Type 1 diabetes (T1D) is associated with premature mortality due to acute and chronic diabetes-related complications (1). Severe hypoglycemic events (SHEs) and impaired awareness of hypoglycemia are significant contributors to the increased morbidity and mortality. Islet transplantation (ITx) has been shown to provide near-normalization of glycemic control, restoration of hypoglycemia awareness, prevention of SHEs, and improved quality of life in a select group of patients with T1D (2).
To analyze overall survival in ITx-alone recipients, we retrospectively evaluated a cohort of 49 T1D subjects who underwent ITx and were followed during 2000–2020. Subjects received ITx in the liver via intrahepatic infusion (n = 46) or on the omentum via laparoscopic approach (n = 3). Major inclusion criteria included age 18 to 65 years, T1D duration >5 years, impaired awareness of hypoglycemia, marked glycemic lability, and history of SHEs in the prior 12 months (2).
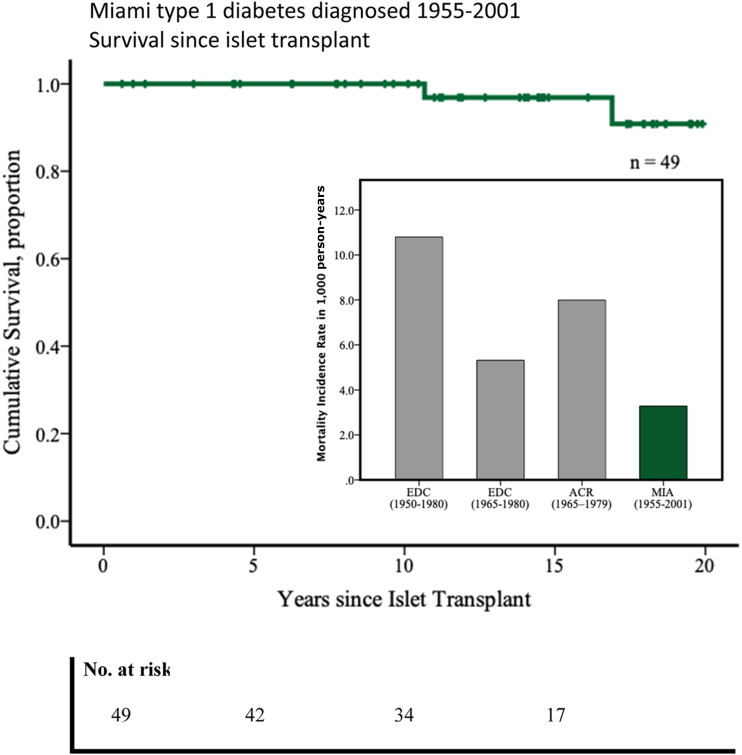
Subjects who did not present the outcome were censored at last encounter. Deaths were reported through contact with family. The study cohort comprised 29 females (59.2%) and 20 males (40.8%). Person-years of follow-up was 610.1, and time after ITx was median (25th–75th) 13.8 (8.2–17.7) years. Median year (minimum–maximum) of T1D diagnosis was 1970 (1955–2001), with 77.6% (n = 38) diagnosed in 1965 or later. Age at T1D diagnosis was median (25th–75th) 13.2 (6.7–20.0) years, mean age ± SD and median duration (25th–75th) of T1D at ITx were 42.8 ± 8.3 years and 29.5 (17.2–38.2) years, respectively. Duration (median [25th–75th]) of graft function while on immunosuppression was 4.4 (1.3–12.2) years. During the follow-up, 31.3% of subjects were censored with allograft function (n = 15). At time of ITx, 86% had no albuminuria, 12% had microalbuminuria, and 2% had macroalbuminuria. Two subjects (4.08%) with T1D diagnosis in 1963 and 1977, respectively, died during the 20-year follow-up. Cause of death for one subject was myocardial infarction (the subject had persistent graft function), while cause of death for the other was likely severe hypoglycemia while sleeping (the subject did not have graft function and was off immunosuppressive drugs for 2 years before death). Kaplan-Meier survival analysis showed a cumulative proportion survival of 100% at 10 years and >80% at 20 years post-ITx, and incidence rate of mortality (95% CI) was 3.28 (2.12–5.05) per 1,000 person-years (Fig. 1).
Figure 1.
Twenty years estimated survival since ITx (Kaplan-Meier). Mortality incidence rate in 1,000 person-years (bar graph inset). EDC, Pittsburgh Epidemiology of Diabetes Complications Study; ACR, Allegheny County Type 1 Diabetes Registry; MIA, Miami cohort ITx Type 1 Diabetes.
The Pittsburgh Epidemiology of Diabetes Complications Study cohort observed a mortality incidence rate of 10.79 per 1,000 person-years (T1D diagnosis between 1950 and 1980) and 5.31 per 1,000 person-years in the subcohort (66.9%) diagnosed between 1965 and 1980. The population-based Allegheny County Type 1 Diabetes Registry cohort, with T1D diagnosed between 1965 and 1979, showed an incidence rate of mortality of 7.99 per 1,000 person-years. This cohort showed a 59% cumulative survival over 20 years for subjects in the same age range (mean ± SD) (43 ± 8 years) as those we report herein (3). In our study, we observed a lower mortality incidence rate, although these comparisons are limited by the eligibility criteria, including absence of albuminuria. Temporal improvement in standardized mortality ratios per diagnosis year is shown and is partly due to improvements in control and management of diabetes clearly seen over the eras.
Intensive therapy in T1D patients, resulting in better glycemic control, has been demonstrated to lower overall mortality risk compared with conventional treatment (hazard ratio 0.67, 95% CI 0.49–0.99, P = 0.045), according to the Diabetes Control and Complications Trial (DCCT) and its observational Epidemiology of Diabetes Interventions and Complications (EDIC) study, with an average of 27 years of follow-up in both groups (4). Our results suggest that ITx is not associated with increased mortality regardless of the use of long-term immunosuppressive therapy. ITx can lead to a near-normal glycemic control and elimination of SHEs, in combination with improvement in patient quality of life already widely reported. Subjects selected for ITx are considered to be a highly vulnerable group with recurrent SHEs, which can lead to higher mortality risk. It is reasonable to consider the hypothesis that the lowest cumulative hyperglycemic exposure and prevention of SHEs provided by ITx represent protection against cardiovascular disease and mortality in patients with T1D. Automation of insulin delivery systems has resulted in marked improvements in glycemic control with reductions in glycemic variability and prevention of SHEs. These technological advancements may help in reducing mortality associated with chronic hyperglycemic exposure and SHEs in T1D (5). However, it remains to be seen whether automated subcutaneous insulin delivery will result in better long-term survival as compared with restoration of endogenous insulin production in patients with T1D. Survival analyses from larger data sets are warranted to confirm our findings in this subset of T1D patients.
Article Information
Acknowledgments. The authors are grateful to the members of the cGMP Human Cell Processing Facility, the preclinical Human Immunology and Immunogenetics Program, the Clinical Cell Transplant Program at the Diabetes Research Institute, and the University of Miami Clinical and Translational Science Institute for their support of this work.
Funding. This work was supported by grants from the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK55347, R01 DK056953, R01 DK025802, DK070460), the Miami Clinical and Translational Science Institute from the National Center for Advancing Translational Sciences (UL1TR000460) and the National Institute on Minority Health and Health Disparities, JDRF International (4-200-946, 4-2004-361, 17-2012-361, 3-SRA-2017-347-M-B), the State of Florida, and the Diabetes Research Institute Foundation.
Duality of Interest. This work was supported in part by Sanofi Genzyme (SA-2014-11258). No other potential conflicts of interest relevant to this article were reported.
Author Contributions. J.R.N.L. wrote the manuscript and participated in statistical analyses. D.A.B. contributed to the discussion and reviewed and edited the manuscript. C.R. reviewed and edited the manuscript. V.F. and A.A. collected data. R.A. reviewed and edited the manuscript. C.R. and R.A participated in research design. R.A. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 80th Scientific Sessions of the American Diabetes Association, 12–16 June 2020.
References
- 1.Feltbower RG, Bodansky HJ, Patterson CC, et al. Acute complications and drug misuse are important causes of death for children and young adults with type 1 diabetes: results from the Yorkshire Register of diabetes in children and young adults. Diabetes Care 2008;31:922–926 [DOI] [PubMed] [Google Scholar]
- 2.Hering BJ, Clarke WR, Bridges ND, et al.; Clinical Islet Transplantation Consortium . Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care 2016;39:1230–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller RG, Secrest AM, Sharma RK, Songer TJ, Orchard TJ. Improvements in the life expectancy of type 1 diabetes: the Pittsburgh Epidemiology of Diabetes Complications study cohort. Diabetes 2012;61:2987–2992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orchard TJ, Nathan DM, Zinman B, et al.; Writing Group for the DCCT/EDIC Research Group . Association between 7 years of intensive treatment of type 1 diabetes and long-term mortality. JAMA 2015;313:45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Senior P, Lam A, Farnsworth K, Perkins B, Rabasa-Lhoret R. Assessment of risks and benefits of beta cell replacement versus automated insulin delivery systems for type 1 diabetes. Curr Diab Rep 2020;20:52. [DOI] [PubMed] [Google Scholar]