Abstract
OBJECTIVE
To investigate the effect on pregnancy outcome of integrating a comprehensive management plan for patients with type 1 diabetes (T1D) into the World Health Organization universal maternal care infrastructure.
RESEARCH DESIGN AND METHODS
A comprehensive preconception-to-pregnancy management plan for women with T1D was implemented in 11 centers from 8 Chinese cities from 2015 to 2017. Sequential eligible pregnant women (n = 133 out of 137 initially enrolled) with T1D and singleton pregnancies attending these management centers formed the prospective cohort. The main outcome was severe adverse pregnancy outcome comprising maternal mortality, neonatal death, congenital malformations, miscarriage in the second trimester, and stillbirth. We compared pregnancy outcomes in this prospective cohort with two control groups with the same inclusion and exclusion criteria: a retrospective cohort (n = 153) of all eligible pregnant women with T1D attending the same management centers from 2012 to 2014 and a comparison cohort (n = 116) of all eligible pregnant women with T1D receiving routine care from 2015 to 2017 in 11 different centers from 7 cities.
RESULTS
The rate of severe adverse pregnancy outcome was lower in the prospective cohort (6.02%) than in either the retrospective cohort (18.30%; adjusted odds ratio [aOR] 0.31 [95% CI 0.13–0.74]) or the contemporaneous comparison cohort (25.00%; aOR 0.22 [95% CI 0.09–0.52]).
CONCLUSIONS
The substantial improvements in the prospective cohort are evidence of a potentially clinically important effect of the comprehensive management plan on pregnancy outcomes among Chinese pregnant women with pregestational T1D. This supports the development of similar approaches in other countries.
Introduction
Women with type 1 diabetes (T1D) are at increased risk of adverse pregnancy outcomes (1). Better glycemic control achieved via technologies such as continuous glycemic monitoring (CGM), preconception folic acid supplementation, and weight-gain management improve pregnancy outcomes in women with T1D (1,2). Comprehensive maternal care management guidelines for pregnancy complicated with T1D have been implemented in Europe (3,4) with an associated decline in stillbirth and congenital malformation rates (1,5), but pregnancy outcomes in patients with T1D remain suboptimal (6—8). In some high-income countries, no improvement in certain adverse outcomes was observed in the past decade (7,8). Many pregnant women with T1D live in low- or middle-income countries that lack the necessary social and medical resources required to implement comprehensive maternal management guidelines during pregnancy (9,10), and the effectiveness of guidelines appropriate for implementation in low- or middle-income countries is unknown. Such evidence is needed as a basis for effective and practicable guidance in developing countries.
The World Health Organization (WHO) has recommended a universal maternal care framework that has been successfully implemented globally, including in China and many low- and middle-income countries (11,12). Previous studies also supported that that integration of disease-specific maternity services into that preexisting framework of the general maternal care can be beneficial for the pregnant women complicated with chronic conditions (13,14). Therefore, the integration of a comprehensive management plan for women with T1D who are planning a pregnancy or are pregnant into the preexisting WHO maternal care infrastructure in China offered a unique opportunity to investigate the effectiveness of such an intervention in a middle-income country. This study, the CARNATION study, was conceived to gather such evidence.
Research Design and Methods
Development of the Comprehensive Pregnancy Management Plan for Women With T1D
In 2014, the National Health and Family Planning Commission of China initiated a project aimed to improve the pregnancy care for women with T1D. A group of experts (the CARNATION committee) reviewed published articles in peer-reviewed journals, consensus statements, and international guidelines (15–18) and proposed a comprehensive, evidence-based preconception-to-pregnancy management plan for women with T1D. The content of the management plan was then adapted to be embedded into the preexisting Chinese national maternal care framework to facilitate implementation.
The management plan is made up of a checklist for the relevant health care providers (HCPs), a one-page educational leaflet for pregnant women with T1D and their families, and educational support for both the patients and the HCPs. The checklist for the HCPs focused on 20 items of care, which covered different stages of care: preconception care, pregnancy care during each trimester, and postpartum care. The items included information on multidisciplinary cooperation and recommendations on diet, management of diabetes and its complications, blood pressure management, prevention and treatment of preeclampsia, and other medical issues (Supplementary Table 1). The one-page educational leaflet for pregnant women with T1D and their families gave specific information on glycemic targets, dietary advice, and important symptoms for identifying potential complications (Supplementary Table 2).
The management plan was approved by the National Health and Family Planning Commission of China. Since 2015, the plan was pilot tested in 11 general medical centers from 8 cities across China (Supplementary Table 3). These centers were selected from member institutions of the Chinese Diabetes Society. They were all public hospitals, had the capability to care for high-risk pregnancies, such as pregnancy in diabetes, and were geographically evenly distributed. At all of these management centers, the management plan was embedded into the preexisting universal Chinese maternal care infrastructure guidelines, which were developed based on recommendations by the WHO (routine antenatal care) (19) (Fig. 1). Briefly, the checklist and the one-page leaflet were distributed to HCPs and women with T1D who were pregnant or planning pregnancy in obstetric/endocrinology clinics at all management centers. The CARNATION committee organized a 2-day workshop to provide educational support for the personnel involved in the delivery of the management plan (Supplementary Material, Management of Care Integration), with written care plans for implementation. The committee also released educational materials via national academic meetings, professional websites, and social media.
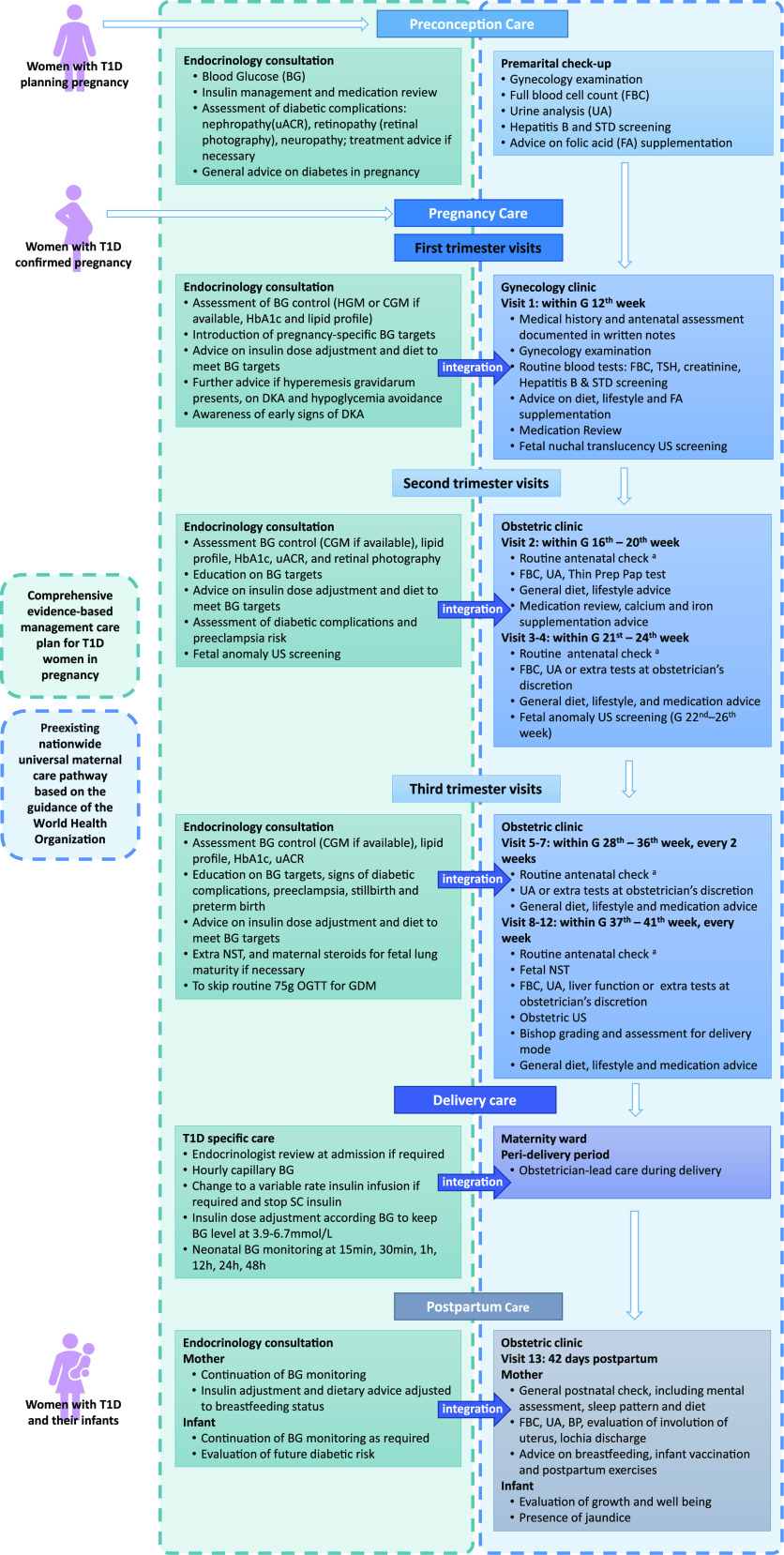
Figure 1.
Integration of the comprehensive preconception-to-pregnancy management plan for T1D to the universal maternal care framework. This figure depicts the integration of the comprehensive evidenced-based preconception-to-pregnancy management plan for women with T1D into the mandatory visits of the Chinese nationwide universal maternal care pathway based on the guidance of the WHO. A routine antenatal check includes measurement of maternal height, weight, blood pressure, uterus fundal height, and abdominal circumference, assessment of edema, and measurement of fetal heart rate. BP, blood pressure; DKA, diabetic ketoacidosis; FA, folic acid; FBC, full blood cell count; G, gestational; HGM, home blood glucose monitoring; NST, nonstress test; OGTT, oral glucose tolerance test; STD, sexually transmitted disease; TSH, thyroid-stimulating hormone; uACR, urinary albumin-to-creatinine ratio; US, ultrasound.
Apart from the educational one-page leaflet, patient education for women with T1D attending the management centers was also given by free online materials released by the CARNATION committee, via a smartphone-based application (20). This application was introduced to women with T1D by their care providers.
Prospective Cohort
Between 1 January 2015 and 31 December 2017, we prospectively and consecutively collected data of all of the pregnant women with pregestational T1D treated in the 11 centers where the comprehensive management plan for T1D was implemented. For analysis, we included those women with pregestational T1D: 1) who had a singleton pregnancy, and 2) who had a live birth or stillbirth or miscarriage during the study period. We excluded those: 1) who had multiple pregnancies, and 2) who had a termination of pregnancy for nonmedical reasons. These pregnant women with T1D in the management centers comprised the prospective cohort. Details of data collection are available in the Supplementary Material. Written informed consent was obtained from each of these women. From 2015 to 2017, among 169,752 pregnancies in these management centers, 137 pregnant women with pregestational T1D were initially enrolled. Two were subsequently found to have multiple pregnancies on ultrasonic examination, and another two elected for pregnancy termination due to nonmedical reasons. All 4 were excluded from the analysis, leaving 133 eligible participants (Fig. 2).
Figure 2.
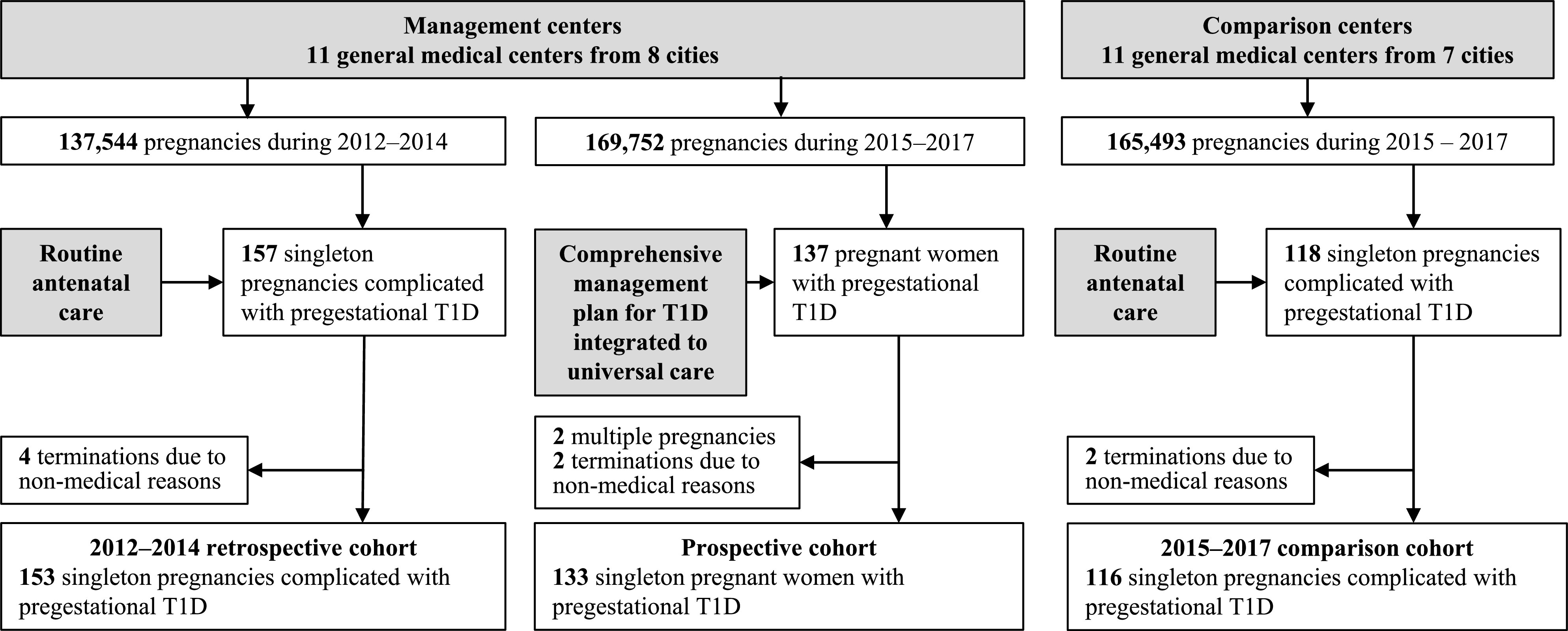
Flowchart of inclusion and exclusion of the participants.
Control Groups
We compared pregnancy outcomes in the prospective cohort with two control groups. The first group is a retrospective cohort of all of the pregnant women with pregestational T1D who received antenatal care preceding the implementation of the management plan for T1D from 2012 to 2014 in the same 11 management centers as the prospective cohort and who fulfilled the same eligibility criteria as for the prospective group. These constituted 153 eligible pregnant women with T1D out of the >137,544 pregnancies who received routine antenatal care from 2012 to 2014 at the management centers, preceding the implementation of the management plan (Fig. 2).
The second group is a comparison cohort of eligible pregnant women with pregestational T1D who received routine antenatal care without the comprehensive management plan in 11 different centers during the study period of 2015–2017. In these comparison centers, the same national maternal care infrastructure as the management centers was applied, but not the management plan for T1D. We selected these comparison centers from seven cities across China (Supplementary Table 4). They were also public hospitals, in cities with similar demographic and economic characteristics, and had similar numbers of annual births and medical facilities as the management centers. We applied the same eligibility criteria to the comparison cohort in the analysis. There was a total of 116 eligible pregnancies with T1D out of 165,493 pregnancies receiving routine care from 2015 to 2017 at the comparison centers, all of whom were included in the analysis (Fig. 2).
Data of eligible pregnancies from the two control groups were compiled from medical records by trained staff. All data were anonymized and reviewed by a trained investigator in the study group (see Supplementary Material, Data collection).
Outcome Measurements
The main outcome was severe adverse pregnancy outcome, which was a composite of maternal mortality, neonatal death, congenital malformations, miscarriage in the second trimester, and stillbirth.
Maternal mortality, neonatal death, and congenital malformations were defined according to a previous report (21) (Supplementary Table 5). Miscarriage in the second trimester and stillbirth were defined as any pregnancy loss or the delivery of a fetus showing no signs of life after the 12th gestational week. The CARNATION committee chose to focus on these miscarriages and stillbirths because these outcomes are unambiguous and uniformly documented in the pregnancy notes and hospital information system throughout 2012 to 2017 at all participating centers.
Other outcomes of interest included: cesarean section (CS), gestational age at birth, preterm birth, birth weight, and other neonatal complications including neonatal hypoglycemia, hyperbilirubinemia, respiratory distress syndrome, and admission to the neonatal intensive care unit (NICU) (21) (Supplementary Table 5).
Preconception diabetes care was assessed by: 1) rates of women having preconception and/or the first trimester hemoglobin A1c (HbA1c) tests, and 2) rates of women on appropriate insulin regimens as recommended by the management plan during the preconception period and/or the first trimester. We defined appropriate insulin regimens as the insulin regimens recommended by the guidelines (17,18,22), including basal-bolus insulin regimens using multiple daily injections or insulin pump. Previous studies showed that many Chinese patients with T1D used self-mixed insulin or twice-daily premixed insulin (23,24), which proved less effective (25). Therefore, we considered using or changing to the appropriate insulin regimen as part of preconception care.
Statistical Analysis
According to a previous report (26) and open data from the local statistics bureaus, pregnancies complicated with T1D account for ∼0.1% of total deliveries. The incidence of a composite outcome of maternal death, neonatal death, and neonatal congenital malformation was 20% in women with T1D. To achieve 90% power at a two-sided 5% significant level, we planned a sample size of 110,000 deliveries and 110 women with pregestational T1D in the prospective cohort study. The comparison between the prospective cohort and each of the comparison population was designed as 1:1 matched case-control study. The incidence of the primary outcome composite of maternal mortality, neonatal death, congenital malformations, miscarriage in the second trimester, and stillbirth was 21.7% in women with T1D in our previous study (26). The incidence of composite adverse pregnancy outcomes in women with T1D could reduce to ∼6% based on other studies (3). We estimated that a sample size of 114 women with pregestational T1D in a matched case-control study would achieve 90% power at a two-sided 5% significant level.
Comparisons were made of differences in maternal characteristics and pregnancy outcomes in the prospective cohort with each of the two comparison populations using Pearson χ2 test, Fisher exact method, or Student t test where appropriate.
Logistic regression analysis was performed to estimate the odds ratios of adverse pregnancy outcomes in the prospective cohort compared with each of the control groups. We adjusted for maternal age at conception, duration of diabetes at conception, preconceptional diabetic complications, and education level. We did not include alcohol consumption or smoking because the prevalence of these among Chinese pregnant women is extremely low (27,28). Analyses were performed using Stata Version.14.0 and IBM SPSS Version.23.0.
Ethics
This study has been approved by the ethics committee of each participating center. These committees agreed that individual patient consent was waived for using anonymized data in the control groups.
Results
Table 1 shows the maternal characteristics of the three cohorts. Overall, the average age at conception was 28.51 ± 4.14 years, with an average duration of diabetes of 6.88 ± 5.65 years in all participants. Except for slight differences in age at diabetes diagnosis and diabetes duration, no significant difference was observed in the preconceptional maternal characteristics between those in the prospective cohort and the comparison populations. For the participation of preconception diabetes care, in the prospective cohort, rates of preconception HbA1c testing and appropriate insulin regimen use were 78.19% and 90.23%, respectively. These rates were significantly higher compared with those either in the 2015–2017 comparison cohort (50.00% and 56.90%, respectively) or those in the 2012–2014 retrospective cohort (20.26% and 61.44%, respectively).
Table 1.
Maternal characteristics of the studied women with T1D
| Prospective cohort (2015–2017) | Retrospective cohort (2012–2014) | Comparison cohort (2015–2017) | Prospective vs. retrospective P value | Prospective vs. comparison P value | |
|---|---|---|---|---|---|
| Number of pregnant women | 133 | 153 | 116 | NA | NA |
| Age at conception, years, mean (SD) | 28.8 (3.9) | 28.3 (4.0) | 28.4 (4.6) | 0.273 | 0.467 |
| Age at diagnosis, years, mean (SD) | 20.5 (6.9) | 22.6 (6.8) | 23.0 (6.8) | 0.010 | 0.005 |
| Duration of diabetes, years, mean (SD) | 8.5 (6.4) | 6.3 (5.3) | 5.8 (4.7) | 0.002 | <0.001 |
| Preconception/1st trimester HbA1c | — | — | — | — | |
| %, mean (SD) | 7.0 (1.3) | 7.6 (2.2) | 7.0 (1.9) | 0.137 | 0.892 |
| mmol/mol, mean (SD) | 53 (14) | 60 (24) | 53 (21) | 0.137 | 0.892 |
| n/N (%) | 104/133 (78.19) | 31/153 (20.26) | 58/116 (50.00) | <0.001 | <0.001 |
| HbA1c ≤6.5% or 48 mmol/mol, % (n/N) | 45.2 (47/104) | 38.7 (12/31) | 51.7 (29/58) | 0.523* | 0.425* |
| HbA1c at delivery | — | — | — | — | — |
| %, mean (SD) | 6.1 (0.75) | 6.8 (1.53) | 7.0 (2.01) | <0.001 | 0.003 |
| mmol/mol, mean (SD) | 43 (8) | 50 (17) | 53 (22) | <0.001 | 0.003 |
| n/N (%) | 86/133 (64.6) | 78/153 (51.0) | 56/116 (48.3) | 0.020 | 0.009 |
| HbA1c ≤6.5% or 48 mmol/mol, % (n/N) | 79.1 (68/86) | 52.6 (41/78) | 55.4 (31/56) | <0.001* | 0.003* |
| Preconception/1st trimester insulin regimen | — | — | — | — | — |
| Insulin pump, n (%) | 52 (39.1) | 21 (13.7) | 14 (12.1) | <0.001 | <0.001 |
| MDI, n (%) | 68 (51.1) | 73 (47.7) | 52 (44.8) | 0.564 | 0.321 |
| Others,† n (%) | 13 (9.8) | 59 (38.6) | 50 (43.1) | <0.001 | <0.001 |
| Preconceptional diabetes complications, n (%) | 11 (8.7) | 21 (13.7) | 12 (10.3) | 0.144 | 0.573 |
| Preconceptional blood pressure, n | 87 | 45 | 115 | NA | NA |
| SBP, mmHg, mean (SD) | 111.0 (11.1) | 116.6 (14.6) | 123.9 (18.8) | 0.025 | <0.001 |
| DBP, mmHg, mean (SD) | 71.0 (8.6) | 74.0 (12.2) | 80.1 (13.7) | 0.141 | <0.001 |
| Blood pressure at 33–36 weeks, n | 111 | 150 | 115 | NA | NA |
| SBP, mmHg, mean (SD) | 112.5 (12.7) | 123.0 (17.5) | 124.3 (18.7) | <0.001 | <0.001 |
| DBP, mmHg, mean (SD) | 70.6 (8.4) | 76.0 (11.6) | 80.6 (14.0) | <0.001 | <0.001 |
| Preconception body weight, kg, mean (SD) | 55.0 (7.9) | NA | NA | NA | NA |
| Body weight at delivery, kg, mean (SD) | 67.8 (7.7) | NA | NA | NA | NA |
| Body weight gain, kg, mean (SD) | 12.2 (5.1) | NA | NA | NA | NA |
| Current smoker, n | 0 | 0 | 2 | NA | NA |
| Current alcohol consumer, n | 0 | 0 | 1 | NA | NA |
| Middle school or higher, n (%) | 131 (98.5) | 93 (95.9) | 73 (97.3) | 0.243 | 0.558 |
| Participation in preconception management,‡ n (%) | 98 (73.7) | 24 (15.7) | 41 (35.3) | <0.001 | <0.001 |
DBP, diastolic blood pressure; MDI, multiple daily injections of basal-bolus insulin; NA, not applicable; SBP, systolic blood pressure.
These comparisons were performed among the patients whose HbA1c values were available.
Other insulin regimens included multiple injections of premixed insulin/insulin analogs, multiple injections of short-acting or rapid-acting insulin, single dose of basal insulin, or other insulin regimens that were not recommended by guidelines for the treatment of pregnant women with T1D.
Participation in preconception management was defined as the proportion of women who had tested HbA1c and using/changing to insulin regimen as guidelines recommended during the preconception period or the first trimester of pregnancy.
Table 2 summarizes the pregnancy outcomes of the three cohorts. The rate of severe adverse pregnancy outcome in the prospective cohort (6.02%) was lower than the 2015–2017 comparison cohort (25.00%; P < 0.001) and the 2012–2014 retrospective cohort (18.30%; P = 0.002). A decline in the rate of miscarriage in the second trimester and stillbirth was observed in the prospective cohort (1.50%) compared with both the 2015–2017 comparison cohort (20.69%; P < 0.001) and the 2012–2014 retrospective cohort (9.80%; P < 0.001). Details of causes of the miscarriage in the second trimester and stillbirth are available in Supplementary Table 6.
Table 2.
Pregnancy outcomes of the studied women with T1D
| Prospective cohort (2012–2014) | Retrospective cohort (2012–2014) | Comparison cohort (2015–2017) | Prospective vs. retrospective P value | Prospective vs. comparison P value | |
|---|---|---|---|---|---|
| Number of pregnant women | 133 | 153 | 116 | NA | NA |
| Severe adverse pregnancy outcome,* n (%) | 8 (6.0) | 28 (18.3) | 29 (25.0) | 0.002 | <0.001 |
| Maternal death, n (%) | 0 (0) | 1 (0.65) | 0 (0) | NA | NA |
| CS, n (%) | 81 (63.3) | 104 (78.8) | 73 (81.1) | 0.006 | 0.004 |
| Preeclampsia, n (%) | 7 (5.26) | 23 (15.03) | 32 (27.59) | 0.007 | <0.001 |
| Miscarriage in the 2nd trimester and stillbirth, n (%) | 2 (1.5) | 15 (9.8) | 24 (20.7) | <0.001 | <0.001 |
| Number of live births | 130 | 132 | 91 | NA | NA |
| Neonatal death, n (%) | 0 (0) | 6 (4.6) | 1 (0.9) | NA | NA |
| Congenital heart malformation, n | 0 | 5 | 0 | NA | NA |
| Neonatal respiratory distress syndrome, n | 0 | 1 | 0 | NA | NA |
| Unknown cause, n | 0 | 0 | 1 | NA | NA |
| Congenital malformation, n (%) | 6 (4.6) | 11 (8.3) | 4 (4.4) | 0.222 | 1 |
| Heart malformation, n | 5 | 7 | 4 | NA | NA |
| Polydactyly, n | 1 | 4 | 0 | NA | NA |
| Cerebral malformation, n | 0 | 1 | 0 | NA | NA |
| Preterm birth, n (%) | 18 (13.9) | 23 (17.4) | 27 (29.7) | 0.425 | 0.006 |
| Early preterm birth (24–30 weeks), n (%) | 3 (2.3) | 5 (3.8) | 6 (6.6) | 0.752 | 0.224 |
| Gestational age at delivery, weeks, mean (SD) | 38.5 (1.8) | 37.5 (1.8) | 36.7 (2.5) | <0.001 | <0.001 |
| Birth weight,# g, mean (SD) | 3,296.0 (592.8) | 3,373.2 (678.1) | 3,063.9 (758.0) | 0.329 | 0.750 |
| SGA, n (%) | 8 (6.2) | 8 (6.2) | 10 (11.5) | 1.000 | 0.162 |
| LGA, n (%) | 19 (14.6) | 42 (32.3) | 22 (25.3) | 0.001 | 0.049 |
| Macrosomia, n (%) | 11 (8.5) | 20 (15.2) | 11 (12.6) | 0.085 | 0.317 |
| Neonatal hypoglycemia, n (%) | 20 (15.4) | 21 (15.9) | 10 (11.0) | 0.907 | 0.348 |
| Neonatal jaundice, n (%) | 33 (25.4) | 29 (22.0) | 24 (26.4) | 0.516 | 0.869 |
| Respiratory distress, n (%) | 6 (4.6) | 7 (5.3) | 9 (9.89) | 0.798 | 0.174 |
| Admission to NICU, n (%) | 37 (28.5) | 55 (41.7) | 40 (44.0) | 0.025 | 0.017 |
NA, not applicable; SGA, small-for-gestational age.
Severe adverse pregnancy outcome comprised maternal death, neonatal death, neonatal malformation, miscarriage in the second trimester, and stillbirth.
The numbers of participants with data available were 130 for the prospective cohort, 130 for the retrospective cohort, and 87 for the comparison cohort.
The prospective cohort had lower rates of CSs (63.28%), preeclampsia (5.26%), and large-for-gestational age babies (LGA; 14.62%) compared with both the 2015–2017 comparison cohort (CS 81.1%, P = 0.004; preeclampsia 27.59%, P < 0.001; and LGA 25.29%, P = 0.049) and the 2012–2014 retrospective cohort (CS 78.79%, P = 0.006; pre-eclampsia 15.03%, P = 0.007; and LGA 32.31%, P = 0.001), respectively.
In the logistic regression models, after adjustment for potential confounding variables, the implementation of the comprehensive management plan for T1D was significantly associated with lower odds of severe adverse pregnancy outcome in the prospective cohort, with 78% lower odds comparing the prospective cohort with the 2015–2017 comparison cohort (adjusted odds ratio 0.22 [95% CI 0.09–0.52]; P = 0.001) and 69% lower odds comparing the prospective cohort with the 2012–2014 retrospective cohort (adjusted odds ratio 0.31 [95% CI 0.13–0.74]; P = 0.009). Similarly, significantly lower odds were also observed for miscarriage in the second trimester and stillbirth and admission to NICU in the prospective cohort (Supplementary Table 7).
Conclusions
The main finding of this study is that the implementation of this comprehensive management plan was associated with a significantly and substantially lower risk of the severe adverse pregnancy outcome and several other adverse outcomes among pregnant women with T1D, under a relatively low-resource setting.
This has major clinical implications. Many low- and middle-income countries with relatively limited health care resource have achieved significant improvement in pregnancy outcomes among the general population (29). This improvement follows the implementation of the maternal care recommended by the WHO. Such improvement following the national implementation of the WHO framework could also be observed in China: maternal and neonatal mortality in the general population has decreased significantly over the past 25 years (12). However, an excess of adverse pregnancy outcomes still occurred for Chinese women with T1D from 2004 to 2014 (26), despite the improvement among the general population. The subsequent improvement in pregnancy outcomes following the implementation of the comprehensive management plan for T1D described in this study could be attributed to several factors.
First, guidance for multiple aspects of differential pregnancy care for T1D is incorporated into our comprehensive management plan. Previous evidence shows that single interventions can improve certain pregnancy outcomes, but may not be enough in themselves to significantly improve the whole range of adverse pregnancy outcomes seen in women with T1D. For example, the ATLANTIC-DIP studies (3) showed that the rates of stillbirth and congenital malformations over a 10-year period were reduced following an increased uptake in preconception care from 28% to 52%. Better control of maternal hyperglycemia is known to lower the risk of fetal and neonatal complications, including neonatal congenital malformations, LGA, macrosomia, preterm birth, miscarriage, and stillbirth (5,30,31). Limiting pregnancy maternal weight gain also lowers the risk of LGA and preterm birth (1). Good blood pressure control throughout the peripregnancy period reduces the risk of preeclampsia (1). Our management plan provided recommendations on multiple aspects of T1D pregnancy care including preconception care, discontinuation of teratogenic medications, the constant emphasis on diet, glycemic targets, insulin regimen adjustments, blood pressure control, and pregnancy weight gain (Supplementary Tables 1 and 2). Our findings supported that, when all of these separate evidence-based strategies are amalgamated into one comprehensive T1D management plan embedded into the country’s universal maternal care package, overall adverse pregnancy outcomes declined.
Second, the integration of the T1D management plan to the country’s universal maternal care framework enhanced the uptake of the management, despite relatively limited health care resource. Improvement in the uptake of specialized care for women with T1D is a challenge worldwide. Take the preconception care as an example: the participation rate worldwide for preconception care in women with T1D ranges from 35% to 71%, as measured by folate acid supplementation rate (3,32).
Likewise, in China, the public hospitals, including our participating centers, are the main HCPs and account for >90% of all hospital admissions (33). These hospitals are typically overcrowded with limited resources. However, by 2012, following collaboration between the public hospitals and government, a universal pregnancy care package became available for the entire Chinese population (12). With this in place, we were able to integrate our T1D management plan into the general framework with the printed checklists for HCPs and the one-page leaflet for women with T1D to follow at different stages of pregnancy. This integration meant that we were able to insert differential care for T1D in pregnancy into the mandatory universal antenatal visits easily, without extra personnel. Also, the one-page leaflet was well accepted by the pregnant women with T1D and mediated multidisciplinary cooperation: the participants would quickly understand when and where to seek assistance if a certain worrying sign occurred (Supplementary Table 2), As a result, the participation of preconception care measured by the rate of preconceptional HbA1c measurement rose from 20.00% to 78.19% in the management centers providing the comprehensive T1D management plan compared with the 2015–2017 comparison centers that remained at 50.00%, highlighting the raise of uptake of preconception care. We must admit that some of the pregnancies in the prospective cohort were unplanned. These women missing preconception care had a higher rate of severe adverse pregnancy outcome (1 out of 10; 10.00%) compared with those who received preconception counseling (7 out of 123; 5.69%; P = 0.582), which was similar to that observed in both the retrospective and the comparison cohorts (Supplementary Table 8). Besides participation in preconception care, women with T1D in the management centers had better glycemic control as measured by the rate achieving glycemic target at delivery and had lower late trimester blood pressure measurements compared with the 2015–2017 comparison cohort or the 2012–2014 retrospective cohort (Table 2). Moreover, results in the prospective cohort showed that 77.08% had a gestational weight gain within the Chinese guideline recommendations (22). Such improvements in multiple aspects of care could explain the decline in pregnancy loss, preeclampsia, preterm birth, neonatal congenital malformations, and LGA birth weight (1). These results suggest that the comprehensive management plan embedded within the preexisting universal pregnancy care framework is feasible and effective. Given that the components in the WHO recommended maternal care that China has adopted as routine antenatal care are universal and have achieved >90% coverage in developed areas and >50% in developing areas (29,34), our management plan could be generalized in other regions after adaption to the local maternal care framework. Potentially, our results may form the basis of changing the current clinical practice and health care policy for pregnancy care for women with T1D. Our findings strengthen the evidence for the integration of a specialized care package for women with manageable chronic diseases, such as T1D, into the preexisting maternal care framework to improve relevant pregnancy outcomes in relatively limited-resource settings.
Additionally, this study is the first to report both a decline in severe adverse pregnancy outcome as well as a decline in CS rates among women with T1D. The ATLANTIC-DIP studies reported an increase in CS rate from 49% (2005–2009) to 68% (2010–2014), despite a reduction in malformations and improved glycemic control following interventions (3). Although still higher than the CS rate among the general population (46.6%, median of 17 supercities in 2014) (35), the implementation of the management plan was followed by a 15% decline of CS among pregnant women with T1D. We hypothesize that the 2-day workshop of education for HCPs and the one-page educational leaflet resulted in an increased awareness of the benefits of vaginal birth. Also, the decreased incidence of LGA might have led to a reduction in CS rates.
A major strength of this study is the careful data collection through a large nationwide collaboration. Although the rate of pregnancy complicated with T1D is low in China, through extensive collaborative efforts, we were able to ensure enough participants to observe a clinically meaningful improvement. The pilot testing of the management plan by the National Health and Family Planning Commission of China offered a unique opportunity for us to observe the effectiveness.
Moreover, we have adopted multiple controls to mitigate potential confounding factors. First, the management and control groups were all treated consistently within the structure of the Chinese national maternal care program, apart from the management plan, limiting the possibility that unrecognized variations in care between the groups contributed to the observed outcomes. Second, one control group was drawn from the same centers as the management group preceding the intervention, limiting the possibility of a contribution from geographic factors. Third, the second control group was contemporaneous with the managed prospective cohort, limiting the possibility that improvements of care over time within China could account for the improved outcomes. Finally, based on our results, we believe that unrecognized confounders are unlikely to have accounted for more than a minor part of the observed difference. First, the change in the main outcome measure is consistent with all of the several other predetermined outcomes of interest among the groups. Second, the two different control groups were consistent with one another. It thus appears likely that most, if not all, of the observed improvements are attributable to an effect of the management plan. The size of such an effect is substantial, with the decline in the main outcome of ∼30% and 80% in the two control groups. But even so, pregnancy outcome in the management group was not as good as in the population without diabetes. This suggests that further refinements to the management plan should be evaluated. Nevertheless, the intervention had a highly favorable number needed to treat (number needed to treat was 8 when compared with the retrospective cohort and 6 when compared with the comparison cohort) to prevent an event of severe adverse pregnancy outcome, which was affordable and accepted by patients with T1D.
There are several limitations to the study. First, given the nature of the observational study design and lack of randomization, we cannot eliminate all unmeasured confounding factors. But we limited impact of biased allocation among groups to the greatest extent by the enrollment of all of the eligible participants in the management centers and comparison centers during the designated period. Second, we did not include aspirin use in our management plan. This is because, when the project was launched, the evidence base for the use of aspirin to prevent preeclampsia in pregnant women with pregestational diabetes was not proven (22). Third, we recommended the use of CGM in our management plan, but the number of women using CGM in this study was limited due to economic constraints. We compared the glycemic control and pregnancy outcomes between the women in the prospective cohort who used CGM during pregnancy and those who did not. No significant difference was observed (Supplementary Table 9). Further improvements in pregnancy outcomes might have been achievable if more participants were able to use CGM, as evidenced by the CONCEPTT study (2).
The findings from this nationwide study suggest that for women with T1D, a comprehensive, preconception-to-pregnancy management plan integrated into the universal WHO maternal care framework is associated with a significantly lower rate of adverse pregnancy outcomes. The reduction of adverse outcomes may be attributable to multiple factors, including better uptake of preconception care, improved glycemic control, better blood pressure control, and limiting excessive gestational weight gain. The management plan achieved these improvements under a relatively resource-limited setting, suggesting that it was feasible and effective and worth development in countries covered by WHO-recommended maternal care framework.
Article Information
Acknowledgments. The authors thank Professor James Ritter, Emeritus Professor of Clinical Pharmacology at King’s College London (London, U.K.), for the editorial assistance.
Funding. This study was funded by the National Key R&D Program of China (2017YFC1309600), the National Foundation for Public Welfare Industry Research (201502011) of the National Health and Family Planning Commission of China, and World Diabetes Foundation (WDF14-921).
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Duality of Interest. All authors have completed the International Committee of Medical Journal Editors uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author). No potential conflicts of interest were reported.
Author Contributions. X.Z., D.Y., S.L., and J.Y. contributed in conceptualization of the idea and helped the corresponding author in project administration, data collection and validation, data analysis, and data interpretation. X.Z. wrote the first draft of the manuscript and was mainly responsible for the designation of methodology. D.Y. was responsible for data management and completed the main part of data analysis. S.L. contributed to the design of the protocol for data collection and data analysis and did the majority of visualization of the data. J.Y. was responsible for the major part of project administration and designed and executed the validation of protocol and data. D.Y., S.L., and J.Y. did critical revision to the draft of the manuscript and edited the manuscript. H.Y. and X.G. contributed to data collection, data interpretation, and the discussion of the manuscript. The following members of the CARNATION Study Group (F.L., X.R., X.X., D.Z., J.H., Z.Z., T.Y., J.Z., Q.H., H.K., Z.L., G.Q., D.C., S.Y., Y.W., J.N., T.P., Q.Z., Y.S., M.L., Y.Z., and B.Y.) also contributed to data collection, data interpretation, and the discussion of the manuscript, and B.Y. contributed to fund acquisition. W.B., L.G., and A.D. mainly contributed to the study design, data interpretation, and did critical revision of the manuscript. J.W. mainly contributed to study design, funding and resources acquisition, project administration and supervision, data interpretation, discussion of results, and critical revision of the manuscript. All authors read and approved the final version. J.W. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. This work was presented at the 80th Scientific Sessions of the American Diabetes Association, 12–16 June 2020.
APPENDIX
Members of the CARNATION Study Group: Xueying Zheng (Department of Endocrinology, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, China); Daizhi Yang (Department of Endocrinology and Metabolism, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China); Sihui Luo (Department of Endocrinology, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, China); Jinhua Yan (Department of Endocrinology and Metabolism, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China); Huixia Yang (Department of Obstetrics and Gynecology, Peking University First Hospital, Beijing, China); Xiaohui Guo (Department of Endocrinology and Metabolism, Peking University First Hospital, Beijing, China); Fang Liu (Department of Endocrinology and Metabolism, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai, China); Xingwu Ran (Department of Endocrinology and Metabolism, West China Hospital, Chengdu, China); Xinhua Xiao (Department of Endocrinology and Metabolism, Peking Union Medical College Hospital, Beijing, China); Dalong Zhu (Department of Endocrinology and Metabolism, Nanjing Drum Tower Hospital, Nanjing, China); Ji Hu (Department of Endocrinology and Metabolism, The Second Affiliated Hospital of Soochow University, Suzhou, China); Zhiguang Zhou (Department of Endocrinology and Metabolism, The Second Xiangya Hospital of Central University, Changsha, China); Tao Yang (Department of Endocrinology and Metabolism, Jiangsu Province Hospital, Nanjing, China); Jiajun Zhao (Department of Endocrinology and Metabolism, Shandong Provincial Hospital, Jinan, China); Qin Huang (Department of Endocrinology and Metabolism, Changhai Hospital, Shanghai, China); Hongyu Kuang (Department of Endocrinology and Metabolism, The First Affiliated Hospital of Harbin Medical University, Harbin, China); Zhen Liang (Department of Endocrinology and Metabolism, Shenzhen Second People’s Hospital, the First Affiliated Hospital of Shenzhen University, Shenzhen, China); Guijun Qin (Department of Endocrinology and Metabolism, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China); Danqing Chen (Department of Obstetrics and Gynecology, Women’s Hospital, Zhejiang University School of Medicine, Hangzhou, China); Shandong Ye (Department of Endocrinology, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, China); Yan Wu (Department of Endocrinology and Metabolism, Shenzhen People’s Hospital, the Second Clinical Medical College of Jinan University, Shenzhen, China); Jianmin Niu (Department of Obstetrics and Gynecology, Shenzhen Maternity and Child Healthcare Hospital Affiliated to Southern Medical University, Shenzhen, China); Tianrong Pan (Department of Endocrinology and Metabolism, The Second Affiliated Hospital of Anhui Medical University, Hefei, China); Qiu Zhang (Department of Endocrinology and Metabolism, The First Affiliated Hospital of Anhui Medical University Hefei, China); Yunfeng Shen (School of Medicine, Nanchang University, Nanchang, China); Minxiang Lei (Department of Endocrinology and Metabolism, Xiangya Hospital of Central South University, Changsha, China); Yan Zhang (School of Medicine, Nanchang University, Nanchang, China); Bin Yao (Department of Endocrinology and Metabolism, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China); Wei Bao (Department of Epidemiology, College of Public Health, University of Iowa, Iowa City, Iowa); Leif Groop (Lund University Diabetes Centre, Department of Clinical Sciences, Lund University, Malmö, Sweden); Anne Dornhorst (Faculty of Medicine, Imperial College London, London, UK); Jianping Weng (Department of Endocrinology, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, China; and Department of Endocrinology and Metabolism, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China).
Footnotes
X.Z., D.Y., S.L., and J.Y. contributed equally to this work.
This article contains supplementary material online at https://doi.org/10.2337/figshare.13623029.
A complete list of the CARNATION Study Group members can be found in the appendix and in the supplementary material online.
Contributor Information
Collaborators: CARNATION Study Group, Xueying Zheng, Daizhi Yang, Sihui Luo, Jinhua Yan, Huixia Yang, Xiaohui Guo, Fang Liu, Xingwu Ran Xinhua Xiao, Dalong Zhu, Ji Hu, Zhiguang Zhou, Tao Yang, Jiajun Zhao, Qin Huang, Hongyu Kuang, Zhen Liang, Guijun Qin, Danqing Chen, Shandong Ye, Yan Wu, Jianmin Niu, Tianrong Pan, Qiu Zhang, Yunfeng Shen, Minxiang Lei, Yan Zhang, Bin Yao, Wei Bao, Leif Groop, Anne Dornhorst, and Jianping Weng
References
- 1.Ringholm L, Damm P, Mathiesen ER. Improving pregnancy outcomes in women with diabetes mellitus: modern management. Nat Rev Endocrinol 2019;15:406–416 [DOI] [PubMed] [Google Scholar]
- 2.Feig DS, Donovan LE, Corcoy R, et al.; CONCEPTT Collaborative Group . Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial [published correction appears in Lancet 2017;390:2346]. Lancet 2017;390:2347–2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Owens LA, Egan AM, Carmody L, Dunne F. Ten years of optimizing outcomes for women with type 1 and type 2 diabetes in pregnancy-the Atlantic DIP experience. J Clin Endocrinol Metab 2016;101:1598–1605 [DOI] [PubMed] [Google Scholar]
- 4.Mathiesen ER. Pregnancy outcomes in women with diabetes-lessons learned from clinical research: the 2015 Norbert Freinkel award lecture. Diabetes Care 2016;39:2111–2117 [DOI] [PubMed] [Google Scholar]
- 5.Murphy HR. Intensive glycemic treatment during type 1 diabetes pregnancy: a story of (mostly) sweet success! Diabetes Care 2018;41:1563–1571 [DOI] [PubMed] [Google Scholar]
- 6.Murphy HR, Bell R, Dornhorst A, Forde R, Lewis-Barned N. Pregnancy in Diabetes: challenges and opportunities for improving pregnancy outcomes. Diabet Med 2018;35:292–299 [DOI] [PubMed] [Google Scholar]
- 7.Mackin ST, Nelson SM, Kerssens JJ, et al.; SDRN Epidemiology Group . Diabetes and pregnancy: national trends over a 15 year period. Diabetologia 2018;61:1081–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feig DS, Hwee J, Shah BR, Booth GL, Bierman AS, Lipscombe LL. Trends in incidence of diabetes in pregnancy and serious perinatal outcomes: a large, population-based study in Ontario, Canada, 1996-2010. Diabetes Care 2014;37:1590–1596 [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto JM, Hughes DJF, Evans ML, et al. Community-based pre-pregnancy care programme improves pregnancy preparation in women with pregestational diabetes. Diabetologia 2018;61:1528–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.GBD 2015 Healthcare Access and Quality Collaborators. Electronic address: cjlm@uw.edu; GBD 2015 Healthcare Access and Quality Collaborators . Healthcare Access and Quality Index based on mortality from causes amenable to personal health care in 195 countries and territories, 1990-2015: a novel analysis from the Global Burden of Disease Study 2015. Lancet 2017;390:231–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quick J, Jay J, Langer A. Improving women’s health through universal health coverage. PLoS Med 2014;11:e1001580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Department of Maternal and Child Health National Health Commission of the People’s Republic of China . Report on the development of maternal and child health in China, 2019. Accessed 29 July 2020. Available from http://www.nhc.gov.cn/fys/jdt/201905/bbd8e2134a7e47958c5c9ef032e1dfa2.shtml
- 13.Semrau KEA, Hirschhorn LR, Marx Delaney M, et al.; BetterBirth Trial Group . Outcomes of a coaching-based WHO safe childbirth checklist program in India. N Engl J Med 2017;377:2313–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Black RE, Levin C, Walker N, Chou D, Liu L, Temmerman M; DCP3 RMNCH Authors Group . Reproductive, maternal, newborn, and child health: key messages from Disease Control Priorities 3rd edition. Lancet 2016;388:2811–2824 [DOI] [PubMed] [Google Scholar]
- 15.Ringholm L, Mathiesen ER, Kelstrup L, Damm P. Managing type 1 diabetes mellitus in pregnancy--from planning to breastfeeding. Nat Rev Endocrinol 2012;8:659–667 [DOI] [PubMed] [Google Scholar]
- 16.Yang H. Diagnosis and therapy guideline of pregnancy with diabetes mellitus (Draft). Chin J Pract Gynecolgy Obsterics 2007;23:475–477 [Google Scholar]
- 17.American Diabetes Association . Standards of Medical Care in Diabetes--2014. Diabetes Care 2014;37(Suppl. 1):S14–S80 [DOI] [PubMed] [Google Scholar]
- 18.National Institute for Health and Care Excellence (NICE) . Diabetes in pregnancy: management of diabetes and its complications from pre-conception to the postnatal period. Clinical guideline [CG63], 2008. Accessed 29 July 2020. Available from https://www.nice.org.uk/Guidance/CG63
- 19.Ministry of Health of the People’s Republic of China . Measures for implementation of the law of the People’s Republic of China on maternal and infant health care, 1995. Accessed 29 July 2020. Available from https://www.chinacourt.org/law/detail/1995/08/id/23394.shtml
- 20.Ling P, Luo S, Yan J, et al. The design and preliminary evaluation of a mobile health application TangTangQuan in management of type 1 diabetes in China. Diabetes 2018;67(Suppl. 1):A224 [Google Scholar]
- 21.Feig DS, Corcoy R, Jensen DM, et al.; International Association of Diabetes in Pregnancy Study Group (IADPSG) Working Group on Outcome Definitions . Diabetes in pregnancy outcomes: a systematic review and proposed codification of definitions. Diabetes Metab Res Rev 2015;31:680–690 [DOI] [PubMed] [Google Scholar]
- 22.Chinese Medical Association, Obstetrics and Gynaecology Society, Obstetrics Group, Chinese Medical Association, Perinatal Medicine Society, Pregnancy Combined Diabetes Collaboration Group . [Diagnosis and therapy guideline of pregnancy with diabetes mellitus]. Chin J Obstetrics Gynecol 2014;49:561–569 [Google Scholar]
- 23.Li J, Yang D, Yan J, Huang B, Zhang Y; Guangdong Type 1 Diabetes Translational Study Group . Secondary diabetic ketoacidosis and severe hypoglycaemia in patients with established type 1 diabetes mellitus in China: a multicentre registration study. Diabetes Metab Res Rev 2014;30:497–504 [DOI] [PubMed] [Google Scholar]
- 24.McGuire HC, Ji L, Kissimova-Skarbek K, et al. Type 1 diabetes mellitus care and education in China: the 3C study of coverage, cost, and care in Beijing and Shantou. Diabetes Res Clin Pract 2017;129:32–42 [DOI] [PubMed] [Google Scholar]
- 25.O’Neill SM, Kenny LC, Khashan AS, West HM, Smyth RM, Kearney PM. Different insulin types and regimens for pregnant women with pre-existing diabetes. Cochrane Database Syst Rev 2017;2:CD011880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng X, Yan J, Yang D, et al. Pregnancy outcomes in patients with type 1 diabetes in China: a retrospective study. Lancet Diabetes Endocrinol 2016;4(Suppl. 1):S19 [Google Scholar]
- 27.Im PK, Millwood IY, Guo Y, et al.; China Kadoorie Biobank (CKB) collaborative group . Patterns and trends of alcohol consumption in rural and urban areas of China: findings from the China Kadoorie Biobank. BMC Public Health 2019;19:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu X, Rao Y, Wang L, et al. Smoking in pregnancy: a cross-sectional study in China. Tob Induc Dis 2017;15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.United Nations . The millennium development goals report 2015, 2015. Accessed 29 July 2020. Available from https://www.undp.org/content/undp/en/home/librarypage/mdg/the-millennium-development-goals-report-2015.html
- 30.Mackin ST, Nelson SM, Wild SH, Colhoun HM, Wood R; SDRN Epidemiology Group and Scottish Diabetes Group Pregnancy subgroup . Factors associated with stillbirth in women with diabetes. Diabetologia 2019;62:1938–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ornoy A, Reece EA, Pavlinkova G, Kappen C, Miller RK. Effect of maternal diabetes on the embryo, fetus, and children: congenital anomalies, genetic and epigenetic changes and developmental outcomes. Birth Defects Res C Embryo Today 2015;105:53–72 [DOI] [PubMed] [Google Scholar]
- 32.Steel A, Lucke J, Adams J. The prevalence and nature of the use of preconception services by women with chronic health conditions: an integrative review. BMC Womens Health 2015;15:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barber SL, Borowitz M, Bekedam H, Ma J. The hospital of the future in China: China’s reform of public hospitals and trends from industrialized countries. Health Policy Plan 2014;29:367–378 [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization; United Nations Population Fund; United Nations Children’s Fund . Pregnancy, Childbirth, Postpartum and Newborn Care: A Guide for Essential Practice. 3rd ed., Geneva, Switzerland, WHO Press, 2015 [PubMed] [Google Scholar]
- 35.Li H-T, Luo S, Trasande L, et al. Geographic variations and temporal trends in cesarean delivery rates in China, 2008-2014. JAMA 2017;317:69–76 [DOI] [PubMed] [Google Scholar]