care (POC) glucose testing protocol to a computerized decision support system for continuous insulin infusion and integrated a validation system for sensor glucose values into the electronic health record. We report our proof-of-concept experience in a COVID-19 intensive care unit.
RESULTS
All nine patients required mechanical ventilation and corticosteroids. During the protocol, 75.7% of sensor values were within 20% of the reference POC glucose with an associated average reduction in POC of 63%. Mean time in range (70–180 mg/dL) was 71.4 ± 13.9%. Sensor accuracy was impacted by mechanical interferences in four patients.
CONCLUSIONS
A hybrid protocol integrating real-time CGM and POC is helpful for managing critically ill patients with COVID-19 requiring insulin infusion.
Introduction
Several studies have confirmed the association of in-hospital hyperglycemia with increased mortality in patients with coronavirus disease 2019 (COVID-19) (1–3). Multiple transformations in inpatient diabetes care are occurring during this pandemic to preserve personal protective equipment (PPE) and reduce exposures while maintaining glucose control (4,5). In early April 2020, the U.S. Food and Drug Administration notified continuous glucose monitoring (CGM) device manufacturers that it would not object to inpatient use of CGM systems (6), and recent reports have shown the feasibility of remote glucose monitoring in patients with COVID-19 using CGM in the hospital (7–10).
While inpatient use of CGM outside of the intensive care unit (ICU) may reduce patient-provider interactions, more significant benefit is likely to be observed among patients receiving continuous insulin infusion (CII) who require hourly point-of-care (POC) glucose testing. This POC glucose testing burden during a public health crisis could lead to the use of subcutaneous insulin regimens in situations where CII would normally be indicated. Moreover, the severity of illness and clinical characteristics of ICU patients with COVID-19 (i.e., vasopressor requirements, medical nutrition therapy, high-dose glucocorticoid therapy, renal failure), make the safety and efficacy of subcutaneous insulin regimens difficult to maintain (10). Therefore, it is paramount to develop protocols that reduce PPE waste, nursing workload, and infectious exposures while maintaining glycemic control and reducing the risk of iatrogenic hypoglycemia.
Based on 1) our preliminary data using CGM in the cardiac ICU (11), 2) inpatient CGM use in non-ICU units (NCT03877068, NCT03832907), 3) experience with computerized decision support algorithms in the ICU (12–14), and 4) our inpatient clinical practice, we emergently implemented a hybrid CGM/POC glucose testing protocol linked to a computerized CII algorithm along with electronic health record (EHR) documentation to care for critically ill patients with active or suspected COVID-19 at Grady Memorial Hospital in Atlanta, GA. We report here our proof-of-concept with our first nine patients.
Research Design and Methods
Diabetes Technology and EHR Documentation in the ICU
POC glucose values were obtained with a Food and Drug Administration-approved POC glucometer (Nova StatStrip) to measure arterial or capillary glucose. We used a factory-calibrated CGM device (Dexcom G6) including three platforms:
Dexcom G6 App for transmission of sensor data to a smartphone (with internet connectivity) placed outside (within 20 feet) the patient’s room.
Follow App for remote monitoring (PharmD and endocrinologists) and glucose telemetry (tablet) at the nursing station (15).
Clarity Dashboard for population-based data monitoring.
We used Glucommander, a computerized algorithm integrated in our hospital’s EHR (EPIC) to provide computer-guided CII adjustments. The software adjusts the multiplier according to glucose trends, insulin sensitivity, and response to therapy and has been associated with faster achievement of glucose targets and a reduction in iatrogenic hypoglycemia (13,14). For documentation and validation of CGM sensor values, we created a flowsheet in EPIC (see Supplementary Material). Initial CGM sensor validation was confirmed if there was <20% variance compared with POC glucose values for 2 consecutive hours. Sensor validation was assessed once every 6 h thereafter. Only POC glucose values were used for glucose levels <100 mg/dL.
We report data for nine patients treated on this hybrid protocol, including clinical characteristics, glycemic control metrics, insulin requirements, sensor interferences, and POC testing frequency. The study was approved by the Emory Institutional Review Board.
Results
Patients with diabetes and active or suspected COVID-19 (seven of nine had confirmed PCR testing) started the hybrid CGM/POC + Glucommander protocol with improvement in glycemic control within 12 h and consistent validation for most sensor values. Figure 1 shows superimposed POC and CGM glucose trends and hourly insulin doses. The arrows show conditions associated with CGM/POC discrepancies.
Figure 1.
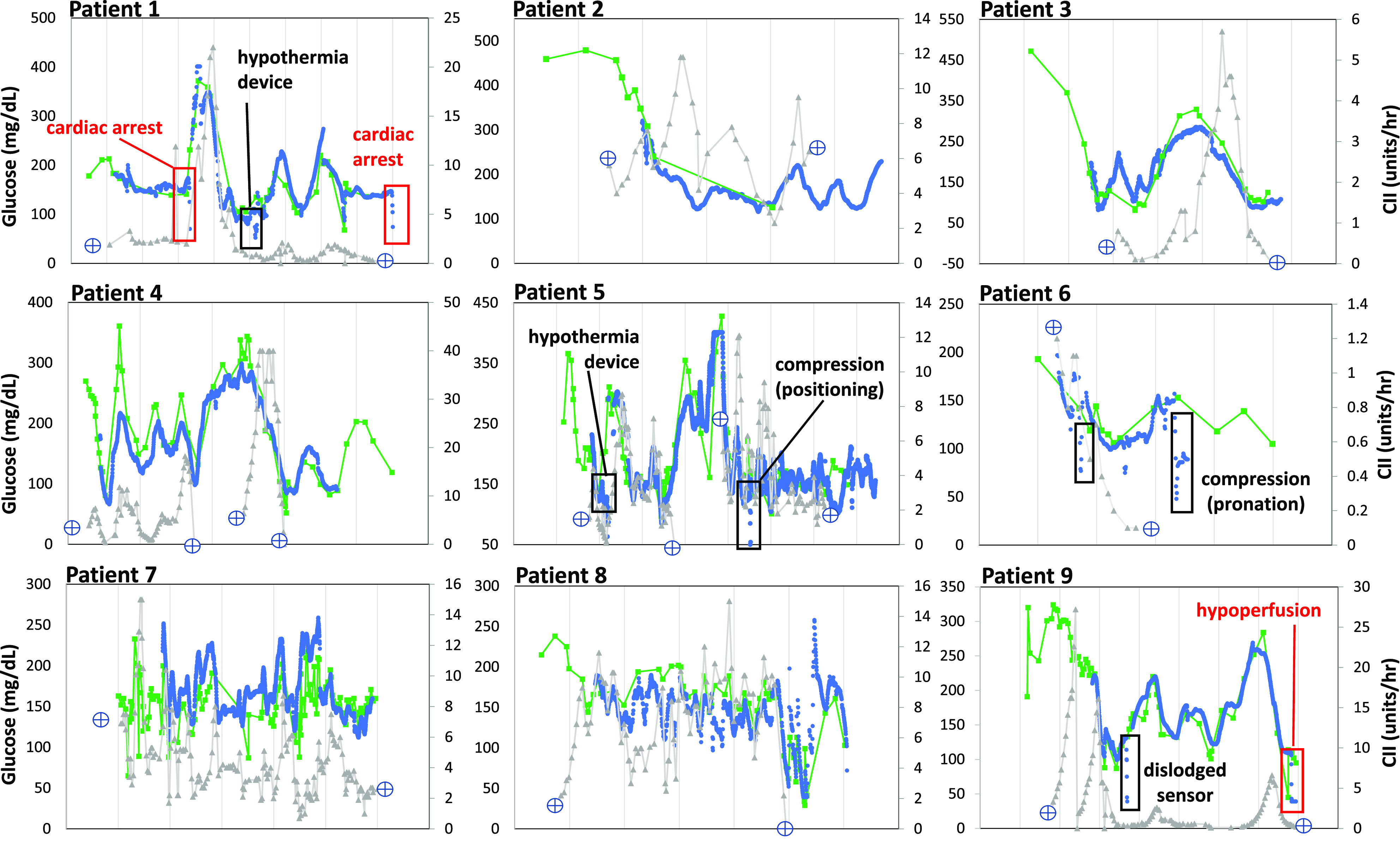
POC (Nova StatStrip glucose meter), real-time CGM (Dexcom G6), and hourly insulin requirements in critically ill patients treated with a computerized CII algorithm (Glucommander). Labeled boxes show identified interferences.
The mean age was 65.9 ± 15.2 years, 67% were men (n = 6), and 89% were African American (n = 8). All patients had type 2 diabetes and blood glucose values >180 mg/dL before starting CII. All patients were on mechanical ventilation and were treated with steroids. Six (67%) were receiving renal replacement therapy. Patient characteristics are summarized in Supplementary Table 1. Eight of nine sensors met initial validation criteria after sensor warm-up (Supplementary Fig. 1).
During hypoperfusion (i.e., pulseless electrical activity, shock), sensor glucose values read lower compared with arterial POC samples, with a sharp decline in sensor values and subsequent signal loss during cardiac arrest and defibrillator use. Similar findings (negative sensor glucose bias) were observed with therapeutic hypothermia protocols and during pronation or position changes causing sensor compression (e.g., bathing). All discrepancies were remotely recognized with alarms. During these interferences, glucose testing reverted to POC testing alone, and the hybrid protocol was resumed after sensor revalidation. Potential interferences are summarized in Supplementary Table 2.
The mean duration on the CGM/POC hybrid protocol with CII was 3.6 ± 2.2 days. Two patients (patients 4 and 5) resumed CII with CGM due to rebound hyperglycemia after initial transition to subcutaneous insulin. During time on the hybrid protocol, 75.7% of sensor glucose values >100 mg/dL were within 20% of the reference POC glucose comparator (validation criteria), with a mean number of POC glucose tests per day of 8.24 ± 3.06. Assuming hourly POC glucose testing requirements during CII, the hybrid protocol resulted in a 63% reduction in required POC tests. Mean time in range (70–180 mg/dL) was 71.4 ± 13.9%. Time between 180 and 250 mg/dL was 19.8 ± 97% and time >250 mg/dL was 7.5 ± 7.3%. Time below range (<70 mg/dL) was 0.6 ± 0.9%. CGM glycemic control metrics are summarized in Supplementary Fig. 2.
Conclusions
Critically ill patients with diabetes and COVID-19 are commonly on vasopressors, steroids, and medical nutrition therapy and are less likely to achieve recommended glucose targets (140–180 mg/dL) on subcutaneous insulin regimens (10,16). A protocol involving multiple stakeholders to implement a hybrid approach (real-time CGM with POC validation every 6 h) with hourly EHR documentation guiding computerized CII is feasible in the ICU and can reduce POC glucose testing without compromising glycemic control.
Although current real-time CGM sensors are not ready to replace POC testing, given the potential for interferences during intensive care leading to discrepancies between POC and sensor glucose values (i.e., signal loss, sensor compression, hypothermia protocols, hypoperfusion, surgery, MRI), the technology has significantly advanced and can provide meaningful improvements in inpatient care. Several advantages to our approach include 1) a reduction in nursing bedside encounters and PPE use; 2) the ability to achieve and maintain adequate glycemic control; 3) minimization of patient discomfort (fingersticks) and blood loss (arterial/venous samples); 4) remote real-time glucose monitoring by additional staff/consult team and glucose telemetry; 5) EHR documentation to easily assess sensor function and facilitate validation; and 6) a comprehensive remote evaluation of overall performance metrics.
A careful and systematic evaluation of emergent care transformations during these unprecedented times is needed (4). As the health care community works toward a new normal, the use of diabetes technology can help alleviate staff concerns related to work burden, exposure, and PPE consumption, while improving glycemic control during this health care crisis.
Article Information
Acknowledgments. The authors would like to thank Maina Karanja, Hang Phang, and Bimpe Umardeen (Grady Health System, Atlanta, GA), who are providing outstanding care to their patients with COVID-19 and used the sensors in the first three patients, and Makeba Lippitt (Grady Health System, Atlanta, GA) for completing the flowsheet build in EPIC. The authors would also like to thank the Dexcom COVID-19 response team for facilitating CGM devices for implementation across hospitals in the U.S. Dexcom had no role in the protocol design or approach.
Funding. This work was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant P30DK111024 from the Georgia Center for Diabetes Translation Research (F.J.P.). G.M.D. is supported in part by National Institutes of Health (NIH) under NIDDK award number 1K23DK122199-01A1. G.U. is partly supported by research grants from the NIH/National Center for Advancing Translational Sciences UL1 TR002378 and NIDDK 1P30DK111024-01. S.A. is supported in part by NIH under NIDDK award numbers K23DK115896 and P30DK111022. F.J.P. is supported in part by NIH National Institute of General Medical Sciences under award number 1K23GM12822101A1 and NIDDK under award numbers P30DK111024 and P30DK11102405S1.
Duality of Interest. G.U. has received unrestricted research support from Sanofi, Novo Nordisk, and Dexcom. K.D. has received research support from Eli Lilly, Novo Nordisk, Abbott, Sanofi Aventis, and Viacyte and consulting/advisory fees from Eli Lilly and Nova Biomedical. F.J.P. has received research support from Dexcom and Merck and consulting fees from Boehringer Ingelheim. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. G.M.D. adapted the protocol, researched the data, trained critical care staff, monitored patients, and edited the first manuscript draft and successive versions. E.F. shared teaching material and her implementation experience at The Ohio State University during multiple virtual meetings and edited the manuscript. T.W. designed the flowsheet and edited the manuscript. D.V., B.M., P.H., N.P., P.S., C.P.-G., S.S.T., and G.S.M. participated in the design of the protocol, supported the implementation, and edited successive drafts. M.R. edited the manuscript and monitored patients. J.H. assisted with data collection and reviewed the manuscript. L.P. performed the statistical analyses and reviewed the manuscript. G.U., S.A., and K.D. critically reviewed and edited the manuscript. F.J.P. adapted and implemented the protocol, researched the data, and wrote the first draft. F.J.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.13570862.
This article is part of a special article collection available at https://care.diabetesjournals.org/collection/diabetes-and-COVID19.
References
- 1.Klonoff DC, Messler JC, Umpierrez GE, et al. Association between achieving inpatient glycemic control and clinical outcomes in hospitalized patients with COVID-19: a multicenter, retrospective hospital-based analysis. Diabetes Care. 15 December 2020 [Epub ahead of print]. DOI: 10.2337/dc20-1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bode B, Garrett V, Messler J, et al. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J Diabetes Sci Technol 2020;14:813–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sardu C, D’Onofrio N, Balestrieri ML, et al. Outcomes in patients with hyperglycemia affected by COVID-19: can we do more on glycemic control? Diabetes Care 2020;43:1408–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pasquel FJ, Umpierrez GE. Individualizing inpatient diabetes management during the coronavirus disease 2019 pandemic. J Diabetes Sci Technol 2020;14:705–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galindo RJ, Aleppo G, Klonoff DC, et al. Implementation of continuous glucose monitoring in the hospital: emergent considerations for remote glucose monitoring during the COVID-19 pandemic. J Diabetes Sci Technol 2020;14:822–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dexcom. Fact Sheet for Healthcare Providers: Use of Dexcom Continuous Glucose Monitoring Systems During the COVID-19 Pandemic. Accessed 14 August 2020. Available from https://www.dexcom.com/hospitalfacts
- 7.Reutrakul S, Genco M, Salinas H, et al. Feasibility of inpatient continuous glucose monitoring during the COVID-19 pandemic: early experience. Diabetes Care 2020;43:e137–e138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shehav-Zaltzman G, Segal G, Konvalina N, Tirosh A. Remote glucose monitoring of hospitalized, quarantined patients with diabetes and COVID-19. Diabetes Care 2020;43:e75–e76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ushigome E, Yamazaki M, Hamaguchi M, et al. Usefulness and safety of remote continuous glucose monitoring for a severe COVID-19 patient with diabetes. Diabetes Technol Ther 2021;23:78–80 [DOI] [PubMed] [Google Scholar]
- 10.Agarwal S, Mathew J, Davis GM, et al. Continuous glucose monitoring in the intensive care unit during the COVID-19 pandemic. Diabetes Care. 23 December 2020 [Epub ahead of print]. DOI: 10.2337/dc20-2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perez-Guzman M, Duggan E, Gibanica S, et al. Continuous glucose monitoring in the operating room and cardiac intensive care unit. Diabetes Care 2021;44:e50–e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newton CA, Smiley D, Bode BW, et al. A comparison study of continuous insulin infusion protocols in the medical intensive care unit: computer-guided vs. standard column-based algorithms. J Hosp Med 2010;5:432–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ullal J, Aloi JA, Reyes-Umpierrez D, et al. Comparison of computer-guided versus standard insulin infusion regimens in patients with diabetic ketoacidosis. J Diabetes Sci Technol 2018;12:39–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Umpierrez G, Cardona S, Pasquel F, et al. Randomized controlled trial of intensive versus conservative glucose control in patients undergoing coronary artery bypass graft surgery: GLUCO-CABG trial. Diabetes Care 2015;38:1665–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spanakis EK, Levitt DL, Siddiqui T, et al. The effect of continuous glucose monitoring in preventing inpatient hypoglycemia in general wards: the glucose telemetry system. J Diabetes Sci Technol 2018;12:20–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gianchandani R, Esfandiari NH, Ang L, et al. Managing hyperglycemia in the COVID-19 inflammatory storm. Diabetes 2020;69:2048–2053 [DOI] [PubMed] [Google Scholar]


