Abstract
The objective was to evaluate the technological processing (protection strategies and storage conditions) influence on viability, on probiotic properties and adsorbent aflatoxin B1 capacity of S. boulardii RC009. Also, the yeast biological safety was evaluated. Lyophilisation (DL) and encapsulation + lyophilisation (EL) were conducted. Yeast protected with maltodextrin (M) or WPC stored at 4 °C reduced 1 and 2 log the viability, respectively. Yeast protected with M stored at 25 °C reduced 1 log after 70 d; with WPC the viability significantly reduced 3 log after 30 d. Technological processing improved the coaggregation’s capacity with pathogens and DL process allowed the greatest AFB1 adsorption. S. boulardii 106 cells/mL were no toxic to Vero cells (p˂0.05). Saccharomyces boulardii RC009 protected with M or WPC maintained viability after technological processing. It possesses a great capacity for AFB1 adsorption and probiotic properties and could be considered a candidate with proven safety for functional food products development.
Keywords: Saccharomyces boulardii, Technological procedures, Probiotic, Aflatoxin B1, Safety
Graphical abstract
Highlights
-
•
Commercial refinery syrup was a good substrate for Saccharomyces boulardii growth.
-
•
Maltodextrin and WPC were efficient protectors in ensuring the yeast viability.
-
•
The lyophilised yeast achieved high percentages of AFB1 adsorption.
-
•
Saccharomyces boulardii cells were non-toxic in Vero cells up to 106 CFU/mL.
1. Introduction
Yeasts have been studied and used to make bread, beer and wine since ancient times. Modern applications involve the production of ethanol, single cell protein, food and fodder, production of heterologous enzymes and proteins. They have also been used as biocontrol, bioremediation agents, as indicators of environmental quality and used as probiotics in animal nutrition to improve productive parameters and health (Rai and Jeyaram., 2017; Poloni et al., 2020). In recent years, interest in its beneficial therapeutic effects such as modulation of the immune system, stimulation of goblet cells, prevention of gastrointestinal tract (GIT) colonization by microbial pathogens adsorption of mycotoxins among others, has been reported (Magnoli et al., 2019).
A problem associated with the microorganisms viability after biotechnological purposes is their low resistance to technological processes and different environmental conditions (Paez et al., 2012). Also, the ability to survive the conditions of the GIT is of vital importance for probiotic microorganisms since to exercise their beneficial effects on the health of host they must remain alive. Probiotics are defined as “live microorganisms which when administered in adequate amounts confer a health benefit on the host” (FAO, 2013). Probiotics included into foodstuffs are subject to stress factors such as temperature, acid and bile, nutrient depletion, oxidative stress along with passage through the GIT transit. Also, technological processes such as freezing process for the development of ice crystals in the intercellular space or inside the cells, drying entails losses in cell viability due to dehydration and osmotic phenomena. These processes may detrimentally affect the probiotic viability and functionality. Maintaining cell viability during drying is critical to the successful production of probiotics. Several strategies have been developed to generate a physical barrier that avoids exposure to adverse environmental conditions, achieving stabilization, both in terms of viability (ability to reproduce) and activity during its useful life. Probiotic microorganisms are generally lyophilised or spray dried with different lyoprotectants whose effectiveness varies according to the microorganism to preserve (Díaz Vergara et al., 2017; Stefanello et al., 2019). Previous studies showed that S. boulardii RC009 had the ability to sequester AFB1 under GIT conditions. Also, it demonstrated to possess beneficial properties to be considered as a probiotic microorganism (Armando et al., 2011). Therefore, the objective of the present work was to evaluate the influence of technological processing that involves protection strategies, protective materials and storage conditions, on the viability, on probiotic properties and adsorbent AFB1 capacity of S. boulardii RC009 after GIT transit. In addition, the biological safety of the yeast was evaluated through cytotoxicity studies in Vero cell cultures.
2. Materials and methods
2.1. Microorganisms
Saccharomyces boulardii RC009 was obtained from the collection centre at the Universidad Nacional de Río Cuarto, Argentina previously isolated from foodstuffs and characterised by molecular techniques. For coaggregation tests, pathogenic Escherichia coli, Salmonella spp., Staphylococcus aureus, Streptococcus uberis y S. haemolyticus strains from the collection of the Collection of the Laboratory of Industrial Microbiology of the National University of Río Cuarto were used (Armando et al., 2011).
2.2. Culture conditions of Saccharomyces boulardii RC009. Kinetic and productive parameters determination
Saccharomyces boulardii RC009 growth conditions were studied in a culture medium with different concentrations of Commercial Refinery Syrup (CRS) composed by levulose and dextrose solution obtained by the cornstarch hydrolysis process, followed by enzymatic conversion and subsequent refining (Table 1).
Table 1.
Composition of commercial refinery syrup (CRS).
| Specifications | Minimum | Maximum |
|---|---|---|
| Solids (%p/p) | 76.5 | 77.5 |
| Brix | 74.8 | 75.8 |
| IR 45 °C | 1.4716 | 1.4740 |
| Density (kg/L) | 1.3717 | 1.3780 |
| SO2 (ppm) | – | 3.0 |
| pH | 3.3 | 4.3 |
| Color (UI) | – | 20 |
| Sulphated ash (%) | – | 0.05 |
| Proteins (%) | ≤0.08 | ≤0.08 |
| Smell – taste | Normal | Normal |
| Viscosity 25 °C (cP) |
– |
1300 |
| Sugar distribution (dry-based) | ||
| Levulosa (%) | 55.0 | – |
| Levulosa + Dextrosa | 95.0 | – |
∗(refractometric, 20 °C).
2.2.1. Growth conditions
An initial yeast inoculum from Yeast Peptone Dextrose agar (YPD - 40 g/L glucose, 5 g/L peptone, 5 g/L yeast extract) was taken with ansa, seeded in YPD broth and incubated at 28 °C for 24 h at 150 rpm. Ten mL of this inoculum were placed in 90 mL broth supplemented with different concentrations of CRS as carbon source (30, 35, 40, 45, 55, 60, 70, 80, and 90 g/L), 5 g/L peptone and 5 g/L yeast extract.
2.2.2. Kinetic parameters
The kinetic parameters (specific growth rate μx (h−1) (Formula (1)) and doubling time td (h) (Formula (2)) were determined. Cell production was monitored by optical density (OD 640 nm), after washing the cells three times with physiological solution, centrifugation (5 min, 5000 rpm) and suspension. Cell viability was measured by counting cells in YPD agar (CFU/g). Dry weight (g/L) determination was performed after centrifugation and washing the cells with physiological solution, and then dried in a forced air oven at 60 °C to constant weight. Reducing sugars were measured using the dinitrosalicylic acid (DNS) method (Miller, 1959).
| (1) |
| (2) |
2.2.3. Productive parameters
Productive parameters (maximum production (Xmax g/L), maximum productivity (Pmax-g/L.h) and Yield (Yx/s - Formula (3)) were determined.
| (3) |
2.3. Technological procedures
2.3.1. Dried by lyophilisation of S. boulardii RC009 (DL)
The yeast biomass was centrifuged for 10 min at 4000 rpm. The following protectants: derivatized chitosan conditioned according to Diaz Vergara et al. (2017), skim milk 10% (w/v), maltodextrin (M) 2.5% (w/v), and whey protein concentrate (WPC) (5% (w/v) were added separately in a 1:1 ratio. The protected biomass was incubated at −80 °C and then, lyophilised in a four-necked drum-type laboratory lyophilizer (Rificor mod L-T4). The process started at −45 °C at a working pressure of 0.001 Pa.
2.3.2. Saccharomyces boulardii RC009 encapsulation by ionic gelation and subsequent lyophilisation (EL)
Yeast encapsulation and subsequent lyophilisation was performed according to Díaz Vergara et al. (2017) with some modifications. Atomisation was achieved by forcing the dispersion of a mixture (sodium alginate 1% + 3 mL 7.7 × 109 CFU/mL S. boulardii RC009) through the spray nozzle over the gelling medium (Cl2Ca 1%). The particles were kept stirred for 30 min in the Cl2Ca solution to ensure complete gelation. Then, they were removed using a sterile sieve and washed with sterile distilled water. The capsules obtained were protected with M 2.5% (w/v) and WPC (5% (w/v) as lyoprotectants in a 1:1 ratio, and subsequently lyophilised as mentioned above.
2.4. Post-technological procedures assays
The influence of technological processes on S. boulardii RC009 viability and functionality was studied. The viability over time was carried out after simulating different storage conditions and the passage through GIT. Then, probiotic and aflatoxin B1 adsorbent properties were evaluated.
2.4.1. Storage conditions
The obtained DL were stored at 4 °C and 25 °C to simulate cooling temperatures and atmosphere, respectively, for 7 and 30 d to determine the viability after the lyophilisation process according to Poloni et al. (2017). Based on the results obtained, the protectants that demonstrated the best ability to preserve cell viability were subjected to a new viability test at 4 °C and 25 °C for 70 d storage. The obtained EL were stored at 4 °C and 25 °C for 90 d and viability was determined according to Díaz Vergara et al. (2017).
2.4.2. Scanning electron microscopy
The DL and EL S. boularddii were processed for Scanning Electron Microscopy (SEM) according to Díaz Vergara et al. (2017). All samples were pre-fixed in 3% (w/v) glutaraldehyde in 0.1 M sodium phosphate buffers, pH 7.2 for 3 h followed by thorough washing with phosphate buffer. Fixed materials were then post-fixed in 1% aqueous osmium tetroxide for 3 h. Dehydration of samples was achieved by transferring to vials containing a graded water–acetone series (10% steps for 30–90% each of 60 min, 100% for 180 min and finally 100% overnight). Dehydrated specimens were embedded with Embed 812 and acetone 100% by 24 h, then were embedded with EMbed 812 with 1.5% hardening agent, DMP-30 at 60 °C by 24 h. Ultra-thin sections (60 nm) were cut and placed on copper grids, counterstained with saturatedmuranyl acetate and aqueous lead citrate. Morphology and size distribution of spray-dried were evaluated by SEM with a ZEISS SIGMA VP Field Emission SEM (FE-SEM) (ZEISS, Germany), using an acceleration voltage of 5 kV. MCs were fixed in stubs containing a double-faced adhesive metallic tape and coated with gold in a CED 010 vacuum evaporator (Balzers Union, Liechtenstein).
2.4.3. Viability maintenance through gastrointestinal tract transit
The effect on DL and EL cell viability against the stress generated by passing through GIT was simulated as follows: tolerance to artificial salivary solution (KCl 15.1 mM; KH2PO4 3.7 mM; NaHCO3 13.6 mM; NaCl 23 mM; MgCl2 0,15 mM; (NH4)2HCO3 0.06 mM; Urea 7.5 mM; HCl 1.1 mM; α-amylase 1500 U/mL; lysozyme 12000 U/mL; CaCl2 0,75 mM), tolerance to artificial gastric juice (KCl 6.9 mM; KH2PO4 0.9 mM; NaHCO3 25.0 mM; NaCl 47.2 mM; MgCl2 0.1 mM; (NH4)2HCO3 0.5 mM; Urea 0.2 mM; HCl 15.6 mM; pepsin 25000 U/mL; lysozyme 12000 U/mL; CaCl2 3.3 mM) and tolerance to artificial intestinal content (KCl 6.8 m M; KH2PO4 0.8 mM; NaHCO3 85.0 mM; NaCl 38.4 mM; MgCl2 0.33 mM; urea 1.8 mM; HCl 8.4 mM; trypsin 1600 U/mL; chymotrypsin 400 U/mL; CaCl2 0,03 mM; biliar juice 833 μl). An ON culture was performed in YPD broth at 28 °C for 24 h which contained an initial inoculum of cells (II). The culture (1 mL) was taken, centrifuged for 10 min at 5000 rpm; the obtained pellet was resuspended in 1.4 mL of saliva solution, and adjusted to pH 6.8 with 0.1 M HCl. The cells were incubated at 37 °C for 2 min at 250 rpm. After incubation, an aliquot of 100 μL was taken (salivary conditions - SC) to which 2.6 mL of gastric solution was added and adjusted to pH 3 with 1 M HCl. The tubes were incubated at 37 °C for 2 h at 250 rpm and 100 μl were taken (gastric conditions - GC). Intestinal solution (4.4 mL) was added to the cells and adjusted to pH 7. The tubes were incubated at 37 °C for 2 h at 250 rpm. At the end of the incubation period an aliquot of 100 μl was taken (intestinal conditions - IC). Serial dilutions in peptone water (0.1%) were obtained with all samples; then, 100 μl aliquots of each dilution were plated on YPD agar for viable cell count. Assays were conducted in triplicate.
2.4.4. Probiotic abilities maintenance of Saccharomyces boulardii RC009
The influence of the technological procedures on probiotic properties of lyophilised cells such as aggregation, coaggregation, and antimicrobial substances production was tested.
2.4.4.1. Autoaggregation ability
Lyophilised cells (0.1 g) were resuspended in 2 mL of PBS buffer (0.2 M pH 7.2). The suspension was vortexed for 30 s and OD at 600 nm was measured in a spectrophotometer. Then, they were incubated at 28 °C for 2 h; the upper layer (1 mL) was transferred to an Eppendorf tube and the OD was determined again.
2.4.4.2. Coaggregation to pathogenic bacteria
Yeast coaggregation capacity with pathogenic microorganisms isolated from the animal ecosystem such as Escherichia coli, Salmonella spp, Staphylococcus aureus, Streptococcus uberis and Streptococcus haemolyticus was evaluated. Lyophilised cells were resuspended in 2 mL PBS buffer (0.2 M pH 7.2) and the final concentration was adjusted to approximately 109 cell/mL by turbidity comparison with tube 4 of the McFarland scale. Pathogenic strains suspensions were carried out in PBS buffer (1 M pH 6.2) and the final concentration was adjusted to approximately 109 cel/mL by turbidity comparison with tube 4 of the McFarland scale. Yeast was mixed (1:1) with each of the pathogens separately in Eppendorf tubes and incubated at 37 °C for 3 h. Suspensions were then observed by optic microscopy after Gram staining to evaluate the aggregation degree and scored according to a scale as follows: (no aggregation), + (little aggregation), ++ (intermediate aggregation) and +++ (maximum aggregation).
2.4.4.3. Antibiotic resistance
Lyophilised cells were resuspended in 2 mL of PBS buffer (0.2 M pH 7.2) and the final concentration adjusted by turbidity comparison with tube 0.5 on the McFarland scale. The suspension was seeded by swabing (in different directions) in a Petri dish with 4 mm YPD agar. The antibiotics used in this trial were gentamicin, kanamycin, amoxicillin + clavulanic acid, and colistin, selected for their frequent use in veterinary medicine. The antibiotic disks were placed on the agar, equidistant from each other and from the edge of the plate. Then, the plates were incubated at 28 °C for 24 h. The antibiograms were read based on the presence or absence of an inhibition halo around the disc.
2.4.5. Aflatoxin B1 adsorption ability maintenance during gastrointestinal tract transit
The AFB1 adsorption assay of DL and EL S. boulardii RC009 was performed according to Armando et al. (2011) with some modifications. Two AFB1 concentrations (20 and 50 ng/mL) ere tested which were in contact with yeast cells (107 cell/mL). An Eppendorf tube containing the SS (1 mL) with each concentration of AFB1 separately was exposed with a pellet of ON yeast culture (107 cell/mL grown in YPD broth at 28 °C for 24 h) and incubated at 37 °C for 2 min. Subsequently, the tubes were centrifuged for 10 min at 5000 rpm, the supernatant was removed and the obtained pellets were exposed to GC at 37 °C for 2 h and then, centrifuged at 5000 rpm for 10 min. The supernatant was removed. Finally, the obtained pellets were exposed to 1 mL IC, mixed with vortex and incubated at 37 °C for 2 h at 250 rpm. The tubes were centrifuged (10 min, 5000 rpm) and the supernatant was removed and stored at −20 °C until its subsequent analysis by HPLC for AFB1 determination.
The analysis of the amount of free AFB1 in the supernatants (non-adsorbed toxin) previously obtained was performed by HPLC with a fluorescence detector (FLD) according to Trucksess et al. (1994). Each sample (200 μL) was placed in 700 μL of acetic acid-trifluoroacetic acid-water (20:10:70) solution. Subsequently, they were incubated at 65 °C for 8.5 min, left to rest for 10 min, and each solution was injected into the HPLC equipment. A control of each sample and standard solutions were included to perform the calibration curve. The conditions of the chromatographic run were C18 Luna column (150 × 4.6 mm, 5 μm); fluorescence detector: 360 ηm excitation, 440 ηm emission; the mobile phase used was water: acetonitrile:methanol (4:1:1 v/v); flow 1.5 mL/min; injection volume 20 μL, the retention time of the samples was 5.5 min. A calibration curve with AFB1 standards (Sigma) was performed. The amount of adsorbed toxin was determined by the difference between the concentration of free toxin in the supernatant and the initial concentration of AFB1 placed.
2.5. Biosafety assays. Citotoxicity
Saccharomyces boulardii RC009 was subjected to cytotoxicity tests, in order to verify that this strain does not have cytotoxic effects. A Vero cell line, clone 76 (continuous line of African green monkey kidney, Chlorocebus sp.) was acquired from the Asociación Banco Argentino de Células (ABAC - Pergamino, Buenos Aires, Argentina). The cells were kept in growth medium forming monolayers until use.
2.5.1. Determination of the Maximum Non-Cytotoxic Concentration
To determine the Maximum Non-Cytotoxic Concentration (MNCC), confluent cell cultures grown in 96-well polycuvettes that were incubated, in independent tests, were carried out with maintenance medium (MM) containing different yeast concentrations according to Escobar et al. (2019) with some modifications. Cell monolayers bathed only with MM without yeasts were used as an indicator of normal cell morphology. Control and treated cultures were kept in an oven at 37 °C for 24 h and examined under an inverted light microscope. The MNCC was defined as the maximum concentration that did not generate any visible alteration in the cellular system used.
2.5.2. Cytotoxic concentration determination
The cytotoxic concentration determination (CC50) was monitored using the Neutral Red (RN) uptake test. Vero cells were seeded in 96-well polycuvettes at a concentration 104 cell/well in minimal essential medium (MEM; Gibco, USA) supplemented with 8% foetal calf serum (FCS; Natocor, Argentina), gentamycin 50 μg/mL and 2 mM glutamine (Sigma-Aldrich, Italy) and incubated at 37 °C for 24 h. The subconfluent cell monolayers were treated for the next 24 h with different concentrations of the yeast (104; 105; 106; 107; 108 cell/mL), in quadruplicate. The solutions were removed from the plates and the cells were washed with 200 μl PBS/well. Then, 200 μl/well of RN solution (30 μg/mL in MM) was added and the plate was incubated at 37 °C for 3 h to favor the incorporation of the dye to the cells. At the end, the cells were washed 3 times with PBS. The dye within the cells was released by extraction with a mixture of acetic acid, ethanol, and water (1:50:49). After stirring the cultures for 20 min, the absorbance values (OD540) were measured in a microplate reader (Labsystems Multiskan MS). The system included untreated cells as control. The relative viability in the treatments was expressed as a percentage decrease in RN uptake in relation to the control cells. The percentage of cell viability was determined according to formula (4):
| (4) |
3. Results and discussion
3.1. Kinetic and productive parameters of Saccharomyces boulardii RC009 growth
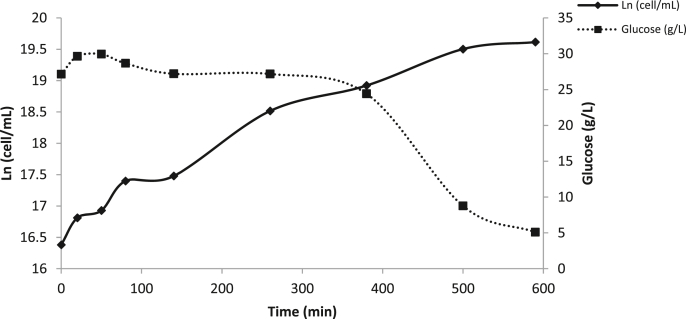
Different CRS concentrations were tested and 30 g/L concentration showed the best results (data not shown). One biotechnological aspects of biomass production involve the design or selection of the culture medium, which must meet all the nutritional requirements that allow an adequate growth. Also, it would be available on the market and obtained as by-product from some industrial process. Fig. 1 shows the growth of S. boulardii RC009 in CRS medium and the consumption of reducing sugars as a function of time. The yeast showed a maximum growth at 10 h incubation (3.3 × 108 cell/mL). The reducing sugars consumption increased significantly after 4 h and reached the maximum consumption when the cells production was maximum (3.9 g/L). The yeast showed a lag phase of approximately 2.5 h allowing it to adapt to the culture medium. Microbial biomass is widely used for different purposes such as fermentation starter cultures and inocula for food and beverage fermentations, as well as a source of protein for human consumption, since it can be formulated in a wide range of foods; and also for the elaboration of animal feed additives, such as probiotics.
Fig. 1.
Growth rate of Saccharomyces boulardii RC009 (Ln) and residual glucose (g/L) as a function of time in Commercial Refinery Syrup (CRS) medium.
Table 2 shows the kinetic and productive parameters obtained from the growth of the yeast in CRS. The kinetic parameters as specific growth rate (μmax) and doubling time (Td) were 0.2860 h−1 and 2.42 h, respectively. Regarding production parameters, the maximum biomass production was 3.9 g/L at 9.83 h. Aguilar et al. (2015) working with S. cerevisiae in a medium with molasses and whey obtained lower kinetic parameters than those obtained in this study. The industrial production of S. boulardii as a probiotic must ensure that the used processing technologies guarantee its stability, both in terms of viability (ability to reproduce) and activity during its useful life. In the present work, the CRS composed by levulose and dextrose allowed achieving high kinetic and productive parameters, making this carbon source promising for industrial use.
Table 2.
Kinetic and productive parameters of Saccharomyces boulardii RC009 growing in commercial refinery syrup (CRS) culture medium as carbon source.
| Kinetic parametersa | Productive parameters | ||
|---|---|---|---|
| Growth rate (μmax) (h−1) | 0.2860 | Maximum harvest (g) | 3.9 |
| Doubling time (Td) (h) | 2.42 | Productivity (g/L/h) | 0.397 (9.83 h) |
| Yield (g/L) | 13.40 | ||
Kinetic parameters were calculated from dry weight determination.
3.2. Saccharomyces boulardii RC009 viability after lyophilisation
Yeasts generally have greater resistance to environmental changes (pH gradients, osmotic changes, etc.) than lactic bacteria, as well as lower nutritional requirements that result in lower costs in production processes. In addition, the greater acid tolerance of yeasts is an important advantage, both in fermentations (since it reduces the risk of bacterial contamination), and in the probiotic quality of the product obtained. All these factors would be beneficial during the production stage, as well as during storage and consumption (Singh et al., 2005).
Protectants such as derivatized chitosan, skim milk; M and WPC were used to eliminate the thermal and osmotic damage caused by the treatments. The lyophilisation process reduced the viability of the yeast cells by 3 logs when they were protected with derivatized chitosan or skim milk, whereas when M or WPC were used, the highest cell viability was achieved (data not shown). Based on these results, it was decided to repeat the viability test with M and WPC at a longer preservation time. Table 3 shows the viability of lyophilised S. boulardii RC009 with M and WPC, stored at 4 °C and 25 °C for 70 d. When the yeast was stored at 4 °C, cell viability was reduced 1 log protected with M and 2 log protected with WPC. When S. boulardii RC009 was stored at 25 °C with M the viability was reduced 1 log after 70 d conservation; however, with WPC the viability was significantly reduced (3 log) after 30 d; no live cells were recovered after 70 d of the test. Trials carried out by Stefanello et al. (2019) showed a marked difference in the ability to maintain cell viability of yeasts and bacteria protected with different materials and lyophilised, the protection of L. fermentum with skim milk supplemented with glutamate achieved a viability of 87% for one year, while for Wickerhamomyces anomalus the viability was reduced. When sucrose was used as a protective agent, it was able to maintain the viability of W. anomalus for one month and 2 months for L. fermentum.
Table 3.
Saccharomyces boulardii RC009 viability after lyophilisation with maltodextrin and whey protein concentrate as protectants and stored at 4 °C y 25 °C during different time periods.
| Time (days) | Lyophilised Saccharomyces boulardii RC009 viability (CFU/g) |
|||
|---|---|---|---|---|
| Maltodextrin |
Whey Protein Concentrate |
|||
| 4 °C | 25 °C | 4 °C | 25 °C | |
| 0 | 6.6 × 1010 | 1.7 × 1010 | 9.3 × 1012 | 9.3 × 1012 |
| 30 | 3.0 × 1010 | 4.5 × 109 | 1.0 × 1011 | 2.3 × 108 |
| 70 | 6.5 × 109 | 1.7 × 109 | 3.5 × 1010 | 0 |
3.2.2. Saccharomyces boulardii RC009 viability of after encapsulation and lyophilisation using different protectants
In this work, two technological processes were carried out, a DL process and EL one. The lyophilisation process was applied both, to DL and EL yeasts. Protection with M and WPC were the most efficient in ensuring that these technological processes did not affect the yeast viability, decreasing in general 2 log after 90 d storage. Table 4 shows S. boulardii RC009 viability (CFU/g) before (2.3 × 1011 CFU/g) and after EL and stored at 4 °C and 25 °C during different time periods. Yeast counts reduced 3 log in all treatments in general after 90 d storage at 4 °C and 25 °C. Similar results were obtained by Díaz Vergara et al. (2017) who obtained a decrease of 2–3 log after 90 d storage of Kluyveromyces marxianus encapsulated with derivatized chitosan. Other authors have shown that microencapsulated yeast strains coated with whey protein obtained 98% viability after 60 d storage (Ragavan and Das, 2018). On the other hand, Chandralekha et al. (2016) evaluated the viability of S. cerevisiae protected with M and then sprayed and observed a reduction of 3 log after 14 d storage.
Table 4.
Saccharomyces boulardii RC009 viability (CFU/g) after encapsulation with different protectants and lyophilisation, and stored at 4 °C and 25 °C during different time periods.
| Time (days) | Encapsulated and lyophilised Saccharomyces boulardii RC009 viability (CFU/g) |
||||
|---|---|---|---|---|---|
| Maltodextrin |
Whey Protein Concentrate |
||||
| 4 °C | 25 °C | 4 °C | 25 °C | ||
| Before encapsulation and lyophilisation (CFU/mL) | 2.3 × 1010 | ||||
| After encapsulation and lyophilisation | 0 | 3.5 × 109 | 7.4 × 109 | 5.5 × 1010 | 7.3 × 109 |
| 5 | 1.6 × 109 | 5.5 × 109 | 1.6 × 1010 | 1.6 × 109 | |
| 30 | 7.0 × 108 | 4.1 × 108 | 5.0 × 109 | 9.0 × 108 | |
| 60 | 6.0 × 108 | 4.0 × 108 | 2.0 × 109 | 9.0 × 108 | |
| 90 | 5.6 × 108 | 9.5 × 107 | 8.0 × 108 | 8.0 × 108 | |
The results are expressed as colony forming units (CFU)/g lyophilisate.
3.2.3. Scanning electron microscopy
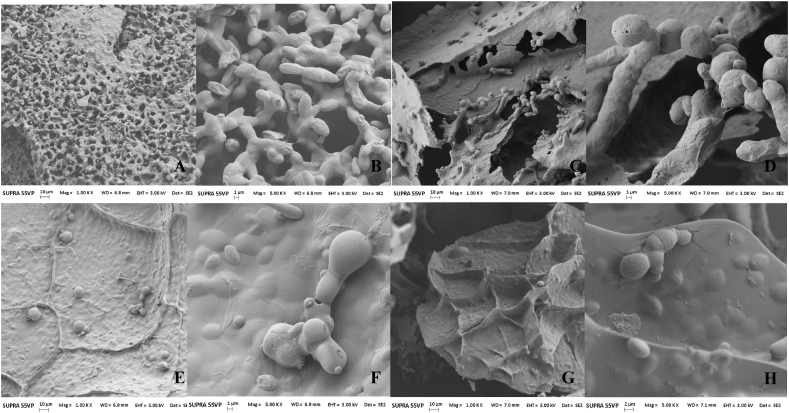
Fig. 2 shows the SEM of yeast protected with M and WPC, after DL or EL. The microphotographs show agglomerations of yeasts arranged in a network with a slightly rough surface (Fig. 2B. and 2.D.). Both protectants used for DL showed the formation of laminae and the cells arranged between them (Fig. 2A. and 2.C.). When S. boulardii RC009 was EL rounded encapsulates were observed with a smooth textured surface (Fig. 2F. and 2.H.), immersed in layers formed by the protective substances (Fig. 2E. and 2.G.). They were found viable and in a budding process.
Fig. 2.
Scanning electron microscopy (SEM) of Saccharomyces boulardii RC009 ∗protected with maltodextrin (M) and whey protein concentrate (WPC) applying lyophilisation (DL) or encapsulation + lyophilisation (EL) methodologies after gastrointestinal tract passage. A) DL + M 1 Kx (scale bar = 10 μm); B) DL + M 5 Kx (scale bar = 1 μm); C) DL + WPC 1 Kx (scale bar = 10 μm); D) DL + WPC 5 Kx (scale bar = 1 μm). E) EL + M 1 Kx (scale bar = 10 μm); F) EL + M 5 Kx (scale bar = 1 μm); G) EL + WPC 1 Kx (scale bar = 10 μm); H) EL + M 5 Kx (scale bar = 2 μm).
3.3. Post-technological processes assays
3.3.1. Dried by lyophilisation or encapsulated and lyophilised Saccharomyces boulardii RC009 viability after gastrointestinal transit
The ability to survive under simulated GIT conditions is an absolute requirement for probiotic microorganisms, and is generally included among the criteria used to select microorganisms with probiotic ability (Sarao and Arora, 2015). Table 5 shows the viability of DL or EL S. boulardii RC009 protected with M or WPC after GIT transit. Yeast without treatment was not significantly reduced after simulated GIT transit. Salivary conditions reduced 2 log the DL yeast counts protected with M and GC followed by IC reduced another 1 log. When cells were DL and protected with WPC also reduced 2 log the yeast counts, which were maintained under GC and IC. Salivary conditions reduced 1 log the EL counts protected with M or WPC wheras GC and IC reduced 1 log the yeast counts. Saliva has significant amounts of lysozyme, a kind of natural enzyme with antimicrobial properties. The functional properties of proteins could be affected in the presence of different polysaccharides. Xu et al. (2018) investigated the influences on structure and activity of lysozyme in the presence of different polysaccharides and provided useful information for its application in food preservation with antimicrobial activity regulation. They indicated the interaction between polysaccharide and lysozyme strengthened as the concentration of lysozyme increased resulting in changing its secondary structure; in the presence of konjac glucomannan and inulin the lysozyme activity remained constant but in the presence of κ-carrageenan the lysozyme activity was reduced probably because strong electrostatic interaction between κ-carrageenan and lysozyme could be induced resulting in spatial structure changing. Studies concerning the interactions between M and chitosan and lysozyme have not been reported but probably an interaction that positively induce the enzyme activity could increase the antimicrobial effect on the yeast that was significantly reduced under the SC in this work.
Table 5.
Influence of simulated gastrointestinal tract transit on Saccharomyces boulardii RC009 viability after technological procedures (lyophilisation or encapsulation + lyophilisation).
| Technological treatments | Log10 CFU during simulated gastrointestinal transit a |
||||
|---|---|---|---|---|---|
| II | SC | GC | IC | ||
| Lyophilisation | M b | 9.10 | 7.66 | 6.93 | 6.30 |
| WPC c | 9.10 | 7.56 | 7.25 | 6.90 | |
| Encapsulation + Lyophilisation | M | 6.75 | 5.60 | 5.02 | 4.78 |
| WPC | 6.93 | 5.70 | 4.95 | 4.40 | |
II: initial inoculum, SC: Salivary conditions tolerance assay, GC: Gastric conditions tolerance assay, IC: Intestinal conditions tolerance assay.
CFU: colony forming units.
M: Maltodextrin.
WPC: Whey protein concentrate.
Based on these results, the levels of viable cells should reach 1010 CFU before the application of protectants to obtain viable levels of S. boulardii RC009 to be considered an effective probiotic. The recommended dose for most probiotic strains is 108–109 CFU/kg food (Simon et al., 2005). These results were similar to those obtained by Armando et al. (2011) who used the same yeast S. boulardii RC009 without technological processing.
Studies carried out Suryabhan et al. (2019), reported that cell counts of yeast strains encapsulated with M and then coated with sucrose or sorbitol increased after passage through gastric and bile juices. Tests performed with lactic acid bacteria sprayed and protected with 20% skim milk + WPC or starch showed an improvement in cell survival of 1.5 log compared to fresh culture in simulated GIT digestion (Páez et al., 2012).
3.3.2. Probiotic properties of dried by lyophilisation or encapsulated and lyophilised Saccharomyces boulardii RC009
3.3.2.1. Autoaggregation
Table 6 shows the autoaggregation ability of S. boulardii RC009 before and after lyophilisation procedure (DL and EL) using M or WPC as protectants. The yeast before lyophilisation procedure obtained a high percentage of aggregation (97.9%) compared to cells after lyophilisation procedure (DL or EL), which obtained very low percentages of aggregation when they were protected with M (28%) or with WPC (22%).
Table 6.
Autoaggregation ability of Saccharomyces boulardii RC009 before and after lyophilisation procedure using maltodextrin or whey protein concentrate as protectants.
| Treatments | OD600 (Ai) | OD600 (Af) | % Auto-aggregation | |
|---|---|---|---|---|
| Yeast before lyophilisation | 1.173 | 0.0253 | 97.9 | |
| Yeast after lyophilisation | M a | 1.184 | 0.8560 | 28.0 |
| WPC b | 0.710 | 0.5550 | 22.0 | |
M: maltodextrin.
WPC: whey protein concentrate.
The ability of autoaggregation or the formation of multicellular groups between microorganisms of the same strain is a measure of the ability to adhere to epithelial cells that could be related to the formation of biofilms. The aggregation property can be shown in two different ways, as autoaggregation, where cells of the same species agglomerate, and as coaggregation, when it gives rise to the union of different microorganisms (Kragh et al., 2016). In the present work, the ability to autoaggregate and coaggregate cells by yeasts after the technological process was studied. It was observed that the strain did not show an autoaggregative phenotype. As far as has been read in the literature, no results related to autoaggregation and coaggregation properties of probiotic yeasts after encapsulation have been reported yet. Armando et al. (2011) had already reported aggregation properties of this same strain, which showed the decrease of this property after technological processing in the present work. The adhesion of probiotic strains varies among strains, depending on the cell surface properties such as hydrophobicity and extracellular protein profiles. The hydrophobic⁄hydrophilic, electron donor (basic) and electron acceptor (acidic) characteristics are attributed to carboxylic groups and Lewis acid – base interactions of bacterial surface (proteins and polysaccharides) (Kos et al., 2003). These groups were probably hidden under the layer of the lyoprotectant or encapsulating material.
3.3.2.2. Coaggregation
Table 7 shows the coaggregation ability of S. boulardii RC009 against pathogenic strains, before and after lyophilisation procedure as protectants. The yeast before lyophilisation procedure was not capable of coaggregating pathogens or has very using M or WPC low coaggregation capacity (Salmonella sp.). After lyophilisation procedure cells (DL or EL) using M or WPC a high capacity to form coaggregates was observed with all the pathogens studied. Furthermore, when cell protection was performed with M, the highest degree of coaggregation for most of the studied pathogens was obtained.
Table 7.
Coaggregation ability of Saccharomyces boulardii RC009 against pathogenic strains, before and after lyophilisation procedure using maltodextrin or whey protein concentrate as protectants.
| Treatments | Pathogenic strains |
|||||
|---|---|---|---|---|---|---|
| E. coli | Salmonella sp. | S. aureus | S. uberis | S. haemolyticus | ||
| Yeast before lyophilisation | – | + | ND | ND | ND | |
| Yeast after lyophilisation | M a | +++ | +++ | +++ | ++ | +++ |
| WPC b | + | +++ | + | +++ | + | |
(−) Absence of coaggregation in 5 randomly observed fields. (+) Presence of coaggregation in 1 of 5 fields observed at random. (++) Presence of coaggregation in 2–3 of 5 fields observed at random. (+++) Presence of coaggregation in 4–5 of 5 fields observed at random. ND: not determined.
M: maltodextrin.
WPC: whey protein concentrate.
The coaggregation property is related to the autoaggregation ability of each strain (Xu et al., 2009). However, in the present work, technological processing improved the coaggregation capacity of pathogens. Probably, the pathogenic microorganisms presented higher co-aggregation than the probiotics, suggesting specific binding capabilities and indirectly preventing their GIT colonization.
3.3.2.3. Antibiotic resistance
Saccharomyces boulardii RC009 (DL and EL) protected with M or with WPC and after the lyophilisation process, demonstrated resistance to all the antibiotics tested (data not shown). The natural antibiotic resistance of yeast is an important characteristic for their use as probiotics (Czerucka et al., 2007). Bacterial resistance can be passing to other bacteria vertical and/or horizontal, through genes transference between bacteria. The main threat associated to probiotic bacteria is the capacity of transfer resistance gene to pathogenic bacteria. Because there is no genetic transference between bacteria and yeasts, its use as probiotic is safe and advantageous. In this work, S. boulardii RC009 showed resistance to all the antibiotics analyzed. It is important to highlight that they must show activity against bacteria, and not against yeasts. This is beneficial since when applying one of these antibiotics to livestock, the yeast would not be affected, so it could remain in the animal’s intestine and develop normally. Previous studies have already shown that antibiotics for veterinary use did not exert a negative influence on probiotic yeasts (Poloni et al., 2017).
3.4. Aflatoxin B1 adsorption by dried by lyophilisation or encapsulated and lyophilised Saccharomyces boulardii RC009 during gastrointestinal tract transit
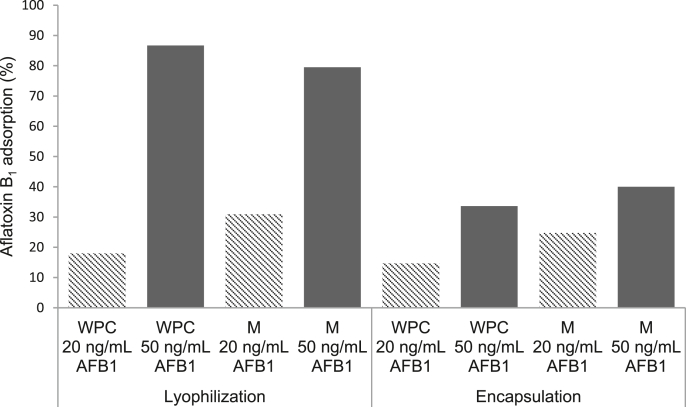
The AFB1 adsorption assay was performed with two concentrations 20 and 50 ng/mL AFB1 using DL or EL of S. boulardii RC009 during GIT transit. Fig. 3 shows DL yeasts protected with M and WPC had the highest adsorption percentages (79.5% and 86.7% respectively), and at the highest AFB1 concentration used (50 ng/mL).
Fig. 3.
Aflatoxin B1 adsorption (20 and 50 ng/mL) by S. bouladii RC009 protected with maltodextrin (M) and whey protein concentrate (WPC) applying lyophilisation or encapsulation + lyophilisation methodologies after gastrointestinal tract passage.
The EL yeasts showed the same tendency but in all cases the adsorption percentages were lower. At 50 ng/mL AFB1, EL yeasts showed the highest adsorption percentage (40%). Mycotoxin adsorbents can be used to control mycotoxins in the diet. The binding of the adsorbents with the mycotoxins reduce their bioavailability (Yanikouris et al., 2006). The results obtained in the present work showed that the yeast S. boulardii RC009 subjected to technological processes achieved high percentages of AFB1 adsorption when it was lyophilised. Previous studies showed that the same strain working 50 ng/g AFB1 without technological processing had adsorption levels of 16.4% (Armando et al., 2011). The increase in the percentage of adsorption observed in this work was related to the cell protectants used. The DL process allowed a greater adsorption of mycotoxin than the EL process; probably the alginate did not allow the exposure of the yeast cells because the conditions of the GIT did not allow its adequate dissolution.
3.5. Biological safety test: maximum non-cytotoxic concentration and cytotoxic concentration 50%
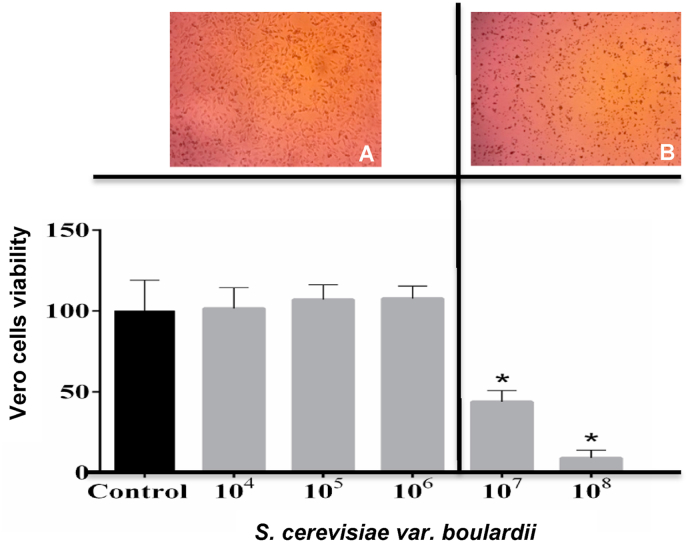
Fig. 4 shows Vero cells viability (%) determined by neutral red uptake after treatment with different concentrations of S. boulardii RC009. Cytopathic effect observed under the light microscope demonstrated that cells treated with 104 cell/mL to 106 cell/mL S. boulardii RC009 did not excise cellular damage, showing the absence of toxicity at these concentrations and percentage viability values similar to the negative control. Fig. 4A. shows S. boulardii RC009 cells without morphological alterations, while the concentrations of 107 cell/mL and 108 cell/mL altered cell morphology (Fig. 4B.) and exhibited significantly lower cell viability values compared to the control treatment cells viability. The Vero cells viability percentage determined by uptake of neutral red (RN) after treatment with S. boulardii RC009 at different concentrations showed 9 × 106 cell/mL as the 50% cytotoxic concentration (CC50) calculated by the intrapolation of the dose-response curve. The results were expressed as percentage of cellular viability compared to the control treatment.
Fig. 4.
Vero cells viability (%) determined by neutral red uptake after treatment with different concentrations of Saccharomyces boulardii RC009 (104-108 cells/mL). A) Vero cells in monolayers after control and Saccharomyces boulardii 104 to 106 cells/mL treatments, B) Vero cells in monolayers after Saccharomyces boulardii 107 and 108 cells/mL treatments. ∗p ˂ 0.05 according to Fisher’s Least Significant Difference test. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
One of the criteria to consider a probiotic microorganism is that it is not toxic to the cell. In this study, Vero cells were exposed to different concentrations of S. boulardii. Assay carried out by Gonzalez-Pereyra et al. (2014) in mice with S. cerevisiae indicated that oral administration (109 CFU/mL) of yeast did not cause cytotoxic effects, the animals did not show any health damage. Also, Zommiti et al. (2018) showed 96% Caco-2/CT7 cell viability with a potential probiotic Enterococcus faecium.
4. Conclusion
This study suggests that substrates that come from the agro-industry can be used for biomass production, contributing to the sustainable development of the process and reducing the costs of large-scale production. Saccharomyces boulardii RC009 after DL or EL technological processing protected with M or WPC could be considered as probiotic candidate with proven safety and promissory for the development of functional products since it possesses great capacity for AFB1 adsorption and, at the same time, effectively preserves the desired probiotic properties.
CRediT authorship contribution statement
Valeria Lorena Poloni: Methodology, Validation, Investigation. María Belén Bainotti: Methodology, Investigation. Ladislao Díaz Vergara: Methodology, Investigation. Franco Escobar: Methodology, Investigation. Mariana Montenegro: Software, Formal analysis. Lilia Cavaglieri: Conceptualization, Writing – original draft, Visualization, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by grants from SECyT-UNRC and PICT 3089/18.
Contributor Information
Valeria Lorena Poloni, Email: vpoloni@exa.unrc.edu.ar.
María Belén Bainotti, Email: bbainotti@gmail.com.
Ladislao Díaz Vergara, Email: ladiazvergara@gmail.com.
Franco Escobar, Email: fescobar@exa.unrc.edu.ar.
Mariana Montenegro, Email: mamontenegro@gmail.com.
Lilia Cavaglieri, Email: lcavaglieri@exa.unrc.edu.ar, lcavaglieri247@gmail.com.
References
- Aguilar J., Espinoza M., Cabanillas J., Avila I., García A., Julca J. Evaluación de la cinética de crecimiento de Saccharomyces cerevisiae utilizando un medio de cultivo a base de melaza de caña y suero lácteo. Agroindustrial Science. 2015;5:37–47. [Google Scholar]
- Armando M., Dogi C., Pizzolitto R., Escobar F., Peirano M., Salvano M., Sabini L., Combina M., Dalcero A., Cavaglieri L. Saccharomyces cerevisiae strains from animal environment with in vitro aflatoxin B1 binding ability and anti-pathogenic bacterial influence. World Mycotoxin J. 2011;4:59–68. doi: 10.3920/WMJ2010.1208. [DOI] [Google Scholar]
- Chandralekha A., Hrishikesh Tavanandi A., Amrutha N., Umesh Hebbar H., Raghavarao K.S.M.S., Ramachandra G. Encapsulation of Yeast (Saccharomyces cerevisiae) by spray drying for extension of shelf life, drying technology. Dry. Technol. 2016;34:1307–1318. doi: 10.1080/07373937.2015.1112808. [DOI] [Google Scholar]
- Czerucka D., Piche T., Rampal P. Yeast as probiotics –Saccharomyces boulardii. Aliment. Pharmacol. Ther. 2007;26:767–778. doi: 10.1111/j.1365-2036.2007.03442.x. [DOI] [PubMed] [Google Scholar]
- Díaz-Vergara L., Pereyra C.M., Montenegro M., Pena G.A., Aminahuel C.A., Cavaglieri L.R. Encapsulated whey–native yeast Kluyveromyces marxianus as a feed additive for animal production. Food Addit. Contam. 2017;34:750–759. doi: 10.1080/19440049.2017.1290830. [DOI] [PubMed] [Google Scholar]
- Escobar F.M., Magnoli A., Sabini M.C., Cariddi L.N., Bagnis G., Soltermann A., Cavaglieri L. Minthostachys verticillata essential oils as potential phytogenic additives and chemoprotective strategy on aflatoxin B1 toxicity. J. Appl. Anim. Res. 2019;47:217–222. doi: 10.1080/09712119.2019.1614929. [DOI] [Google Scholar]
- FAO . FAO; Rome, Italy: 2013. Guidelines for the Evaluation of Probiotics in Food: Report of a Joint FAO. [Google Scholar]
- Gonzalez Pereyra M.L., Dogi C., Torres Lisa A., Wittouck P., Ortíz M., Escobar F., Bagnis G., Yaciuk R., Poloni L., Torres A., Dalcero A.M., Cavaglieri L. Genotoxicity and cytotoxicity evaluation of probiotic Saccharomyces cerevisiae RC016: a 60-day subchronic oral toxicity study in rats. J. Appl. Microbiol. 2014;117:824–833. doi: 10.1111/jam.12552. [DOI] [PubMed] [Google Scholar]
- Kos B., Susković J., Vuković S., Simpraga M., Frece J., Matosic S. Adhesion and aggregation ability of probiotic strain Lactobacillus acidophilus M92. J. Appl. Microbiol. 2003;94:981–987. doi: 10.1046/j.1365-2672.2003.01915.x. [DOI] [PubMed] [Google Scholar]
- Kragh K.N., Hutchison J.B., Melaugh G., Rodesney C., Roberts A.E.L., Irie Y., Jensen P., Diggle S.P., Allen R.J., Gordon V., Bjarnsholt T. Role of multicellular aggregates in biofilm formation. mBio. 2016;7 doi: 10.1128/mBio.00237-16. e00237-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnoli A.P., Poloni V.L., Cavaglieri L. Impact of mycotoxin contamination in the animal feed industry. Current Opinion in Food Science. 2019;29:99–108. doi: 10.1016/j.cofs.2019.08.009. [DOI] [Google Scholar]
- Miller G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Paez R., Lavari L., Vinderola G., Audero G., Cuatrin A., Zaritzky N., Reinheimer J. Effect of heat treatment and spray drying on lactobacilli viability and resistance to simulated gastrointestinal digestion. Food Res. Int. 2012;48:748–754. doi: 10.1016/j.foodres.2012.06.018. [DOI] [Google Scholar]
- Poloni V., Salvato L., Pereyra C., Oliveira A., Rosa C., Cavaglieri L., Keller K.M. Bakery by-products based feeds borne-Saccharomyces cerevisiae strains with probiotic and antimycotoxin effects plus antibiotic resistance properties for use in animal production. Food Chem. Toxicol. 2017;107:630–636. doi: 10.1016/j.fct.2017.02.040. [DOI] [PubMed] [Google Scholar]
- Poloni V., Magnoli A., Fochesato A., Cristofolini A., Caverzan M., Merkis C., Montenegro M., Cavaglieri L. Saccharomyces cerevisiae RC016-based feed additive reduces liver toxicity, residual aflatoxin B1 levels and positively influences intestinal structure in broiler chickens fed on chronic aflatoxin B1 levels-contaminated diets. Animal Nutrition. 2020;6:31–38. doi: 10.1016/j.aninu.2019.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragavan M.L., Das N. Process optimization for microencapsulation of probiotic yeasts. Front. Biol. 2018;13:197–207. doi: 10.1007/s11515-018-1495-1. [DOI] [Google Scholar]
- Rai A.K., Jeyaram K. Yeast Diversity in Human Welfare. Springer; Singapore: 2017. Role of yeasts in food fermentation; pp. 83–113. [Google Scholar]
- Sarao L.K., Arora M. Probiotics, prebiotics, and microencapsulation: a review. Crit. Rev. Food Sci. Nutr. 2015;57:344–371. doi: 10.1080/10408398.2014.887055. [DOI] [PubMed] [Google Scholar]
- Simon O., Vahjen W., Scharek L. Microorganisms as feed additives-probiotics. Adv. Pork Prod. 2005;16:161–167. [Google Scholar]
- Singh V., Johnston D.B., Naidu K., Rausc h K.D., Belyea R.L., Tumbleson M.E. Comparison of modified dry grind processes for fermentation characteristics and DDGS composition. Cereal Chem. 2005;82:187–190. doi: 10.1094/cc-82-0187. [DOI] [Google Scholar]
- Stefanello R.F., Nabeshima E.H., Iamanaka B.T., Ludwig A., Fries L.L.M., Bernardi A.O., Copetti M.V. Survival and stability of Lactobacillus fermentum and Wickerhamomyces anomalus strains upon lyophilisation with different cryoprotectant agents. Food Res. Int. 2019;115:90–94. doi: 10.1016/j.foodres.2018.07.044. [DOI] [PubMed] [Google Scholar]
- Suryabhan P., Lohith K., Anu-Appaiah K.A. Sucrose and sorbitol supplementation on maltodextrin encapsulation enhance the potential probiotic yeast survival by spray drying. LWT-Food Science and Technology. 2019;107:243–248. doi: 10.1016/j.lwt.2019.03.002. [DOI] [Google Scholar]
- Trucksess M.W., Stack M.E., Nesheim S., Albert R.H., Romer T.R. Multifunctional column coupled with liquid chromatography for determination of aflatoxins B1, B2, G1 and G2 in corn, almonds, Brazil nuts, peanuts and pistachio nuts: collaborative study. J. AOAC Int. 1994;77:1512–1521. [PubMed] [Google Scholar]
- Xu H., Jeong H.S., Lee H.Y., Ahn J. Assessment of cell surface properties and adhesion potential of selected probiotic strains. Lett. Appl. Microbiol. 2009;49:434–442. doi: 10.1111/j.1472-765X.2009.02684.x. [DOI] [PubMed] [Google Scholar]
- Xu W., Jin W., Wang Y., Li J., Huang K., Shah B.R., Li B. Effect of physical interactions on structure of lysozyme in presence of three kinds of polysaccharides. J. Food Sci. Technol. 2018;55:3056–3064. doi: 10.1007/s13197-018-3228-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiannikouris A., André G., Poughon L., François J., Dussap C., Jeminet G., Jouany J. Chemical and conformational study of the interactions involved in mycotoxin complexation with β-D-glucans. Biomacromolecules. 2006;7:1147–1155. doi: 10.1021/bm050968t. [DOI] [PubMed] [Google Scholar]
- Zommiti M., Cambronel M., Maillot O., Barreau M., Sebei K., Feuilloley, Ferchichi M., Connil N. Evaluation of probiotic properties and safety of Enterococcus faecium isolated from artisanal Tunisian meat “Dried Ossban”. Front. Microbiol. 2018;9:1685. doi: 10.3389/fmicb.2018.01685. [DOI] [PMC free article] [PubMed] [Google Scholar]