Abstract
OBJECTIVE
We estimated the cost-effectiveness of the Program ACTIVE (Adults Coming Together to Increase Vital Exercise) II community-based exercise (EXER), cognitive behavioral therapy (CBT), and EXER+CBT interventions in adults with type 2 diabetes and depression relative to usual care (UC) and each other.
RESEARCH DESIGN AND METHODS
Data were integrated into the Michigan Model for Diabetes to estimate cost and health outcomes over a 10-year simulation time horizon from the health care sector and societal perspectives, discounting costs and benefits at 3% annually. Primary outcome was cost per quality-adjusted life-year (QALY) gained.
RESULTS
From the health care sector perspective, the EXER intervention strategy saved $313 (USD) per patient and produced 0.38 more QALY (cost saving), the CBT intervention strategy cost $596 more and gained 0.29 more QALY ($2,058/QALY), and the EXER+CBT intervention strategy cost $403 more and gained 0.69 more QALY ($585/QALY) compared with UC. Both EXER and EXER+CBT interventions dominated the CBT intervention. Compared with EXER, the EXER+CBT intervention strategy cost $716 more and gained 0.31 more QALY ($2,323/QALY). From the societal perspective, compared with UC, the EXER intervention strategy saved $126 (cost saving), the CBT intervention strategy cost $2,838/QALY, and the EXER+CBT intervention strategy cost $1,167/QALY. Both EXER and EXER+CBT interventions still dominated the CBT intervention. In comparison with EXER, the EXER+CBT intervention strategy cost $3,021/QALY. Results were robust in sensitivity analyses.
CONCLUSIONS
All three Program ACTIVE II interventions represented a good value for money compared with UC. The EXER+CBT intervention was highly cost-effective or cost saving compared with the CBT or EXER interventions.
Introduction
The personal and economic burden of type 2 diabetes is considerable and growing worldwide. More than 34.1 million adults in the U.S. have diabetes (1), resulting in annual costs exceeding $327 billion (2). Low-income urban and rural areas bear a disproportionate burden of the U.S. type 2 diabetes epidemic (1). Patients with type 2 diabetes are two times more likely to experience depressive symptoms than their peers without diabetes, with one in four patients reporting elevated depressive symptoms and 11.4% meeting criteria for major depressive disorder (MDD) (3). Depressive symptoms are associated with worse diabetes outcomes including higher blood glucose levels, greater rates and severity of diabetes complications (4), decreased adherence to diabetes care regimens, increased functional disability, decreased quality of life (QoL), and earlier all-cause mortality (5).
Multiple studies have documented the considerable medical costs and associated decrements in QoL for patients with depression (6–9) and type 2 diabetes (10,11), as individual disease states and comorbid conditions (12–14). In the general population, the cognitive behavioral therapy (CBT) (15), exercise (16), and antidepressant (17) interventions have been widely demonstrated to be effective treatments for depression, and a recent decision-analytic modeling analysis (18) showed that over a 5-year simulation time horizon, CBT versus the second-generation antidepressant treatment could be cost-effective or even cost saving as the initial treatment of depression for adults with newly diagnosed MDD. However, only randomized controlled behavioral intervention trials using the Collaborative Care model tailored for the treatments of depression and type 2 diabetes have studied the cost-effectiveness of these interventions (14,19,20). Moreover, many patients in rural and underserved communities lack access to health care systems offering collaborative care, depending instead on community-based programs to extend access to the depression treatment. To date, no studies have evaluated the comparative cost-effectiveness of community-based behavioral interventions for treating depression among patients with type 2 diabetes.
Program ACTIVE (Adults Coming Together to Increase Vital Exercise) II was a multicenter repeated-measures randomized controlled trial conducted in three U.S. states including Ohio, West Virginia, and Indiana (21,22). The study used a community-engaged research approach in which community organizations participated in recruitment, intervention implementation, and dissemination of findings. The study protocol was approved by the institutional review boards of Indiana University, Ohio University, and West Virginia University. The study design and outcomes have previously been published (21–23). Overall, Program ACTIVE II (22) demonstrated that compared with usual care (UC), the community-based exercise (EXER), and CBT interventions delivered individually (EXER alone or CBT alone) and concurrently (EXER+CBT) resulted in significant improvements in depression, diabetes distress, or cardiometabolic outcomes among rural and urban adults with type 2 diabetes and MDD.
Based on results of Program ACTIVE II (22), we extended the analyses with a computer simulation model to estimate the longer-term costs and health outcomes and to assess the longer-term cost-effectiveness of the community-based EXER and CBT interventions delivered individually (EXER alone or CBT alone) and in combination (EXER+CBT) to treat MDD in adults with type 2 diabetes in comparison with UC and with each other.
Research Design and Methods
Program ACTIVE II
In Program ACTIVE II (22), the comparative effectiveness of the community-based EXER, CBT, and EXER+CBT interventions relative to UC was assessed in a sample of 140 adults with type 2 diabetes who met the DSM-IV, Text Revision (DSM-IV-TR), criteria for MDD at the baseline assessment time point. Participants were followed over a total of 15 months’ follow-up, including a 3-month intervention period followed by a 12-month follow-up assessment period. The DSM-IV-TR criteria for MDD include depressed mood and/or a loss of interest or pleasure in daily activities for at least 2 weeks and at least five of nine specific symptoms (e.g., hypo-/hypersomnia, changes in appetite or weight, fatigue, decrements to concentration, psychomotor retardation or agitation, feelings of worthlessness or excessive guilt, and suicidal ideation, intent, or plan) that cause clinically significant impairment in social, work, or other important areas of functioning nearly every day. At baseline, the mean age of the study population was 56 years, diabetes duration 12 years, HbA1c 7.9%, and BMI 37 kg/m2; 77% of the study population was female and 71% White.
At the postintervention assessment visit (3 months after randomization), all three active intervention groups showed significant remission rates of MDD in comparison with UC. The EXER+CBT group showed a significant improvement in HbA1c in comparison with UC. Specifically, in the whole study cohort, the EXER+CBT group showed a 0.4% improvement in HbA1c (P = 0.016) after adjustment for baseline education status, baseline HbA1c levels, and postintervention changes in glycemic control medications in comparison with UC at the postintervention assessment visit. An exploratory subgroup analysis for the majority subsample of participants with clinically elevated baseline HbA1c values ≥7.0% at baseline revealed that the EXER+CBT group had a 1.1% improvement in HbA1c (P < 0.0001) in comparison with UC at the postintervention assessment visit, and the improvement persisted through the 15-month follow-up assessment visit after randomization. Moreover, compared with UC at the postintervention assessment visit, participants in all three active intervention groups showed greater improvements in depressive symptoms (P < 0.05), negative automatic thoughts (P < 0.05), and diabetes-related distress (P < 0.01) and those in the EXER and EXER+CBT groups showed significant improvements in physical QoL (P < 0.05) and diabetes-specific QoL (P < 0.01).
Simulation Model
We used a validated microsimulation model for type 2 diabetes, the Michigan Model for Diabetes (MMD), version 2.0, to simulate the longer-term cost-effectiveness of the EXER, CBT, and EXER+CBT interventions compared with UC and with each other. Disease progression in the MMD is based on six discrete-time discrete-event submodels that simulate diabetes-related complications (coronary heart disease, cerebrovascular disease, retinopathy, nephropathy, and neuropathy) and death. Transition probabilities in these submodels are functions of individual characteristics, risk factor levels, and current disease and treatment states. The model also estimates the costs of diabetes and its complications and the health-related QoL associated with health states. Details of the MMD have previously been published (24–29).
Simulation Population Characteristics and Modeled Health Outcomes
We incorporated empirical information about the characteristics of the Program ACTIVE II trial participants and the effectiveness of the trial interventions into the MMD. The time horizon for the simulation began at the end of the follow-up in Program ACTIVE II, i.e., 15 months after randomization. For risk factors (i.e., HbA1c, systolic blood pressure [SBP], and lipids) and health utilities that had within- or between-group differences, the intervention group–specific summary statistics at 15 months after randomization were used as the initial simulation population characteristics. For other variables (e.g., BMI, diastolic blood pressure) that did not have within- or between-group differences, the summary statistics based on the pooled population at 15 months after randomization were used for the model simulation (Supplementary Table 1).
Continuous measures at 15 months following randomization were simulated with a Gaussian distribution with the given mean (median) and SD but truncated at 3 SDs. Height and weight were simulated with a correlation of 0.51. SBP and diastolic blood pressure were simulated with a correlation of 0.82 (29). Highly skewed distributions such as HbA1c and triglyceride levels were simulated using log normal distributions.
To evaluate the long-term effectiveness and cost-effectiveness of the interventions, we estimated the incidence of clinical outcomes, life expectancy, and quality-adjusted life expectancy (QALE). The seven clinical outcomes included three-point major adverse cardiovascular event (MACE) (including nonfatal myocardial infarction [MI], nonfatal stroke, or cardiovascular death), fatal or nonfatal MI, fatal or nonfatal stroke, coronary revascularization procedures, hospitalization for heart failure, death from cardiovascular causes, and all-cause mortality.
Intervention-Related Costs and Outcome Costs
We considered both the intervention-related costs and the outcome costs in the cost-effectiveness analyses. To estimate intervention-related costs (Supplementary Table 2), we included the costs of 1) implementing the CBT and EXER interventions by CBT therapists and exercise trainers, 2) passes/memberships to fitness facilities provided for participants in the EXER and EXER+CBT intervention groups, and 3) participant time spent on participation in CBT and EXER sessions. Moreover, we recognized that either by causing side effects or by improving health, the Program ACTIVE II interventions might affect the resource use and cost of medical care outside the study. To estimate the medical costs of care incurred or averted by the Program ACTIVE II interventions during the 15-month trial for each of the four intervention groups, we surveyed participants and estimated the cost of using outpatient, urgent care, emergency room, and hospitalization services; laboratory testing; and self-monitoring of blood glucose. Taken together, these costs were summed to derive the total intervention-related costs. In consideration of these costs in the formal health care sector, the per-participant intervention-related costs were $1,615, $1,346, $1,757, and $1,736 for the UC, EXER, CBT, and EXER+CBT groups, respectively, over 15 months. As recommended by the Second Panel on Cost-Effectiveness in Health and Medicine (30), resources and costs for the purpose of cost-effectiveness analysis in health and medicine can be categorized as those that fall within the formal health care sector and those that fall outside of it, the latter including the informal health care sector. Costs within the informal health care sector include time costs of patients in seeking and receiving health care, time costs of informal (unpaid) caregivers in caring for patients, or transportation costs. In further consideration of the costs in both the formal health care sector and the informal health care sector (e.g., costs of patient time spent on participation in CBT and EXER sessions), the per-participant intervention-related costs over 15 months were $1,615, $1,532, $1,983, and $2,138 for the UC, EXER, CBT, and EXER+CBT groups, respectively (Supplementary Table 2).
Outcome costs refer to the direct medical costs of type 2 diabetes and its complications. The MMD incorporates a cost module to estimate outcome costs based on the published literature. Costs were assessed according to sex, BMI, glucose-lowering therapy, and diabetes-related complications. The costs of complications were estimated as the cost incurred during the 1st year that a complication occurred (event cost) and the cost in each subsequent year after the complication occurred (ongoing cost). We also considered the costs of death. All costs were expressed in 2014 U.S. dollars.
Health Utilities
Health utility scores are a measure of health-related QoL, in which perfect health is assigned a value of 1.0 and death is assigned a value of 0.0. In economic analyses, the health utility score for each health state is multiplied by the time a subject spends in that health state. These are then summed to calculate the QALE, as expressed in quality-adjusted life-years (QALYs), which is accrued over a specified period of time. The MMD incorporates a health utility module to calculate yearly and cumulative QALYs based on subjects’ demographic, glucose-lowering therapy, and complication and comorbidity status.
Base-Case and Sensitivity Analyses
In the base-case analysis, we assumed that no additional interventions were implemented after the end of the follow-up in Program ACTIVE II (i.e., 15 months after randomization) and that the intervention effects observed at 15 months of the trial diminished over the simulation time horizon. Under this scenario, intervention-related costs only include the costs incurred over the 15-month trial period. We projected trajectories of HbA1c, blood pressure, and lipids after 15 months according to the equations derived from the UK Prospective Diabetes Study Outcomes Model (UKPDS-OM) (31), which demonstrated gradual convergence of each risk factor as the treatment effects wore off. We assessed the cumulative incidence of diabetes complications, life expectancy, costs, and QALYs over 10 years of the simulation time horizon. We then estimated the 10-year cost-effectiveness of interventions by calculating the incremental cost-effectiveness ratios (ICERs) as incremental total costs divided by incremental QALYs. In these analyses, following the recommendations of the Second Panel on Cost-Effectiveness in Health and Medicine (30), we adopted a health care sector perspective for consideration of formal health care sector medical costs (i.e., the intervention-related and outcome costs) borne by third-party payers. The impact inventory for components considered in the cost-effectiveness analyses is provided in Supplementary Table 3.
In sensitivity analyses, we first assessed the 10-year cost-effectiveness of interventions from a societal perspective by including both formal and informal health care sector medical costs. Then, we ran the MMD simulation for 5 years to assess the shorter-term cost-effectiveness of interventions from a health care sector perspective. Third, we assumed that the intervention effects observed at 15 months persisted over the simulation time horizon and the levels of risk factors (i.e., HbA1c, blood pressure, and lipids) for predicting diabetes complications did not change over time, and then we assessed the 10-year cost-effectiveness of interventions from a health care sector perspective. Last, we assessed the effects of increasing the intervention-related costs on the 10-year cost-effectiveness of interventions from a health care sector perspective. Specifically, we recalculated the costs of the EXER, CBT, and EXER+CBT interventions, assuming that there was a 400% increase of the fee for passes/memberships to fitness facilities, a 50% increase of the hourly rate for CBT therapists, and a 50% increase of the hourly rate for exercise trainers.
In all analyses, we simulated 3,000 individuals for each of the four intervention groups and repeated the simulations 1,000 times. Results from the simulation of the four intervention groups were evaluated with SAS (SAS Institute, Cary, NC). We assessed the cost-effectiveness of interventions by calculating the ICERs of the three interventions (EXER, CBT, and EXER+CBT) relative to UC and to each other. We discounted both costs and QALYs at 3% per year.
Results
Population Characteristics
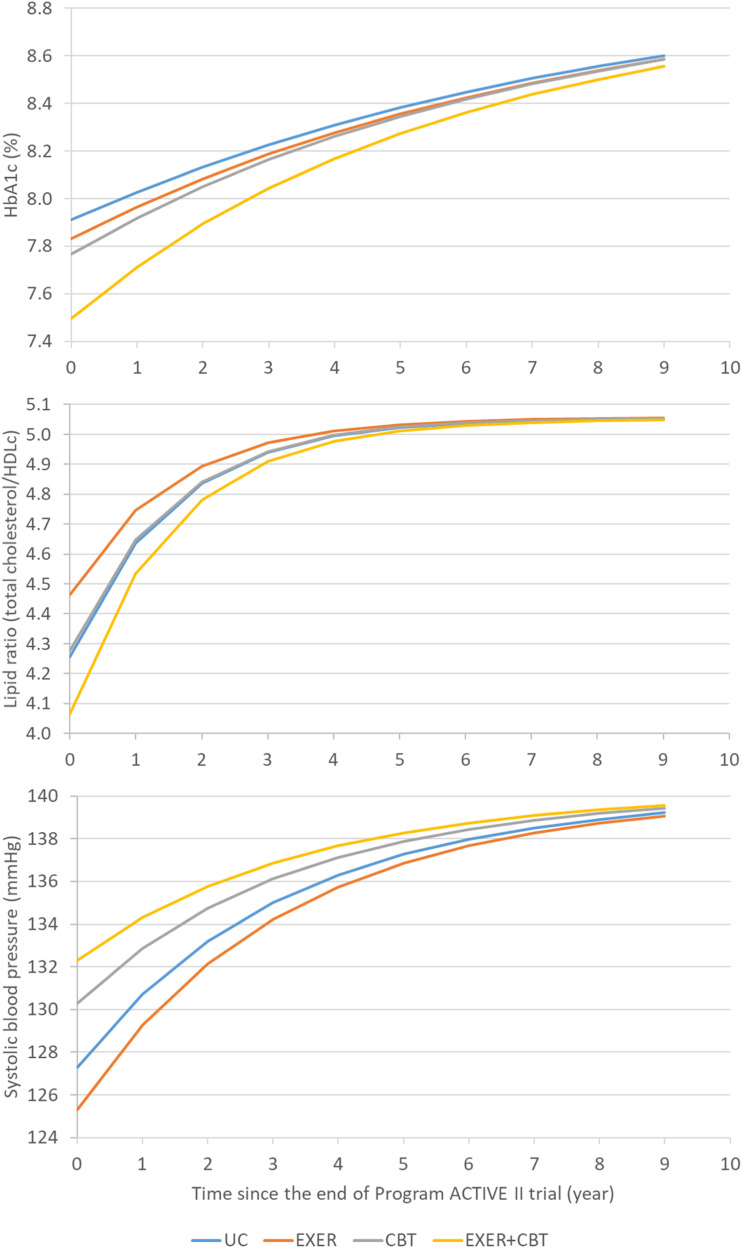
Supplementary Table 1 shows the characteristics of the four study groups at the end of the 15-month trial and the characteristics of the four simulated groups at the initiation of the model simulation. The simulated population of adults with type 2 diabetes and depression reflected the pooled sample means from the four trial groups. The mean age of the simulated population was 57.3 years, 24% were male, 70.7% were White, mean type 2 diabetes duration was 13.3 years, and mean BMI was 36.6 kg/m2. At the end of the trial, the UC group had the highest HbA1c, total cholesterol (TC), HDL cholesterol (HDLc), and LDL cholesterol; the EXER group had the lowest TC, HDLc, LDL cholesterol, and SBP; and the EXER+CBT group had the lowest HbA1c but the highest SBP. The UC group had the lowest health utility score, while the EXER+CBT group had the highest health utility score. Figure 1 shows the gradual convergence of risk factors for diabetes progression over the simulation period in the base-case analysis scenario. The magnitude of the difference in risk factors among the four intervention groups becomes smaller over time.
Figure 1.
Simulated risk factor trajectories for the scenario assuming the diminishing intervention effects after the end of Program ACTIVE II and over the simulation period.
Health Outcomes
Table 1 summarizes the simulated 10-year cumulative incidence of diabetes complications by intervention group. In the base-case analysis scenario in which we modeled diminishing intervention effects over time, clinical outcomes were generally best for the EXER+CBT or EXER groups and worse for the CBT and UC groups. For instance, the cumulative incidence of cardiovascular death was lower and similar in the EXER+CBT (9.00%) and EXER (8.99%) groups but higher in the CBT (9.16%) and UC (9.02%) groups. In the sensitivity analysis scenario in which we modeled persistent intervention effects over time, in general, clinical outcomes were best for the EXER+CBT group and worst for the CBT group. For example, the cumulative incidence of three-point MACE was lowest in the EXER+CBT group (11.3%), followed by the EXER (12.2%), UC (12.2%), and CBT (12.9%) groups.
Table 1.
Cumulative incidence of complications when the intervention effects diminish or persist over 10 years of the simulation time horizon by intervention group
| Scenario with the diminishing intervention effects∗ | Scenario with the persistent intervention effects† | |||||||
|---|---|---|---|---|---|---|---|---|
| UC | EXER | CBT | EXER+CBT | UC | EXER | CBT | EXER+CBT | |
| MI | 6.15 (0.36) | 6.17 (0.35) | 6.15 (0.35) | 6.03 (0.33) | 4.80 (0.31) | 4.97 (0.30) | 4.88 (0.31) | 4.64 (0.29) |
| Stroke | 6.49 (0.34) | 6.45 (0.34) | 6.67 (0.34) | 6.61 (0.35) | 4.92 (0.31) | 4.70 (0.31) | 5.51 (0.32) | 4.49 (0.29) |
| Cardiovascular death | 9.02 (0.39) | 8.99 (0.40) | 9.16 (0.40) | 9.00 (0.40) | 6.92 (0.35) | 6.92 (0.37) | 7.37 (0.36) | 6.11 (0.34) |
| Three-point MACE | 15.7 (0.52) | 15.7 (0.53) | 15.9 (0.50) | 15.7 (0.51) | 12.2 (0.46) | 12.2 (0.46) | 12.9 (0.47) | 11.3 (0.45) |
| Revascularization procedure | 13.9 (0.49) | 14.0 (0.51) | 13.9 (0.48) | 13.7 (0.48) | 11.1 (0.44) | 11.5 (0.45) | 11.2 (0.44) | 10.8 (0.43) |
| Congestive heart failure | 24.2 (0.60) | 24.1 (0.60) | 24.5 (0.61) | 24.6 (0.62) | 21.0 (0.57) | 21.1 (0.57) | 21.9 (0.55) | 13.6 (0.48) |
| All-cause death | 17.1 (0.51) | 17.1 (0.53) | 17.2 (0.52) | 17.1 (0.52) | 15.1 (0.50) | 15.1 (0.50) | 15.5 (0.49) | 14.3 (0.48) |
Data are % (SD).
We used the UKPDS-OM risk equations to project gradual convergence trajectories of the postintervention levels of all risk factors for diabetes complications (i.e., HbA1c, SBP, and lipid ratio [TC divided by HDLc]) as the treatment effect wears off over the simulation time horizon.
We assumed that the postintervention levels of all risk factors for diabetes complications (i.e., HbA1c, SBP, and lipid ratio [TC divided by HDLc]) did not change over the simulation time horizon.
Base-Case Analyses
Table 2 summarizes the simulated cost-effectiveness outcomes for the base-case analysis of comparing the EXER, CBT, and EXER+CBT interventions with the UC intervention. Over a 10-year period, the EXER+CBT intervention was associated with the longest QALE (5.355 QALYs). The EXER intervention resulted in the lowest total costs over 10 years ($75,714). Compared with with UC, the EXER intervention cost $313 less, resulted in a gain of 0.382 QALY, and was cost saving. Thus, the EXER intervention dominated the UC intervention. Compared with UC, the CBT intervention cost $596 more and gained 0.290 more QALY, leading to an ICER of ∼$2,100 per QALY gained, and EXER+CBT cost $403 more and gained 0.690 more QALY, resulting in an ICER of ∼$600 per QALY gained. Supplementary Table 4 summarizes the simulated cost-effectiveness outcomes for the base-case analysis of comparison among the EXER, CBT, and EXER+CBT interventions. Compared with the CBT intervention, EXER and EXER+CBT cost $908 and $192 less and gained 0.092 and 0.401 more QALY, respectively. Therefore, both the EXER and EXER+CBT interventions were cost saving and dominated the CBT intervention. Compared with EXER, EXER+CBT cost $716 more and gained 0.308 more QALY, leading to an ICER of ∼$2,300 per QALY gained.
Table 2.
Base-case analysis from the health care sector perspective for the 10-year simulation cost-effectiveness∗
| UC | EXER | CBT | EXER+CBT | |
|---|---|---|---|---|
| Intervention-related cost ($)† | 1,615 | 1,346 | 1,757 | 1,736 |
| Cost of the EXER or CBT intervention | 0 | 320 | 694 | 1,033 |
| Resource use and cost of medical care outside Program ACTIVE II | 1,615 | 1,026 | 1,063 | 704 |
| Outcome cost ($)‡ | 74,411 | 74,368 | 74,865 | 74,693 |
| Total cost ($) | 76,026 | 75,714 | 76,622 | 76,429 |
| QALYs | 4.665 | 5.047 | 4.955 | 5.355 |
| Incremental total cost vs. UC ($) | −313 | 596 | 403 | |
| Incremental QALY vs. UC | 0.382 | 0.290 | 0.690 | |
| ICER vs. UC ($) | Cost saving | 2,058 | 585 |
Cost-effectiveness of the Program ACTIVE II interventions assuming the diminishing intervention effects after the end of the trial.
Intervention-related cost refers to the per-participant costs over the 15-month intervention period of Program ACTIVE II and includes the costs of the EXER intervention, CBT intervention, and EXER+CBT intervention and the resource use and cost of medical care outside Program ACTIVE II.
Outcome cost refers to the per-participant medical costs of type 2 diabetes and its complications.
Sensitivity Analyses
We used sensitivity analyses to assess the effects of the analytic perspective, simulation time horizon, and plausible changes in intervention effectiveness and costs on the cost-effectiveness of the Program ACTIVE II interventions (Table 3 and Supplementary Table 4). When a societal perspective was adopted to also include informal health care sector costs, the ICER of the EXER+CBT intervention versus the UC and EXER interventions increased to ∼$1,200 and $3,000 per QALY gained, respectively. Adopting a societal perspective had little effect on the cost-effectiveness of the EXER and CBT interventions versus the UC intervention and that of the EXER and EXER+CBT interventions versus the CBT intervention. Adopting a shorter simulation time horizon (5 years) had little effect on the cost-effectiveness of the EXER, CBT, and EXER+CBT interventions versus the UC intervention. Both the EXER and EXER+CBT interventions still dominated the CBT intervention over a 5-year simulation time horizon, while the ICER of the EXER+CBT intervention versus the EXER intervention increased to ∼$3,500 per QALY gained.
Table 3.
Sensitivity analyses for cost-effectiveness of the EXER, CBT, and EXER+CBT interventions versus the UC intervention
| EXER vs. UC | CBT vs. UC | EXER+CBT vs. UC | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Incremental total cost ($) | Incremental QALY | ICER ($) | Incremental total cost ($) | Incremental QALY | ICER ($) | Incremental total cost ($) | Incremental QALY | ICER ($) | |
| Base-case analysis∗ | −313 | 0.382 | Cost saving | 596 | 0.290 | 2,058 | 403 | 0.690 | 585 |
| Societal perspective | −126 | 0.382 | Cost saving | 822 | 0.290 | 2,838 | 805 | 0.690 | 1,167 |
| 5-year simulation time horizon | −340 | 0.214 | Cost saving | 473 | 0.163 | 2,904 | 266 | 0.387 | 687 |
| Effectiveness: persistent intervention effects | −182 | 0.384 | Cost saving | 1,379 | 0.286 | 4,822 | −4,266 | 0.712 | Cost saving |
| Cost | |||||||||
| 400% increase of the cost for passes/memberships to fitness facilities† | 204 | 0.382 | 533 | 596 | 0.290 | 2,058 | 919 | 0.690 | 1,332 |
| 50% increase of the hourly rate for CBT therapists | −313 | 0.382 | Cost saving | 943 | 0.290 | 3,256 | 773 | 0.690 | 1,120 |
| 50% increase of the hourly rate for exercise trainers | −217 | 0.382 | Cost saving | 596 | 0.290 | 2,058 | 486 | 0.690 | 705 |
| 400% increase of the cost for passes/memberships to fitness facilities† and 50% increase of the hourly rate for both CBT therapists and exercise trainers | 299 | 0.382 | 784 | 943 | 0.290 | 3,256 | 1,371 | 0.690 | 1,987 |
The base-case analysis was from the health care sector perspective over a 10-year simulation time horizon for the cost-effectiveness of the Program ACTIVE II interventions assuming the diminishing intervention effects after the end of the trial.
The cost of passes/memberships to fitness facilities was assumed to increase by 400%, which would provide participants with free access to fitness facilities for the first 3 months of the study period ($129 per participant in the base-case analysis) vs. for the total 15 months of the study period ($645 per participant in the sensitivity analysis).
When we assumed that the intervention effects observed at 15 months persisted over 10 years, compared with the UC intervention, the EXER intervention was still cost saving, while the CBT intervention produced an increased ICER to ∼$4,800 per QALY gained and the EXER+CBT intervention became cost saving. Both the EXER and EXER+CBT interventions still dominated the CBT intervention, and the EXER+CBT intervention would dominate the EXER intervention. Increasing the intervention-related costs increased the ICERs. Compared with the UC intervention, the ICER of the EXER, CBT, and EXER+CBT interventions would increase up to ∼$800, $3,300, and $2,000 per QALY gained, respectively. Compared with the CBT intervention, the EXER intervention was still cost saving, while the ICER for the EXER+CBT intervention could increase up to ∼$1,100 per QALY gained. Compared with the EXER intervention, the ICER for the EXER+CBT intervention could increase up to ∼$3,500 per QALY gained.
Conclusions
Type 2 diabetes with comorbid depression is common, but access to treatment remains limited. This is the first study to examine the comparative cost-effectiveness of two community-based behavioral treatment strategies, EXER and CBT, for depression and type 2 diabetes using the community fitness and mental health partners who provided the interventions. In Program ACTIVE II (22), we observed significant improvements in depression in all three active intervention groups compared with UC. The EXER+CBT group showed a significant improvement in HbA1c compared with UC. While prior trials tested individual interventions (32–34), this trial demonstrated that the EXER alone, CBT alone, and combination therapy (EXER+CBT) interventions were comparable in improving MDD diagnosis in an underserved type 2 diabetes population drawn from both rural and urban regions. These findings demonstrate that depression and hyperglycemia can be effectively controlled in adults with type 2 diabetes with MDD by use of multiple behavioral strategies.
In the current study, the cost-effectiveness analyses of the Program ACTIVE II interventions demonstrated that from the health care sector perspective over a 10-year simulation time horizon, the EXER, CBT, and EXER+CBT interventions were either cost saving (∼$300 less) or highly cost-effective (less than ∼$2,100 per QALY gained) relative to the UC intervention, the EXER and EXER+CBT interventions were cost saving (∼$190 to $900 less) relative to the CBT intervention, and the EXER+CBT intervention was highly cost-effective (less than ∼$2,400 per QALY gained) compared with the EXER intervention. The results were robust in the analyses from a societal perspective and in several sensitivity analyses.
There are no absolute criteria for cost-effectiveness in the U.S., and the long-cited benchmark of $50,000 per QALY gained for an intervention to be deemed cost-effective is largely unsupported (35). Nevertheless, in general, interventions costing <$20,000 per QALY gained may be considered to have strong evidence for adoption, those costing $20,000–$100,000 per QALY gained to have moderate evidence for adoption, and those >$100,000 per QALY gained to have weaker evidence for adoption (36). Recently, it has been suggested that a threshold of $100,000 or $150,000 per QALY gained may better reflect the modern U.S. health care environment (37) and represent good value for money.
Three randomized controlled behavioral intervention trials tailored for the treatment of MDD among patients with type 2 diabetes using the Collaborative Care model have assessed the cost-effectiveness of the interventions (14,19,20). In the Pathways Study by Simon et al. (14), the systematic depression intervention program with a combination of the structured antidepressant pharmacotherapy program, problem-solving treatment psychotherapy, and specialty mental health consultation was integrated within primary care clinics to treat depression in adults with diabetes for 12 months compared with UC. They reported that over a 24-month follow-up period, the intervention produced a lower average outpatient health services cost of $314 per patient relative to UC, but they did not report the intervention impact on QALYs. In the TEAMcare study by Katon et al. (19), the nurse care manager–led multicondition collaborative care intervention program, which was aimed to improve disease control of depression, diabetes, hypertension, and dyslipidemia, was added to the primary care team for the systematic management of adults with depression and elevated HbA1c or coronary heart disease for 12 months compared with UC. They reported that over a 24-month follow-up period, the intervention that produced a lower mean outpatient health cost of $594 and a gain of 0.335 additional QALY per patient was cost saving relative to UC. In another study by Hay et al. (20), the socioculturally adapted Multifaceted Diabetes and Depression Program (MDDP), which was aimed at increasing patient exposure to evidence-based depression psychotherapy and/or pharmacotherapy, was integrated within public safety net clinics to manage depression among low-income, predominantly Hispanic patients with diabetes for 12 months compared with UC. They reported that over an 18-month follow-up period, the MDDP intervention relative to UC cost $515 more and gained 0.13 more QALY, leading to an ICER of ∼$4,000 per QALY gained.
Comparison of the cost-effectiveness results between our study and previous studies could not be made directly and must take into account significant discrepancies in the nature of study populations, context of study interventions and their deliveries, intervention and follow-up periods, and analytical design, approaches, and assumptions. For instance, participants in Program ACTIVE II had overall higher depression severity, meeting the full criteria for MDD per DSM-IV-TR criteria (38) at baseline compared with those with the elevated depressive symptoms measured using a self-report questionnaire. The Program ACTIVE II interventions were delivered in community settings with providers drawn from multiple health care systems and practice settings compared with the collaborative care interventions led by nurse case managers in primary care clinics. In addition, the total contact for the Program ACTIVE II interventions could be considered more intensive for both providers and patients over a shorter intervention period of 3 months compared with the case management approach involving the extended brief contact with patients over a 12-month intervention period. Moreover, our study was a modeling-based cost-effectiveness analysis over a 10-year simulation time horizon, while prior studies were the trial-based cost-effectiveness analysis over a 1.5-year or 2-year follow-up period.
Several limitations to our study should be acknowledged. First, no simulation model can perfectly represent reality, and all models have inherent limitations (39). The validity of our modeling results is contingent on data quality and model assumptions. Misspecifications of model parameters (e.g., uncertainty about the estimation of the model parameters) and model structure (e.g., uncertainty about the structure or assumptions of the model) are generally the most important sources of uncertainty. The SDs provided with our simulated results account for Monte Carlo uncertainty and population uncertainty but not model parameter and structure uncertainty. For this study, we updated the structure of the MMD (version 2), incorporated the UKPDS hazard equations and recalibrated the model parameters to recent clinical studies, and performed both internal and external validation studies to verify the model’s performance, and the validation studies demonstrated very good performance of the model (25). Second, the study included a predominantly female and White sample and relatively small sample sizes that may skew findings of cost-effectiveness. Future studies may be warranted to explore whether heterogeneity of treatment effectiveness and cost-effectiveness from the Program ACTIVE II interventions may exist by sex or race. Sensitivity analyses conducted to evaluate the stability of estimates in light of small sample sizes demonstrated the consistency of findings when assuming a 400% increase in facility fees for exercise and a 50% hourly rate increase for CBT therapists and/or exercise trainers. Third, the sample represented socioeconomic, ethnic, and geographic diversity but did not include Latinos and Asian Americans, thereby limiting the generalizability of the findings to these populations. Consistent with many other trials, participants in this study may not be generalized to all adults with type 2 diabetes and MDD. Finally, the effects reported in this study and across Program ACTIVE II more generally describe the impacts of the CBT and EXER interventions in the absence of diabetes self-management education and support (DSMES). Prior studies have demonstrated significant positive effects of DSMES interventions on mood and diabetes-related distress in adults with type 2 diabetes (40). Future studies could evaluate the incremental effectiveness of this standard treatment in conjunction with the Program ACTIVE II interventions.
In summary, Program ACTIVE II used a set of manualized interventions for the treatment of depression in adults with type 2 diabetes and demonstrated the cost-effectiveness of the EXER, CBT, and EXER+CBT interventions at 5 and 10 years beyond the end of the trial from both health care sector and societal perspectives. These findings demonstrate the value for health care systems to partner with community-based fitness and mental health professionals to extend the availability of depression treatment options that are complementary to medical care for patients with type 2 diabetes in order to achieve improvements in depression and diabetes outcomes.
Article Information
Acknowledgments. The Program ACTIVE Research Team thanks the participants of Program ACTIVE II who gave generously of their time and energy to be involved in this study. The authors also thank their community partners who delivered the interventions to participants and for referring health care providers for their collaboration. The Program ACTIVE Research Team thanks the following individuals for their many contributions to this study: Barb Myers, Chelsea Holbert, Debby Wimer, Kelly Chaudoin, Sarah Mielens, Ellen Knapp, Kent Crick, Tracey Garrett, Trenity Taylor, Danielle Epler, Michelle Weinstein, Kasey Goodpaster, Brett McKinney, and Kisha Wilkerson (Indiana University); Rachel Clift, Frank Schwartz, Cammie Starner, Lynn Petrik, and Melinda Ruberg (Heritage College of Osteopathic Medicine, Ohio University); Susan Eason, Alex Tylka, David Donley, Lindsey Sams, Jaclyn Babe, Christian Abildso, and Daniel Bonner (West Virginia University); and Bernadette Heckman (University of Georgia). The authors also acknowledge their community partners who delivered interventions to participants: Richard Nulter, Kim Johnson, Adrianne Garrett, Bonnie de Lange, and Cassandra Watt (Hopewell Health Center, Belpre and Athens, OH); Suzy Zumwalde and Dave Vogel (Marietta Family YMCA, Marietta, OH); Sharon Sheets, Jason Weber, and Sheila Williams (private practitioners, Athens, OH); Priscilla Leavitt, Stephen Givens, and Rick Stanley (Counseling & Wellness Center, Parkersburg, WV); Noah Albrecht, Joe Leaman, Erin Weber, Allison Burner, Dan Braatz, Cassy Offenberger, and Jonathan Rodriquez (Mountain River Physical Therapy, Parkersburg and Vienna, WV); Pat Perine and Louie Haer (Family Fitness, Parkersburg, WV); Josh Christen (Wellworks, Athens, OH); McKenzie Walter, Flynt Smathers, and Rich Campitelli (Athens Community Center, Athens, OH); Karen Newton, Eric Murphy, Jennifer Murray, Lauren Prinzo, and Rebecca Smith (West Virginia University County Extension Program); Kathy Dodrill (The Ohio State University Washington County Extension Program); Christina Ferroli and Lydia Armstrong (Purdue University Marion County Extension Program); Brian Sharp, Dana Nugent, and Mark Tipton (United Summit Center, Clarksburg, WV); Kimberly Yingling (private practitioner, Morgantown, WV); Eric Shaw and Rick Williams (Tygart Valley Rehabilitation and Fitness Center, Grafton, WV); Kellie Snyder (Fairmont General HealthPlus, Fairmont, WV); Shane Trivigno and Beth Burleson (Pro Performance, Morgantown, WV); Rob Cress and Jesse Halldin (Rob’s Fitness Factory, Morgantown, WV); Whitney Hickman (Harrison County YMCA, Lodgeville Branch, Bridgeport, WV); James Brummett, Kathi Bledsoe, Allison White, Ella Vinci, Cynthia Donel, Monica Staples, Tina Wiesert, Carol Hendricks, Krisha MacDonald, and Cindy Wilson (Midtown Community Mental Health, Indianapolis, IN); Gary Brown, Chelsy Winters, Anne Graves, Evan Heald, Latisha Idlewine, Julie Kenny, Matt Larson, and LaRona Dixon (YMCA of Greater Indianapolis, Indianapolis, IN); Allison Plopper, Natalie Johann, and Denise Sayasit (Physically Active Residential Communities and Schools [PARCS] Program, Indianapolis, IN); and Ben Jones (Chase Near Eastside Legacy Center, Indianapolis, IN).
Funding. Program ACTIVE II was funded by the National Institute of Diabetes and Digestive and Kidney Diseases (R18DK092765 and R34DK071545). The project described was supported by grant P30DK092926 (Michigan Center for Diabetes Translational Research, Methods and Measurement Core) from the National Institute of Diabetes and Digestive and Kidney Diseases.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Duality of Interest. M.d.G. is a faculty consultant to the Lifescan Diabetes Institute. K.J.M. is a consultant with Merck & Co. and Novo Nordisk. J.H.S. is a consultant for Eli Lilly, Novo Nordisk, and Intarcia. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. All authors contributed to the study design, writing the manuscript, and reviewing and editing all portions of the manuscript. S.K., W.Y., M.d.G., C.S., and W.H.H., had full access to all the data in the study. S.K., W.Y., and W.H.H. conducted and are responsible for the data analyses. S.K., M.d.G., and W.H.H. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 80th Scientific Sessions of the American Diabetes Association, 12–16 June 2020.
Footnotes
Clinical trial reg. no. NCT03371940, clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.13622906.
S.K. and W.Y. share equal authorship.
References
- 1.Centers for Disease Control and Prevention . National Diabetes Statistics Report, 2017. Atlanta, GA, Centers for Disease Control and Prevention, U.S. Department of Health and Human Services, 2017 [Google Scholar]
- 2.American Diabetes Association . Economic costs of diabetes in the U.S. in 2017. Diabetes Care 2018;41:917–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holt RI, de Groot M, Golden SH. Diabetes and depression. Curr Diab Rep 2014;14:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nouwen A, Adriaanse MC, van Dam K, et al.; European Depression in Diabetes (EDID) Research Consortium . Longitudinal associations between depression and diabetes complications: a systematic review and meta-analysis. Diabet Med 2019;36:1562–1572 [DOI] [PubMed] [Google Scholar]
- 5.Zhang X, Norris SL, Gregg EW, Cheng YJ, Beckles G, Kahn HS. Depressive symptoms and mortality among persons with and without diabetes. Am J Epidemiol 2005;161:652–660 [DOI] [PubMed] [Google Scholar]
- 6.Henk HJ, Katzelnick DJ, Kobak KA, Greist JH, Jefferson JW. Medical costs attributed to depression among patients with a history of high medical expenses in a health maintenance organization. Arch Gen Psychiatry 1996;53:899–904 [DOI] [PubMed] [Google Scholar]
- 7.Simon GE, Manning WG, Katzelnick DJ, Pearson SD, Henk HJ, Helstad CS. Cost-effectiveness of systematic depression treatment for high utilizers of general medical care. Arch Gen Psychiatry 2001;58:181–187 [DOI] [PubMed] [Google Scholar]
- 8.Simon GE, VonKorff M, Rutter C, Wagner E. Randomised trial of monitoring, feedback, and management of care by telephone to improve treatment of depression in primary care. BMJ 2000;320:550–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simon GE, Von Korff M, Ludman EJ, et al. Cost-effectiveness of a program to prevent depression relapse in primary care. Med Care 2002;40:941–950 [DOI] [PubMed] [Google Scholar]
- 10.Hayes A, Arima H, Woodward M, et al. Changes in quality of life associated with complications of diabetes: results from the ADVANCE study. Value Health 2016;19:36–41 [DOI] [PubMed] [Google Scholar]
- 11.Rushing J, Wing R, Wadden TA, et al.; Look AHEAD Research Group . Cost of intervention delivery in a lifestyle weight loss trial in type 2 diabetes: results from the Look AHEAD clinical trial. Obes Sci Pract 2017;3:15–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egede LE, Zheng D, Simpson K. Comorbid depression is associated with increased health care use and expenditures in individuals with diabetes. Diabetes Care 2002;25:464–470 [DOI] [PubMed] [Google Scholar]
- 13.Simon GE, Katon WJ, Lin EH, et al. Diabetes complications and depression as predictors of health service costs. Gen Hosp Psychiatry 2005;27:344–351 [DOI] [PubMed] [Google Scholar]
- 14.Simon GE, Katon WJ, Lin EH, et al. Cost-effectiveness of systematic depression treatment among people with diabetes mellitus. Arch Gen Psychiatry 2007;64:65–72 [DOI] [PubMed] [Google Scholar]
- 15.Cuijpers P, Berking M, Andersson G, Quigley L, Kleiboer A, Dobson KS. A meta-analysis of cognitive-behavioural therapy for adult depression, alone and in comparison with other treatments. Can J Psychiatry 2013;58:376–385 [DOI] [PubMed] [Google Scholar]
- 16.Cooney GM, Dwan K, Greig CA, et al. Exercise for depression. Cochrane Database Syst Rev 2013;9:CD004366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freeman MP, Mischoulon D, Tedeschini E, et al. Complementary and alternative medicine for major depressive disorder: a meta-analysis of patient characteristics, placebo-response rates, and treatment outcomes relative to standard antidepressants. J Clin Psychiatry 2010;71:682–688 [DOI] [PubMed] [Google Scholar]
- 18.Ross EL, Vijan S, Miller EM, Valenstein M, Zivin K. The cost-effectiveness of cognitive behavioral therapy versus second-generation antidepressants for initial treatment of major depressive disorder in the United States: a decision analytic model. Ann Intern Med 2019;171:785–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katon W, Russo J, Lin EH, et al. Cost-effectiveness of a multicondition collaborative care intervention: a randomized controlled trial. Arch Gen Psychiatry 2012;69:506–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hay JW, Katon WJ, Ell K, Lee PJ, Guterman JJ. Cost-effectiveness analysis of collaborative care management of major depression among low-income, predominantly Hispanics with diabetes. Value Health 2012;15:249–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Groot M, Shubrook J, Schwartz F, Hornsby WG Jr., Pillay Y, Saha C. Program ACTIVE II: design and methods for a multi-center community-based depression treatment for rural and urban adults with type 2 diabetes. J Diabetes Res Ther 2015;1:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Groot M, Shubrook JH, Hornsby WG Jr., et al. Program ACTIVE II: outcomes from a randomized, multistate community-based depression treatment for rural and urban adults with type 2 diabetes. Diabetes Care 2019;42:1185–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Myers BA, Pillay Y, Guyton Hornsby W Jr., et al. Recruitment effort and costs from a multi-center randomized controlled trial for treating depression in type 2 diabetes. Trials 2019;20:621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou H, Isaman DJ, Messinger S, et al. A computer simulation model of diabetes progression, quality of life, and cost. Diabetes Care 2005;28:2856–2863 [DOI] [PubMed] [Google Scholar]
- 25.Ye W, Brandle M, Brown MB, Herman WH. The Michigan model for coronary heart disease in type 2 diabetes: development and validation. Diabetes Technol Ther 2015;17:701–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brandle M, Zhou H, Smith BR, et al. The direct medical cost of type 2 diabetes. Diabetes Care 2003;26:2300–2304 [DOI] [PubMed] [Google Scholar]
- 27.Coffey JT, Brandle M, Zhou H, et al. Valuing health-related quality of life in diabetes. Diabetes Care 2002;25:2238–2243 [DOI] [PubMed] [Google Scholar]
- 28.Zhang P, Brown MB, Bilik D, Ackermann RT, Li R, Herman WH. Health utility scores for people with type 2 diabetes in U.S. managed care health plans: results from Translating Research Into Action for Diabetes (TRIAD). Diabetes Care 2012;35:2250–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.University of Michigan Center of Diabetes Translational Research (MCDTR) Disease Modeling Group . The Michigan Model for Diabetes user manual, 2015. Accessed 1 June 2018. Available from http://diabetesresearch.med.umich.edu/peripherals/DiseaseModel/MDRTC%20Diabetes%20Model/UserManual_MichiganModel_for_Diabetes_ver2.pdf
- 30.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA 2016;316:1093–1103 [DOI] [PubMed] [Google Scholar]
- 31.Clarke PM, Gray AM, Briggs A, et al.; UK Prospective Diabetes Study (UKDPS) Group . A model to estimate the lifetime health outcomes of patients with type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68). Diabetologia 2004;47:1747–1759 [DOI] [PubMed] [Google Scholar]
- 32.Piette JD, Richardson C, Himle J, et al. A randomized trial of telephonic counseling plus walking for depressed diabetes patients. Med Care 2011;49:641–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lustman PJ, Griffith LS, Freedland KE, Kissel SS, Clouse RE. Cognitive behavior therapy for depression in type 2 diabetes mellitus. A randomized, controlled trial. Ann Intern Med 1998;129:613–621 [DOI] [PubMed] [Google Scholar]
- 34.Johnson JA, Al Sayah F, Wozniak L, et al. Collaborative care versus screening and follow-up for patients with diabetes and depressive symptoms: results of a primary care–based comparative effectiveness trial. Diabetes Care 2014;37:3220–3226 [DOI] [PubMed] [Google Scholar]
- 35.Braithwaite RS, Meltzer DO, King JT Jr., Leslie D, Roberts MS. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Med Care 2008;46:349–356 [DOI] [PubMed] [Google Scholar]
- 36.Laupacis A, Feeny D, Detsky AS, Tugwell PX. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. CMAJ 1992;146:473–481 [PMC free article] [PubMed] [Google Scholar]
- 37.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness--the curious resilience of the $50,000-per-QALY threshold. N Engl J Med 2014;371:796–797 [DOI] [PubMed] [Google Scholar]
- 38.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC, American Psychiatric Association, 2000 [Google Scholar]
- 39.Herman WH. Diabetes modeling. Diabetes Care 2003;26:3182–3183 [DOI] [PubMed] [Google Scholar]
- 40.McGowan P. The relative effectiveness of self-management programs for type 2 diabetes. Can J Diabetes 2015;39:411–419 [DOI] [PubMed] [Google Scholar]