Abstract
OBJECTIVE
Despite advances in exogenous insulin therapy, many patients with type 1 diabetes do not achieve acceptable glycemic control and remain at risk for ketosis and insulin-induced hypoglycemia. We conducted a randomized controlled trial to determine whether TTP399, a novel hepatoselective glucokinase activator, improved glycemic control in people with type 1 diabetes without increasing hypoglycemia or ketosis.
RESEARCH DESIGN AND METHODS
SimpliciT1 was a phase 1b/2 adaptive study. Phase 2 activities were conducted in two parts. Part 1 randomly assigned 20 participants using continuous glucose monitors and continuous subcutaneous insulin infusion (CSII). Part 2 randomly assigned 85 participants receiving multiple daily injections of insulin or CSII. In both parts 1 and 2, participants were randomly assigned to 800 mg TTP399 or matched placebo (fully blinded) and treated for 12 weeks. The primary end point was change in HbA1c from baseline to week 12.
RESULTS
The difference in change in HbA1c from baseline to week 12 between TTP399 and placebo was −0.7% (95% CI −1.3, −0.07) in part 1 and −0.21% (95% CI −0.39, −0.04) in part 2. Despite a greater decrease in HbA1c with TTP399, the frequency of severe or symptomatic hypoglycemia decreased by 40% relative to placebo in part 2. In both parts 1 and 2, plasma β-hydroxybutyrate and urinary ketones were lower during treatment with TTP399 than placebo.
CONCLUSIONS
TTP399 lowers HbA1c and reduces hypoglycemia without increasing the risk of ketosis and should be further evaluated as an adjunctive therapy for the treatment of type 1 diabetes.
Introduction
Despite the advent of more physiologic insulin analogs, continuous glucose monitoring (CGM), and continuous subcutaneous insulin infusion (CSII) therapy, glycemic control in type 1 diabetes remains suboptimal. Only a minority of patients with type 1 diabetes achieve the American Diabetes Association goal of glycated hemoglobin (HbA1c) <7% (<53 mmol/mol), and hospitalizations for diabetic ketoacidosis (DKA) and hypoglycemia are increasing globally (1–3). An unmet need exists for adjunctive therapies that improve glycemic control in type 1 diabetes without increasing the risk of hypoglycemia or ketoacidosis.
Glucokinase (GK), a hexokinase that catalyzes glucose phosphorylation, has long been a target of interest because of the observation that human mutations lead to dysglycemia (4). Heterozygous inactivating mutations in GK raise the set point for insulin secretion, resulting in maturity onset diabetes of the young type 2, and homozygous mutations in GK result in severe neonatal diabetes (5–7). Conversely, activating mutations cause hyperinsulinemic hypoglycemia (8).
Compared with other hexokinases, the unique kinetics of glucose binding and lack of inhibition by downstream products allow GK to act as a glucose sensor (4,9). In pancreatic islet cells, GK is constitutively expressed and sets the threshold for glucose-stimulated insulin release (10). In the hepatocyte, GK regulates the Rg and glycolysis (10–13). At low glucose concentrations, hepatic-specific GK regulatory protein (GKRP) binds GK into an inactive form and sequesters it within the nucleus (14). At high glucose concentrations, GK is released by GKRP into its active form in the cytosol, thus restricting hepatic GK activity to periods of hyperglycemia (15,16).
Additionally, hepatic GK expression is insulin dependent. Individuals with type 1 diabetes who have little or no endogenous insulin secretion have low portal vein concentrations of insulin, less GK expression, and impaired liver uptake and metabolism of glucose (17). In insulinopenic mice, restoring GK expression in the liver improves glycemic control without increasing hypoglycemia or ketoacidosis (18).
Numerous small-molecule GK activators have been developed, but their use has been limited by severe hypoglycemia and hypertriglyceridemia, because unfettered activation of GK results in unregulated release of insulin and increased lipogenesis. We previously described the development of TTP399, which selectively targets hepatic GK without disrupting the GKRP/GK interaction (19). Treatment with TTP399 improves glycemic control in both animal models and individuals with type 2 diabetes, primarily through lowering postprandial glucose levels, without increasing hypoglycemia, plasma triglycerides, or hepatic fat (19). It is unknown how GK activation will affect glycemia in individuals who have minimal endogenous insulin secretion.
The SimpliciT1 study, an adaptive three-part phase 1b/2 proof-of-concept study, was designed to explore the effect of TTP399 as an adjunctive therapy for the treatment of type 1 diabetes. We hypothesized that hepatoselective GK activator treatment, adjunctive to insulin, would improve glycemic control in patients with type 1 diabetes without increasing the risk of hypoglycemia or ketoacidosis.
Research Design and Methods
Study Design
SimpliciT1 (TTP399–203) was a phase 1b/2 adaptive study conducted in three sequential parts under a single protocol. The phase 1b portion was termed the sentinel phase. The phase 2 portions were referred to as part 1 and part 2. The sentinel phase was not placebo controlled and was performed at one clinical site to establish safety and guidance on TTP399 dosing. The placebo-controlled phase 2 portions included 14 sites in the U.S. Part 1, designed to determine feasibility, randomly assigned 20 participants using CSII and CGM and was performed at four centers. Part 2 randomly assigned 85 participants at 13 sites. Participants in part 2 used either multiple daily injections of insulin or CSII and monitored glycemia with either CGM or a capillary glucose meter.
All phases were conducted as treat-to-target studies in which basal insulin was optimized to achieve fasting and premeal glucose levels between 80 and 130 mg/dL. Detailed methods and study design schema are found in the Supplementary Material. The study was conducted in accordance with the Declaration of Helsinki on Ethical Principles for Medical Research Involving Human Participants and applicable local regulatory requirements and laws and was approved by the Western Institutional Review Board–Copernicus Group Institutional Review Board.
Participants
Eligible participants were adults diagnosed with type 1 diabetes before 40 years of age and at least 1 year before screening. Entry criteria included HbA1c between 7.0 and 9.5% (53 and 80 mmol/mol), BMI ≤39 kg/m2, estimated glomerular filtration rate ≥50 mL/min/1.73 m2, liver enzymes <1.5-fold the upper limit of normal, and generally good health in the opinion of the investigators. All participants provided written informed consent before any study procedure. Basal insulin was optimized to achieve targets as described above during screening and the insulin adjustment period. Participants who did not meet the fasting/premeal glucose targets within 3 weeks were considered screen failures.
Randomization
In this adaptive study, parts 1 and 2 were conducted sequentially and randomization occurred separately in accordance with guidance for adaptive trials (20,21). Each part of the study started with a single-blinded 2-week placebo run-in period. Subsequently, participants were randomly assigned into the fully blinded treatment period at a ratio of one to one (TTP399 to placebo). The randomization scheme for part 1 balanced active drug to placebo within each site. The randomization scheme for part 2 included a stratification based on whether participants used CGM to ensure similar numbers of CGM users in each treatment arm. Participants, investigators, all site personnel, and the sponsor were blinded to treatment code. The randomizing statistician at EarlyPhase International (Magnolia, TX) was the only unblinded person until the time of study unblinding. The first dose of investigational product was observed at the randomization visit. Participants were instructed to take two 400-mg tablets of TTP399 or matching placebo once daily with the morning mealtime insulin dose for 12 weeks.
Part 1
In part 1, participants completed a 3-week insulin dose-adjustment period at the investigators’ discretion based on stability of fasting premeal glucose measurements. All participants underwent a 2-week single-blind placebo run-in period, 12-week double-blind dosing period, and follow-up safety visit. CSII and CGM data were downloaded from participant devices at study visits and uploaded into the electronic data capture system.
HbA1c (primary end point) and safety laboratory measurements were performed at a central laboratory (Covance, Inc., Princeton, NJ) from collections at screening; before dosing at weeks 0, 2, 4, 6, 8, and 12; and at the follow-up visit (7–10 days off investigational product). Pharmacokinetic samples were collected before week 0 dosing and ∼24 h after last dose at visits during the double-blind dosing period. Participants were contacted by phone as outlined in Supplementary Fig. 1B. Adjustments in insulin dosing were recorded. On the 1st day of randomized product, bolus insulin was reduced by adjusting the insulin-to-carbohydrate ratio by 10–30% and the insulin sensitivity factor by 10–20%. Basal and bolus insulin were subsequently titrated to achieve fasting and premeal glucose levels between 80 and 130 mg/dL and postmeal peak <180 mg/dL.
Part 2
In part 2, participants completed a 3-week basal adjustment period as in part 1. Participants using multiple daily injections for insulin delivery were provided with a smart pen to collect bolus insulin dosing using a phone app (InPen; Companion Medical, San Diego, CA). Participants also manually entered basal doses into the InPen app. CSII and InPen data were downloaded from participant devices at study visits with data collected from week −2 to 12. Masked CGM devices (Abbott Freestyle Libre Pro CGM, Alameda, CA) were worn from week −2 to 0 and week 10 to 12. Outcomes, adverse events (AEs), and laboratory data were collected at screening, weeks 0, 2, 6, and 12; and 7–10 days after stopping investigational product. Patients were contacted by phone as outlined in Supplementary Fig. 1C.
Outcomes
Body weight, blood pressure, pulse, HbA1c, safety laboratories (hematology, metabolic panel, lactate, β-hydroxybutyrate, lipids, and urinalysis), and electrocardiograms were collected at all visits before dosing. A single-item quality-of-life Likert scale score was determined at study completion. The primary efficacy end point was change from baseline HbA1c at 12 weeks. For part 1, a responders’ analysis included participants with improved HbA1c who did not experience severe or symptomatic hypoglycemia, abnormal β-hydroxybutyrate, or lactic acid. The adaptive protocol allowed for modification of the responder definition based on what was learned from part 1. Therefore, the responder definition was modified in part 2 to exclude participants who required significant increases in bolus insulin dose (>3 units/day was considered to be rescue medication). Prespecified secondary end points included change from baseline in CGM parameters (daytime [6:00 a.m.–10:00 p.m.) time in range [TIR] [70–180 mg/dL], time in hyperglycemia levels 1 [>180 mg/dL] and 2 [>250 mg/dL], and time in hypoglycemia levels 1 [<70 mg/dL] and 2 [<54 mg/dL]) and absolute and relative changes in insulin (basal, bolus, or total daily dose). An analysis comparing subgroups based on insulin changes (average change in bolus and total insulin separately) was prespecified and is described in the Supplementary Material. Safety end points included reported AEs, vital signs, electrocardiography, and safety laboratory measurements. Patient-reported hypoglycemia and DKA events were considered AEs of special interest, for which additional information was collected. A pharmacovigilance team and safety monitors who were blinded to treatment assignment evaluated these events. The study provided Freestyle Precision Neo glucose meters (Abbott Diabetes Care) and urine ketone strips for additional self-monitoring. Participants were instructed to report any symptoms of hypoglycemia, measure blood glucose, and provide narratives for each of the reported hypoglycemia events; they were also instructed to measure ketones if they exhibited any symptoms of DKA.
Statistical Methods
On the basis of data from part 1, sample size estimation was performed for part 2. An SD of 1% was assumed for HbA1c. Thirty-four participants per group provided 80% power to detect a difference in HbA1c between a group treated with TTP399 and group treated with placebo of 0.7% using α = 0.049.
Statistical analysis plans for parts 1 and 2 were separate plans. The analysis results for part 1 were used to guide the analysis plan for part 2. The efficacy analysis in each part used α = 0.05; α control in part 1 used Bauer closed procedures, and α control in part 2 used a combination of Bauer closed procedures and the Hochberg method. The details of conditional analysis sequence are provided in the Supplementary Material. Safety data were pooled across parts 1 and 2 because both were double blinded, and data were collected similarly. Efficacy data are reported separately in parts 1 and 2 because the statistical model differed between the parts.
Efficacy analysis included all randomly assigned participants who received at minimum the observed dose of doubleblind study medication and had at least one postbaseline value of HbA1c. Safety analysis included all participants who received at minimum the observed dose of randomized study medication. For part 2, the second estimand excluded participants who were randomly assigned to TTP399 but whose drug concentrations were below the limit of quantitation at all time points or who increased bolus insulin >3 units/day. Multiple imputation was performed for missing data using Monte Carlo methods with 100 invocations.
Analysis of change in HbA1c in part 1 used a main-effects ANCOVA statistical model with baseline HbA1c as a covariate. On the basis of what was learned from part 1, the part 2 analysis of HbA1c used a main-effects ANCOVA with baseline HbA1c and baseline total insulin use as covariates. Least-squares means, SEs, and 95% CIs were calculated for HbA1c changes and secondary outcomes.
Supportive analyses were performed to ensure the robustness of analysis conclusions against use of parametric or nonparametric methods, use of covariables, and methodology for handling missing data. Descriptive statistics were calculated for HbA1c, related laboratory variables, CGM variables, and quality-of-life measures to evaluate consistency of the primary and key secondary analysis results with related variables.
Part 1 analysis of the responders used the Fisher exact test. On the basis of what was learned from part 1, the part 2 analysis of responders used logistic regression.
Safety was monitored by a blinded safety committee that included the medical monitor, clinical study lead, sponsor chief scientific officer, and responsible medical officer. The study was registered with ClinicalTrials.gov.
Role of the Funding Source
vTv was the sponsor of the study. The study was funded by vTv Therapeutics, LLC (High Point, NC), and JDRF International (New York, NY). J.B.B.’s efforts were supported in part through grants from the National Institutes of Health (UL1TR002489 and P30DK124723). All authors and the funders provided input on the trial design. Site monitoring was performed by Cato Research (Durham, NC), and data management was provided by Target Health, LLC (New York, NY). Analysis was performed by Cato Research. All authors participated in data interpretation and drafting and editing of the report and had final responsibility for the decision to submit for publication.
Results
Five adults with type 1 diabetes using CGM and CSII underwent dose escalation to ensure safety during the sentinel phase; 800 mg TTP399 was selected as the dose for subsequent phases. Sentinel phase results are provided in Supplementary Tables 1 and 2.
Participant disposition for parts 1 and 2 is shown in Supplementary Fig. 2. Twenty participants in part 1 and 85 participants in part 2 were randomly assigned. One participant in part 1 was randomly assigned in error but completed the study and contributed safety data only. All randomly assigned participants took at least one dose of study medication, which was observed at the first study visit.
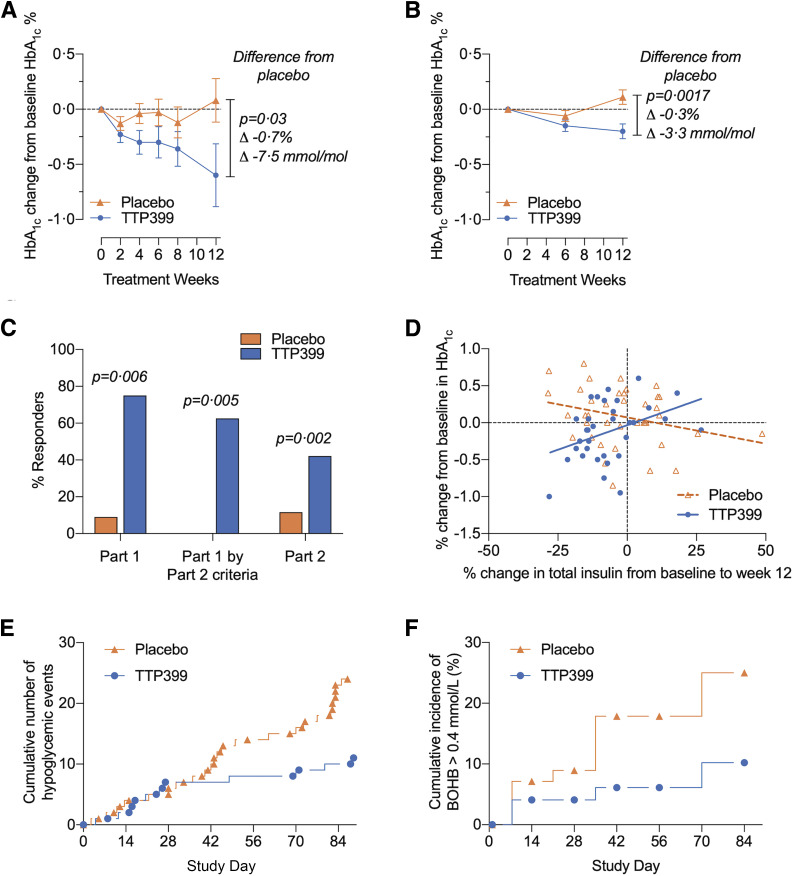
Baseline characteristics and demographics were comparable in both parts (Table 1). Prespecified outcomes are shown in Table 2. In part 1, the change in HbA1c from baseline after 12 weeks of treatment was 0.08 percentage points (SE 0.2) with placebo and −0.60 percentage points (SE 0.2) with TTP399, resulting in a placebo-adjusted change in HbA1c from baseline to 12 weeks of −0.69% (95% CI −1.3, 0.07%; P = 0.032) (Fig. 1A). In part 2, the change from baseline after 12 weeks of treatment was 0.07 percentage points (SE 0.06) with placebo and −0.14 percentage points (SE 0.06) with TTP399, resulting in a placebo-adjusted change of −0.21% (95% CI −0.39, −0.04%; P = 0.018) (Table 2). Analysis of the second estimand resulted in a placebo-adjusted change in HbA1c of −0.32% (95% CI −0.50, −0.13%; P = 0.0017) (Fig. 1B).
Table 1.
Baseline characteristics for the full analysis set of parts 1 and 2
| Part 1 | Part 2 | |||
|---|---|---|---|---|
| Placebo (n = 11) | TTP399 (n = 8) | Placebo (n = 43) | TTP399 (n = 38) | |
| Female sex, n (%) | 8 (73) | 5 (63) | 24 (56) | 14 (37) |
| Age (years), mean (SD) | 47 (10) | 38 (15) | 42 (13) | 43 (15) |
| Race, n (%) | ||||
| White | 11 (100) | 7 (87) | 41 (95) | 36 (95) |
| Black or African American | 0 | 1 (13) | 1 (2) | 0 |
| Asian | 0 | 0 | 1 (2) | 2 (5) |
| Non-Hispanic or non-Latino ethnicity, n (%) | 11 (100) | 8 (100) | 41 (95) | 37 (97) |
| Weight (kg), mean (SD) | 82.8 (15.1) | 80.2 (14.3) | 83.6 (15.0) | 83.1 (18.4) |
| BMI (kg/m2), mean (SD) | 29.0 (4.1) | 28.4 (3.3) | 28.3 (3.8) | 27.6 (4.0) |
| Age at type 1 diabetes diagnosis (years), mean (SD) | 18 (11) | 9 (7) | 16 (10) | 16 (9) |
| Duration of diabetes (years), mean (SD) | 29 (17) | 29 (16) | 26 (14) | 26 (13) |
| Insulin pump user, n (%) | 11 (100) | 8 (100) | 27 (63) | 20 (53) |
| CGM user, n (%) | 11 (100) | 8 (100) | 25 (58) | 24 (63) |
| Fasting plasma glucose (mg/dL), mean (SD) | ND | ND | 153 (49) | 141 (59) |
| HbA1c, mean (SD) | ||||
| % | 7.4 (0.4) | 7.2 (0.4) | 7.5 (0.60) | 7.6 (0.6) |
| mmol/mol | 57 (4.5) | 55 (4.7) | 59 (6.5) | 60 (5.8) |
| β-Hydroxybutyrate (mmol/L), mean (SD) | 0.19 (0.32) | 0.12 (0.21) | 0.14 (0.25) | 0.11 (0.12) |
| C-peptide, mean (SD) | ||||
| ng/ml | 0.10 (0.10) | 0.10 (0.05) | 0.09 (0.17) | 0.05 (0.09) |
| nmol/L | 0.03 (0.03) | 0.03 (0.02) | 0.03 (0.06) | 0.02 (0.03) |
| Undetectable (<0.004 ng/mL), n (%) | 5 (45) | 5 (63) | 22 (51) | 20 (53) |
| Daily insulin dose (IU), mean (SD) | ||||
| Total | 48.9 (13.9) | 52.6 (14.1) | 55.8 (22.2) | 57.5 (29.3) |
| Basal | 26.0 (7.5) | 30.0 (4.7) | 29.8 (13.9) | 30.4 (13.9) |
| Bolus | 22.8 (8.8) | 22.6 (12.3) | 26.0 (12.5) | 27.0 (18.7) |
| Daily insulin dose/mass (IU/kg), mean (SD) | ||||
| Total | 0.59 (0.14) | 0.65 (0.11) | 0.65 (0.19) | 0.68 (0.27) |
| Basal | 0.31 (0.07) | 0.38 (0.06) | 0.35 (0.13) | 0.37 (0.15) |
| Bolus | 0.28 (0.10) | 0.27 (0.13) | 0.30 (0.12) | 0.32 (0.17) |
| CGM average glucose reading (mg/dL), mean (SD) | 159 (14) | 155 (15) | 158 (26) | 167 (30) |
| CGM TIR, % (SD) | ||||
| <54 mg/dL | 1 (1) | 1 (1) | 4 (4) | 4 (4) |
| <70 mg/dL | 3 (3) | 3 (3) | 9 (6) | 9 (9) |
| In target (70–180 mg/dL) | 64 (10) | 67 (10) | 57 (11) | 52 (13) |
| >180 mg/dL | 33 (11) | 30 (11) | 34 (14) | 39 (16) |
| >250 mg/dL | 6 (4) | 6 (4) | 11 (8) | 15 (10) |
Baseline CGM values are for the 14 days before the day-1 visit. Data from CGM were obtained from Dexcom (unblinded) and Abbott Freestyle Libre Pro in parts 1 and 2, respectively. ND, not done.
Table 2.
Prespecified outcomes for the full analysis set
| Part 1 | Part 2 | |||
|---|---|---|---|---|
| Placebo (n = 11) | TTP399 (n = 8) | Placebo (n = 43) | TTP399 (n = 38) | |
| HbA1c (mmol/mol) | ||||
| Baseline, mean (SD) | 57 (4.5) | 55 (4.7) | 59 (6.5) | 60 (5.8) |
| Week 12 change from baseline, mean (SE) | 0.9 (2.2) | −6.6 (2.3) | 0.8 (0.7) | −0.15 (0.7) |
| Treatment effect, mean (95% CI) | NA | −7.5 (−14.2, −0.8; P = 0.032) | NA | −2.3 (−4.3, −0.4; P = 0.018) |
| HbA1c (%) | ||||
| Baseline, mean (SD) | 7.4 (0.4) | 7.2 (0.4) | 7.5 (0.6) | 7.6 (0.6) |
| Week 12 change from baseline, mean (SE) | 0.08 (0.2) | −0.60 (0.2) | 0.07 (0.06) | −0.14 (0.06) |
| Treatment effect, mean (95% CI) | NA | −0.69 (−1.3, −0.07; P = 0.032) | NA | −0.21 (−0.39, −0.04; P = 0.018) |
| Responders | ||||
| Proportion with composite response, n (%) | 0 | 5 (62) | 5 (12) | 16 (42) |
| Treatment effect, OR (95% CI) | NA | 11.8 (2.58, 20.98; P = 0.016) | NA | 7.8 (0.93, 14.68; P = 0.027) |
| Time in target range (daytime*) (%) | ||||
| Baseline, mean (SD) | 65 (13) | 70 (9) | 57 (11) | 51 (14) |
| Week 12 change from baseline, mean (SD) | −9.2 (2.5) | 2.6 (3.4) | −7.8 (2.3) | 0.1 (2.6) |
| Treatment effect, mean (95% CI) | NA | 11.8 (2.58, 20.98; P = 0.016) | NA | 7.8 (0.93, 14.68; P = 0.027) |
| Time in target range (24 h†) (%), mean (SD) | ||||
| Baseline | 64 (10) | 67 (10) | 57 (11) | 52 (13) |
| Week 12 change from baseline | −8 (10) | −3 (8) | −7 (14) | 0.5 (16) |
| Time in hypoglycemia <70 mg/mL (%), mean (SD) | ||||
| Baseline | 3 (3) | 3 (3) | 9 (6) | 9 (6) |
| Week 12 change from baseline | −1 (3) | −1 (3) | −2 (5) | −2 (8) |
| Time in hypoglycemia <54 mg/mL (%), median (min, max) | ||||
| Baseline | 0.2 (0, 2) | 0.1 (0, 3) | 2 (0, 15) | 3 (0, 13) |
| Week 12 change from baseline | −0.1 (−2, 2) | −0.1 (−3, 0) | −0.5 (−8, 8) | −0.5 (−8, 22) |
| Time in hyperglycemia >180 mg/mL (%), mean (SD) | ||||
| Baseline | 33 (11) | 30 (11) | 34 (14) | 39 (16) |
| Week 12 change from baseline | 9 (12) | 5 (8) | 10 (17) | 1 (18) |
| Quality of life slightly or significantly improved, n (%) | 5 (45) | 6 (75) | 18 (42) | 20 (53) |
| Total insulin use (units/kg/day), mean (SD) | ||||
| Baseline | 0.59 (0.14) | 0.65 (0.10) | 0.65 (0.19) | 0.68 (0.27) |
| Change from baseline to week 12 (%) | −0.1 (8.7) | −1.7 (14.7) | −1.6 (16.0) | −7.6 (10.1) |
| Bolus insulin use (units/kg/day), mean (SD) | ||||
| Baseline | 0.28 (0.10) | 0.27 (0.13) | 0.30 (0.12) | 0.32 (0.17) |
| Change from baseline to week 12 (%) | −5.9 (17.5) | −1.0 (31.8) | −3.9 (28.4) | −8.4 (22.7) |
| Basal insulin use (units/kg/day), mean (SD) | ||||
| Baseline | 0.31 (0.07) | 0.38 (0.06) | 0.35 (0.13) | 0.37 (0.15) |
| Change from baseline to week 12 (%) | 4.4 (7.7) | −2.3 (10.3) | −0.5 (11.6) | −5.4 (7.1) |
NA, not applicable; OR, odds ratio.
*Daytime was defined as 6:00 a.m. to 10:00 p.m.
†24-h TIR was not prespecified in the statistical analysis plan.
Figure 1.
The effect of TTP399 on HbA1c, insulin dosing, and safety in parts 1 and 2 of the SimpliciT1 study. A and B: Mean change in HbA1c from baseline to week 12 in parts 1 (A) and 2 (B). C: Percentages of responders in parts 1 and 2 are shown. Part 1 data were analyzed by the responder criteria outlined in the statistical analysis plan from parts 1 and 2. D: Change in HbA1c is plotted against change in total insulin. The positive correlation between reduction in HbA1c and reduction in total insulin was significant (P = 0.008). Data analyzed comprise the full analysis set. E: Cumulative number of episodes of severe or symptomatic hypoglycemia in part 2. Post hoc analysis demonstrated nominal P < 0.05. F: Cumulative incidence of β-hydroxybutyrate >0.4 mmol/L. Data in F represent the pooled cohort from parts 1 and 2 of the study. BOHB, β-hydroxybutyrate.
Responder analysis revealed a greater percentage of responders among participants treated with TTP399 as compared with placebo in both parts 1 (75% vs. 9%; P = 0.006) and 2 (42% vs. 12%; P = 0.001) (Fig. 1C). CGM data are provided in Table 2. Participants randomly assigned to TTP399 demonstrated increased daytime TIR from before randomization to week 12, whereas participants in the placebo arm experienced decreased daytime TIR. The difference between the effect of TTP399 on daytime TIR compared with placebo was significant (Table 2) (part 1: 11.8%; 95% CI 2.58, 20.98%; P = 0.016; part 2: 7.8% 95% CI 0.93, 14.68%; P = 0.027). Time in hypoglycemia was numerically lower in both treatment groups after 12 weeks.
In part 1, total insulin was numerically reduced by 1.7% with TTP399 compared with 0.1% with placebo. In part 2, total insulin was numerically reduced by 7.6% with TTP399 compared with 1.6% with placebo. Bolus insulin was numerically reduced by 8.4% and 3.9% in the TTP399 and placebo groups, respectively (Table 2).
To explore the effect of alterations in insulin dose in part 2, we conducted a prespecified subgroup analysis based on changes in total insulin (Supplementary Fig. 3A). Numerically more participants in the TTP399 arm decreased their insulin dose, and fewer participants in the TTP399 arm increased insulin. In the subgroup that reduced total insulin dose by >15%, TTP399 significantly reduced HbA1c relative to placebo by 0.4% (Supplementary Fig. 3B left) (P = 0.017). In the subgroup that maintained the same insulin dose, TTP399 reduced HbA1c by 0.35% relative to placebo (Supplementary Fig. 3B middle; P = 0.041). Pharmacokinetic samples suggested adherence in at least 95% of patients randomly assigned to TTP399. Two of 43 patients randomly assigned to TTP399 had no detectable levels of TTP399 in their plasma when measured at any of the study visits. Both of these participants experienced numerically increased insulin.
A post hoc analysis was conducted to better understand the relationship of change in insulin dose to change in HbA1c. A positive correlation was observed between reduction in HbA1c and reduction in total insulin (Fig. 1D blue circles) (P = 0.008) and bolus insulin (P = 0.004) in the TTP399-treated group. Conversely, an inverse correlation was observed in the placebo group.
No events of severe or symptomatic hypoglycemia were reported in part 1. In part 2, one patient in the placebo group reported an event of severe hypoglycemia. Fewer participants in the TTP399 group reported events of symptomatic hypoglycemia compared with the placebo-treated group. In total, 27 severe or symptomatic patient-reported hypoglycemic events were identified in the placebo-treated group compared with 12 in the TTP399-treated arm (Table 3 and Fig. 1E) (nominal P < 0.05). Twenty percent of the participants in the placebo group and 12% of participants in the TTP399 group experienced at least one hypoglycemic event, resulting in a 40% reduction in severe and symptomatic events compared with placebo in part 2 (Table 3).
Table 3.
Adverse events
| Part 1 | Part 2 | Combined | ||||
|---|---|---|---|---|---|---|
| Placebo (n = 11) | TTP399 (n = 9) | Placebo (n = 45) | TTP399 (n = 40) | Placebo (n = 56) | TTP399 (n = 49) | |
| AEs reported | 16 | 13 | 83 | 58 | 99 | 71 |
| Participants with at least one AE | 7 (64) | 6 (67) | 29 (64) | 26 (65) | 36 (64) | 32 (65) |
| SAEs | 0 | 0 | 1 | 1 | 1 | 1 |
| Participants with at least 1 SAE | 0 | 0 | 1 (2) | 1 (2) | 1 (2) | 1 (2) |
| Coronary artery disease | 0 | 0 | 1 (2) | 0 | 1 (2) | 0 |
| Noncardiac chest pain | 0 | 0 | 0 | 1 (2) | 0 | 1 (2) |
| Participants with AE leading to death | 0 | 0 | 0 | 0 | 0 | 0 |
| Participants with at least one drug-related AE | 2 (18) | 1 (11) | 3 (7) | 2 (5) | 5 (9) | 3 (6) |
| Hypoglycemia* | ||||||
| Participants with hypoglycemic AEs (week 1 to EOS) | 0 | 0 | 9 (20) | 5 (12) | 9 (16) | 5 (10) |
| Total hypoglycemic AEs (weeks 1–12) | 0 | 0 | 27 | 12 | 27 | 12 |
| Severe hypoglycemia | 0 | 0 | 1 | 0 | 1 | 0 |
| Symptomatic hypoglycemia | 0 | 0 | 26 | 12 | 26 | 12 |
| Events per person-exposure month | 0 | 0 | 0.2 | 0.1 | ||
| Participants with hypoglycemic AEs (week 2 to EOS) | 0 | 0 | 8 (18) | 2 (5) | 8 (14) | 2 (4) |
| Events per person-exposure month | 0 | 0 | 0.15 | 0.04 | 0.15 | 0.04 |
| Ketone events* | ||||||
| DKA AEs | 0 | 0 | 0 | 0 | 0 | 0 |
| Ketosis AEs | 0 | 0 | 1 | 1 | 1 | 1 |
| Participants with at least one elevated serum BOHB level | 3 (27) | 0 | 11 (24) | 5 (13) | 14 (25) | 5 (10) |
| >1 mmol/L | 0 | 0 | 3 (7) | 1 (3) | 3 (5) | 1 (2) |
| >0.4 and ≤1 mmol/L | 3 (27) | 0 | 8 (18) | 4 (10) | 11 (20) | 4 (8) |
| Participants with change from baseline ≥1 mmol/L | 0 | 0 | 2 (4) | 0 | 2 (4) | 0 |
| Liver function* | ||||||
| ALT, AST, ALP >1.5× ULN and/or bilirubin >2× ULN | 0 | 0 | 2 (4) | 1 (2) | 2 (4) | 1 (2) |
| AST or ALT >3× ULN and bilirubin >1.5× ULN | 0 | 0 | 0 | 0 | 0 | 0 |
Data presented as n or n (%). Population includes any person randomly assigned at study start. Severe hypoglycemia was defined as the participant having blood glucose <49 mg/dL and neurologic impairment requiring assistance to actively administer carbohydrate, glucagon, or other resuscitative actions. If blood glucose was not measured, the clinical manifestations must have been reversed by oral carbohydrate, subcutaneous glucagon, or intravenous glucose. ALP, alkaline phosphatase; BOHB, β-hydroxybutyrate; EOS, end of study; SAE, significant AE; ULN, upper limit of normal.
AE of special interest.
Two treatment-emergent events of ketosis occurred in part 2 of the study: one participant receiving placebo and one participant receiving TTP399. The ketosis event in the participant on TTP399 occurred during hospitalization for worsening chronic obstructive pulmonary disease and was not considered drug related. A trend toward reduction in ketone events in the TTP399 group was observed. In part 1, three participants (27%) had at least one elevated serum β-hydroxybutyrate level, compared with no participants in the TTP399 group (Table 3). In part 2, 11 participants in the placebo group compared with five participants in the TTP399 group had an elevated serum β-hydroxybutyrate. In the pooled cohort, 25% and 10% of participants treated with placebo and TTP399, respectively, experienced one elevated serum β-hydroxybutyrate during dosing (Table 3 and Fig. 1F). When participants were grouped by change in insulin, regardless of insulin subgroup, the incidence of hypoglycemia and ketone events was lower in participants treated with TTP399 (Supplementary Tables 3 and 4). The incidence of treatment-emergent AEs was otherwise similar between the groups, with no change in liver function or plasma lipids (Table 3 and Supplementary Tables 5–7).
Conclusions
The SimpliciT1 study evaluated and confirmed the safety and efficacy of TTP399 as an adjunctive therapy to insulin in type 1 diabetes. We used an adaptive proof-of-concept design to enhance efficiency and a hierarchic testing strategy to evaluate regulatory end points, clinical benefits, and safety.
In part 1, TTP399 therapy was associated with a 0.7% greater reduction from baseline HbA1c to a mean end-of-trial HbA1c of 6.7% (50 mmol/mol) while numerically reducing total insulin. In part 2, the second estimand demonstrated a 0.3% reduction in HbA1c. Although the reductions in HbA1c observed in part 2 were more modest, the sample sizes in both parts of the study were small, and the CIs overlapped. The observed reductions in HbA1c were coupled with a favorable safety profile. At 6 and 12 weeks in both parts 1 and 2 of the study, participants randomly assigned to TTP399 had continued reductions in HbA1c, despite a numeric reduction in bolus insulin, whereas participants on placebo returned to baseline HbA1c.
Glycemic benefits were confirmed by analysis of CGM data, where treatment with TTP399 improved daytime TIR. Because GK is critical for glucose metabolism in the postprandial state, physiologic GK activation is expected to disproportionally affect postprandial hyperglycemia (19). To proactively prevent hypoglycemia, bolus insulin was reduced on the 1st day of randomized drug in all participants. Despite an aggressive treat-to-target design, participants randomly assigned to placebo experienced less daytime TIR at the end of the treatment period. Conversely, consistent with the mechanism of GK activation, daytime TIR was improved in participants randomly assigned to TTP399 despite numerically decreased bolus insulin.
Adjunctive therapies in type 1 diabetes, if effective, require insulin dose adjustment. Therefore, the change in HbA1c observed in part is a function of study product and in part results from the blinded adjustment of insulin therapy to achieve desired glycemic control. On average, participants randomly assigned to TTP399 achieved better glycemic control while numerically reducing insulin doses. The observed positive correlation between the largest responses in HbA1c reduction and the largest reduction in insulin suggests that there is a subpopulation of people who respond particularly well to TTP399. In contrast, a negative correlation was observed with participants on placebo, such that reduction in insulin was associated with increases in HbA1c. At baseline, HbA1c was near the American Diabetes Association–recommended target of 7% (53 mmol/mol) in both parts 1 (7.3%, 56.3 mmol/mol) and 2 (7.6%, 59.6 mmol/mol). Small reductions in HbA1c therefore represent improvement in already good glycemic control. It is unknown whether TTP399 would have a larger impact in participants with higher baseline HbA1c.
Importantly, despite further reduction in HbA1c, TTP399 administration was associated with a nominal reduction in patient-reported hypoglycemia events. Tight glycemic control is often compromised by patient and provider fear of hypoglycemia (22). Given that hypoglycemia remains a leading cause of morbidity in the treatment of insulin-dependent diabetes, an adjunctive therapy that improves glycemia while reducing hypoglycemia would represent a significant advance.
The smaller numbers of hypoglycemic events may be related to the lower insulin dose with TTP399; however, subgroup analysis does not support this idea, because hypoglycemia events were not generally observed in patients on increased insulin doses. Furthermore, the incidence of hypoglycemia was lower in participants randomly assigned to TTP399 regardless of the magnitude of change in insulin dose. Instead, we speculate that by restoring hepatic glycogen stores, TTP399 enhances the ability of the liver to release glucose in response to falling glucose concentrations.
There were no drug-related DKA events, and treatment-emergent events of ketosis in urine or blood were few. However, abnormal β-hydroxybutyrate and urine ketones were detected more frequently in participants on placebo than on TTP399. This finding is compatible with the hypothesis that GK activation has a direct metabolic effect that prevents hepatic ketogenesis in the setting of insulin deficiency (23). GK activation increases postprandial hepatic glycogen synthesis. Increased glycogen stores in the fasting state may increase the proportion of energy provided to the liver via glycolysis rather than through free fatty acid oxidation, thus protecting against ketosis. Consistently, preclinical studies in insulin-dependent Göttingen mini-pigs demonstrate that GK activation prevents ketosis in this animal model during insulin withdrawl (24).
The favorable safety profile of TTP399 stands in contrast to what has been observed in trials of other promising adjunctive agents, such as sodium-glucose co-transporter 1 and 2 inhibitors and glucagon-like peptide-1 receptor agonists, for the treatment of type 1 diabetes (25–27). Although both classes improve glycemic control with placebo-adjusted changes in HbA1c comparable to those observed with TTP399, sodium-glucose co-transporter inhibitors are associated with increased rates of ketosis and DKA, and glucagon-like peptide-1 receptor agonists are associated with increased rates of hypoglycemia and frequent hyperglycemic episodes associated with ketosis (25–30). The scarcity of AEs during treatment with TTP399 underscores the potential for TTP399 in the treatment of type 1 diabetes. Although the study was limited to 12 weeks, TTP399 has been tested in patients with type 2 diabetes for 6 months without substantial AEs. Longer clinical trials are necessary to confirm the long-term safety and efficacy of TTP399 in type 1 diabetes.
The SimpliciT1 study suggests that TTP399 is a candidate for further development as an adjunctive therapy for the treatment of type 1 diabetes. The study, although conducted under one adaptive protocol, is representative of two independent trials showing that TTP399 significantly, although modestly, lowers HbA1c when used as an adjunctive therapy to insulin. The improvement in glycemia was achieved despite a rigorous treat-to-target approach. Treatment with TTP399 also displayed a favorable safety profile and demonstrated potential benefit in reduction of hypoglycemia and ketosis events. An adjunctive therapy that protects against these complications would be a substantial advance in the treatment of type 1 diabetes. To our knowledge, this observation is unique. If confirmed in larger and longer phase 3 studies, these data suggest that TTP399 will both improve glycemia and reduce risks of hypoglycemia and ketosis in patients with type 1 diabetes.
Article Information
Acknowledgments. The authors are grateful to JDRF and their reviewers for funding and helpful suggestions. The authors also greatly appreciate the support of Dr. Robert Rizza throughout the study, including review of the manuscript. The successful conduct of the study was the product of the skill and dedication of the participants and study personnel, for which the authors are extremely appreciative.
Funding. JDRF and vTv co-funded this study. K.R.K. was supported in this work by the University of North Carolina Department of Medicine Physician Scientist Training Program. J.B.B. was supported in part through grants from the Center for Scientific Review of the National Institutes of Health (UL1TR002489 and P30DK124723), Patient-Centered Outcomes Research Institute, and American Diabetes Association.
Duality of Interest. J.B.B. reports contracted consulting fees and travel support for contracted activities paid to the University of North Carolina by Adocia, AstraZeneca, Dance Biopharm, Dexcom, Eli Lilly, Fractyl, GI Dynamics, Intarcia Therapeutics, Lexicon, MannKind, Metavention, NovaTarg, Novo Nordisk, Orexigen, PhaseBio, Sanofi, Senseonics, vTv Therapeutics, and Zafgen; grant support from AstraZeneca, Eli Lilly, Intarcia Therapeutics, Johnson & Johnson, Lexicon, Medtronic, NovaTarg, Novo Nordisk, Sanofi, Theracos, Tolerion, and vTv Therapeutics; consultancy for Cirius Therapeutics, Inc., CSL Behring, Fortress Biotech, Mellitus Health, Moderna, Neurimmune AG, Pendulum Therapeutics, Stability Health, and Zealand Pharma; and stock/options in Mellitus Health, Pendulum Therapeutics, PhaseBio, and Stability Health. J.L.R.F., I.D., C.D., and C.V. are employees of vTv. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. K.R.K. participated in data collection, wrote the first draft, and edited and approved subsequent versions. J.L.R.F., I.D., C.D., and C.V. participated in protocol development and editing of the manuscript. I.D. was primarily responsible for development of the statistical analysis plan and supervision of the analyses reported. M.S.K. and J.B.B. participated in protocol development, data collection, and editing the manuscript. All authors approved the final manuscript for submission and publication. C.V. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented at the 78th Scientific Sessions of the American Diabetes Association (ADA), Orlando, FL, 22–26 June 2018; the 55th Annual Meeting of the European Association for the Study of Diabetes (EASD), Barcelona, Spain, 16–20 September 2019; the 80th Scientific Sessions of the ADA, 12–16 June 2020; and the 56th Annual Meeting of the EASD, 21–25 September 2020.
Footnotes
Clinical trial reg. no. NCT03335371, clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.13635230.
A complete list of the SimpliciT1 research group investigators is provided in the supplementary material online.
Contributor Information
Collaborators: SimpliciT1 research group, John B. Buse, Klara R. Klein, M. Sue Kirkman, Katherine A. Bergamo, Elizabeth H. Harris, Jean M. Dostou, Laura A. Young, Sriram Machineni, Alex M. Kass, Jamie C. Diner, Milana Dezube, Virginia C. Purrington, Julie M. Uehling, Rachael M. Fraser, Katherine R. Schuch, Jennifer V. Rowell, Ali Qamar, K. Jean Lucas, Luke Snedaker, Stephanie Hoover, Justin Smith, Paul Becton, Jeffrey Hainsworth, Timothy S. Bailey, Juan Pablo Garcia-Naranjo, Niki Nguyen, Bruce W. Bode, Jennifer M. Boyd, Betsy Childs, Pablo Mora, Allison Camacho, Carl D. Vance, Karen Lugo, Anuj Bhargava, Kirstie Stifel, Lisa B. Connery, Birjis Khan, Simone D. Smith, John Parker, Kathryn Zweier, Emily Kronenfeld, Brittany Savoca, Viral N. Shah, Prakriti Joshee, Shivani Dixit, Hal Joseph, Halis Kaan Akturk, Subbulaxami Trikudanathan, Dori Khakpour, Julia Chang, Anne Peters, Pejman Cohan, Mark Harmel, and Wendy S . Lane
References
- 1.Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016-2018. Diabetes Technol Ther 2019;21:66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Diabetes Association . 6. Glycemic targets: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020;43(Suppl. 1):S66–S76 [DOI] [PubMed] [Google Scholar]
- 3.Vellanki P, Umpierrez GE. Increasing hospitalizations for DKA: a need for prevention programs. Diabetes Care 2018;41:1839–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iynedjian PB. Molecular physiology of mammalian glucokinase. Cell Mol Life Sci 2009;66:27–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Froguel P, Vaxillaire M, Sun F, et al. Close linkage of glucokinase locus on chromosome 7p to early-onset non-insulin-dependent diabetes mellitus. Nature 1992;356:162–164 [DOI] [PubMed] [Google Scholar]
- 6.Hattersley AT, Turner RC, Permutt MA, et al. Linkage of type 2 diabetes to the glucokinase gene. Lancet 1992;339:1307–1310 [DOI] [PubMed] [Google Scholar]
- 7.Njølstad PR, Sagen JV, Bjørkhaug L, et al. Permanent neonatal diabetes caused by glucokinase deficiency: inborn error of the glucose-insulin signaling pathway. Diabetes 2003;52:2854–2860 [DOI] [PubMed] [Google Scholar]
- 8.Glaser B, Kesavan P, Heyman M, et al. Familial hyperinsulinism caused by an activating glucokinase mutation. N Engl J Med 1998;338:226–230 [DOI] [PubMed] [Google Scholar]
- 9.Matschinsky FM, Glaser B, Magnuson MA. Pancreatic beta-cell glucokinase: closing the gap between theoretical concepts and experimental realities. Diabetes 1998;47:307–315 [DOI] [PubMed] [Google Scholar]
- 10.Matschinsky FM, Wilson DF. The central role of glucokinase in glucose homeostasis: a perspective 50 Years after demonstrating the presence of the enzyme in islets of langerhans. Front Physiol 2019;10:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vandercammen A, Van Schaftingen E. The mechanism by which rat liver glucokinase is inhibited by the regulatory protein. Eur J Biochem 1990;191:483–489 [DOI] [PubMed] [Google Scholar]
- 12.Vandercammen A, Van Schaftingen E. Competitive inhibition of liver glucokinase by its regulatory protein. Eur J Biochem 1991;200:545–551 [DOI] [PubMed] [Google Scholar]
- 13.Vandercammen A, Van Schaftingen E. Species and tissue distribution of the regulatory protein of glucokinase. Biochem J 1993;294:551–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de la Iglesia N, Veiga-da-Cunha M, Van Schaftingen E, Guinovart JJ, Ferrer JC. Glucokinase regulatory protein is essential for the proper subcellular localisation of liver glucokinase. FEBS Lett 1999;456:332–338 [DOI] [PubMed] [Google Scholar]
- 15.Brown KS, Kalinowski SS, Megill JR, Durham SK, Mookhtiar KA. Glucokinase regulatory protein may interact with glucokinase in the hepatocyte nucleus. Diabetes 1997;46:179–186 [DOI] [PubMed] [Google Scholar]
- 16.Hale C, Lloyd DJ, Pellacani A, Véniant MM. Molecular targeting of the GK-GKRP pathway in diabetes. Expert Opin Ther Targets 2015;19:129–139 [DOI] [PubMed] [Google Scholar]
- 17.McCarty MF. In type 1 diabetics, high-dose biotin may compensate for low hepatic insulin exposure, promoting a more normal expression of glycolytic and gluconeogenic enyzymes and thereby aiding glycemic control. Med Hypotheses 2016;95:45–48 [DOI] [PubMed] [Google Scholar]
- 18.Morral N, McEvoy R, Dong H, et al. Adenovirus-mediated expression of glucokinase in the liver as an adjuvant treatment for type 1 diabetes. Hum Gene Ther 2002;13:1561–1570 [DOI] [PubMed] [Google Scholar]
- 19.Vella A, Freeman JLR, Dunn I, Keller K, Buse JB, Valcarce C. Targeting hepatic glucokinase to treat diabetes with TTP399, a hepatoselective glucokinase activator. Sci Transl Med 2019;11:eaau3441. [DOI] [PubMed] [Google Scholar]
- 20.European Medicines Agency Committee for Medicinal Products for Human Use . Reflection paper on methodological issues in confirmatory clinical trials planned with an adaptive design. Accessed 22 December 2020. Available from https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-methodological-issues-confirmatory-clinical-trials-planned-adaptive-design_en.pdf
- 21.Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research , Eds. Adaptive design for clinical trials of drugs and biologics: guidance for industry. Accessed 22 December 2020. Available from https://www.fda.gov/media/78495/download
- 22.Cryer PE. Glycemic goals in diabetes: trade-off between glycemic control and iatrogenic hypoglycemia. Diabetes 2014;63:2188–2195 [DOI] [PubMed] [Google Scholar]
- 23.Ferre T, Pujol A, Riu E, Bosch F, Valera A. Correction of diabetic alterations by glucokinase. Proc Natl Acad Sci U S A 1996;93:7225–7230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valcarce C. Selective activation of glucokinase (GK) in the liver: improves glycemic control and reduces insulin need as well as risk of ketoacidosis in type 1 diabetic minipigs. Accessed 19 August 2020. Available from https://vtvtherapeutics.com/wp-content/uploads/2020/08/GKA-Poster-Keystone-2017_01182017_final-minipigs.pdf
- 25.Karras SN, Koufakis T, Zebekakis P, Kotsa K. Pharmacologic adjunctive to insulin therapies in type 1 diabetes: the journey has just begun. World J Diabetes 2019;10:234–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahrén B, Hirsch IB, Pieber TR, et al.; ADJUNCT TWO Investigators . Efficacy and safety of liraglutide added to capped insulin treatment in subjects with type 1 diabetes: the ADJUNCT TWO randomized trial. Diabetes Care 2016;39:1693–1701 [DOI] [PubMed] [Google Scholar]
- 27.Taylor SI, Blau JE, Rother KI, Beitelshees AL. SGLT2 inhibitors as adjunctive therapy for type 1 diabetes: balancing benefits and risks. Lancet Diabetes Endocrinol 2019;7:949–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathieu C, Zinman B, Hemmingsson JU, et al.; ADJUNCT ONE Investigators . Efficacy and safety of liraglutide added to insulin treatment in type 1 diabetes: the ADJUNCT ONE treat-to-target randomized trial. Diabetes Care 2016;39:1702–1710 [DOI] [PubMed] [Google Scholar]
- 29.Wang W, Liu H, Xiao S, Liu S, Li X, Yu P. Effects of insulin plus Glucagon-Like Peptide-1 Receptor Agonists (GLP-1RAs) in treating type 1 diabetes mellitus: a systematic review and meta-analysis. Diabetes Ther 2017;8:727–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peters AL, McGuire DK, Danne T, et al. Diabetic ketoacidosis and related events with sotagliflozin added to insulin in adults with type 1 diabetes: a pooled analysis of the inTandem 1 and 2 studies. Diabetes Care 2020;43:2713–2720 [DOI] [PMC free article] [PubMed] [Google Scholar]