Abstract
OBJECTIVE
Although elevated glucose levels are reported to be associated with adverse outcomes of coronavirus disease 2019 (COVID-19), the optimal range of glucose in patients with COVID-19 and diabetes remains unknown. This study aimed to investigate the threshold of glycemia and its association with the outcomes of COVID-19.
RESEARCH DESIGN AND METHODS
Glucose levels were assessed through intermittently scanned continuous glucose monitoring in 35 patients for an average period of 10.2 days. The percentages of time above range (TAR), time below range (TBR), time in range (TIR), and coefficient of variation (CV) were calculated. Composite adverse outcomes were defined as either the need for admission to the intensive care unit, need for mechanical ventilation, or morbidity with critical illness.
RESULTS
TARs using thresholds from 160 to 200 mg/dL were significantly associated with composite adverse outcomes after adjustment of covariates. Both TBR (<70 mg/dL) and TIR (70–160 mg/dL), but not mean sensor glucose level, were significantly associated with composite adverse outcomes and prolonged hospitalization. The multivariate-adjusted odds ratios of the CV of sensor glucose across tertiles for composite adverse outcomes of COVID-19 were 1.00, 1.18, and 25.2, respectively.
CONCLUSIONS
Patients with diabetes and COVID-19 have an increased risk of adverse outcomes with glucose levels >160 mg/dL and <70 mg/dL and a high CV. Therapies that improve these metrics of glycemic control may result in better prognoses for these patients.
Introduction
Epidemiological studies have reported that approximately one-half of patients with coronavirus disease 2019 (COVID-19) have comorbidities, of which diabetes is the second most common (1,2). There was more than a fourfold chance of patients with diabetes and COVID-19 meeting the primary composite end point of need for intensive care, need for mechanical ventilation, or death (26.9% vs. 6.1%) (3). On the basis of self-monitoring of blood glucose, patients with good glycemic control appear to have lower mortality during hospitalization than those with suboptimal control (4). Unlike self-monitoring of blood glucose, continuous glucose monitoring (CGM) provides a comprehensive view of patients’ interstitial glucose levels, permitting quantification of hyperglycemia, hypoglycemia, and glycemic variability (5). CGM might allow better glycemic control, with a reasonable target of glycemic control in patients with diabetes and COVID-19 (6).
Although elevated glucose levels are reported to be associated with adverse outcomes of COVID-19, the optimal range of glucose in patients with diabetes and COVID-19 remains unknown. Limited evidence has supported the close link between thresholds of glycemia and the adverse outcomes of COVID-19 among patients with diabetes. Thus, our study was designed to analyze the glycemic profiles of patients with diabetes with confirmed COVID-19 using intermittently scanned CGM (isCGM) and to determine the association of various glycemia metrics with adverse outcomes of COVID-19.
Research Design and Methods
Study Design and Participants
Wuhan Leishenshan Hospital is a newly built, temporary, 1,500-bed facility designed for patients with COVID-19. It contains several medical isolation zones with negative pressure ventilation, a living zone for medical staff, and a logistics area. The authors traveled from two hospitals in Shanghai to assist in treating patients in one of the 48-bed quarantine areas. The presence of COVID-19 was defined as a positive result on high-throughput sequencing or real-time RT-PCR assay of nasal and pharyngeal swab specimens (7,8). The study protocol was approved before its initiation by the institutional review boards of both Shanghai Jiao Tong University Affiliated Sixth People’s Hospital and Zhongnan Hospital of Wuhan University and was subsequently registered in the Chinese Clinical Trial Registry. Informed consent was obtained from all patients involved in this study.
Demographic, Anthropometric, and Laboratory Measurements
Patients’ demographic and clinical data were extracted from electronic medical records and included date of birth, diabetes duration, sex, admission dates, weight, height, heart rate, respiratory rate, symptoms on admission, sensor pressure, smoking status, comorbidities, fasting plasma glucose (FPG), hemoglobin A1c (HbA1c), ALT, AST, total cholesterol, triglycerides, HDL cholesterol, LDL cholesterol, serum creatinine, uric acid, C-reactive protein, interleukin 6 (IL-6), brain natriuretic peptide (BNP), and medication prescriptions, including antihypertensive drugs, glucose-lowering drugs, lipid-lowering drugs, and glucocorticoids. On the basis of smoking status, patients were classified into three groups: current smokers, ever smokers, and never smokers. BMI was calculated as weight (kg) / height squared (m2). The estimated glomerular filtration rate was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation (9). The average length of stay during hospitalization was 21.8 days. CGM was initiated during admission but was discontinued when a patient was admitted to the intensive care unit (ICU) because of limited application and accuracy of CGM during ICU stay. The routine protocol for glucose monitoring during hospitalization was fixed at seven swipes daily (fasting, premeal, postmeal, and bedtime) according to the 2019 standards of medical care for type 2 diabetes in China (10). When a patient developed symptoms of hypoglycemia, such as palpitations, tremulousness, hunger, and cold sweats, a subsequent sensor glucose level would be measured. Additionally, swipes were performed when a patient required or encountered any other discomfort. Since patients were all under quarantine, the meal regimen was standardized, and all participants engaged in daily mild to moderate physical exercise according to their physical capacity.
isCGM and a Cloud Platform–Based CGM System
All patients with diabetes were equipped with an isCGM sensor (FreeStyle Libre Flash glucose monitoring system; Abbott Diabetes Care, Alameda, CA). The nursing staff swiped the sensor and uploaded the data to a hospital-wide cloud platform that was specifically established for uploading isCGM data with the capability of incorporating the clinical characteristics of patients. The receiver was placed at the patient’s bedside and not allowed to be taken outside the room; a nurse wearing personal protective equipment did the swipes at the bedside. On average, patients wore the sensor for 10.2 days during their hospitalization. An ambulatory glucose profile was provided for each patient, with various metrics calculated according to the isCGM records. Time above range (TAR) was defined as the percentage of time in the glucose ranges of >140, 150, 160, 170, 180, 190, and 200 mg/dL during the whole glucose monitoring period for each patient. Time below range (TBR) was defined as the percentage of time in the glucose range of <70 mg/dL. Time in range (TIR) was defined as the percentage of time between 70 mg/dL and the time above target ranges. The mean sensor glucose level and coefficient of variation (CV) of glucose levels were also calculated according to the isCGM data.
Composite Adverse Outcomes
The composite adverse outcomes were defined as either the need for admission to the ICU, need for mechanical ventilation, or critical illness defined either as vasopressor-requiring hypotension and/or multiple organ dysfunction (11). A prolonged hospitalization was defined as a length of stay >30 days.
Statistical Analyses
The general characteristics (continuous and categorical variables) of patients with diabetes with or without composite adverse outcomes were compared using the χ2 test or Student t test. Restricted cubic splines nested in the multivariate-adjusted logistic regression model were used to test for a dose-response or nonlinear association of mean glucose, TIR, or CV of glucose levels as continuous variables with the odds of the composite adverse outcomes of COVID-19. To screen for the covariates to be included in the multivariate analysis, a univariate logistic regression was performed first (see Supplementary Table 1). Candidate covariates were selected with a univariate P < 0.1. The multivariate-adjusted logistic regression models were then used for assessing the association between the TAR, TBR, and TIR using isCGM and composite adverse outcomes. Odds ratios (ORs) with 95% CIs were presented. All analyses were adjusted for age and sex (model 1) and then for age, sex, BMI, symptoms on admission, systolic blood pressure, BNP, IL-6, and the use of glucocorticoids (model 2). P < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 24.0 for Windows (IBM Corporation, Armonk, NY) and SAS 9.3 for Windows (SAS Institute, Cary, NC).
Data and Resource Availability
Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will, on request, detail the restrictions and any conditions under which access to some data may be provided.
Results
CGM data from 35 patients with diabetes complicated with COVID-19 were analyzed. The mean age was 62.5 ± 10.2 years. There were no deaths during the study period. Fifteen of the 35 patients had a composite adverse outcome of either mechanical ventilation, admission to the ICU, or critical illness. The characteristics of these patients are listed in Table 1. All TARs of sensor glucose were different among the groups (all P < 0.05). Patients with composite adverse outcomes showed significantly higher TBR (P < 0.01) than those without composite adverse outcomes.
Table 1.
Characteristics and isCGM data of patients with diabetes and COVID-19
| Presence of the composite adverse outcome | ||
|---|---|---|
| Yes | No | |
| n | 15 | 20 |
| Age (years) | 63.2 ± 10.7 | 62.6 ± 10.7 |
| Sex | ||
| Male | 33.3 | 45.0 |
| Female | 66.7 | 55.0 |
| BMI (kg/m2) | 21.9 ± 2.06 | 22.4 ± 3.86 |
| Current smoker | 6.67 | — |
| Heart rate (beats/min) | 87.9 ± 11.0 | 87.1 ± 12.1 |
| Respiratory rate (breaths/min) | 20.0 ± 3.3 | 19.8 ± 1.7 |
| Systolic blood pressure (mmHg) | 142.7 ± 17.4 | 132.3 ± 19.0 |
| Diastolic blood pressure (mmHg) | 86.7 ± 13.7 | 78.4 ± 8.81* |
| Symptoms on admission | ||
| Fever | 66.7 | 60.0 |
| Cough | 80.0 | 50.0 |
| Chest tightness | 46.7 | 35.0 |
| Fatigue | 46.7 | 30.0 |
| Gastrointestinal | — | 10.0 |
| Comorbidities on admission | ||
| Hypertension | 60.0 | 60.0 |
| Dyslipidemia | 13.3 | 20.0 |
| Coronary heart disease | 15.0 | 6.67* |
| Stroke | — | 5.00 |
| COPD | — | 5.00 |
| Chronic kidney disease | 6.67 | 10.0 |
| Hepatic diseases | — | — |
| Laboratory measurements | ||
| FPG (mg/dL) | 147 ± 70.6 | 136 ± 42.9 |
| HbA1c (%) | 7.26 ± 1.35 | 6.85 ± 1.48 |
| ALT (units/L) | 23.0 ± 17.3 | 24.2 ± 18.2 |
| AST (units/L) | 19.4 ± 8.9 | 20.7 ± 11.6 |
| Triglycerides (mmol/L) | 1.77 ± 0.89 | 2.24 ± 2.51 |
| LDL cholesterol (mmol/L) | 2.76 ± 0.69 | 2.49 ± 0.99 |
| HDL cholesterol (mmol/L) | 1.04 ± 0.15 | 1.05 ± 0.24 |
| eGFR (mL/min/1.73 m2) | 91.7 ± 27.2 | 91.0 ± 28.1 |
| Uric acid (mmol/L) | 272.7 ± 89.2 | 320.5 ± 85.9 |
| C-reactive protein (mmol/L) | 2.05 ± 2.34 | 7.46 ± 15.93 |
| BNP (mmol/L) | 84.9 ± 200.5 | 50.2 ± 61.8* |
| IL-6 (pg/mL) | 14.50 ± 3.10 | 7.67 ± 8.13* |
| Sensor glucose (mg/dL) | 174 ± 49.0 | 144 ± 21.2† |
| Coefficient of variation (%) | 30.8 ± 5.54 | 25.2 ± 5.73† |
| TAR (mg/dL) by isCGM (%) | ||
| >140 | 63.3 ± 28.5 | 47.7 ± 22.9* |
| >150 | 57.9 ± 27.2 | 38.7 ± 20.3* |
| >160 | 52.1 ± 26.2 | 29.8 ± 18.0† |
| >170 | 47.4 ± 25.6 | 23.9 ± 15.6† |
| >180 | 42.4 ± 25.3 | 18.3 ± 12.9† |
| >190 | 37.2 ± 24.2 | 13.8 ± 10.6† |
| >200 | 32.9 ± 22.9 | 10.7 ± 9.0† |
| TBR (mg/dL) by isCGM (%) | ||
| <70 | 4.43 ± 11.4 | 0.54 ± 0.65† |
| Glucose-lowering medications | ||
| Metformin | 33.3 | 40.0 |
| Sulfonylureas | 33.3 | 5.00 |
| Thiazolidinediones | — | 5.00 |
| DPP-4 inhibitors | — | 5.00 |
| Insulin | 20.0 | 15.0 |
| α-Glycosidase inhibitors | 40.0 | 50.0 |
| Blood pressure–lowering medications | ||
| ACEI/ARB | 20.0 | 20.0 |
| CCB | 33.3 | 25.0 |
| β-Blocker | — | — |
| Diuretic | 6.67 | 5.00 |
| Lipid-lowering medications | — | 20.0 |
| Use of glucocorticoid | 6.67 | 20.0 |
Data are mean ± SD or % unless otherwise indicated. ACEI, ACE inhibitor; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; COPD, chronic obstructive pulmonary disease; DPP-4, dipeptidyl peptidase 4; eGFR, estimated glomerular filtration rate.
P < 0.05.
P < 0.01.
Glycemic Metrics Derived From isCGM, Outcomes of COVID-19, and Prolonged Hospitalization
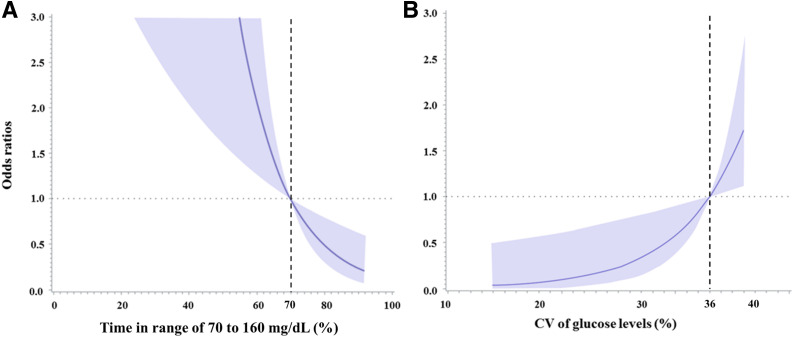
To examine the association between TAR, TBR, and TIR with various thresholds and composite adverse outcomes of COVID-19, two different models were used for the analysis (Table 2). In model 1 with adjustments for age and sex, TARs with thresholds of 160–200 mg/dL (OR 1.05 [95% CI 1.01–1.09], 1.05 [1.02–1.07], 1.07 [1.02–1.14], 1.11 [1.03–1.19], and 1.12 [1.04–1.20]) and increasing time <70 mg/dL were significantly associated with increased odds of composite adverse outcomes of COVID-19 (2.45 [1.45–4.24]). In model 2 with multiple adjusted covariates, TARs with thresholds of 160–200 mg/dL (1.06 [1.02–1.11], 1.07 [1.02–1.13], 1.12 [1.04–1.20], 1.12 [1.05–1.21], 1.14 [1.02–1.26]) and increasing time <70 mg/dL were significantly associated with increased odds of composite adverse outcomes of COVID-19 (6.56 [1.38–16.4]). Thus, TIR of 70–160 mg/dL was significantly associated with the composite adverse outcomes of COVID-19 in both models, and a graded inverse association was observed through the restricted cubic spline analysis, with TIR of 70% as the reference value (P for linearity < 0.001) (Fig. 1A). Similar results were observed when prolonged hospitalization was considered as the outcome. TARs with thresholds of 160–200 mg/dL and increasing time <70 mg/dL were significantly associated with an increased odds of prolonged hospitalization in both models 1 and 2 (Supplementary Table 2).
Table 2.
Association between glycemic metrics derived from isCGM and the composite outcome using logistic regression analysis
| Model 1 | Model 2 | |
|---|---|---|
| Sensor glucose levels (mg/dL) | ||
| TAR | ||
| >140 | 1.05 (0.92–1.17) | 1.04 (0.97–1.11) |
| >150 | 1.04 (1.00–1.07) | 1.03 (0.99–1.08) |
| >160 | 1.05 (1.01–1.09) | 1.06 (1.02–1.11) |
| >170 | 1.05 (1.02–1.07) | 1.07 (1.02–1.13) |
| >180 | 1.07 (1.02–1.14) | 1.12 (1.04–1.20) |
| >190 | 1.11 (1.03–1.19) | 1.12 (1.05–1.21) |
| >200 | 1.12 (1.04–1.20) | 1.14 (1.02–1.26) |
| TBR | ||
| <70 | 2.45 (1.45–4.24) | 6.56 (1.38–16.4) |
Data are OR (95% CI). Model 1 adjusted for age and sex. Model 2 adjusted for age, sex, BMI, symptoms on admission, systolic blood pressure, BNP, IL-6, and the use of glucocorticoids.
Figure 1.
The ORs of percentage of TIR of 70–160 mg/dL (A) and CV of sensor glucose levels (B) for composite adverse outcomes of COVID-19 using restricted cubic spline analysis. For TIR of 70–160 mg/dL, 70% was set as the reference value, while for CV of sensor glucose levels, 36% was set as the reference value. Adjustments were made for age, sex, BMI, symptoms on admission, systolic blood pressure, BNP, IL-6, and the use of glucocorticoids. Shaded areas represent 95% CIs.
Mean Glucose Levels Derived From isCGM and Outcomes of COVID-19
The mean sensor glucose level assessed using isCGM was significantly higher among patients with composite adverse outcomes than those without (174 ± 49.0 vs. 144 ± 21.2 mg/dL, P < 0.01). However, the overall linear association between mean sensor glucose level and composite adverse outcomes did not reach statistical significance (age- and sex-adjusted OR 1.60 [95% CI 0.87–2.43], multivariate-adjusted OR 2.00 [0.77–3.15]). A U-shaped curve was presented using the restricted cubic spline analysis, with mean glucose levels of 120 mg/dL as the reference value (Supplementary Fig. 1).
Glycemic Variability Derived From isCGM and Outcomes of COVID-19
The association of glycemic variability, as measured using CV, with composite adverse outcomes of COVID-19 was analyzed using the logistic regression model (Table 3). The multivariate-adjusted ORs of CV across tertiles for composite adverse outcomes of COVID-19 were 1.00, 1.18 (95% CI 0.19–9.82), and 25.2 (3.15–340). A significantly positive spline association was observed between the CV of sensor glucose and composite adverse outcomes of COVID-19, with a CV of 36% as the reference value (P for linearity < 0.001) (Fig. 1B).
Table 3.
Association between CV of sensor glucose derived from isCGM and the composite outcome using logistic regression analysis
| First tertile | Second tertile | Third tertile | P value for trend | As continuous variable | |
|---|---|---|---|---|---|
| Patients, n | 12 | 12 | 11 | ||
| Cases, n | 3 | 3 | 9 | ||
| CV of sensor glucose | |||||
| Model 1 | 1.00 | 1.34 (0.19–9.11) | 18.2 (2.01–172) | 0.019 | 1.19 (1.05–1.37) |
| Model 2 | 1.00 | 1.18 (0.19–9.82) | 25.2 (3.15–340) | 0.020 | 1.17 (1.04–1.31) |
Data are OR (95% CI) unless otherwise indicated. Model 1 adjusted for age and sex. Model 2 adjusted for age, sex, BMI, symptoms on admission, systolic blood pressure, BNP, IL-6, and use of glucocorticoids.
Conclusions
In this retrospective study among patients with diabetes complicated with COVID-19, both glucose levels of >160 mg/dL and <70 mg/dL were associated with a significantly high risk of composite adverse outcomes of COVID-19 as well as with a prolonged hospitalization. Higher glycemic variability was significantly associated with a poorer outcome of COVID-19. In contrast, the mean sensor glucose level was not a significant predictor of adverse outcomes of COVID-19.
In our study, the association of various blood glucose thresholds of patients with diabetes and COVID-19 with adverse outcomes was demonstrated. The major strength of our study was the use of the isCGM system, which can assess the exposure to hyper- and hypoglycemia as well as glycemic variability. Given that TAR is the direct measure of exposure to hyperglycemia, the significant association between TARs (with thresholds of 160–200 mg/dL) and the adverse outcomes of COVID-19 further supported the tight link between hyperglycemia per se and diabetes-related end points. On the other hand, HbA1c is a marker of mean glucose level but may not be an optimal marker of hyperglycemia. For instance, in certain patients with both hypoglycemia and hyperglycemia, HbA1c can be in the falsely normal range and be misleading, and this phenomenon is most often observed in patients with a high degree of glycemic variability. Compared with the results reported by Zhu et al. (4), who only used point-of-care measures of fasting blood glucose and 2-h postprandial blood glucose, our findings on the basis of the isCGM system rely on more robust data. Previous studies have confirmed that diabetes is associated with an increased risk of viral pneumonia–associated adverse outcomes (12,13). Studies during the severe acute respiratory syndrome (SARS) pandemic in 2003 showed that FPG levels and diabetes were independent predictors of high morbidity and mortality in patients with SARS (14). Similar results were observed during this COVID-19 pandemic. One study in 72,314 patients with COVID-19 in China showed that the mortality rate of patients with diabetes was three times higher than that of those without diabetes (7.3% vs. 2.3%) (7). Another retrospective study of 132 patients with COVID-19 found high levels of HbA1c associated with inflammation, hypercoagulability, and hypoxia (15). The possible mechanism of the increased risk of COVID-19 and poor prognosis in patients with diabetes is reported to be related to neutrophil dysfunction, decreased T-cell immune response, and abnormal humoral immunity (16). Furthermore, SARS coronavirus 2 can bind to the ACE2 receptor expressed on the surface of various tissues and cells, including pancreatic islets, causing pancreatic damage and a series of inflammatory cascades, resulting in severe hyperglycemic events and increased glycemic fluctuations (15,17). Finally, a stress-induced rise in glucocorticoids and catecholamines can further negatively affect glycemic control (18). Therefore, the combination of diabetes and COVID-19 may lead to a toxic milieu, resulting in more severe infection and death.
In recent years, especially with the increasing usage of CGM, TIR has become the focus in clinical practice and research owing to its simple calculation and intuitive results (19). Although several previous studies have demonstrated the detrimental effect of hyperglycemia on patients with COVID-19, none were designed to explore the threshold of hyperglycemia, above which the risk of adverse outcomes can be detected. Thus, the target range of glucose remains poorly understood in patients with COVID-19, which hampers the optimal management of patients with COVID-19 and preexisting diabetes. In our study, we showed that TARs with thresholds of 160–200 mg/dL and TBR with the threshold <70 mg/dL were associated with the occurrence of composite adverse outcomes, suggesting that a glucose level of 70–160 mg/dL may be the appropriate target for minimizing the risk of adverse outcomes of COVID-19.
A significant association of mean glucose with adverse outcomes of COVID-19 was not observed, which was further confirmed by the nonsignificant association between HbA1c and COVID-19 outcomes (see univariate analysis in Supplementary Table 1). A possible explanation for this observation could be that the mean glucose level does not provide information on the magnitude of hyperglycemia, hypoglycemia, and glycemic variability (19). There is a wide range of possible TAR, TBR, and TIR values for a given mean glucose/HbA1c level as previously described by Rodbard (20). Glycemic variability itself may account for the discrepancy between mean glucose levels and various clinical outcomes (20,21). In our study, patients with greater glycemic variability were at a significantly higher risk of composite adverse outcomes. Consistent with our findings, previous studies have shown that glycemic fluctuations are related to the severity of disease and prognosis. A retrospective study by Krinsley et al. (22) found that great glycemic fluctuations (defined as CV ≥20%) were associated with an increased risk of death among ICU patients. One study of 20,375 ICU inpatients with blood glucose data in the Netherlands found that the SD and mean amplitude of glycemic excursions of glucose levels were significantly associated with the hospital mortality rate (23). Additionally, Atamna et al. (24) found that a high CV was significantly associated with the 30-day and 5-year mortality of patients hospitalized with acute infectious diseases. Moreover, the Netherlands study reported that glycemic fluctuations can be related to the severity of influenza, as indicated by damage of the alveolar epithelial-endothelial barrier in the mouse model of influenza A virus infection, thus promoting apoptosis (23). This supports the possibility that glycemic fluctuations may be associated with alveolar membrane damage in patients with COVID-19 and suggests that the metric of glycemic variabilities, such as CV, may be an important prognostic indicator of adverse outcomes. Additionally, large glycemic variability presented by a high CV may be associated with a high risk of hypoglycemia, of which most cases are asymptomatic. Another hypothesis is that wide glycemic variability induces oxidative stress, reduces the production of endothelial progenitor cells, and results in a progressive inflammatory state. One study suggests that glycemic fluctuations, especially postprandial excursions, have a stronger trigger effect on oxidative stress than persistent hyperglycemia (25). Inflammation is an important downstream reaction of oxidative stress, which can further accelerate the process of oxidative stress. In vivo studies using human kidney cells have found that production of inflammatory cytokines, such as transforming growth factor-β and IGF-I binding protein-3, can be increased more by glycemic fluctuations than by persistent hyperglycemia (26).
The strength of our study is that there were limited data supporting the association of CGM-derived metrics obtained from patients with diabetes and adverse outcomes from COVID-19 infection. Moreover, the data suggest glycemic targets for the management of patients with diabetes hospitalized with COVID-19. A major limitation of this study is the small sample size. The single-center design with Chinese patients may limit the generalizability of our findings. Furthermore, this is a cross-sectional study of patients during hospitalization, and the findings may be insufficient for confirming the causal relationship between the goal of glycemic control and the outcome of patients with diabetes and COVID-19. In conclusion, patients with diabetes with sensor glucose levels of >160 mg/dL, <70 mg/dL, or high glycemic variability may have an increased risk of adverse outcomes from COVID-19 infection.
Article Information
Acknowledgments. The authors appreciate the assistance of Dr. Robert A. Vigersky, Medtronic Diabetes, with the language of this manuscript. The authors also appreciate all the doctors and nurses of the Wuhan medical team from Shanghai Jiao Tong University Affiliated Sixth People’s Hospital and Fifth People’s Hospital of Shanghai Fudan University and all the patients who participated in this study.
Funding. This work was supported by the Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant (20161430) and Shanghai Municipal Key Clinical Specialty.
Duality of Interest. The isCGM and cloud platform–based system were partly supported by Abbott Diabetes Care, Inc. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. X.F., J.Z., and W.J. designed the study. Y.S., L.Z., B.Z., Y.Wu, and X.C. collected the data. Y.S., L.Z., Y.Wa., and C.L. cleaned the data. Y.S. and J.Z. performed the statistical analysis. Y.S., Y.Wa., and J.Z. wrote the draft of the manuscript. X.F., J.L., and W.J. rescanned and edited the manuscript. All authors read and approved the final manuscript. J.Z. and W.J. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. ChiCTR2000030436, chictr.org.cn
This article contains supplementary material online at https://doi.org/10.2337/figshare.13636796.
This article is part of a special article collection available at https://care.diabetesjournals.org/collection/diabetes-and-COVID19.
Y.S., X.F., L.Z., and Y.Wa. contributed equally to this work.
References
- 1.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan WJ, Ni ZY, Hu Y, et al.; China Medical Treatment Expert Group for Covid-19 . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu L, She ZG, Cheng X, et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab 2020;31:1068–1077.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bao Y, Chen L, Chen L, et al.; Chinese Diabetes Society . Chinese clinical guidelines for continuous glucose monitoring (2018 edition). Diabetes Metab Res Rev 2019;35:e3152. [DOI] [PubMed] [Google Scholar]
- 6.Bornstein SR, Rubino F, Khunti K, et al. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol 2020;8:546–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239–1242 [DOI] [PubMed] [Google Scholar]
- 8.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levey AS, Stevens LA, Schmid CH, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jia W, Weng J, Zhu D, et al.; Chinese Diabetes Society . Standards of medical care for type 2 diabetes in China 2019. Diabetes Metab Res Rev 2019;35:e3158. [DOI] [PubMed] [Google Scholar]
- 11.Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. an official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med 2019;200:e45–e67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carey IM, Critchley JA, DeWilde S, Harris T, Hosking FJ, Cook DG. Risk of infection in type 1 and type 2 diabetes compared with the general population: a matched cohort study. Diabetes Care 2018;41:513–521 [DOI] [PubMed] [Google Scholar]
- 13.Peleg AY, Weerarathna T, McCarthy JS, Davis TM. Common infections in diabetes: pathogenesis, management and relationship to glycaemic control. Diabetes Metab Res Rev 2007;23:3–13 [DOI] [PubMed] [Google Scholar]
- 14.Yang JK, Feng Y, Yuan MY, et al. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med 2006;23:623–628 [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181:271–280.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geerlings SE, Hoepelman AI. Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol Med Microbiol 1999;26:259–265 [DOI] [PubMed] [Google Scholar]
- 17.Yang JK, Lin SS, Ji XJ, Guo LM. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol 2010;47:193–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang A, Zhao W, Xu Z, Gu J. Timely blood glucose management for the outbreak of 2019 novel coronavirus disease (COVID-19) is urgently needed. Diabetes Res Clin Pract 2020;162:108118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care 2017;40:1631–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodbard D. Glucose time in range, time above range, and time below range depend on mean or median glucose or HbA1c, glucose coefficient of variation, and shape of the glucose distribution. Diabetes Technol Ther 2020;22:492–500 [DOI] [PubMed] [Google Scholar]
- 21.Lu J, Ma X, Zhang L, et al. Glycemic variability modifies the relationship between time in range and hemoglobin A1c estimated from continuous glucose monitoring: a preliminary study. Diabetes Res Clin Pract 2020;161:108032. [DOI] [PubMed] [Google Scholar]
- 22.Krinsley JS, Maurer P, Holewinski S, et al. Glucose control, diabetes status, and mortality in critically ill patients: the continuum from intensive care unit admission to hospital discharge. Mayo Clin Proc 2017;92:1019–1029 [DOI] [PubMed] [Google Scholar]
- 23.Marshall RJ, Armart P, Hulme KD, et al. Glycemic variability in diabetes increases the severity of influenza. MBio 2020;11:e02841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atamna A, Ayada G, Akirov A, Shochat T, Bishara J, Elis A. High blood glucose variability is associated with bacteremia and mortality in patients hospitalized with acute infection. QJM 2019;112:101–106 [DOI] [PubMed] [Google Scholar]
- 25.Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006;295:1681–1687 [DOI] [PubMed] [Google Scholar]
- 26.Jones SC, Saunders HJ, Qi W, Pollock CA. Intermittent high glucose enhances cell growth and collagen synthesis in cultured human tubulointerstitial cells. Diabetologia 1999;42:1113–1119 [DOI] [PubMed] [Google Scholar]