Highlights
-
•
Radiotherapy may alter silicone shell permeability and predispose gel bleeding.
-
•
Adjuvant radiotherapy to silicone implants resulted in earlier detected complications on BMRI.
-
•
Radiotherapy and silicone implants are associated to a new disease in patients cured from breast cancer.
Keywords: Radiotherapy, Silicone, Breast implant, Granuloma
Abstract
Breast reconstructive surgery with silicone implants is routinely one of the techniques performed immediately after a mastectomy and before adjuvant radiotherapy. Implant shell degradation may result in gel bleeding that can trigger capsular disease. The silicone corpuscle in contact with the implant fibrous capsule can promote an inflammatory reaction, identified as silicone-induced granuloma which is, related to clinical complaints referred to as breast implant illness.
This short communication aims to demonstrate and discuss the impact of radiotherapy's side effects on patients with post-mastectomy reconstructive breast surgery with silicone implants followed by adjuvant radiation therapy.
Introduction
Breast reconstruction surgery (BRS) procedures, delayed or immediate, have been rising since the beginning of the century. The use of silicone implants and the improvement of surgical techniques contributed to offer these stablished procedures as an alternative for postmastectomy for patients with breast cancer diagnosis. Post mastectomy followed by immediate breast reconstructive surgery and adjuvant radiation therapy is an option [1]. Radiotherapy is recommended to reduce locoregional recurrence in this patient group [2], [3]. The American Society of Surgical Oncology (SSO) and the American Society of Radiation Oncology (ASTRO) guideline recommends adjuvant radiotherapy in patients with T1-2 tumors and positive 1–3 axillary lymph nodes [1]. It is also recommended for patients with T1-2 stage tumors with one positive sentinel node, without axillary dissection [2], [3], [4]. Radiotherapy reduces the local recurrence of the tumor and increases the survival rate [3], [4], [5].
The primary complications of RT reported by patients are skin reactions, breast edema, local pain, alopecia, sore throat, fatigue, lymphedema, breast morphological changes (shape, size, and color), tenderness in the ribs. Late side effects refer to: thickening of the skin and subcutaneous tissue, vessels alterations like telangiectasias, and cardiovascular and lung effects [6]. There are few published studies in the medical literature regarding the possible effects of radiotherapy concerning breast implants. Some studies indicate that radiotherapy is harmless to silicone implants, while others have reported complications related to the procedure [7], [8]. However, there seems to be no evidence that correlates silicone implant complications with adjuvant radiotherapy.
Among the main complications reported in BRS with silicone implants, capsular contracture, gel leakage, and the implants' rupture are the most reported [7]. Studies speculate that capsular contracture process results from the organism’s reaction to the foreign body in a multifactorial process [9]. Capsular contracture can cause pain, distortion of the breast, and unsatisfactory aesthetic results. The pathological mechanism for capsular contracture remains uncertain.
In our service, postmastectomy patients with immediate breast reconstructive surgery and who meet the adjuvant RT indication criteria are referred for adjuvant radiotherapy of the surgical site. On average, patients are treated with 25–28 radiation therapy sessions accumulated dose ranging from 45.0 to 50.0 Gy.
After adjuvant radiotherapy sessions, patients with clinical complaints, at our institution are referred for further investigation by magnetic resonance imaging (BMRI) for better diagnostic interpretation. Our patients' most frequent clinical complaints reported after radiotherapy is breast enlargement and hardening, skin rash, joint pain, and inflammatory changes in the irradiated breast.
Recently, we demonstrated that silicone's extravasation in macroscopic intact breast implants could be diagnosed by BMRI [10]. We describe the intracapsular finding of extravasated silicone as silicone induced granuloma of breast implant capsule (SIGBIC). The diagnosis of SIGBIC is performed adopting three diagnostic criteria: intracapsular mass with high signal in the T2-weighted sequence, a late enhancement to intravenous contrast, and black-drop signal. The BMRI granuloma image reflects the granulation tissue formed by the fibrous capsule's reaction to the gel bleeding [10], [11].
Our published manuscripts discussed that gel bleeding and SIGBIC were generally related to permeability loss of breast implants, pericapsular edema, and intracapsular collection. The Magnetic Resonance signals that allow diagnosing the implants' permeability loss are water-droplets (when there are foci of signal change inside the implants) and double-gel sign (when a double gel signal is observed inside the silicone implants). SIGBIC is considered an unequivocal diagnosis finding of silicone leakage, while water- droplet, pericapsular edema, and intracapsular collection are considered equivocal findings. The implant permeability loss has clinical significance because it infers the potential toxicity risk of silicone leakage into the intracapsular space. The primary pathogen particle of silicone implants is polydimethylsiloxane 4 (PDMS4), the smallest molecular structure of silicone, with the most significant toxicity potential (see Figs. 1 and 2). Our study also observed that patients diagnosed with SIGBIC were generally accompanied by the clinical symptoms reported in breast implant illness.
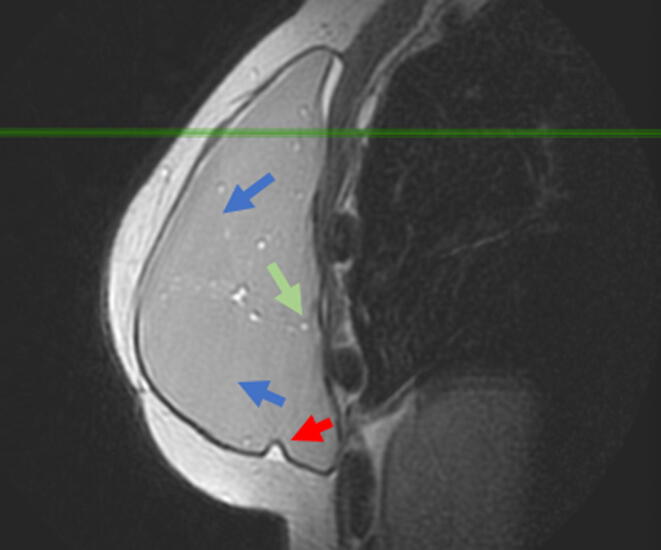
Fig. 1.
Sagittal proton density image of the right breast. 45 women with breast cancer oncologic reconstruction with silicone breast implant (Allergan 510 MX) and latissimus dorsi myocutaneous flap for 3 years. In the image, the water droplets (green arrow) and the double gel (blue arrow) are shown. There is also segmentary thickening of the myocutaneous flap (red arrow) and intracapsular collection (withe arrow). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
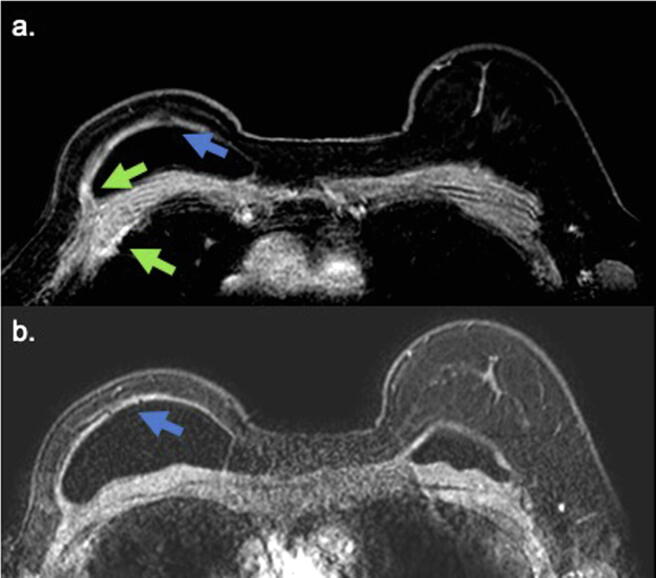
Fig. 2.
Axial pos contrast sequence of the same patient. The blue arrow shows the black-drop signal and enhancement of the myocutaneous flap associated with pericapsular edema. These findings are compatible with infiltrative extension of the capsular granuloma. Inflammatory changes in the myocutaneous FLAP is also observed (green arrow). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
This study aims to determine the impact of adjuvant radiotherapy on patients with immediate breast reconstructive surgery post mastectomy detected on BMRI.
Materials and methods
Since the beginning of the year 2017, we have started a research protocol in our service, a referral cancer center, to research breast implants' complications using BMRI. The study's initial objective was to research the frequency of breast implant-associated anaplastic large cell lymphoma (BIA-ALCL). However, the results showed the prevalence of complications related to gel leakage and clinical symptoms related to this leakage, described as silicone induced granuloma of breast implant capsule.
This manuscript aims to evaluate the first follow-up BMRI assessment findings of patients with clinical complaints related to silicone breast implants treated with BRS from May/2017 to March/2019. Patients who underwent adjuvant radiotherapy were compared with those with no adjuvant RT. The association between the time to perform the MRI study after the surgical procedure, the implant position (sub-pectoral or pre-pectoral), the signs of implant permeability loss (water-droplet and double-gel), pericapsular edema, and SIGBIC were compared with patients who underwent adjuvant radiotherapy and who did not expose to radiation therapy. We further compared the association between the time to perform the MRI study after the surgical procedure, the implant position (sub-pectoral or pre-pectoral), the signs of implant permeability loss (water-droplet and double-gel), pericapsular edema, and exposure to adjuvant therapy with patients with and without SIGBIC diagnosis in BMRI images.
The Pearson χ2 test or Fischer exact test was used to analyze categorical variables, and an unpaired, 2-tailed t test or Mann-Whitney test was used to analyze continuous variables.
Statistical analyses were conducted with MedCalc Statistical Software version 16.4.3 (MedCalc Software bv, Ostend, Belgium), and a 2-sided P < .05 was deemed to be statistically significant. Data analysis was performed from November 15, 2020, to December 3, 2020.
Results
Table 1, Table 2 shows our study results, in which 173 patients were evaluated, 68 without RT, and 105 with adjuvant RT.
Table 1.
Characteristics of the study population, patients with and without radiotherapy.
| No. (%) |
||||
|---|---|---|---|---|
| Characteristic | Overall | Without radiotherapy | With radiotherapy | P value |
| No. of cases | 173 | 68 | 105 | |
| Age, mean (SD), 95% CI, y |
49.4 (10.2) 47.936–51.011 |
49.9 (10.3) 47.400–52.424 |
49.1 (10.2) 47.217–51.164 |
0.652 |
| First magnetic resonance scan | ||||
| Time from surgery (years) | 3.7 (3.8) 3.142–4.302 |
4.1 (4.7) 2.962–5.272 |
3.4 (3.1) 2.858–4.074 |
0. < 001 |
| Location | ||||
| Subglandular | 27 (15.6) | 7 (10.3) | 20 (19.0) | 0.122 |
| Subpectoral | 146 (84.4) | 61 (89.7) | 85 (81.0) | |
| Silicone induced granuloma of breast implant capsule (SIGBIC) | ||||
| Without SIGBIC | 61 (35.3) | 32 (47.1) | 29 (27.6) | 0.0092 |
| With SIGBIC | 112 (64.7) | 36 (52.9) | 76 (72.4) | |
| Pericapsular edema | ||||
| Without edema | 153 (88.4) | 62 (91.2) | 91 (86.7) | 0.366 |
| With edema | 20 (11.6) | 6 (8.8) | 14 (13.3) | |
| Intracapsular collection | ||||
| Without collection | 152 (87.9) | 62 (1.2) | 90 (85,7) | 0.284 |
| With collection | 21 (12.1) | 6 (8.8) | 15 (14.3) | |
| Implant permeability loss (water droplet and double gel) | ||||
| Without | 90 (52.0) | 36 (52.9) | 54 (51.4) | 0.846 |
| With | 83 (48) | 32 (47.1) | 51 (48.6) | |
Table 2.
Characteristics of the study comparing patients with and without SIGBIC.
| No. (%) |
|||||
|---|---|---|---|---|---|
| Characteristic | Overall | Without SIGBIC | WITH SIGBIC | P value | |
| No. of cases | 173 | 61 | 112 | ||
| FIRST magnetic resonance scan | |||||
| Time from surgery | Without radiation | 4.12 (4.77) 2.062–5.272 |
4.09 (4,79) 2.365–5,822 |
4.138 (4.817) 2.508–5,768 |
0.984 |
| With radiation | 3.466 (3.141) 2.858–4.704 |
4,00 (3.305) 2.742–5.257 |
3.263 (3.704) 2.560–3.965 |
0.001 | |
| P value | 0.05 | 0.001 | |||
| Location | |||||
| Subglandular | 27 (15.6) | 8 (13.1) | 19 (17.0) | 0.507 | |
| Subpectoral | 146 (84.4) | 53 (86.9) | 93 (83.0) | ||
| Implant permeability loss (water droplet and double gel) | |||||
| Without | 90 (52.0) | 51 (83.6) | 39 (34.8) | <0.0001 | |
| With | 83 (48.0) | 10 (16.4) | 73 (65.2) | ||
| Pericapsular edema | |||||
| Without edema | 153 (88.4) | 59 (96.7) | 94 (83.9) | <0.001 | |
| With edema | 20 (11.6) | 2 (3.3) | 18 (16.1) | ||
| Intracapsular collection | |||||
| Without collection | 142 (82.1) | 57 (93.4) | 95 (84.8) | 0.098 | |
| With collection | 21 (17.9) | 4 (6.6) | 17 (15.2) | ||
| Radiation | |||||
| Without | 68 (39.3) | 32 (52.5) | 36 (32.1) | 0.008 | |
| With | 105 (60.7) | 29 (47.5) | 76 (67.9) | ||
All patients included in the study had capsular contracture. For this reason, we decided to exclude the capsular contracture data in the data evaluation.
The mean time for BMRI was 3.7 years, where patients with adjuvant RT having earlier scans, 3.4 vs. 4.1 years, with a statistically significant P (0.001). Most patients had a submuscular implant (84.4%). Among the breast implants reported complications related to RT, the only statistically relevant association was the SIGBIC (0.0092). 76 (72.4%) of the patients who underwent RT presented the three unequivocal diagnostic criteria. RT patients showed an even higher rate of pericapsular edema (13.3% vs. 8.8%), intracapsular collection (14.3% vs. 8.8%), and permeability loss of breast implant (48.6% vs. 47.1%), but with no “true” difference.
As a complementary assessment, we determined the other MRI findings associated with the SIGBIC formation. Patients with SIGBIC had a higher rate of breast implant permeability loss (65.2% vs. 16.4%) and pericapsular edema (16.1% vs. 3.1%). Although the intracapsular collection is more associated with SIGBIC (15.2% vs. 6.6%), this difference was not statistically significant.
When assessing the association between the examination time with SIGBIC presence and radiotherapy's performance, the patients who were diagnosed with SIGBIC and underwent radiotherapy were those who performed the procedure earlier, 3.2 years, which was statistically significant.
Discussion
Our results show that the association of BRS with silicone breast implants and adjuvant radiotherapy are related to silicone implants complications on BMRI images. Among the complications observed, the silicone gel bleeding with the intracapsular silicone granuloma formation is the most frequent. These changes are often related to surgical approaches, unsatisfactory aesthetic results, locoregional complications, and systemic symptoms, in which the breast implant illness (BII) stands out.
Complications related to silicone implants impacts patients' outcomes. These patients, usually cured of breast cancer, start to live with a new disease resulting from the primary cancer treatment. A human-made disease replaces the constitutional disease. It is common to hear statements in clinical practice that we should prevent patient mutilation from breast cancer treatment. However, what is the primary purpose in cancer treatment, cancer eradication, or aesthetic results? The answer is not binary and trivial. We should evaluate each case individually to propose the best therapeutic options. However, it seems not logical to trigger a new and complex disease in a patient healing from breast cancer.
According to the latest Food and Drug Administration (FDA) BI screening recommendations update, a BMR is recommended for symptomatic patients at any time postoperatively [12]. In our previous published studies, we associated SIGBIC with BII [10]. SIGBIC is the radiological marker of BII, related to gel bleeding/shell deterioration from macroscopic intact implants. BMRI may show indirect signs of implant permeability loss such as black drop-signal, water-droplet, and double-gel. The inflammatory process and the fibrous capsule's silicone corpuscles could invade the contiguous muscle plane and cause edema/pericapsular infiltration. This process makes the complications surgical treatment challenging as it is necessary to en bloc removal of the diseased capsule. When there is a residual fibrous capsule in the surgical site, local inflammatory processes' recurrence can be observed.
This new human-made disease has a direct impact on the patient's care. The patient cured of the primary neoplasia are often referred to complementary exams for the cancer treatment complications diagnosis. Patients are subjected to BMRI and US, percutaneous diagnostic biopsies, and new surgical procedures. Moreover, the psychological impact of the new disease should not be ignored.
In addition to the patient recovery outcome, there is a public health impact. The implant-related complications diagnosis and treatment represent additional costs that are not included in the breast cancer standard treatment. These expenses are expendable. Our results have some limitations. The main limitation is that the patients underwent an MRI study in the postoperative period as indicated by the requesting physician, without pre-established criteria to perform the scan. Our data were obtained opportunistically instead of organized. However, an organized BMRI annual screening study to assess complications related to breast implants would require the paramagnetic agent's injection, which risks would not justify the benefit of the study. This study only evaluated the variables associated with radiotherapy and SIGBIC.
Our study's data show an association between silicone breast implant complications in cancer patients undergoing adjuvant radiation therapy. The side effects of radiation on breast implants and the surgical site's complications should be exhaustively discussed in the therapeutic management of breast cancer patients eligible for conservative treatments.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Eduardo de Faria Castro Fleury, Email: eduardo.fleury@prof.saocamilo-sp.br.
Jose Carlos Vendramini Fleury, Email: zkfleury@uol.com.br.
References
- 1.Smith B.D., Bellon J.R., Blitzblau R., Freedman G., Haffty B., Hahn C. Radiation therapy for the whole breast: Executive summary of an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Practical Radiation Oncol. 2018;8(3):145–152. doi: 10.1016/j.prro.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 2.Salerno K.E. NCCN guidelines update: evolving radiation therapy recommendations for breast cancer. J Natl Comprehensive Cancer Network : JNCCN. 2017;15(5S):682–684. doi: 10.6004/jnccn.2017.0072. [DOI] [PubMed] [Google Scholar]
- 3.Lo Torto F., Relucenti M., Familiari G., Vaia N., Casella D., Matassa R. The effect of postmastectomy radiation therapy on breast implants: material analysis on silicone and polyurethane prosthesis. Ann Plast Surg. 2018;81(2):228–234. doi: 10.1097/SAP.0000000000001461. [DOI] [PubMed] [Google Scholar]
- 4.Frasier L.L., Holden S., Holden T., Schumacher J.R., Leverson G., Anderson B. Temporal trends in postmastectomy radiation therapy and breast reconstruction associated with changes in national comprehensive cancer network guidelines. JAMA Oncology. 2016;2(1):95–101. doi: 10.1001/jamaoncol.2015.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lo Torto F., Parisi P., Casella D., Di Taranto G., Cigna E., Ribuffo D. Impact of evolving radiation therapy techniques on implant-based breast reconstruction. Plast Reconstr Surg. 2018;141(1):182e–183e. doi: 10.1097/PRS.0000000000003972. [DOI] [PubMed] [Google Scholar]
- 6.Chang J.M., Kosiorek H.E., Dueck A.C., Casey W.J., Rebecca A.M., Mahabir R. Trends in mastectomy and reconstruction for breast cancer; a twelve year experience from a tertiary care center. Am J Surg. 2016;212(6):1201–1210. doi: 10.1016/j.amjsurg.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 7.Berry M.G., Cucchiara V., Davies D.M. Breast augmentation: Part II—Adverse capsular contracture. J Plastic, Reconstructive & Aesthetic Surg : JPRAS. 2010;63(12):2098–2107. doi: 10.1016/j.bjps.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Ribuffo D., Lo Torto F., Giannitelli S.M., Urbini M., Tortora L., Mozetic P. The effect of post-mastectomy radiation therapy on breast implants: Unveiling biomaterial alterations with potential implications on capsular contracture. Mater Sci & Eng C, Mater Biol Appl. 2015;57:338–343. doi: 10.1016/j.msec.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Magill L.J., Ricketts K., Keshtgar M., Mosahebi A., Jell G. Impact of post mastectomy radiotherapy on the silicone breast implant. Mater Sci Eng C, Mater Biol Appl. 2019;98:288–292. doi: 10.1016/j.msec.2018.12.047. [DOI] [PubMed] [Google Scholar]
- 10.Fleury E. Silicone Induced Granuloma of Breast Implant Capsule (SIGBIC) diagnosis: Breast Magnetic Resonance (BMR) sensitivity to detect silicone bleeding. PLoS ONE. 2020;15(6) doi: 10.1371/journal.pone.0235050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Faria Castro Fleury E, D’Alessandro GS, Lordelo Wludarski SC. Silicone-induced granuloma of breast implant capsule (SIGBIC): Histopathology and radiological correlation. J Immunology Res, 2018, 2018, 6784971. https://doi.org/10.1155/2018/6784971 [DOI] [PMC free article] [PubMed]
- 12.Food and Drug Administration. Breast Implants - Certain Labeling Recommendations to Improve Patient Communication - Guidance for Industry and Food and Drug Administration Staff (fda.gov). https://www.fda.gov/media/131885/download. Accessed 25 February 2021.