Abstract
Delineation of the genomic complexities of malignant pleural mesothelioma (MPM) has lagged behind other malignancies. Zhang et al. meaningfully add to our understanding of MPM, and their findings emphasize the need to combine drug development efforts with appropriate predictive biomarkers.
Subject terms: Cancer genomics, Mesothelioma
Historical beliefs about mesothelioma
For far too long, malignant pleural mesothelioma (MPM), a cancer of the mesothelial cells lining the pleural cavity, has been approached as a single homogeneous disease entity. Our relatively basic classification based on histology1 helps us prognosticate but does not meaningfully inform management decisions, except perhaps for surgery in sarcomatoid disease2. Because of a variety of beliefs surrounding the etiology, prognosis, and treatment of MPM, efforts to delineate the genomic complexities of this disease have lagged behind those of other malignancies. With rational drug development successes in other common malignancies, such as lung cancer, as well as the advent of increasingly powerful next-generation sequencing and analytic tools, we are beginning to identify and define the complex genomic landscape of MPM so personalized therapies can be developed.
The genomics of MPM
A decade ago, Dr. Ladanyi and colleagues reported the first integrated genomic analysis of MPM3. They examined 25 potential driver genes and found a high rate of non-synonymous mutation in BAP1, confirmed frequent inactivating mutations in NF2, and described missense mutations in LATS1 and LATS2. Subsequently, in 2016, Dr. Bueno and colleagues analyzed RNA sequencing data from 216 MPM tumors which confirmed and expanded prior findings4. In addition to common alterations in BAP1 and NF2, alterations were identified in TP53, SETD2, DDX3X, ULK2, RYR2, CFAP45, SETDB1, and DDX51. Based on the robust genomic heterogeneity observed in MPM, Bueno et al. proposed four distinct molecular disease subtypes: sarcomatoid, epithelioid, biphasic-epithelioid, and biphasic-sarcomatoid. The Cancer Genome Atlas’ (TCGA) comprehensive integrated genomic study of MPM, reported by Dr. Hmeljak and colleagues in 2018, identified prognostic molecular subsets independent of histology5, and also defined a novel subtype with extensive loss of heterozygosity and mutations in TP53 and SETDB1. Taken together, these studies paint a picture of the common recurring alterations in mesothelioma and begin to define disease subsets based on genomic characteristics.
Multiple novel targets and pathways of interest have been identified from genomic studies of MPM. Unlike many malignancies with mutations in growth-regulating kinases, the genomic landscape of MPM is primarily characterized by alterations in tumor suppressor genes. Unfortunately, therapeutic targeting of these tumor suppressor alterations remains elusive. Furthermore, no predictive subsets or markers have been identified to help with patient selection for various treatments, and intra-tumoral heterogeneity has not been evaluated. It is in this context that Zhang et al. present a body of work examining clonal architecture and its influence on the tumor microenvironment in MPM6.
Modeling MPM
Zhang et al. describe their creation of a platform, entitled MEDUSA (Mesothelioma Evolution: Deciphering Drugable Somatic Alterations), used to infer a model of MPM tumorigenesis6. Through multi-regional exome sequencing of 90 tumor samples collected at the time of extended pleurectomy/decortication from anatomically stereotyped regions in 22 patients with MPM, and with whole blood germline controls, Zhang and colleagues created phylogenetic tree topology in order to identify potential evolutionary constraints that could be exploited for drug development. Extensive inter-patient and intra-tumor heterogeneity was identified.
Linear evolutionary trees, comprised of monophyletic clones arising from a common node, modeled 64% of the cases. Branched trees, comprised of polyphyletic clones arising from subclonal nodes, described the remaining 36% of cases. The evolutionary analysis suggested that BAP1 events occur early in the evolution of MPM as evidenced by their presence in almost all subclones. By contrast, NF2 events occur late and are therefore only evident in some branches. Clonal positive selection was demonstrated for NF2, BAP1, SETD2, FBXW7, and PRELID1. Subclonal selection was only identified for NF2. No whole-genome haploidization or whole-genome doubling events were noted. Twenty-four percent of samples had copy number alterations with losses more significant than gains, and only 5% of these were subclonal. There was also some evidence of allelic heterogeneity. Homozygous deletion of CDKN2A/B and MTAP occurred only as clonal events. Detectable circulating free DNA was associated with inferior survival. Taken together, these findings demonstrate that in addition to substantial intra- and inter-tumoral heterogeneity, there are common key genomic events for clonal and subclonal evolution which lend themselves to focused drug development.
Zhang et al. also explored how intra-tumoral heterogeneity modulates host immune surveillance or immune escape. High clonal copy number burden was associated with greater systemic inflammation as measured by neutrophil:lymphocyte and platelet:lymphocyte ratios. Additionally, the most highly branched tumors had higher T-cell infiltration. Highly branched tumors also had higher neoantigen burden which is associated with immunoediting via HLA loss of heterozygosity and can lead to immune escape. These data are compelling evidence that clonal architecture modulates immune surveillance and is a provocative potential mechanism of resistance to immune checkpoint inhibitor therapy in MPM.
Moving beyond the limited therapeutic options in MPM
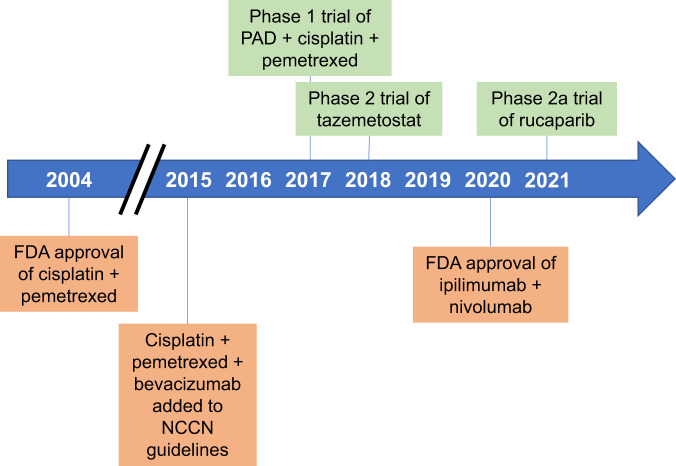
Approved therapeutic options in MPM had remained largely unchanged for the last 16 years (Fig. 1). This study by Zhang et al. comes at an opportune time as the treatment approach in MPM is now evolving. Based on the positive results of the Checkmate-743 trial7, the combination of ipilimumab and nivolumab has received FDA approval and is included in the NCCN guidelines as a first-line treatment option for patients with MPM. While this is an important advance for the treatment of MPM, many questions remain unanswered. Responses differ by histology and PD-L1, but no compelling predictive marker to facilitate treatment selection has been identified. This underscores the importance of identifying reliable predictive biomarkers based on the underlying biologic mechanisms of response and resistance for all treatment options.
Fig. 1. Timeline of drug approvals/recommendations and personalized clinical trials in malignant mesothelioma.
Genome-informed clinical trials are shown above the timeline in green. Drug approvals and recommendations are shown below the timeline in orange. FDA U.S. Food and Drug Administration, NCCN National Comprehensive Cancer Network, PAD pegylated arginine deaminase.
Future clinical trials need to focus on predictive biomarkers for novel therapies. In order to meaningfully advance outcomes in this disease, we must develop effective therapies and identify the patients most and least likely to benefit from them. Exceptional responders were identified in many early phase clinical trials in MPM, but without a biomarker, these compounds were abandoned for lack of efficacy. In other cancers, biomarkers have been identified that predict a patient’s response to drugs; for instance, larotrectinib for TRK fusion-positive cancers8. A parallel approach in MPM is possible, but only with more studies like those described here by Zhang et al.6.
The tremendous diversity of MPM described by Zhang et al. and others means that similar rational drug development and trial design is needed for this disease. To do this, we must abandon the long-held, mistaken assumptions of homogeneity within and between MPM tumors as well as the nihilism around conducting MPM trials in selected populations enriched for response. There have been some efforts in this space to establish the feasibility of selecting patients based on histology9 or BAP1 loss10,11. Now, we need more biologic inquiry to fuel the next generation of potential therapies and their target populations. This body of work from Zhang et al. is an important step toward those goals with its characterization of the extensive exomic variation within patients that has long been overlooked due to the low metastatic potential of MPM. By building on these discoveries and the relationships identified between intratumoral heterogeneity and immune surveillance, we can better identify potentially druggable alterations and create personalized therapies for patients with MPM. Work like this hails the dawn of a new age for drug discovery and development in MPM, and I am optimistic for what lies ahead.
Acknowledgements
This work was partially supported by a National Cancer Institute Cancer Center Support Grant to Memorial Sloan Kettering Cancer Center [P30 CA008748]. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. I thank Clare Wilhelm for his editorial assistance in the preparation of this manuscript.
Author contributions
M.G.Z. wrote and conceived the manuscript.
Competing interests
In the last 3 years, Dr. Zauderer has received consulting fees from Takeda, GlaxoSmithKline, Aldeyra Therapeutics, Novocure, and Atara and honoraria from Research to Practice, Medical Learning Institute and OncLive. Memorial Sloan Kettering receives research funding from the Department of Defense, the National Institutes of Health, GlaxoSmithKline, Epizyme, Polaris, Sellas Life Sciences, Bristol Myers Squibb, Millenium, Curis, Atara, and Roche for research conducted by Dr. Zauderer. Dr. Zauderer serves as Chair of the Board of Directors of the Mesothelioma Applied Research Foundation.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Galateau-Salle F, Churg A, Roggli V, Travis WD. The 2015 World Health Organization classification of tumors of the pleura: advances since the 2004 classification. J. Thorac. Oncol. 2016;11:142–154. doi: 10.1016/j.jtho.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Kindler HL, et al. Treatment of malignant pleural mesothelioma: American society of clinical oncology clinical practice guideline. J. Clin. Oncol. 2018;36:1343–1373. doi: 10.1200/JCO.2017.76.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bott M, et al. The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nat. Genet. 2011;43:668–672. doi: 10.1038/ng.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bueno R, et al. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat. Genet. 2016;48:407–416. doi: 10.1038/ng.3520. [DOI] [PubMed] [Google Scholar]
- 5.Hmeljak J, et al. Integrative molecular characterization of malignant pleural mesothelioma. Cancer Discov. 2018;8:1548–1565. doi: 10.1158/2159-8290.CD-18-0804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang, M. et al. Clonal architecture in mesothelioma is prognostic and shapes the tumour microenvironment. Nat. Commun10.1038/s41467-021-21798-w (2021). [DOI] [PMC free article] [PubMed]
- 7.Baas P, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet. 2021;397:375–386. doi: 10.1016/S0140-6736(20)32714-8. [DOI] [PubMed] [Google Scholar]
- 8.Drilon A, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N. Engl. J. Med. 2018;378:731–739. doi: 10.1056/NEJMoa1714448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beddowes E, et al. Phase 1 dose-escalation study of pegylated arginine deiminase, cisplatin, and pemetrexed in patients with argininosuccinate synthetase 1–deficient thoracic cancers. J. Clin. Oncol. 2017;35:1778–1785. doi: 10.1200/JCO.2016.71.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zauderer MG, et al. Phase 2, multicenter study of the EZH2 inhibitor tazemetostat as monotherapy in adults with relapsed or refractory (R/R) malignant mesothelioma (MM) with BAP1 inactivation. J. Clin. Oncol. 2018;36:8515–8515. doi: 10.1200/JCO.2018.36.15_suppl.8515. [DOI] [Google Scholar]
- 11.Fennell D. A. et al. Rucaparib in patients with BAP1-deficient or BRCA1-deficient mesothelioma (MiST1): an open-label, single-arm, phase 2a clinical trial. Lancet Respir. Med.10.1016/S2213-2600(20)30390-8 (2021). [DOI] [PubMed]