Abstract
We aimed to determine if plasma levels of bacterial lipopolysaccharide (LPS) and lipoteichoic acid (LTA) are associated with different causes of stroke and correlate with C-reactive protein (CRP), LPS-binding protein (LBP), and the NIH stroke scale (NIHSS). Ischemic stroke (cardioembolic (CE), large artery atherosclerosis (LAA), small vessel occlusion (SVO)), intracerebral hemorrhage (ICH), transient ischemic attack (TIA) and control subjects were compared (n = 205). Plasma LPS, LTA, CRP, and LBP levels were quantified by ELISA. LPS and CRP levels were elevated in ischemic strokes (CE, LAA, SVO) and ICH compared to controls. LBP levels were elevated in ischemic strokes (CE, LAA) and ICH. LTA levels were increased in SVO stroke compared to TIA but not controls. LPS levels correlated with CRP and LBP levels in stroke and TIA. LPS, LBP and CRP levels positively correlated with the NIHSS and WBC count but negatively correlated with total cholesterol. Plasma LPS and LBP associate with major causes of ischemic stroke and with ICH, whereas LPS/LBP do not associate with TIAs. LTA only associated with SVO stroke. LPS positively correlated with CRP, LBP, and WBC but negatively correlated with cholesterol. Higher LPS levels were associated with worse stroke outcomes.
Subject terms: Stroke, Infection, Inflammation
Introduction
Stroke incidence increases with infection and inflammation prior to stroke1. C-reactive protein (CRP) levels after stroke correlate with stroke severity2; and, there is a whole genome immune response after stroke that differs for each stroke cause3. This response includes TNF, IL1, IL6 and other cytokines downstream of TLR4 and TLR2 pathways. Thus, we explored whether LPS (Lipopolysaccharide) or LTA (Lipoteichoic acid) levels are elevated in different causes of stroke and correlated with CRP levels since TLR4 is the receptor for Gram-negative bacterial LPS and TLR2 is the receptor for Gram-positive bacterial LTA, respectively.
LPS is increased in acute stroke and associated with poor short term outcome and long term mortality4,5. However, LPS levels were measured with the limulus lysate enzymatic assay which detects total LPS activity without identifying LPS molecules6. To solve this problem, we used a LPS specific ELISA for human plasma to quantify LPS, combined with an LPS binding protein (LBP) ELISA. LBP measurements, unlike LPS, are not subject to contamination.
Thus, this study assessed plasma levels of LPS, LBP, LTA, and CRP in patients with different causes of ischemic stroke, intracerebral hemorrhage (ICH) and transient ischemic attacks (TIAs) compared to controls. We hypothesize that levels of LPS and LTA, the inflammatory molecules from Gram-negative bacteria and Gram-positive bacteria, respectively, might change in some types of stroke and the LPS and LTA levels might correlate with LBP or CRP levels since LBP and CRP are acute phase proteins whose plasma concentrations change in response to inflammation.
Methods
Subject recruitment
Subjects with ischemic stroke (cardioembolic (CE, n = 33), large artery atherosclerosis (LAA, n = 42), small-vessel/lacunar (SVO, n = 41), intracerebral hemorrhage (ICH, n = 36), transient ischemic attacks (TIAs, n = 31), and controls (n = 22) were recruited at the University of California, Davis. The study was approved by the UC Davis Institutional Review Board and adhered to all federal and state regulations related to the protection of human research subjects, including the Common Rule, the principles of the Belmont Report, and institutional policies and procedures. Written informed consent for participation was obtained from all participants or their proxy. Ischemic stroke, ICH, and TIA subjects were recruited within 72 h of symptom onset. Exclusion criteria for all subjects were cancer, recent infection (< 4 weeks) or chronic infection including HIV.
Ischemic stroke, ICH, and TIA diagnoses were determined by two board-certified vascular neurologists. NIHSS, WBC count, lipid panel ((triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C)) were measured at admission. Non-HDL-C levels were calculated using TC levels subtracted from HDL-C levels. All TIA patients had their neurological symptoms resolve within 24 h and their brain MRIs were normal including DWI-MRI. Cause of ischemic stroke was determined using TOAST criteria from medical history, blood tests, Doppler and vascular angiography, cardiac investigations, and brain imaging. CE stroke required at least 1 source of cardiac embolus to be identified as well as the exclusion of LAA or SVO causes of stroke. CE sources included chronic atrial fibrillation, acute myocardial infarction, prosthetic valve, and cardiomyopathy. LAA stroke required > 50% stenosis of ipsilateral extracranial or major intracranial artery (middle cerebral artery, posterior cerebral artery or basilar artery) presumed to be due to atherosclerosis, and exclusion of CE or SVO causes of stroke. SVO strokes were defined as subcortical infarction less than 15 mm in longest diameter on brain imaging. Cryptogenic strokes (no cause, or more than one cause) or strokes of other determined causes such as arterial dissection, vasculitis or hypercoagulable states were excluded from this study. Intracerebral Hemorrhage (ICH) was confirmed by CT brain scan or gradient echo MRI. Patients with subarachnoid hemorrhage and hemorrhagic infarction were excluded. Controls were 22 healthy subjects similar in age, gender, and risk factors to stroke subjects. They had no history of stroke, cardiovascular disease or hematological disease, and had no infection within four weeks manifested either by clinical symptoms or temperature > 100° F.
Plasma samples were obtained on admission within 72 h of a stroke or TIA from each subject via venipuncture. Samples were collected in endotoxin free K2 EDTA 10 mL tubes (Becton Dickinson). Each sample was centrifuged at 1200×g for 10 min, plasma aliquoted and stored at − 80 °C. Extreme care was taken to keep all samples sterile and endotoxin/LPS free, and all processing was performed using sterile, LPS free reagents and glass/plastic ware.
Measurement of LPS, LTA, LBP, and CRP
Sandwich ELISA kits were used to quantify LPS, LTA, LBP, and CRP. A standard curve was generated from known amounts of LPS, LTA, LBP, and CRP, and this was used to derive the values from patient plasma samples. Duplicate samples of plasma at different dilutions (1:100 dilution for LTA, 1:1000 dilution for LPS and LBP, and 1:4000 dilution for CRP) were measured according to protocol and the average used for statistical analyses. Human LPS ELISA kits (MBS266722, MyBioSource, Inc., San Diego, CA, USA), LTA ELISA kits (MBS772314, MyBioSource, Inc., San Diego, CA, USA), LBP ELISA kits (HK315, Hycult Biotech Inc. Wayne, USA), and CRP ELISA kits (KHA0031, Invitrogen, Carlsbad, CA, USA) were used to measure plasma levels. The monoclonal antibody against LPS used in the ELISA kits was raised to LPS from E. coli O111:B4, though it is unknown whether it cross-reacts to LPS from other Gram-negative bacteria.
Statistics
A one-way ANOVA was performed with a Fisher LSD post hoc test to evaluate the differences of continuous variables among the groups if they passed normality test (age and LBP). For those continuous variables which failed normality test, Kruskal–Wallis test was employed (NIHSS, LPS, LTA, and CRP) followed by Dunn’s multiple comparison Post-hoc test. Chi-square test was used to evaluate the differences in sex, vascular risk factors and underlying diseases for different type of strokes among all groups followed by Fisher Exact test to evaluate the differences between stroke/TIA and controls. Pearson Correlation analysis was used to determine the correlations between the levels of the different analytes. Age was expressed as Mean ± standard error of the mean (SE) and other continuous variables were expressed as Median (IQR). A p value ≤ 0.05 was considered statistically significant. SigmaStat 2.03 software was used (San Jose, USA).
Results
There were no significant differences in age, sex, hypertension, diabetes, and hyperlipidemia among the groups (Table 1). The incidences of atrial fibrillation and coronary artery disease were higher in some stroke groups compared to control (Table 1). As expected, the NIHSS on admission was significantly lower in the TIA group than stroke (p < 0.001) (Table 1). By 24 h the NIHSS was 0 in all TIA subjects who had normal brain MRI scans including DWI-MRI.
Table 1.
Demographic variables in different type of stroke, TIA, and healthy controls.
| CE (n = 33) | LAA (n = 42) | SVO (n = 41) | ICH (n = 36) | TIA (n = 31) | Control (n = 22) | p value | |
|---|---|---|---|---|---|---|---|
| Sex male n (%) | 28 (85) | 30 (71) | 28 (68) | 27 (75) | 19 (61) | 14 (64) | 0.232 |
| Age: years ± SE | 65.2 ± 2.0 | 61.6 ± 1.4 | 65.3 ± 1.1 | 62.5 ± 2.4 | 65.1 ± 2.1 | 63.2 ± 2.0 | 0.496 |
| Hypertension n (%) | 27 (81.8) | 35 (83.3) | 34 (82.9) | 31 (86.1) | 24 (77.4) | 12 (54.6) | 0.069 |
| Diabetes n (%) | 9 (27.3) | 18 (42.9) | 23 (56.1) | 11 (30.6) | 10 (32.3) | 7 (31.8) | 0.095 |
| Hyperlipidemia n (%) | 16 (48.5) | 22 (52.4) | 25 (61.0) | 11 (30.6) | 20 (64.5) | 9 (40.9) | 0.054 |
| AF (%) | 18 (54.5) | 2 (4.8) | 5 (12.2) | 3 (8.3) | 3 (9.7) | 0 (0) | < 0.001* |
| CAD (%) | 10 (30.3) | 10 (23.8) | 3 (7.3) | 6 (16.7) | 7 (22.6) | 0 (0) | 0.023** |
| CHF (%) | 2 (6.1) | 1 (2.4) | 1 (2.4) | 1 (2.8) | 4 (12.9) | 0 (0) | 0.181 |
| Dementia n (%) | 1 (3.0) | 0 (0) | 0 (0) | 1 (2.8) | 0 (0) | 0 (0) | 0.551 |
| Parkinson’s disease n (%) | 1 (3.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.388 |
| NIHSS: median (IQR) | 5 (1–10.25) | 3 (1–5) | 4 (2–7) | 4 (1–7) | 0 (0–1) | – | < 0.001 |
CE cardioembolic, LAA large artery atherosclerosis, SVO small-vessel occlusion, TIA transient ischemic attack, AF atrial fibrillation, CAD coronary artery disease, CHF congestive heart failure, SE standard error of the mean, NIHSS National Institutes of Health Stroke Scale, IQR Interquartile range.
*p value for chi-square of total 6 groups. CE to control: p < 0.001; LAA to control: p = 0.542; SVO to control: p = 0.153; ICH to control: p = 0.281; TIA to control: p = 0.258.
**p value for chi square of total 6 groups. CE to control: p = 0.004; LAA to control: p = 0.012; SVO to control: p = 0.546; ICH to control: p = 0.073; TIA to control: p = 0.033.
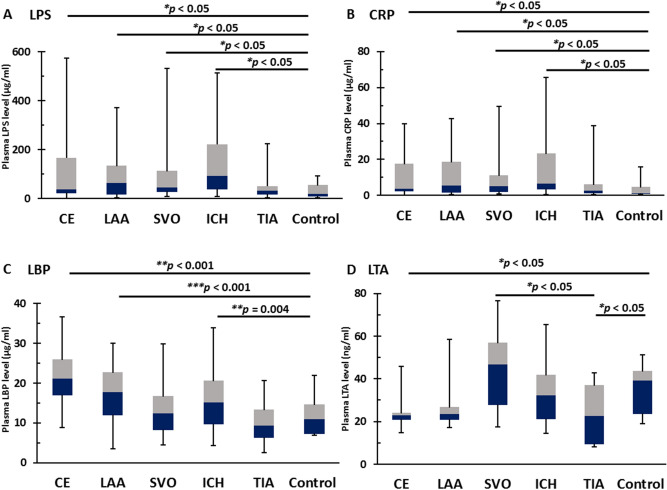
LPS levels in ICH (94.2 (38.9–223.7) µg/ml), CE strokes (37.6 (20.6–168.9) µg/ml), SVO stroke (45.7 (27.0–115.1) µg/ml), and LAA stroke (64.7 (16.0–136.2) µg/ml) but not TIA (31.9 (16.5–53.4) µg/ml) were significantly greater than controls (18.4 (8.6–56.8) µg/ml) (Fig. 1A). CRP levels in ICH (6.7 (3.3–23.6) µg/ml), CE strokes (3.6 (2.1–17.6) µg/ml), LAA stroke (5.4 (1.4–18.6) µg/ml), and SVO stroke (5.2 (1.8–11.2) µg/ml) but not TIA (2.7 (1.2–6.1) µg/ml) were significantly greater than controls (1.3 (0.8–4.7) µg/ml) (Fig. 1B).
Figure 1.
ELISA for LPS, LBP, CRP, and LTA for different causes of stroke and TIA vs controls. (A) The plasma levels of LPS, a component of the cell wall of all Gram-negative bacteria, were significantly higher in patients with intracerebral hemorrhage (ICH), CE stroke, LAA stroke, and SVO stroke compared to age-, sex- and vascular risk factor-matched healthy controls. The difference between LPS levels in TIA and healthy control was not significant. (B) The plasma levels of CRP, the classical acute-phase protein, were significantly higher in ICH, CE stroke, LAA stroke, and SVO stroke than healthy controls. The difference of CRP levels between TIA and healthy controls was not significantly different. (C) The plasma levels of LBP, an acute-phase protein that binds to LPS, were significantly higher in patients with CE stroke, LAA stroke, and ICH compared to healthy controls. The difference between LBP levels in SVO stroke and TIA were not significantly different from controls. (D) The plasma levels of LTA, a Gram-positive bacterial component, were significantly higher in SVO stroke compared to TIA. LTA levels were lower in CE stroke and TIA compared to controls. The difference of LTA levels between LAA stroke and ICH and healthy controls was not significant. LPS lipopolysaccharide, LBP lipopolysaccharide binding protein, CRP C-reactive protein, LTA lipoteichoic acid, CE cardioembolic, LAA large artery atherosclerosis, SVO small-vessel occlusion, ICH intracerebral hemorrhagic, TIA transient ischemic attack, VRFs vascular risk factors.
LBP levels in CE stroke (21.1 (17.0–25.9) µg/ml), LAA strokes (17.8 (11.9–22.7) µg/ml), and ICH (15.1 (9.6–20.7) µg/ml) but not SVO stroke (12.4 (8.2–16.7) µg/ml) or TIA (9.4 (6.3–13.4) µg/ml) were significantly greater than controls (10.9 (7.3–14.7) µg/ml) (Fig. 1C). LTA levels in SVO stroke (46.7 (27.7–56.9) ng/ml) were not significantly greater than controls (39.1 (23.6–43.6) ng/ml) but were significantly greater than TIA (22.6 (9.4–37.2) ng/ml). LTA levels in CE strokes (23.0 (20.7–24.2) ng/ml) and TIA were significantly lower than controls (Fig. 1D). LTA levels in LAA stroke (23.5 (20.7–26.8) ng/ml) and ICH (32.3 (21.0–41.8) ng/ml) did not differ from controls and TIA (Fig. 1D).
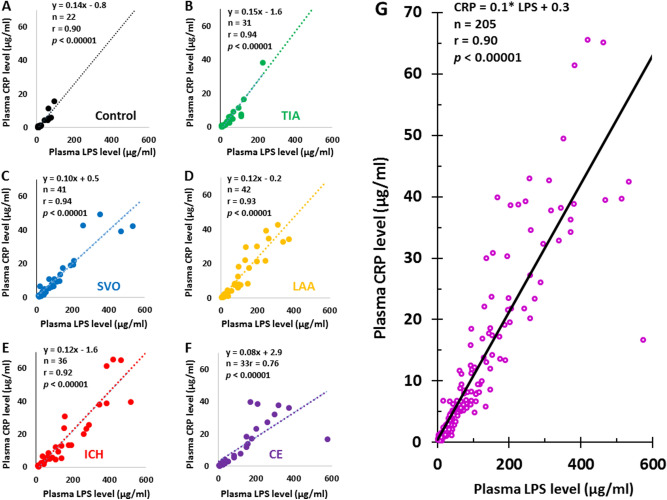
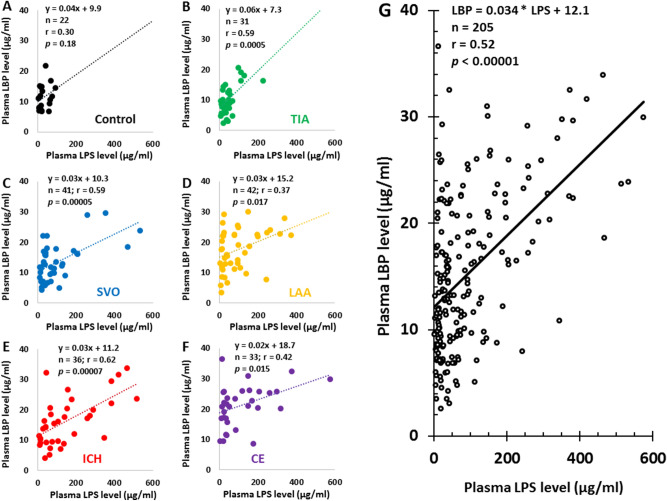
Pearson Correlation analyses showed LPS levels correlated with CRP levels in controls (Fig. 2A), TIA (Fig. 2B), SVO stroke (Fig. 2C), LAA stroke (Fig. 2D), ICH (Fig. 2E), CE stroke (Fig. 2F), and all 205 subjects (Fig. 2G, r = 0.90, and p < 0.00001). The intercept for 0 LPS on the CRP axis was near zero (CRP, 0.3 µg/ml) (Fig. 2). Although LPS levels did not correlate with LBP levels in controls (Fig. 3A), LPS levels correlated with LBP in TIA (Fig. 3B), SVO stroke (Fig. 3C), LAA stroke (Fig. 3D), ICH (Fig. 3E), CE stroke (Fig. 3F), and all 205 subjects (Fig. 3G). The intercept for 0 LPS on the LBP axis was 12.1 µg/ml (Fig. 3G). LTA did not correlate with CRP levels in controls (Supplementary Fig. S1A), TIA (Supplementary Fig. S1B), SVO stroke (Supplementary Fig. S1C), LAA stroke (Supplementary Fig. S1D), ICH (Supplementary Fig. S1E), CE stroke (Supplementary Fig. S1F), and in all 205 subjects (Supplementary Fig. S1G).
Figure 2.
Pearson Correlation Analysis for plasma LPS levels and plasma CRP levels in different causes of stroke, TIA, and controls. The plasma LPS levels correlated with plasma CRP levels in controls (A), TIA (B), SVO stroke (C), LAA stroke (D), ICH (E), CE stroke (F) and for all subjects (G). Note that the intercepts of LPS trendlines on Y axis (CRP levels) in control, TIA, SVO stroke, LAA stroke, ICH stroke and CE strokes were − 0.8, − 1.6, 0.5, − 0.2, − 1.6 and 2.9 µg/ml, respectively (A–F). The intercept of LPS trendline on Y axis (CRP levels) for all 205 subjects was + 0.3 as indicated in (G). LPS lipopolysaccharide, CRP C-reactive protein, CE cardioembolic, LAA large artery atherosclerosis, SVO small-vessel occlusion, ICH intracerebral hemorrhagic, TIA transient ischemic attack.
Figure 3.
Pearson Correlation Analysis for plasma LPS levels and plasma LBP levels in different causes of stroke, TIA, and controls. The plasma LPS levels correlated with plasma LBP levels in TIA patients (B), SVO stroke (C), LAA stroke (D), ICH (E), CE stroke (F) and in all subjects (G) though not in controls (A). Note that the intercepts of LPS trendlines on Y axis (LBP levels) in control, TIA, SVO stroke, LAA stroke, ICH stroke and CE strokes were 9.9, 7.3, 10.3, 15.2, 11.2, and 18.7 µg/ml, respectively (A–F). The intercept of LPS trendline on Y axis (LBP levels) for all 205 subjects was 12.1 as indicated in (G). LPS lipopolysaccharide, LBP lipopolysaccharide binding protein, CE cardioembolic, LAA large artery atherosclerosis, SVO small-vessel occlusion, ICH intracerebral hemorrhagic, TIA transient ischemic attack.
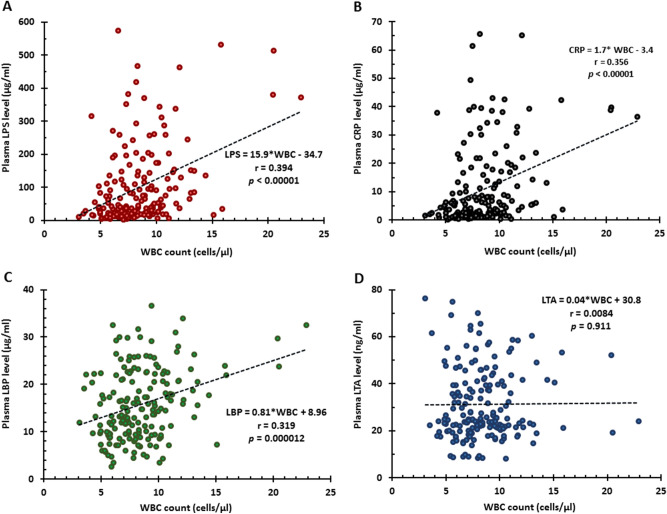
The levels of LPS (Fig. 4A), CRP (Fig. 4B), LBP (Fig. 4C) but not LTA (Fig. 4D) positively correlated with WBC counts. Similarly, the levels of LPS (Supplementary Fig. 2A), CRP (Supplementary Fig. S2B), LBP (Supplementary Fig. S2C) but not LTA (Supplementary Fig. S2D) positively correlated with percentage of neutrophils. In contrast to neutrophils, lymphocytes had negative correlations with the levels of LPS (Supplementary Fig. S3A), CRP (Supplementary Fig. S3B), LBP (Supplementary Fig. S3C) but not LTA (Supplementary Fig. S3D).
Figure 4.
Pearson Correlation Analysis for WBC and levels of LPS, LBP, CRP, and LTA. WBC correlated with plasma LPS levels (A), CRP levels (B), LBP levels (C) but not with LTA levels (D). WBC white blood cell, LPS lipopolysaccharide, LBP lipopolysaccharide binding protein, CRP C-reactive protein, LTA lipoteichoic acid.
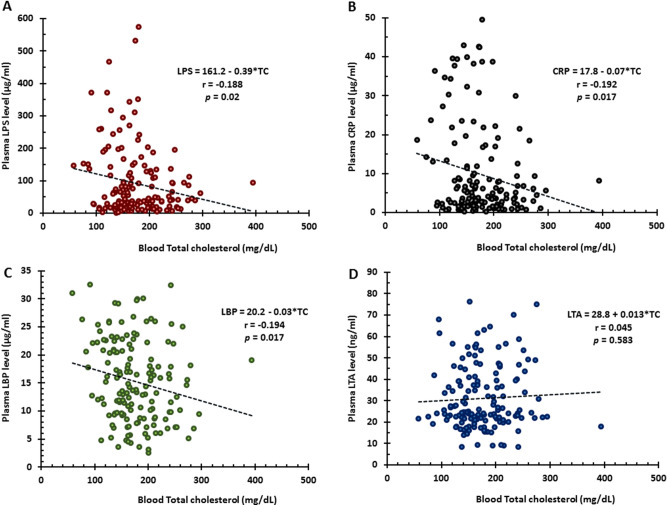
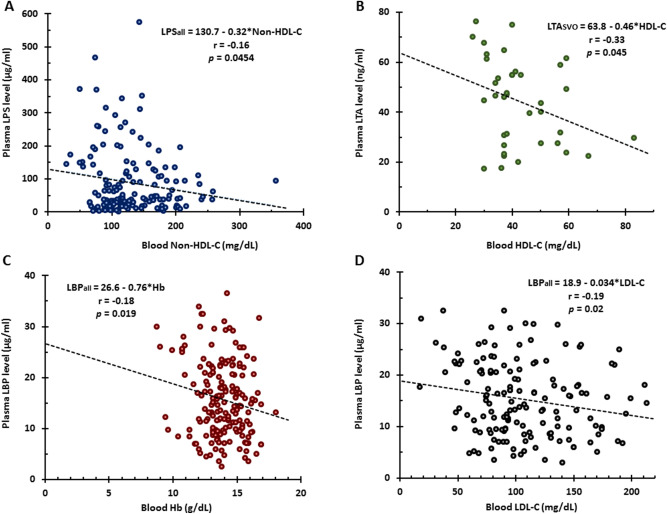
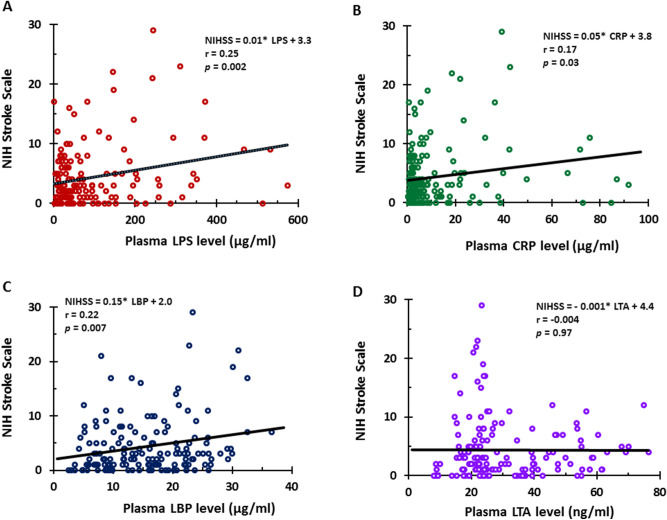
The levels of LPS (Fig. 5A), CRP (Fig. 5B), LBP (Fig. 5C) but not LTA (Fig. 5D) negatively correlated with total cholesterol. Furthermore, LPS levels negatively correlated with Non-HDL-C (Fig. 6A) but not with HDL-C (not shown), LDL-C (not shown) and triglycerides (not shown). LTA levels negatively correlated with HDL-C only in SVO (Fig. 6B) stroke. In addition, hemoglobin (Fig. 6C) and LDL-C (Fig. 6D) negatively correlated with LBP. NIHSS values were significantly greater in CE, LAA, SVO strokes and ICH compared to TIA subjects (Supplementary Fig. S4). LPS levels (Fig. 7A), CRP levels (Fig. 7B), and LBP levels (Fig. 7C) positively correlated with the NIHSS. LTA levels did not correlate with the NIHSS (Fig. 7D).
Figure 5.
Pearson Correlation Analysis for total cholesterol and levels of LPS, LBP, CRP, and LTA. Total cholesterol negatively correlated with plasma LPS levels (A), CRP levels (B), LBP levels (C) but not with LTA levels (D). TC total cholesterol, LPS lipopolysaccharide, LBP lipopolysaccharide binding protein, CRP C-reactive protein, LTA lipoteichoic acid.
Figure 6.
Pearson Correlation Analysis for Non-HDL-C and LPS levels, HDL-C and LTA levels, as well as hemoglobin and LDL-C and LBP levels. Non-HDL-C negatively correlated with plasma LPS levels (A). HDL-C negatively correlated with plasma LTA levels (B). Hb (C) and LDL-C (D) negatively correlated with plasma LBP. Hb hemoglobin, LDL-C low density lipoprotein-cholesterol, HDL-C high density lipoprotein-cholesterol, Non HDL-C non high density lipoprotein-cholesterol, LPS lipopolysaccharide, LBP lipopolysaccharide binding protein, LTA Lipoteichoic acid.
Figure 7.
Pearson Correlation Analysis for NIHSS and levels of LPS, LBP, CRP, and LTA from TIA and different causes of stroke. NIHSS correlated with plasma LPS levels (A), CRP levels (B), LBP levels (C) but not with LTA levels (D). NIHSS NIH stroke scale, LPS lipopolysaccharide, LBP lipopolysaccharide binding protein, CRP C-reactive protein, LTA lipoteichoic acid.
Discussion
This is the first study to evaluate plasma levels of both Gram-negative bacterial LPS and Gram-positive bacterial LTA in patients with different causes of stroke compared to controls. LPS levels are elevated in ischemic stroke (CE, LAA, and SVO) and ICH compared to controls. Since there might be some concern about LPS contamination, these findings were supported by elevated LPS-binding protein (LBP) levels in ischemic stroke (CE and LAA) and ICH compared to controls. In contrast, LTA levels only increased in SVO stroke compared to TIA but not controls. LPS levels correlate with CRP and LBP for all causes of stroke. LPS, LBP and CRP levels correlate with NIHSS. Since the half-life of LPS/LTA is short, we postulate that LPS and LTA in plasma are continually derived from the gut and other body organs like the gums and skin.
LPS levels have been associated with several risk factors for stroke and myocardial infarction including diabetes, hypertension, and high fat diet7. Since the risk factors in stroke compared to control subjects were not significantly different in our study, the elevated LPS levels associated with stroke suggests that LPS promotes inflammation that begins either prior to a stroke and/or following a stroke. Previous studies have demonstrated that LPS levels following human ischemic stroke correlate with worse short-term outcomes and mortality at 90 days4,5. This is consistent with LPS and LBP levels correlating with the NIHSS in the current study.
LPS may play different roles in different causes of stroke. LPS and LBP levels correlate with atherosclerosis in LAA stroke and with carotid intima-medial thickness (CIMT) in humans8 and experimentally LPS promotes atherosclerosis8. Indeed, LPS is found in carotid atherosclerotic plaque and blood LPS levels are higher in patients with carotid plaques compared to those without plaques9. LPS induces dysfunction of serum lipids that then accumulate on the aortic walls to form atherosclerotic plaques in ApoE−/− mice10, which might provide some insight into why LPS levels are higher in LAA patients.
Though there are no previous studies of LPS in cardioembolic stroke per se, a recent study did find that atrial fibrillation was associated with higher LPS levels, and that LPS levels were predictive of atrial fibrillation patients who developed MACE (Major Adverse Cardiovascular Events) that included a composite of myocardial infarction, ischemic stroke and TIAs, cardiac revascularization and cardiovascular death11. No data were given for stroke per se, however. Experimentally, LPS can promote atrial fibrillation and thus could contribute to human CE stroke. Finally, LPS can contribute to clot formation12 which plays a role in CE stroke as well as LAA and SVO stroke.
LBP and LPS have an intricate, interrelated biology. LBP binds LPS in blood. The LPS-LBP complex binds CD14 and triggers TLR4 on monocytes, macrophages, and microglia to bind LPS directly. This causes TLR4 internalization and downstream activation of MyD88-independent activation of NFκB and induces the production of IL1, IL6 and TNF13. Of the genes activated in blood following CE and LAA stroke, a number are down-stream of LPS14. In addition, LPS/TLR4 downstream genes were also activated following ICH stroke15. Our data are consistent with suggestions that there is a pro-inflammatory state either before a stroke and/or after a stroke which could contribute to causing a stroke and/or worsening outcome.
LPS induction of IL1 and particularly IL6 activate APRF/STAT3 which binds to the LBP gene promoter to increase the synthesis of LBP in hepatocytes and intestinal epithelial cells16. This likely explains the correlation of LPS levels with LBP levels with ischemic stroke, TIA, and ICH in the current study. That LPS drives LBP expression in stroke is supported by human studies where LPS infusion increases LBP levels17. However, when all of the subjects are considered, the LPS trendline for zero LPS intercepts the LBP axis at 12.1 µg/ml, which means LBP is expressed even when LPS is very low. Therefore, LBP is likely activated by and binds other molecules in addition to LPS. Indeed, LBP has been reported to recognize different ligands such as live bacteria18, glycolipids19, lipoproteins20 and peptidoglycan21.
CRP, an acute-phase protein, rapidly rises in serum in response to tissue injury and infection22. CRP is synthesized in hepatocytes in response to proinflammatory cytokines such as IL1, IL6 and IL1723, and mediates elimination of pathogens by recruiting complement and phagocytic cells22. CRP levels are increased in stroke and are associated with stroke incidence and outcomes. Since phosphorcholine (PC) is the principal ligand of CRP and PC is present in bacterial LPS23, we hypothesize that elevated LPS levels might directly drive CRP expression. Indeed, our results show CRP levels increase proportional to LPS levels in controls, TIA, ischemic stroke and ICH subjects, and the correlation coefficient is 0.90 (p < 0.00001) with the trendline for LPS vs CRP intercepting near zero on both axes. The proposal that LPS drives CRP expression is also consistent with LPS infusions in humans elevating plasma CRP17.
Lipoteichoic Acid (LTA) is found in the cell wall of almost all Gram-positive bacteria24, and LTA functions in parallel to LPS. LTA induces inflammatory responses by binding to TLR2 and inducing the innate immune system via different downstream regulators25. TLR2 signaling also has overlapping downstream pathways to TLR4. LTA binds TLR2 which signals to MyD88. However, TLR2-MyD88 activates the IRAK 1,2,4 complex. This then activates TRAF6 via a ubiquitin-linked pathway to induce downstream cytokines including IL1, IL1R, TNF and iNOS26. One of the most novel findings of the current study is that LTA levels are elevated only in SVO stroke, but not in LAA or CE stroke, or ICH, compared to TIA subjects. SVO stroke mainly involves penetrating arterioles, capillaries and venules, causes 25% of strokes and contributes to vascular dementia27. Though inflammation and infection have been implicated in SVO disease27, the causes have not been identified. The current study suggests that both LTA (TLR2) as well as LPS (TLR4) pathways are activated in SVO stroke and likely contribute to inflammation either before and/or after the SVO strokes. LTA activates bronchoalveolar coagulation via increases in the levels of thrombin-antithrombin complexes, d-dimer, and soluble tissue factor28 and induces similar changes in coagulation and fibrinolysis as LPS28. LTA activates the coagulation pathway in the lungs through TLR2-dependent pathway and the process is likely amplified by endogenous TLR4 ligands29. Of interest is a finding from human umbilical vein endothelia cells (ECs) that shows peptidoglycan rather than LTA induces EC ICAM-1 and VCAM-1 to facilitate the adhesion of monocytes, whereas both LTA and peptidoglycan significantly increase the expression of pro-coagulant Tissue Factor in ECs30. Further studies are required to evaluate any role for peptidoglycan in SVO or other causes of stroke.
Our data raise the interesting question of why LTA is only associated with SVO whereas LPS is associated with both SVO and LAA? Thus, we hypothesize that LPS/TLR4 is expressed in both small and large vessels whereas LTA/TLR2 may be expressed only in small vessels. In a previous study we have shown that LPS is localized in both small and large vessels of both Alzheimer’s Disease and normal control brains31. Future studies will need to determine if LTA/TLR2 is expressed exclusively in small vessels, and thus potentially explain its specific role in SVO but not other causes of stroke.
Notably, LTA levels did not correlate with CRP levels, in spite of the fact that Gram- positive infections have been shown to increase CRP32. This might be because of different CRP isoforms32, only one of which is detected by ELISA in this study. Alternatively, it is possible that Gram-positive bacteria induce CRP via different molecules than LTA, and these molecules are not increased with stroke since there probably are no live Gram-positive bacteria in blood following most strokes.
TIA subjects had LPS, LBP and CRP levels that were similar to controls and LTA levels in TIA patients were lower than control. The TIA subjects in these analyses were MRI DWI negative, and thus did not have minor strokes. About ~ 6.4% of TIAs go on to have a cardiovascular event including stroke33. Whether TIA subjects who go on to have strokes have higher LPS levels requires further study, particularly since we identified two types of TIA based upon gene expression in blood34.
LPS positively correlated with WBC counts and neutrophil percentage, which is consistent with an animal study showing LPS increases WBC and neutrophil counts35. Moreover, LPS challenge in humans also increases WBC/neutrophil counts associated with increased levels of CRP36,37 and LBP38. As mentioned earlier, CD14 acts as a co-receptor along with TLR4 to bind LPS in the presence of LBP. CD14 is a 55-kDa glycoprotein which exists in two forms: membrane-bound CD14 (mCD14) which is found on monocytes, macrophages, and neutrophils, and soluble CD14 (sCD14) which is present in plasma39,40. Both mCD14 and sCD14 mediate biological responses to LPS. It has been demonstrated that neutrophils respond to LPS/LBP complexes via CD14 to release TNFα41. Our study demonstrates a direct relationship between the levels of inflammatory markers of LPS, LBP and CRP and the percentage of neutrophils. Higher WBC counts and higher percentage of neutrophil without infection are likely a result of a LPS-induced proinflammatory state with elevated LBP and CRP as well. Given the critical role of neutrophils in mediating clot formation, this provides an additional reason for considering LPS as a risk factor for strokes.
An unexpected finding of the current study was that LPS levels negatively correlated with total cholesterol and non-HDL-C. Because cholesterol lipid has minimal solubility in water, free cholesterol levels in blood are exceedingly small. Instead, cholesterol is packaged within lipoproteins complexes which allow cholesterol to travel through blood via emulsification. Several types of lipoproteins exist in blood including chylomicrons, very-low-density lipoprotein (VLDL), intermediate-density lipoprotein, LDL, and HDL. Thus, cholesterol exists in blood in a free form and as cholesterol esters within lipoprotein particles. Our study demonstrates a significant inverse relationship between LPS levels and total cholesterol levels as well as non-HDL-C levels. Decreases in cholesterol during inflammatory diseases have been observed for more than a century in patients with bacterial and viral infections, critically ill patients, trauma patients, postoperative patients and patients with sepsis42. Thus, our findings are consistent with the previous literature showing that pro-inflammatory states, including the presence of LPS in our study, are associated with decreases of cholesterol in blood.
The mechanism by which LPS lowers cholesterol, however, is less clear. LPS is a large molecule consisting of a lipid and a polysaccharide. LPS can bind lipid transfer protein including cholesteryl ester transfer protein (CETP), lipoproteins and LBP. LPS markedly increases LBP expression and decreases hepatic CETP mRNA expression, plasma CETP concentration, plasma total cholesterol levels and non-HDL-C levels43, which might explain the direct relationship between LPS levels and LBP levels and inverse relationship between LPS levels and total cholesterol levels as well as non-HDL-C levels found in our study. Animal and human studies demonstrate that LPS decreases plasma CETP concentration43. Lipoproteins, particularly HDL44,45 and LDL46, bind and neutralize LPS and attenuate LPS induced biological responses in vivo and in vitro47,48. In fact, a large fraction of LPS enters the bloodstream by binding to HDL as a LPS-HDL complex49. Since HDL binds both cholesterol and LPS, plasma LPS might compete with cholesterol to bind HDL and cause an inverse relationship of LPS with total cholesterol and non-HDL-C found in our study. Mechanistic studies are required to assess this hypothesis.
Our study also demonstrated that LBP levels positively correlated with LPS but negatively correlated with LDL-C. As noted above, LPS increases LBP expression and decreases hepatic CETP mRNA expression, plasma CETP concentration, plasma total cholesterol levels and non-HDL-C levels in animal studies. CETP and LBP together with bactericidal/ permeability-increasing (BPI) and phospholipid transfer protein (PLTP) belong to members of a lipid transfer/LBP gene family50–53 which are produced in liver and participate in the innate immune response. Proteomic studies of human plasma demonstrate that LBP is present in the VLDL but not in LDL54. LBP is carried on lipoprotein and transfers LPS to lipoproteins in addition to CD1440. These studies suggest that both LBP and lipoproteins may be involved in LPS associated cholesterol changes in stroke patients. Since we did not measure lipoprotein levels, the relationship between LPS, LBP, lipoproteins and cholesterol in stroke patients remains to be elucidated.
Endothelial cells exposed to LPS show prothrombotic properties combined with enhanced Tissue Factor activity55. This may be partly associated with hemoglobin binding to LPS. Hb is present in high concentrations in red blood cells (RBCs). Hb has the capacity to bind LPS and enhance LPS biological activity56,57. Addition of Hb to cultured macrophages significantly enhanced LPS-dedicated cytokine production. Normally, LPS activity is regulated by LBP. However, Hb also binds LPS and forms a stable Hb-LPS complex which enhances the procoagulant activity of peripheral blood cells and tissue factor activity of endothelia58. Our current study demonstrates an inverse relationship between Hb concentrations and plasma LBP levels. Further study is required to determine whether Hb-LPS-LBP interactions are a risk factor for stroke.
The NIHSS correlated with LPS levels, CRP levels, and LBP levels. This finding would suggest that higher LPS levels produced a greater “pro-inflammatory” state that was associated with worse stroke outcomes. Indeed, higher LPS levels and higher CRP levels have previously been associated with increased morbidity and mortality following stroke4,5.
LPS has a short half-life of a few hours in plasma. The finding of increased LPS levels in stroke suggests there is a continuous release of LPS into blood. Recent evidence suggests that gut bacterial molecules or bacterial molecules from other sites like the gums and skin leak into the blood, and that stroke risk factors including hypertension, high fat diet, diabetes and others increase the permeability of gut and gum endothelial cells and thus contribute to inflammation caused by a “leaky gut” or inflamed gums11. LPS identified in this study was from E. coli O111:B4, which indicates a possible gut source. Our findings suggest that the gut may be leakier in stroke patients. Leakage of LPS from Gram-negative bacteria in the gut or gums to blood provides one model of how the gut or gum microbiome may affect stroke and other CNS diseases59. Stroke risk factors and stroke itself might lead to a “leaky gut” and increases of blood LPS via the autonomic nervous system, brain-gut endocrine interactions, and miRNA released from brain and brain-gut immune interactions. The finding that Gram-negative LPS is elevated in all causes of stroke including ICH, whereas Gram-positive LTA is elevated only in SVO stroke, suggests complex brain-gut-immune interactions that may be specific for molecules from specific classes of bacteria.
Limitations to the current studies include the fact that other TLR were not examined, so they have an uncertain role in stroke. The lower than control levels of LTA in CE and TIA subjects is of uncertain significance but could relate to factors not controlled for in our analyses including drugs given post-stroke. The study will need to be replicated in a larger cohort, and though age, hypertension, diabetes, and lipids were controlled for, future studies should consider additional variables like smoking, alcohol, BMI, and other vascular risk factors. Future studies will need to compare males and females and examine the effects of race and increasing age on LPS and LTA.
Supplementary Information
Acknowledgements
We thank the patients and families who participated in the study. We also thank Dr. Charles DeCarli and Dr. Lee-Way Jin for their intellectual partnership in this study. These studies were supported by grants from the California Department of Public Health (18-10924 to XZ), National Institutes of Health (NS097000, NS101718, NS075035, NS079153, and NS106950 to FRS, BSS, BPA, and GCJ) and grants from the American Heart Association (BSS).
Author contributions
X.Z. and F.R.S. designed the studies. M.H. performed all ELISA under the supervision of X.Z. E.F. collected the informed consents and samples. M.H., E.F., N.A. and B.P.A. stored samples and collected the data. G.C.J. and F.R.S. reviewed subjects for correct diagnosis. M.H., B.P.A., H.A., B.S. and X.Z. performed statistical analysis. M.H., E.F., F.R.S. and X.Z. wrote the manuscript. All authors made changes to the manuscript.
Data availability
The corresponding author has access to all the data. The data will be made available upon written request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors jointly supervised this work: Frank R. Sharp and Xinhua Zhan.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-86083-8.
References
- 1.Dziedzic T. Systemic inflammation as a therapeutic target in acute ischemic stroke. Expert Rev. Neurother. 2015;15:523–531. doi: 10.1586/14737175.2015.1035712. [DOI] [PubMed] [Google Scholar]
- 2.Mobarra N, Morovatdar N, Di Napoli M, Stranges S, Behrouz R, Amiri A, et al. The association between inflammatory markers in the acute phase of stroke and long-term stroke outcomes: Evidence from a population-based study of stroke. Neuroepidemiology. 2019;53:20–26. doi: 10.1159/000494685. [DOI] [PubMed] [Google Scholar]
- 3.Sharp FR, Jickling GC, Stamova B, Tian Y, Zhan X, Liu D, et al. Molecular markers and mechanisms of stroke: RNA studies of blood in animals and humans. J. Cereb. Blood Flow Metab. 2011;31:1513–1531. doi: 10.1038/jcbfm.2011.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klimiec E, Pera J, Chrzanowska-Wasko J, Golenia A, Slowik A, Dziedzic T. Plasma endotoxin activity rises during ischemic stroke and is associated with worse short-term outcome. J. Neuroimmunol. 2016;297:76–80. doi: 10.1016/j.jneuroim.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Klimiec E, Pasinska P, Kowalska K, Pera J, Slowik A, Dziedzic T. The association between plasma endotoxin, endotoxin pathway proteins and outcome after ischemic stroke. Atherosclerosis. 2018;269:138–143. doi: 10.1016/j.atherosclerosis.2017.12.034. [DOI] [PubMed] [Google Scholar]
- 6.Duquenne P, Marchand G, Duchaine C. Measurement of endotoxins in bioaerosols at workplace: A critical review of literature and a standardization issue. Ann. Occup. Hyg. 2013;57:137–172. doi: 10.1093/annhyg/mes051. [DOI] [PubMed] [Google Scholar]
- 7.Cox AJ, Zhang P, Bowden DW, Devereaux B, Davoren PM, Cripps AW, et al. Increased intestinal permeability as a risk factor for type 2 diabetes. Diabetes Metab. 2017;43:163–166. doi: 10.1016/j.diabet.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Trojova I, Kozarova M, Petrasova D, Malachovska Z, Paranicova I, Joppa P, et al. Circulating lipopolysaccharide-binding protein and carotid intima-media thickness in obstructive sleep apnea. Physiol. Res. 2018;67:69–78. doi: 10.33549/physiolres.933632. [DOI] [PubMed] [Google Scholar]
- 9.Carnevale R, Nocella C, Petrozza V, Cammisotto V, Pacini L, Sorrentino V, et al. Localization of lipopolysaccharide from Escherichia coli into human atherosclerotic plaque. Sci. Rep. 2018;8:3598. doi: 10.1038/s41598-018-22076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou L, Long J, Sun Y, Chen W, Qiu R, Yuan D. Resveratrol ameliorates atherosclerosis induced by high-fat diet and LPS in ApoE(-/-) mice and inhibits the activation of CD4(+) T cells. Nutr. Metab. (Lond.) 2020;17:41. doi: 10.1186/s12986-020-00461-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pastori D, Carnevale R, Nocella C, Novo M, Santulli M, Cammisotto V, et al. Gut-derived serum lipopolysaccharide is associated with enhanced risk of major adverse cardiovascular events in atrial fibrillation: Effect of adherence to mediterranean diet. J. Am. Heart Assoc. 2017;6:e005784. doi: 10.1161/JAHA.117.005784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang Y, Wu LC, Ma K, Pan G, Yang S, Zheng Y, et al. Paeoniflorin alleviates lipopolysaccharide-induced disseminated intravascular coagulation by inhibiting inflammation and coagulation activation. Drug Dev. Res. 2020;81:517. doi: 10.1002/ddr.21647. [DOI] [PubMed] [Google Scholar]
- 13.Zhan X, Stamova B, Sharp FR. Lipopolysaccharide associates with amyloid plaques, neurons and Oligodendrocytes in Alzheimer's disease brain: A review. Front. Aging Neurosci. 2018;10:42. doi: 10.3389/fnagi.2018.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jickling GC, Xu H, Stamova B, Ander BP, Zhan X, Tian Y, et al. Signatures of cardioembolic and large-vessel ischemic stroke. Ann. Neurol. 2010;68:681–692. doi: 10.1002/ana.22187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stamova B, Ander BP, Jickling G, Hamade F, Durocher M, Zhan X, et al. The intracerebral hemorrhage blood transcriptome in humans differs from the ischemic stroke and vascular risk factor control blood transcriptomes. J. Cereb. Blood Flow Metab. 2019;39:1818–1835. doi: 10.1177/0271678X18769513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schumann RR. Old and new findings on lipopolysaccharide-binding protein: A soluble pattern-recognition molecule. Biochem. Soc. Trans. 2011;39:989–993. doi: 10.1042/BST0390989. [DOI] [PubMed] [Google Scholar]
- 17.Hudgins LC, Parker TS, Levine DM, Gordon BR, Saal SD, Jiang XC, et al. A single intravenous dose of endotoxin rapidly alters serum lipoproteins and lipid transfer proteins in normal volunteers. J. Lipid Res. 2003;44:1489–1498. doi: 10.1194/jlr.M200440-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Zweigner J, Gramm HJ, Singer OC, Wegscheider K, Schumann RR. High concentrations of lipopolysaccharide-binding protein in serum of patients with severe sepsis or septic shock inhibit the lipopolysaccharide response in human monocytes. Blood. 2001;98:3800–3808. doi: 10.1182/blood.V98.13.3800. [DOI] [PubMed] [Google Scholar]
- 19.Schroder NW, Opitz B, Lamping N, Michelsen KS, Zahringer U, Gobel UB, et al. Involvement of lipopolysaccharide binding protein, CD14, and Toll-like receptors in the initiation of innate immune responses by Treponema glycolipids. J. Immunol. 2000;165:2683–2693. doi: 10.4049/jimmunol.165.5.2683. [DOI] [PubMed] [Google Scholar]
- 20.Schroder NW, Heine H, Alexander C, Manukyan M, Eckert J, Hamann L, et al. Lipopolysaccharide binding protein binds to triacylated and diacylated lipopeptides and mediates innate immune responses. J. Immunol. 2004;173:2683–2691. doi: 10.4049/jimmunol.173.4.2683. [DOI] [PubMed] [Google Scholar]
- 21.Weber JR, Freyer D, Alexander C, Schroder NW, Reiss A, Kuster C, et al. Recognition of pneumococcal peptidoglycan: An expanded, pivotal role for LPS binding protein. Immunity. 2003;19:269–279. doi: 10.1016/S1074-7613(03)00205-X. [DOI] [PubMed] [Google Scholar]
- 22.Volanakis JE. Human C-reactive protein: Expression, structure, and function. Mol. Immunol. 2001;38:189–197. doi: 10.1016/S0161-5890(01)00042-6. [DOI] [PubMed] [Google Scholar]
- 23.Di Napoli M, Elkind MS, Godoy DA, Singh P, Papa F, Popa-Wagner A. Role of C-reactive protein in cerebrovascular disease: A critical review. Expert Rev. Cardiovasc. Ther. 2011;9:1565–1584. doi: 10.1586/erc.11.159. [DOI] [PubMed] [Google Scholar]
- 24.Rajagopal M, Walker S. Envelope structures of gram-positive bacteria. Curr. Top. Microbiol. Immunol. 2017;404:1–44. doi: 10.1007/82_2015_5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 26.Reinhardt C. The involvement of toll-like receptor-2 in arterial thrombus formation. Hamostaseologie. 2018;38:223–228. doi: 10.1055/s-0038-1668164. [DOI] [PubMed] [Google Scholar]
- 27.Cannistraro RJ, Badi M, Eidelman BH, Dickson DW, Middlebrooks EH, Meschia JF. CNS small vessel disease: A clinical review. Neurology. 2019;92:1146–1156. doi: 10.1212/WNL.0000000000007654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoogerwerf JJ, de Vos AF, Levi M, Bresser P, van der Zee JS, Draing C, et al. Activation of coagulation and inhibition of fibrinolysis in the human lung on bronchial instillation of lipoteichoic acid and lipopolysaccharide. Crit. Care Med. 2009;37:619–625. doi: 10.1097/CCM.0b013e31819584f9. [DOI] [PubMed] [Google Scholar]
- 29.Dessing MC, Schouten M, Draing C, Levi M, von Aulock S, van der Poll T. Role played by Toll-like receptors 2 and 4 in lipoteichoic acid-induced lung inflammation and coagulation. J. Infect. Dis. 2008;197:245–252. doi: 10.1086/524873. [DOI] [PubMed] [Google Scholar]
- 30.Mattsson E, Heying R, van de Gevel JS, Hartung T, Beekhuizen H. Staphylococcal peptidoglycan initiates an inflammatory response and procoagulant activity in human vascular endothelial cells: A comparison with highly purified lipoteichoic acid and TSST-1. FEMS Immunol. Med. Microbiol. 2008;52:110–117. doi: 10.1111/j.1574-695X.2007.00350.x. [DOI] [PubMed] [Google Scholar]
- 31.Zhan X, Stamova B, Jin LW, DeCarli C, Phinney B, Sharp FR. Gram-negative bacterial molecules associate with Alzheimer disease pathology. Neurology. 2016;87:2324–2332. doi: 10.1212/WNL.0000000000003391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 2018;9:754. doi: 10.3389/fimmu.2018.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amarenco P, Lavallee PC, Monteiro Tavares L, Labreuche J, Albers GW, Abboud H, et al. Five-year risk of stroke after TIA or minor ischemic stroke. N. Engl. J. Med. 2018;378:2182–2190. doi: 10.1056/NEJMoa1802712. [DOI] [PubMed] [Google Scholar]
- 34.Zhan X, Jickling GC, Tian Y, Stamova B, Xu H, Ander BP, et al. Transient ischemic attacks characterized by RNA profiles in blood. Neurology. 2011;77:1718–1724. doi: 10.1212/WNL.0b013e318236eee6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee SY, Cho SS, Bae CS, Bae MS, Park DH. Socheongryongtang suppresses COPD-related changes in the pulmonary system through both cytokines and chemokines in a LPS COPD model. Pharm. Biol. 2020;58:538–544. doi: 10.1080/13880209.2020.1770808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boos CJ, Lip GY, Jilma B. Endotoxemia, inflammation, and atrial fibrillation. Am. J. Cardiol. 2007;100:986–988. doi: 10.1016/j.amjcard.2007.04.039. [DOI] [PubMed] [Google Scholar]
- 37.Michel O, Duchateau J, Plat G, Cantinieaux B, Hotimsky A, Gerain J, et al. Blood inflammatory response to inhaled endotoxin in normal subjects. Clin. Exp. Allergy. 1995;25:73–79. doi: 10.1111/j.1365-2222.1995.tb01005.x. [DOI] [PubMed] [Google Scholar]
- 38.Calvano SE, Thompson WA, Marra MN, Coyle SM, de Riesthal HF, Trousdale RK, et al. Changes in polymorphonuclear leukocyte surface and plasma bactericidal/permeability-increasing protein and plasma lipopolysaccharide binding protein during endotoxemia or sepsis. Arch. Surg. 1994;129:220–226. doi: 10.1001/archsurg.1994.01420260116016. [DOI] [PubMed] [Google Scholar]
- 39.Hailman E, Vasselon T, Kelley M, Busse LA, Hu MC, Lichenstein HS, et al. Stimulation of macrophages and neutrophils by complexes of lipopolysaccharide and soluble CD14. J. Immunol. 1996;156:4384–4390. [PubMed] [Google Scholar]
- 40.Wurfel MM, Kunitake ST, Lichenstein H, Kane JP, Wright SD. Lipopolysaccharide (LPS)-binding protein is carried on lipoproteins and acts as a cofactor in the neutralization of LPS. J. Exp. Med. 1994;180:1025–1035. doi: 10.1084/jem.180.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haziot A, Tsuberi BZ, Goyert SM. Neutrophil CD14: Biochemical properties and role in the secretion of tumor necrosis factor-alpha in response to lipopolysaccharide. J. Immunol. 1993;150:5556–5565. [PubMed] [Google Scholar]
- 42.Fraunberger P, Schaefer S, Werdan K, Walli AK, Seidel D. Reduction of circulating cholesterol and apolipoprotein levels during sepsis. Clin. Chem. Lab. Med. 1999;37:357–362. doi: 10.1515/CCLM.1999.059. [DOI] [PubMed] [Google Scholar]
- 43.van der Tuin SJL, Li Z, Berbee JFP, Verkouter I, Ringnalda LE, Neele AE, et al. Lipopolysaccharide lowers cholesteryl ester transfer protein by activating F4/80(+)Clec4f(+)Vsig4(+)Ly6C(-) Kupffer cell subsets. J. Am. Heart Assoc. 2018 doi: 10.1161/JAHA.117.008105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ulevitch RJ, Johnston AR, Weinstein DB. New function for high density lipoproteins. Their participation in intravascular reactions of bacterial lipopolysaccharides. J. Clin. Investig. 1979;64:1516–1524. doi: 10.1172/JCI109610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Munford RS, Hall CL, Dietschy JM. Binding of Salmonella typhimurium lipopolysaccharides to rat high-density lipoproteins. Infect. Immun. 1981;34:835–843. doi: 10.1128/IAI.34.3.835-843.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Flegel WA, Baumstark MW, Weinstock C, Berg A, Northoff H. Prevention of endotoxin-induced monokine release by human low- and high-density lipoproteins and by apolipoprotein A-I. Infect. Immun. 1993;61:5140–5146. doi: 10.1128/IAI.61.12.5140-5146.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Skarnes RC. In vivo interaction of endotoxin with a plasma lipoprotein having esterase activity. J. Bacteriol. 1968;95:2031–2034. doi: 10.1128/JB.95.6.2031-2034.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berbee JF, Havekes LM, Rensen PC. Apolipoproteins modulate the inflammatory response to lipopolysaccharide. J. Endotoxin Res. 2005;11:97–103. doi: 10.1177/09680519050110020501. [DOI] [PubMed] [Google Scholar]
- 49.Freudenberg MA, Bog-Hansen TC, Back U, Galanos C. Interaction of lipopolysaccharides with plasma high-density lipoprotein in rats. Infect. Immun. 1980;28:373–380. doi: 10.1128/iai.28.2.373-380.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bingle CD, Craven CJ. Meet the relatives: A family of BPI- and LBP-related proteins. Trends Immunol. 2004;25:53–55. doi: 10.1016/j.it.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 51.Alva V, Lupas AN. The TULIP superfamily of eukaryotic lipid-binding proteins as a mediator of lipid sensing and transport. Biochim. Biophys. Acta. 2016;1861:913–923. doi: 10.1016/j.bbalip.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 52.Yamashita S, Hirano K, Sakai N, Matsuzawa Y. Molecular biology and pathophysiological aspects of plasma cholesteryl ester transfer protein. Biochim. Biophys. Acta. 2000;1529:257–275. doi: 10.1016/S1388-1981(00)00164-5. [DOI] [PubMed] [Google Scholar]
- 53.Yamashita S, Sakai N, Hirano K, Ishigami M, Maruyama T, Nakajima N, et al. Roles of plasma lipid transfer proteins in reverse cholesterol transport. Front. Biosci. 2001;6:D366–D387. doi: 10.2741/A616. [DOI] [PubMed] [Google Scholar]
- 54.Dashty M, Motazacker MM, Levels J, de Vries M, Mahmoudi M, Peppelenbosch MP, et al. Proteome of human plasma very low-density lipoprotein and low-density lipoprotein exhibits a link with coagulation and lipid metabolism. Thromb. Haemost. 2014;111:518–530. doi: 10.1160/TH13-02-0178. [DOI] [PubMed] [Google Scholar]
- 55.Moore KL, Andreoli SP, Esmon NL, Esmon CT, Bang NU. Endotoxin enhances tissue factor and suppresses thrombomodulin expression of human vascular endothelium in vitro. J. Clin. Investig. 1987;79:124–130. doi: 10.1172/JCI112772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roth RI, Kaca W, Levin J. Hemoglobin: A newly recognized binding protein for bacterial endotoxins (LPS) Prog. Clin. Biol. Res. 1994;388:161–172. [PubMed] [Google Scholar]
- 57.Kaca W, Roth RI, Levin J. Hemoglobin, a newly recognized lipopolysaccharide (LPS)-binding protein that enhances LPS biological activity. J. Biol. Chem. 1994;269:25078–25084. doi: 10.1016/S0021-9258(17)31501-6. [DOI] [PubMed] [Google Scholar]
- 58.Roth RI. Hemoglobin enhances the production of tissue factor by endothelial cells in response to bacterial endotoxin. Blood. 1994;83:2860–2865. doi: 10.1182/blood.V83.10.2860.2860. [DOI] [PubMed] [Google Scholar]
- 59.Winek K, Dirnagl U, Meisel A. The gut microbiome as therapeutic target in central nervous system diseases: Implications for stroke. Neurotherapeutics. 2016;13:762–774. doi: 10.1007/s13311-016-0475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The corresponding author has access to all the data. The data will be made available upon written request.