Abstract
OBJECTIVE
Obesity and metabolic syndrome are associated with major adverse cardiovascular events (MACE). However, whether distinct metabolic phenotypes differ in risk for coronary artery disease (CAD) and MACE is unknown. We sought to determine the association of distinct metabolic phenotypes with CAD and MACE.
RESEARCH DESIGN AND METHODS
We included patients from the Prospective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE) who underwent coronary computed tomography (CT) angiography. Obesity was defined as a BMI ≥30 kg/m2 and metabolically healthy as less than or equal to one metabolic syndrome component except diabetes, distinguishing four metabolic phenotypes: metabolically healthy/unhealthy and nonobese/obese (MHN, MHO, MUN, and MUO). Differences in severe calcification (coronary artery calcification [CAC] ≥400), severe CAD (≥70% stenosis), high-risk plaque (HRP), and MACE were assessed using adjusted logistic and Cox regression models.
RESULTS
Of 4,381 patients (48.4% male, 60.5 ± 8.1 years of age), 49.4% were metabolically healthy (30.7% MHN and 18.7% MHO) and 50.6% unhealthy (22.3% MUN and 28.4% MUO). MHO had similar coronary CT findings as compared with MHN (severe CAC/CAD and HRP; P > 0.36 for all). Among metabolically unhealthy patients, those with obesity had similar CT findings as compared with nonobese (P > 0.10 for all). However, both MUN and MUO had unfavorable CAD characteristics as compared with MHN (P ≤ 0.017 for all). A total of 130 events occurred during follow-up (median 26 months). Compared with MHN, MUN (hazard ratio [HR] 1.61 [95% CI 1.02–2.53]) but not MHO (HR 1.06 [0.62–1.82]) or MUO (HR 1.06 [0.66–1.72]) had higher risk for MACE.
CONCLUSIONS
In patients with stable chest pain, four metabolic phenotypes exhibit distinctly different CAD characteristics and risk for MACE. Individuals who are metabolically unhealthy despite not being obese were at highest risk in our cohort.
Introduction
Metabolic syndrome (MetS) components, including arterial hypertension, diabetes, and hyperlipidemia, are established risk factors for cardiovascular disease (CVD) and major adverse cardiovascular events (MACE) and are closely linked to obesity (1,2). However, the association of distinct metabolic phenotypes and coronary artery disease (CAD) characteristics is unknown, and the role of obesity as risk modifier in the absence of MetS is controversial (3,4).
To account for the fact that obesity alone may not reflect the actual metabolic health state, the term metabolically healthy obesity (MHO) was introduced to characterize patients with obesity without metabolic alterations. Use of this definition distinguishes four distinct metabolic phenotypes: MHO, metabolically healthy and unhealthy nonobese (MHN and MUN, respectively), and metabolically unhealthy obese (MUO), based on: 1) MetS components, and 2) a BMI <30 or ≥30 kg/m2 for western populations.
Conflicting data, predominantly deriving from population-based cohorts focusing on MHO, have reported on whether metabolic phenotypes are associated with a higher risk of CVD and MACE (3–6). So far, a limited number of studies evaluated the association of metabolic phenotypes and coronary computed tomography (CT) and CT angiography (CTA) findings. Coronary CT provides a noninvasive, precise evaluation of severity and extent of CAD and is increasingly used in the workup for patients with stable or acute chest pain, and its role has been emphasized by current guidelines (7,8).
Screening programs in asymptomatic South Korean individuals suggest that MHO is associated with higher risk for prevalent coronary artery calcification (CAC) and CAD (9–11). These studies, however, do not reflect a western population and investigated individuals with low risk for CVD. The role of obesity as risk modifier for CAD characteristics and future MACE in metabolically healthy or unhealthy individuals has not been studied in patients with intermediate or high risk for CVD.
We therefore aimed to investigate the association of distinct metabolic phenotypes and coronary CT characteristics in the Prospective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE) trial. This cohort provides the unique opportunity to investigate a detailed core laboratory–based CAD assessment in a large symptomatic cohort, in which >90% were at intermediate or high risk for CVD according to the Framingham risk score and 68% had an atherosclerotic CVD (ASCVD) risk score of ≥7.5%.
Research Design and Methods
Patient Population
We enrolled all patients from the PROMISE trial who underwent CTA and had interpretable scans. The study protocol has previously been described in detail. In short, 10,003 individuals with stable chest pain, whose physicians considered noninvasive cardiovascular testing, were included and randomized on a 1:1 ratio to either functional stress test or anatomic testing using CTA. The median follow-up was 25 months, and use of initial CTA did not change clinical outcomes compared with stress testing, defined as composite of death, myocardial infarction, hospitalization for unstable angina, or major procedural complication (12).
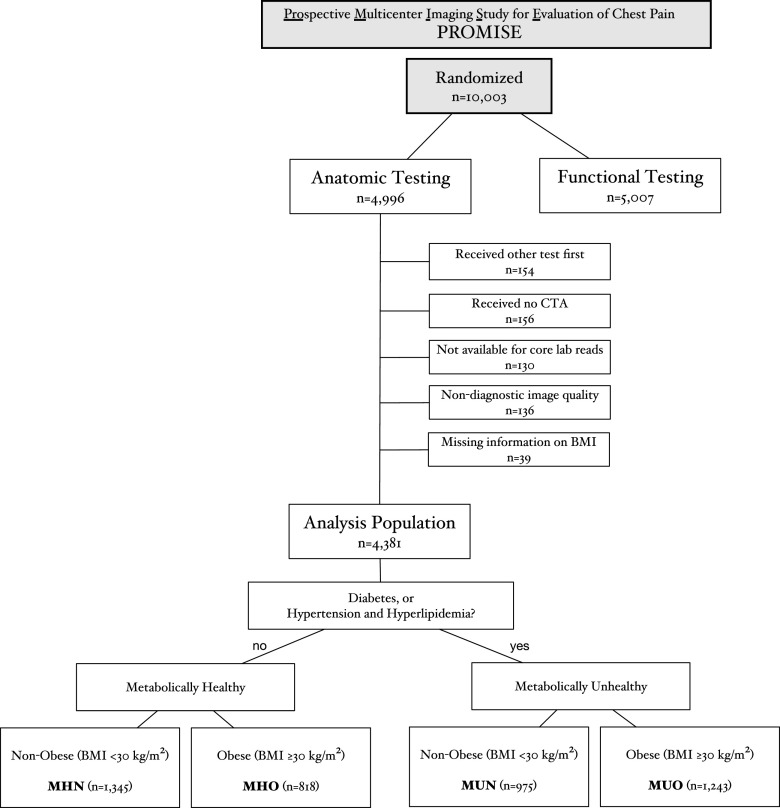
For this analysis, we only included patients assigned for initial CTA (n = 4,996) excluding those in whom: 1) another test was performed as first test (n = 154), 2) no CTA was performed (n = 156), 3) CTA was not available for core laboratory reads (n = 130), 4) image quality was nondiagnostic in core laboratory reads (n = 136), and 5) data on BMI was missing (n = 39). The final cohort, thus, comprised 4,381 individuals for this analysis (Fig. 1).
Figure 1.
Flow diagram of study population.
All patients gave written informed consent, and the Institutional Review Board approved the study protocol.
Definitions of Obesity and Metabolic Health
Definitions for MetS components were adapted from current recommendations. We defined being metabolically unhealthy as presence of diabetes or hypertension and hyperlipidemia. Hypertension was defined as a blood pressure ≥140/90 mmHg on at least two occasions (≥130/80 mmHg for patients with diabetes or chronic kidney disease) or requiring antihypertensive therapy. Diabetes was defined as a history of diabetes, an elevated fasting blood glucose ≥126 mg/dL (7 mmol/L), or the need for antidiabetic medication. Hyperlipidemia was defined as an elevated triglyceride level (≥150 mg/dL) or cholesterol level (total cholesterol ≥200 mg/dL [5.18 mmol/L], LDL ≥130 mg/dL [3.37 mmol/L], or HDL <40 mg/dL [1.04 mmol/L] in men and <50 mg/dL [1.30 mmol/L] in women) or cholesterol-lowering agents. Smoking was defined as current or past smoking.
All patients with diabetes, irrespective of additional risk factors, were considered metabolically unhealthy. Obesity was defined as a BMI ≥30 kg/m2, as recommended by the World Health Organization. Figure 1 displays patient stratification into four distinct metabolic phenotypes: metabolically healthy nonobese (MHN) and obese (MHO) and metabolically unhealthy nonobese (MUN) and obese (MUO). Waist circumference was not included due to previously described collinearity with BMI (13) and lack of data.
In an exploratory step, we defined metabolically healthy as absence of any MetS components (MHN-II and MHO-II) and metabolically unhealthy as one or more MetS components (MUN-II and MUO-II).
CT
CT scans were performed on 64-slice or greater multidetector scanners across 193 enrolling sites in North America. Detailed protocols have been reported elsewhere (12). CAC was defined as regions of ≥130 Hounsfield units in the coronary artery system on noncontrast scans. CAC burden was stratified according to Agatston score (AS) into none (AS 0), mild (AS 1–99), moderate (AS 100–400), and severe (AS >400).
We used CTA core laboratory reads as previously described (14). Nonobstructive CAD was defined as lumen narrowing of <50%, obstructive CAD as ≥50%, and severe CAD as ≥70% in any coronary artery segment or ≥50% in the left main coronary artery.
High-risk plaque (HRP) features were defined as at least one of the following: napkin-ring sign (ringlike peripheral higher attenuation with central low CT attenuation), positive remodeling (remodeling index >1.1), low attenuation plaque (mean Hounsfield units <30), and spotty calcium, as previously reported (14,15). The segment involvement score (SIS) and the CT-adapted Leaman score were calculated as previously described (16,17).
Statistical Analysis
Baseline characteristics are presented as total numbers and percentages as well as means and SDs. Differences of baseline characteristics among the metabolic phenotypes were described using the Kruskal-Wallis and Fisher exact test (across all groups) and the Student t test or Wilcoxon rank sum test and Fisher exact test when comparing two groups (MHN compared with MHO and MUN compared with MUO).
We used logistic regression models to investigate the association between metabolic phenotypes and 1) severe CAC, 2) severe CAD, and 3) HRPs.
Cox regression models were used to identify the prognostic value of the distinct metabolic phenotypes using a composite end point of death, nonfatal myocardial infarction, and hospitalization for unstable angina.
MHN was set as reference group for all regression analyses, which were adjusted for age, sex, smoking, and total cholesterol. In a secondary step, MUN was set as reference group to investigate the effect of obesity among metabolically unhealthy individuals.
In an exploratory step, we additionally performed analyses using BMI as continuous variable instead of the binary information obese/nonobese to investigate differences among metabolically healthy and unhealthy individuals.
All P values are two-sided and considered significant at the nominal 0.05 level. All statistical analyses were performed using Stata 14.2 (StataCorp LP, College Station, TX).
Results
Baseline Characteristics
The baseline characteristics of 4,381 patients (60.5 ± 8.1 years old; 51.6% female) are displayed in Table 1, stratified by metabolic phenotypes. In total, 2,163 (49.4%) were metabolically healthy (MHN: n = 1,345 [30.7%]; MHO: n = 818 [18.7%]) and 2,218 (50.6%) metabolically unhealthy (MUN: n = 975 [22.3%]; MUO: n = 1,243 [28.4%]).
Table 1.
Clinical baseline characteristics stratified by metabolic phenotypes
| Clinical parameters | MHN (n = 1,345) | MHO (n = 818) | P value | MUN (n = 975) | MUO (n = 1,243) | P value |
|---|---|---|---|---|---|---|
| Age (years), mean ± SD | 60.2 ± 8.3 | 58.6 ± 7.6 | <0.001 | 62.9 ± 8.6 | 60.1 ± 7.8 | <0.001 |
| Male sex, n (%) | 673 (50.0) | 412 (50.4) | 0.894 | 453 (46.5) | 579 (46.6) | 0.966 |
| BMI (kg/m2), mean ± SD | 25.7 ± 2.9 | 34.9 ± 4.5 | <0.001 | 26.4 ± 2.6 | 35.6 ± 4.6 | <0.001 |
| Racial or ethnic minority, n (%) | 256 (19.1) | 159 (19.6) | 0.778 | 272 (28.1) | 290 (23.4) | 0.014 |
| Cardiac risk factors | ||||||
| Hypertension, n (%) | 378 (28.1) | 389 (47.6) | <0.001 | 893 (91.6) | 1,144 (92.0) | 0.755 |
| Diabetes, n (%) | 0 (0.0) | 0 (0.0) | — | 303 (31.1) | 593 (47.7) | <0.001 |
| Hyperlipidemia, n (%) | 627 (46.6) | 307 (37.5) | <0.001 | 899 (92.2) | 1,111 (89.4) | 0.028 |
| Family history of premature CAD, n (%) | 443 (33.1) | 249 (30.6) | 0.235 | 305 (31.3) | 435 (35.1) | 0.069 |
| Peripheral or cerebrovascular disease, n (%) | 45 (3.4) | 26 (3.2) | 0.901 | 80 (8.2) | 69 (5.6) | 0.016 |
| Current or former tobacco use, n (%) | 719 (53.5) | 427 (52.2) | 0.594 | 487 (50.0) | 600 (48.3) | 0.441 |
| Sedentary lifestyle, n (%) | 522 (38.9) | 417 (51.1) | <0.001 | 458 (47.1) | 714 (57.4) | <0.001 |
| History of depression, n (%) | 233 (17.3) | 156 (19.1) | 0.326 | 183 (18.8) | 289 (23.3) | 0.010 |
| Mean combined Diamond-Forrester and Coronary Artery Surgery Study risk score* | 52.1 ± 21.0 | 51.7 ± 21.7 | 0.665 | 55.4 ± 21.1 | 53.5 ± 21.3 | 0.038 |
| ASCVD risk score | 10.7 ± 8.2 | 10.5 ± 7.4 | 0.424 | 18.3 ± 13.1 | 17.5 ± 13.2 | 0.149 |
| Relevant medication, n (%) | ||||||
| Aspirin | 468 (37.8) | 327 (42.8) | 0.031 | 481 (50.3) | 620 (50.5) | 0.931 |
| ACE inhibitor or ARB | 195 (15.8) | 235 (30.7) | <0.001 | 557 (58.2) | 807 (65.7) | <0.001 |
| β-Blocker | 175 (14.1) | 168 (22.0) | <0.001 | 297 (31.0) | 393 (32.0) | 0.643 |
| Statin | 364 (29.4) | 186 (24.3) | 0.013 | 619 (64.7) | 741 (60.3) | 0.037 |
| Primary presenting symptom, n (%) | ||||||
| Chest pain | 1,027 (76.5) | 599 (73.2) | 0.100 | 700 (71.8) | 891 (71.7) | 1.000 |
| Dyspnea on exertion | 157 (11.7) | 121 (14.8) | 0.040 | 141 (14.5) | 204 (16.4) | 0.215 |
| Others | 159 (11.8) | 98 (12.0) | 0.945 | 134 (13.7) | 147 (11.8) | 0.198 |
| Type of angina, n (%) | 0.532 | 0.988 | ||||
| Typical | 127 (9.4) | 89 (10.9) | 125 (12.8) | 157 (12.6) | ||
| Atypical | 1,050 (78.1) | 632 (77.3) | 761 (78.1) | 971 (78.1) | ||
| Nonanginal pain | 168 (12.5) | 97 (11.9) | 89 (9.1) | 115 (9.3) |
Racial or ethnic minority group was self-reported, with the status of “minority” being defined by the patient. A family history of premature CAD was defined as diagnosis of the disease in a male first-degree relative before 55 years of age or in a female first-degree relative before 65 years of age.
ARB, angiotensin receptor blocker.
Combined Diamond-Forrester and Coronary Artery Surgery Study risk scores range from 0 to 100, with higher scores indicating a greater likelihood of obstructive CAD.
Among metabolically healthy individuals, those with obesity were younger (58.6 ± 7.6 vs. 60.2 ± 8.3 years old; P < 0.001), more frequently had a sedentary lifestyle (51.1% vs. 38.9%; P < 0.001) and hypertension (47.6% vs. 28.1%; P < 0.001), but had lower rates of hyperlipidemia (37.5% vs. 46.6%; P < 0.001). We observed a similar pattern for obese versus nonobese patients among metabolically unhealthy subjects in terms of age (60.1 ± 7.8 vs. 62.9 ± 8.6 years old; P < 0.001), sedentary lifestyle (57.4% vs. 47.1%; P < 0.001), and hyperlipidemia (89.4% vs. 92.2%; P = 0.028). Patients with diabetes (n = 896; 20.5%) presented more frequently with severe calcification (AS >400; 19.2% vs. 11.7%), severe CAD (8.0% vs. 5.8%), and HRP (54.4% vs. 48.4%) as compared to patients free of diabetes (P < 0.05 for all).
Across the four metabolic phenotypes, we found significant racial differences, with lower rates of racial or ethnic minorities among metabolic healthy and higher rates in metabolically unhealthy individuals (MHN: 19.1%; MHO: 19.6%; MUN: 28.1%; and MUO: 23.4%, P < 0.001 for all).
Coronary CT Findings
Coronary CT findings are summarized in Table 2. On noncontrast CT scans, CAC was present (AS >0) in 2,892 (66.0%), and in 507 (11.6%), AS was >400. On CTA, 2,256 (51.5%) patients were diagnosed with nonobstructive CAD, 336 (7.7%) with obstructive CAD, 273 (6.2%) with severe CAD, and 2,175 (49.6%) had HRP. Across all patients, mean Leaman score and SIS were 5.0 ± 5.0 and 3.7 ± 3.9, respectively.
Table 2.
Baseline coronary CT findings according to metabolic phenotypes
| Coronary CT findings | MHN (n = 1,345) | MHO (n = 818) | P value | MUN (n = 975) | MUO (n = 1,243) | P value |
|---|---|---|---|---|---|---|
| Noncontrast CT, n (%) | 0.080 | 0.917 | ||||
| Normal CAC (AS 0) | 527 (44.7) | 327 (46.2) | 278 (32.2) | 357 (32.7) | ||
| Mild CAC (AS 1–100) | 340 (28.8) | 228 (32.2) | 262 (30.4) | 340 (31.1) | ||
| Moderate CAC (AS 101–400) | 200 (17.0) | 92 (13.0) | 170 (19.7) | 214 (19.6) | ||
| Severe CAC (AS >400) | 112 (9.5) | 61 (8.6) | 153 (17.7) | 181 (16.6) | ||
| Plaque observed on CTA, n (%) | ||||||
| None | 526 (39.1) | 333 (40.7) | 0.469 | 286 (29.3) | 371 (29.9) | 0.815 |
| Any plaque | 819 (60.9) | 485 (59.3) | 0.469 | 689 (70.7) | 872 (70.2) | 0.815 |
| Calcified | 107 (8.0) | 60 (7.3) | 0.619 | 66 (6.8) | 124 (10.0) | 0.007 |
| Noncalcified | 58 (4.3) | 33 (4.0) | 0.825 | 33 (3.4) | 37 (3.0) | 0.625 |
| Mixed | 654 (48.6) | 392 (47.9) | 0.756 | 590 (60.5) | 711 (57.2) | 0.118 |
| HRP | 619 (46.0) | 364 (44.5) | 0.504 | 542 (55.6) | 650 (52.3) | 0.123 |
| Stenosis observed on CTA | 0.099 | 0.174 | ||||
| No CAD (0%), n (%) | 526 (39.1) | 333 (40.7) | 286 (29.3) | 371 (29.9) | ||
| Nonobstructive CAD (1–49%), n (%) | 663 (49.3) | 407 (49.8) | 506 (51.9) | 680 (54.7) | ||
| Obstructive CAD (≥50%), n (%) | 90 (6.7) | 34 (4.2) | 107 (11.0) | 105 (8.5) | ||
| Severe CAD (≥70%*), n (%) | 66 (4.9) | 44 (5.4) | 76 (7.8) | 87 (7.0) | ||
| Leaman score | 4.4 ± 4.9 | 4.0 ± 4.6 | 0.032 | 5.9 ± 5.4 | 5.5 ± 5.2 | 0.056 |
| SIS | 2.3 ± 2.7 | 2.1 ± 2.7 | 0.106 | 3.2 ± 3.1 | 3.1 ± 3.1 | 0.221 |
| CAD-RADS | 0.198 | 0.106 | ||||
| CAD-RADS 0 | 519 (38.6) | 330 (40.3) | 276 (28.3) | 362 (29.1) | ||
| CAD-RADS 1 | 446 (33.2) | 267 (32.6) | 281 (28.8) | 412 (33.2) | ||
| CAD-RADS 2 | 224 (16.7) | 143 (17.5) | 235 (24.1) | 277 (22.3) | ||
| CAD-RADS 3 | 90 (6.7) | 34 (4.2) | 107 (11.0) | 105 (8.5) | ||
| CAD-RADS 4 | 55 (4.1) | 39 (4.8) | 54 (5.5) | 67 (5.4) | ||
| CAD-RADS 4b and 5 | 11 (0.8) | 5 (0.6) | 22 (2.3) | 20 (1.6) |
Metric data are displayed as mean ± SD.
CAD-RADS, CAD reporting and data system; CAD-RADS 0, no plaque/stenosis; CAD-RADS 1, 1–29% stenosis; CAD-RADS 2, 30–49% stenosis; CAD-RADS 3, 50–69% stenosis; CAD-RADS 4a, 70–99% stenosis; CAD-RADS 4b and 5, ≥50% left main coronary artery stenosis or ≥70% stenosis in three vessels or total occlusion.
≥70% in any coronary artery or ≥50% in the left main coronary artery.
In general, MHN and MHO shared similar CAD findings, as did MUN and MUO (Fig. 2). By logistic regression analysis adjusting for age, sex, smoking, and total cholesterol (Supplementary Table 1), MHO had a similar likelihood as MHN for presence of severe CAC (adjusted odds ratio [adj. OR] 1.06 [95% CI 0.75–1.49]; P = 0.743), severe CAD (adj. OR 1.22 [0.82–1.81]; P = 0.335), and HRP (adj. OR 1.04 [0.86–1.25]; P = 0.671). Among metabolically unhealthy individuals, patients with obesity also had similar odds for severe CAC (adj. OR 1.22 [0.94–1.58]; P = 0.137), severe CAD (adj. OR 1.07 [0.77–1.50]; P = 0.674), and HRP (adj. OR 1.03 [0.86–1.24]; P = 0.735) as compared with nonobese patients.
Figure 2.
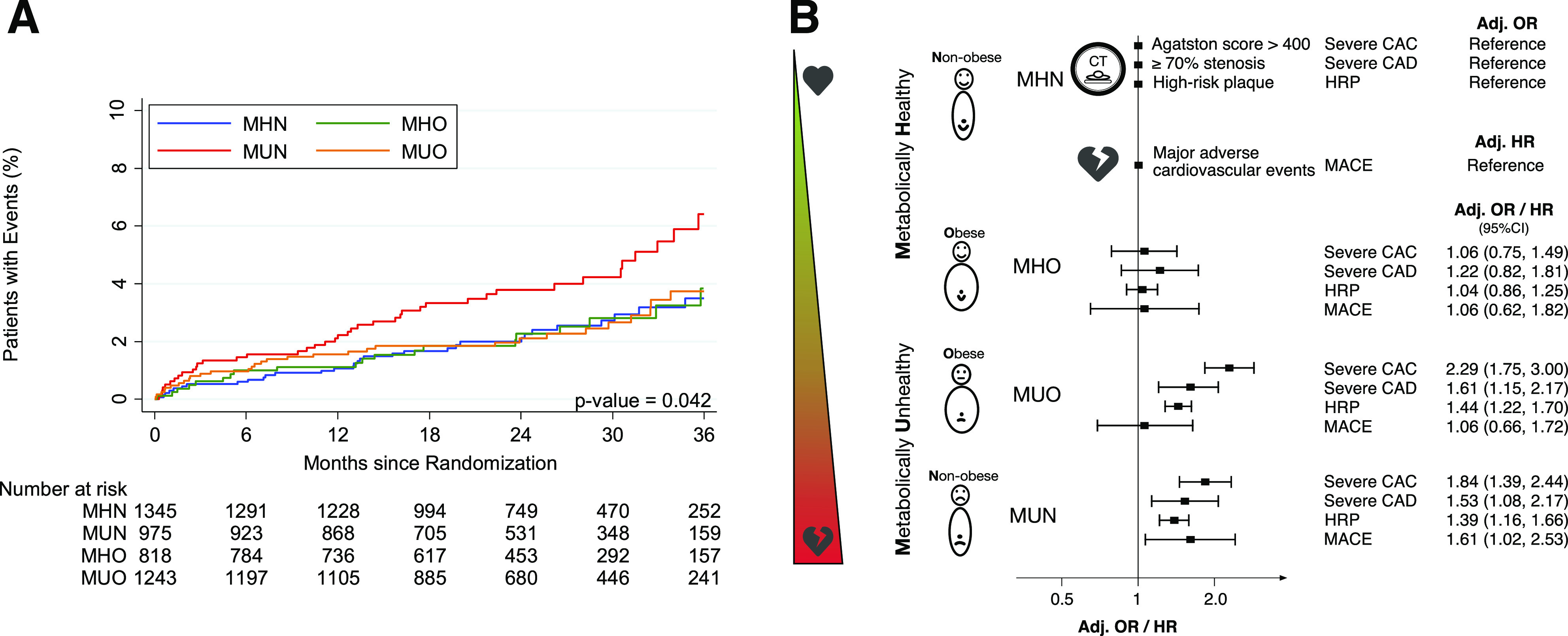
A: Kaplan-Meier estimates demonstrating significant differences between metabolic phenotypes in event-free survival using a composite end point of death, nonfatal myocardial infarction, and hospitalization for unstable angina, with higher event rates among MUN (log-rank across all four metabolic phenotypes, P = 0.042). B: Metabolic phenotypes exhibit different CAD phenotypes. In patients with stable chest pain, obesity does not increase risk for unfavorable CAD characteristics or cardiovascular events. ORs and HRs are adjusted for age, sex, smoking, and total cholesterol.
However, both MUN and MUO had unfavorable CT findings as compared with MHN (severe CAC: adj. OR 1.84 [1.39–2.44] and 2.29 [1.75–3.00], P < 0.001 for both; severe CAD: adj. OR 1.53 [1.08–2.17], P = 0.017 and 1.61 [1.15–2.17], P = 0.005; HRP: adj. OR 1.39 [1.16–1.66] and 1.44 [1.22–1.70], P < 0.001 for both).
Investigating BMI as a continuous variable instead of binary classification based on a 30 kg/m2 threshold, we found no influence of BMI on severe CAC, severe CAD, and HRP in adjusted analyses (P > 0.15 for all) (Supplementary Table 2). Also, adjusting logistic regression models additionally for race (model A), statin treatment (model B), and sedentary lifestyle (model C) did not influence our main findings (Supplementary Table 3).
Using an extreme definition of metabolically healthy, defined as absence of any MetS component, we found a similar trend (Supplementary Table 4). MHO-II had similar CT findings compared with MHN-II (P > 0.16 for all), whereas MUN-II and MUO-II had significantly higher odds for an unfavorable CT phenotype as compared with MHN-II. Among patients with one or more MetS components, obesity was associated with a higher risk for severe CAC (adj. OR 1.26 [1.02–1.56]; P = 0.032) but not with a higher risk for severe CAD or HRP (MUO-II vs. MUN-II: adj. OR 1.10 [0.84–1.43], P = 0.485 and adj. OR 1.06 [0.93–1.22], P = 0.373, respectively).
Outcome
During a median follow-up of 26 months, a total of 130 (3.0%) events occurred, including 25 nonfatal myocardial infarctions, 47 hospitalizations for unstable angina, and 60 deaths. Kaplan-Meier estimates revealed significant differences in survival across metabolic phenotypes (log-rank, P = 0.042) (Fig. 2). By Cox regression analysis adjusting for age, sex, smoking, and total cholesterol (Supplementary Table 5), MHO was at no higher risk for the combined end point compared with MHN (adjusted hazard ratio [adj. HR] 1.05 [95% CI 0.61–1.81]; P = 0.862). MUN but not MUO had higher event rates as compared with MHN (adj. HR 1.60 [1.02–2.52], P = 0.041 and adj. HR 1.03 [0.64–1.67], P = 0.892, respectively). Among metabolically unhealthy subjects, patients with obesity had a significantly lower risk for MACE compared with nonobese patients on a univariable level (HR 0.56 [0.37–0.93]; P = 0.024). This effect was attenuated after adjustment for age, sex, smoking, and total cholesterol, however, with a similar trend (adj. HR 0.65 [0.41–1.03]; P = 0.068).
BMI as a continuous variable had no statistically significant effect on the combined outcome in both the metabolically healthy and unhealthy subgroup (Supplementary Table 6). Supplementary Table 7 displays additional adjustment of Cox regression models for race (model A), statin and aspirin treatment at day 60 (model B), and sedentary lifestyle (model C). In all of these models, MUN was the only group with a significant, or trend toward, higher risk compared with MHN (adj. HR for model A: 1.62 [1.03–2.55], P = 0.037; model B: 1.52 [0.92–2.49], P = 0.101; and model C: 1.53 [0.97–2.41], P = 0.069). Consistent with the main analysis, MUO was at lower risk for events than MUN (adj. HR for model A: 0.64 [0.49–1.02], P = 0.061; model B: 0.66 [0.40–1.08], P = 0.098; and model C: 0.62 [0.39–0.99], P = 0.047).
Supplementary Table 8 displays the influence of the number of MetS components on coronary CT findings and the composite end point. Whereas a gradual increase in adverse CT findings could be observed with an increasing number of MetS factors, this association was not observed for the composite end point of nonfatal myocardial infarctions, hospitalizations for unstable angina, and death.
Conclusions
In this large prospectively studied cohort with stable chest pain, core laboratory CTA analysis, and adjudicated events, we report three main findings. Firstly, distinct metabolic phenotypes exhibit different CAD characteristics and subsequent risk for MACE. Secondly, obesity alone was not associated with unfavorable CT findings and did not significantly increase risk for MACE among metabolically healthy or unhealthy individuals. Thirdly, among metabolically unhealthy individuals, only nonobese patients were at higher risk for events.
The biggest strength of our study is the systematic CAD characterization on CTA in patients with stable chest pain across a wide spectrum of cardiometabolic risk. The role of coronary CT is increasingly emphasized by current guidelines, both in the setting of primary prevention (18) and in symptomatic patients with stable or acute chest pain (7,8). In contrast to functional stress testing, detection of nonobstructive CAD on CTA may additionally improve risk stratification and identify individuals eligible for statin therapy (19).
Obesity and Metabolic Health
Obesity is an epidemic burden, affecting >90 million adults in the U.S. (20), and by 2030, the estimated prevalence of obesity is 48.9% (21). There is universal agreement that obesity is associated with an increased risk for CVD and MACE. However, this may be driven by its risk for and coexistence with MetS components, including hypertension, hyperlipidemia, and diabetes (1–4). This is also reflected by that fact that obesity is not incorporated in quantitative risk assessment tools such as the ASCVD risk score (22) or the Framingham Risk Score (23).
We previously reported in the PROMISE cohort that the level of obesity had an impact on the physicians’ preferred functional test and that the diagnostic yield of CTA was less adversely affected by obesity compared with nuclear myocardial perfusion imaging (24). Also, increasingly obese patients had obstructive CAD less frequently than predicted by their health care providers and a better clinical outcome (25). Among patients with diabetes, a CTA strategy resulted in fewer MACE compared with those without diabetes (26).
The association of distinct metabolic phenotypes with CAD characteristics or CV outcome, however, has not been evaluated. The present analysis is the first to comprehensively investigate the interaction of obesity and metabolic health status in patients with stable chest pain.
Metabolic Healthy Phenotypes and Cardiovascular Risk
Most community-based studies showed that obesity, irrespective of metabolic health state, is associated with adverse cardiovascular outcome, including the Framingham Heart Study (27) and data from the UK Biobank (28), whereas other authors report conflicting results (29). In symptomatic intermediate or high-risk patients, limited data on the association of metabolic phenotypes and outcome are available. Registry data from 1,118 patients referred for CTA found no association of metabolic phenotypes on MACE, whereas MetS was an independent predictor for reduced event-free survival (6). Also, in 8,397 patients with stable CAD, MHO had no significant effect on cardiovascular death during a 4-year follow-up period. Interestingly, in that study, MUO had a lower relative risk for cardiovascular death as compared with MUN (30). This is in line with our findings that MHO was at no higher risk for MACE compared with MHN and that, among metabolically unhealthy individuals, patients with obesity had a trend toward better outcomes as compared with nonobese. Failure to reach the significance level could result from a limited number of events in our cohort (n = 130). However, when adjusting our Cox regression model additionally for race and sedentary lifestyle, MUO was at significantly lower risk for events compared with MUN.
A paradoxically benign effect of obesity on outcome has been reported in heart failure (31), CAD (32), and cancer (33). This “obesity paradox” describes a protective effect of a higher BMI once a disease is established, although obesity per se is a risk factor for developing the disease. Of note, other studies did not find a protective effect of obesity in patients with CAD (34). Several explanations for the obesity paradox have been proposed, including a lack of cachexia, higher muscle mass and metabolic reserve, and protective cytokines among patients with obesity (35). Notably, other factors have been discussed as potential confounders, including a more aggressive therapy regimen in patients with obesity (36) and a lead-time bias, describing the phenomenon that CVD occurs at younger age in obese individuals when they have fewer comorbidities compared with nonobese subjects (37). Indeed, MUO patients were younger than MUN in our study. However, MUO did not have more favorable characteristics in terms of comorbidities; in contrast, diabetes was significantly more prevalent among MUO compared with MUN. Of note, we cannot exclude a selection bias, caused by a potentially earlier onset of disease and death at younger age, preventing these patients from being included in PROMISE.
Regardless of possible confounding factors, PROMISE data capture a real-life scenario of symptomatic patients in which MUO showed a lower risk for MACE compared with MUN, consistent with previous reports of the obesity paradox, although not statistically significant after adjustment for age, sex, smoking, and total cholesterol.
Metabolic Phenotypes and CAD
So far, a limited number of studies evaluated the association of MHO and coronary imaging findings, primarily in asymptomatic community cohort settings. In both healthy South Korean individuals and in a study of 1,107 participants of the Framingham Heart Study, MHO was associated with higher risk for subclinical CAC and CAD (9–11,27). In contrast, our results suggest that MUN, but not MHO and MUO, carries a higher risk for prevalent severe CAC and severe CAD as compared with MHN. These differences may be primarily due to differences in the population (stable chest pain vs. screening), cardiovascular risk profile (lower CVD risk), and ethnicity of the cohorts (9–11,38). PROMISE targeted a population at intermediate or high risk for CVD with a median ASCVD score of 11. Moreover, patients had to present with symptoms suggestive of CAD and were recommended to undergo noninvasive testing by their physicians. In addition, inclusion criteria were an age of >54 years in men (or 45 years with at least one risk factor) or >64 years in women (or 50 years and at least one risk factor).
Supporting this hypothesis that differences in the study populations are the culprit for conflicting findings are the results of a retrospective registry of 1,118 symptomatic patients referred to coronary CTA, which, similar to our results, found that an increasing BMI is associated with MetS components but not with CAD severity (6). Also, among 77 patients with acute chest pain in the Rule Out Myocardial Infarction by Computed tomography Angiography Trial (ROMICAT), MetS was associated with the presence and extent of atherosclerotic plaques, independent of BMI and other risk factors (39). A large international registry of patients without known CAD undergoing CTA found that diabetes was associated with higher prevalence and extent of CAD compared with propensity-matched individuals without diabetes (40), which is in line with our findings.
However, the current study goes beyond the separation of BMI and MetS and is the first to comprehensively characterize the CAD characteristics in four distinct metabolic phenotypes (based on both BMI and metabolic health).
Limitations
Several limitations merit comment. In PROMISE, the overall MACE rate was low, limiting the strengths of our observations. Our findings, consistent with the obesity paradox, may be confounded by a lead time and/or a selection bias. Also, we did not measure waist circumference, which is used for the definition of MetS, and waist-to-hip ratio, both of which may carry additional information for risk stratification in patients across metabolic phenotypes. However, a collinearity between waist circumference and BMI was previously shown; hence, the value of waist circumference in context of distinct metabolic phenotypes is questionable and not used by most authors (5,13). Furthermore, our study does not reflect the full spectrum of obesity, as patients with a BMI >40 kg/m2 were excluded from the PROMISE trial to increase likelihood of diagnostic image quality of testing. Given a limited follow-up of a median of 26 months, we cannot assess the long-term impact of metabolic phenotypes in these patients beyond our observation period.
Conclusion
Our findings show that primarily metabolic health status, but not obesity, is associated with adverse CAD findings in symptomatic patients. Health care professionals and patients should pay special attention in case of a metabolically unhealthy status despite the lack of obesity.
Article Information
Funding. The PROMISE trial was funded by the National Heart, Lung, and Blood Institute (grants R01HL098237, R01HL098236, R01HL98305, and R01HL098235).
The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Duality of Interest. A.A.K. reports receiving a grant from the Austrian Society of Cardiology during the time of this work. M.F. reports receiving a grant from the American Heart Association. N.J.P. reports research grants from Amarin Pharmaceutical Inc., Amgen, AstraZeneca, Baseline Study LLC, Boehringer Ingelheim, Duke Clinical Research Institute, Eli Lilly & Company, Novo Nordisk, Regeneron Pharmaceuticals, Inc., Sanofi, and Verily Life Sciences and received consulting fees from AstraZeneca, Boehringer Ingelheim, and Esperion Therapeutics, Inc., outside the submitted work. M.T.L. reports research funding from the NVIDIA Corporation Academic Program; research funding as a coinvestigator to Massachusetts General Hospital from Kowa Company, Ltd. and MedImmune/AstraZeneca; and personal fees from PQ Bypass unrelated to this work. U.H. reports research support not related to the present research: research support on behalf of the institution: Kowa Company, Ltd., MedImmune, HeartFlow, Duke University (Abbott Laboratories), Oregon Health & Science University (American Heart Association, 13FTF16450001), Columbia University (National Institutes of Health [NIH] grant 5R01-HL109711), NIH/National Heart, Lung, and Blood Institute grants 5K24HL113128, 5T32HL076136, and 5U01HL123339; and consulting fees: Duke University (NIH) and ReCor Medical, Inc. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. A.A.K., P.S.D., and U.H. were responsible for study concept and design. A.A.K., T.M., M.F., M.T.L., D.O.B., S.B.P., N.M.M., H.E., P.S.D., and U.H. were responsible for acquisition, analysis, or interpretation of data. A.A.K. and U.H. were responsible for drafting of the manuscript. All authors performed critical revision of the manuscript for important intellectual content. T.M. was responsible for statistical analysis. P.S.D. and U.H. obtained funding. P.S.D. and U.H. were responsible for study supervision. U.H. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT01174550, clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.13574531.
This article is featured in a podcast available at https://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
References
- 1.Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA 1979;241:2035–2038 [DOI] [PubMed] [Google Scholar]
- 2.Kivimäki M, Kuosma E, Ferrie JE, et al. Overweight, obesity, and risk of cardiometabolic multimorbidity: pooled analysis of individual-level data for 120 813 adults from 16 cohort studies from the USA and Europe. Lancet Public Health 2017;2:e277–e285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kramer CK, Zinman B, Retnakaran R. Are metabolically healthy overweight and obesity benign conditions?: a systematic review and meta-analysis. Ann Intern Med 2013;159:758–769 [DOI] [PubMed] [Google Scholar]
- 4.Hamer M, Stamatakis E. Metabolically healthy obesity and risk of all-cause and cardiovascular disease mortality. J Clin Endocrinol Metab 2012;97:2482–2488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mongraw-Chaffin M, Foster MC, Anderson CAM, et al. Metabolically healthy obesity, transition to metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol 2018;71:1857–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hulten EA, Bittencourt MS, Preston R, et al. Obesity, metabolic syndrome and cardiovascular prognosis: from the Partners coronary computed tomography angiography registry. Cardiovasc Diabetol 2017;16:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J.29 August 2020 [Epub ahead of print]. DOI: 10.1093/eurheartj/ehaa575 [DOI] [PubMed] [Google Scholar]
- 8.Knuuti J, Wijns W, Saraste A, et al.; ESC Scientific Document Group . 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020;41:407–477 [DOI] [PubMed] [Google Scholar]
- 9.Jung CH, Lee MJ, Hwang JY, et al. Association of metabolically healthy obesity with subclinical coronary atherosclerosis in a Korean population. Obesity (Silver Spring) 2014;22:2613–2620 [DOI] [PubMed] [Google Scholar]
- 10.Yoon JW, Jung CH, Kim MK, et al. Influence of the definition of “metabolically healthy obesity” on the progression of coronary artery calcification. PLoS One 2017;12:e0178741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang Y, Kim BK, Yun KE, et al. Metabolically-healthy obesity and coronary artery calcification. J Am Coll Cardiol 2014;63:2679–2686 [DOI] [PubMed] [Google Scholar]
- 12.Douglas PS, Hoffmann U, Patel MR, et al.; PROMISE Investigators . Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med 2015;372:1291–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hinnouho GM, Czernichow S, Dugravot A, et al. Metabolically healthy obesity and the risk of cardiovascular disease and type 2 diabetes: the Whitehall II cohort study. Eur Heart J 2015;36:551–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferencik M, Mayrhofer T, Bittner DO, et al. Use of high-risk coronary atherosclerotic plaque detection for risk stratification of patients with stable chest pain: a secondary analysis of the PROMISE randomized clinical trial. JAMA Cardiol 2018;3:144–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puchner SB, Liu T, Mayrhofer T, et al. High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: results from the ROMICAT-II trial. J Am Coll Cardiol 2014;64:684–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Min JK, Shaw LJ, Devereux RB, et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol 2007;50:1161–1170 [DOI] [PubMed] [Google Scholar]
- 17.Leaman DM, Brower RW, Meester GT, Serruys P, van den Brand M. Coronary artery atherosclerosis: severity of the disease, severity of angina pectoris and compromised left ventricular function. Circulation 1981;63:285–299 [DOI] [PubMed] [Google Scholar]
- 18.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;74:1376–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emami H, Takx RAP, Mayrhofer T, et al. Nonobstructive coronary artery disease by coronary CT angiography improves risk stratification and allocation of statin therapy. JACC Cardiovasc Imaging 2017;10:1031–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hales CM, Carroll MD, Fryar CD, Odgden CL. Prevalence of Obesity Among Adults and Youth: United States, 2015-2016. NCHS Data Brief, No 288. Hyattsville, MD, National Center for Health Statistics, 2017 [PubMed] [Google Scholar]
- 21.Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. State-level prevalence of adult obesity and severe obesity. N Engl J Med 2019;381:2440–2450 [DOI] [PubMed] [Google Scholar]
- 22.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;74:e177–e232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97:1837–1847 [DOI] [PubMed] [Google Scholar]
- 24.Litwin SE, Coles A, Pagidipati N, et al.; PROMISE Investigators . Effects of obesity on noninvasive test results in patients with suspected cardiac ischemia: insights from the PROMISE trial. J Cardiovasc Comput Tomogr 2019;13:211–218 [DOI] [PubMed] [Google Scholar]
- 25.Litwin SE, Coles A, Hill CL, et al. Discordances between predicted and actual risk in obese patients with suspected cardiac ischaemia. Heart 2020;106:273–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma A, Coles A, Sekaran NK, et al. Stress testing versus CT angiography in patients with diabetes and suspected coronary artery disease. J Am Coll Cardiol 2019;73:893–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Echouffo-Tcheugui JB, Short MI, Xanthakis V, et al. Natural history of obesity subphenotypes: dynamic changes over two decades and prognosis in the Framingham heart study. J Clin Endocrinol Metab 2019;104:738–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iliodromiti S, Celis-Morales CA, Lyall DM, et al. The impact of confounding on the associations of different adiposity measures with the incidence of cardiovascular disease: a cohort study of 296 535 adults of white European descent. Eur Heart J 2018;39:1514–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ortega FB, Lee DC, Katzmarzyk PT, et al. The intriguing metabolically healthy but obese phenotype: cardiovascular prognosis and role of fitness. Eur Heart J 2013;34:389–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daly CA, Hildebrandt P, Bertrand M, et al.; EUROPA investigators . Adverse prognosis associated with the metabolic syndrome in established coronary artery disease: data from the EUROPA trial. Heart 2007;93:1406–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pokharel Y, Sun W, Virani SS, et al. Myocardial injury, obesity, and the obesity paradox: the ARIC study. JACC Heart Fail 2017;5:56–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biswas S, Andrianopoulos N, Dinh D, et al. Association of body mass index and extreme obesity with long-term outcomes following percutaneous coronary intervention. J Am Heart Assoc 2019;8:e012860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lennon H, Sperrin M, Badrick E, Renehan AG. The obesity paradox in cancer: a review. Curr Oncol Rep 2016;18:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rana JS, Mittleman MA, Ho KK, Cutlip DE. Obesity and clinical restenosis after coronary stent placement. Am Heart J 2005;150:821–826 [DOI] [PubMed] [Google Scholar]
- 35.Lavie CJ, Laddu D, Arena R, Ortega FB, Alpert MA, Kushner RF. Healthy weight and obesity prevention: JACC health promotion series. J Am Coll Cardiol 2018;72:1506–1531 [DOI] [PubMed] [Google Scholar]
- 36.Costanzo P, Cleland JG, Pellicori P, et al. The obesity paradox in type 2 diabetes mellitus: relationship of body mass index to prognosis: a cohort study. Ann Intern Med 2015;162:610–618 [DOI] [PubMed] [Google Scholar]
- 37.Khan SS, Ning H, Wilkins JT, et al. Association of body mass index with lifetime risk of cardiovascular disease and compression of morbidity. JAMA Cardiol 2018;3:280–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.WHO Expert Consultation . Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies [published correction appears in Lancet 2004;363:902]. Lancet 2004;363:157–163 [DOI] [PubMed] [Google Scholar]
- 39.Butler J, Mooyaart EA, Dannemann N, et al. Relation of the metabolic syndrome to quantity of coronary atherosclerotic plaque. Am J Cardiol 2008;101:1127–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rana JS, Dunning A, Achenbach S, et al. Differences in prevalence, extent, severity, and prognosis of coronary artery disease among patients with and without diabetes undergoing coronary computed tomography angiography: results from 10,110 individuals from the CONFIRM (COronary CT Angiography EvaluatioN For Clinical Outcomes): an InteRnational Multicenter Registry. Diabetes Care 2012;35:1787–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]