Abstract
Background/aims
The aim of this study is to share autopsy findings of COVID-19-positive cases and autopsy algorithms for safely handling of suspicious bodies during this pandemic.
Methods
COVID-19-positive cases of Istanbul Morgue Department were retrospectively analyzed. Sampling indications for PCR tests in suspicious deaths, macroscopic and microscopic findings obtained in cases with positive PCR tests were evaluated.
Results
In the morgue department, 345(25.8%) of overall 1336 autopsy cases were tested for COVID-19. PCR test was found positive in 26 cases. Limited autopsy procedure was performed in 7 cases, while the cause of death was determined by external examination in the remaining 19 cases. Male-to-female ratio was found 3.3:1 and mean age was 60.0 ± 13.6 among all PCR-positive cases. Cause of death was determined as viral pneumonia in fully autopsied cases. Most common findings were sticky gelatinous fluid in cavities and firm and swollen lungs, varying degrees of consolidation. In microscopy, diffuse alveolar epithelial damage, type-II pneumocyte hyperplasia, hyaline membrane formation, fibrinous exudate, and fibrinous plaques in the alveoli were the most common findings.
Conclusions
In COVID-19 autopsies, pulmonary findings were found to be prominent and the main pathology was pneumonia. Older age and findings of chronic diseases indicate that the cases were in the multirisk group in terms of COVID-19 mortality.
Keywords: Autopsy, COVID-19, Pulmonary findings, Risk factors
Introduction
Several coronaviruses (human coronaviruses) belonging to zoonotic human coronavirus family resulted in severe respiratory tract infections globally in recent times. Despite the fact that six types of human coronaviruses were defined, this family gained particular attention by Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS) [1, 2].
Coronavirus family is an enveloped, helical symmetry, positive-polar RNA virus family that causes disease in mammals and birds. In humans, they cause respiratory infections ranging from mild cold to pneumonia and rarely gastrointestinal complaints [3]. Although the history of coronaviruses began in the 1940s, the first human coronaviruses, HCoV229E and HCoV-OC43, were reported in the 1960s. After 2000s, HKU1, HCoV-NL63, SARS-CoV, and MERSCoV were shown as other members of coronavirus infecting humans [4]. The first four CoVs mentioned are universally constantly in circulation and are causative agents of about one-third of common colds in humans. SARSCoV and MERS-CoV differ from other coronaviruses in that they cause severe lung infections, and the fatality rates are 10% and 34%, respectively [5].
In December 2019, a group of patients with a new type of coronavirus was reported to be detected in Wuhan, China. International Committee of Taxonomy of Viruses (ICTV) named the new virus as SARS-CoV-2 [6].
After documented first cases in China, the infection has rapidly spread across the world and WHO declared “pandemic” on the 11th day of March. The first confirmed case of SARS-CoV-2 infection in Turkey was reported on March 10, 2020 and first mortality resulting from this infection on March 15. Though the infection has spread across the country after the report of first case, at the beginning, Istanbul was reported to have about two-thirds of total positive cases. Istanbul was one of the epicenters of the pandemic in Turkey.
Council of Forensic Medicine is an official organ of Ministry of Justice and provides expertise in medico-legal issues all over the country. Almost all Forensic autopsies are performed in the facilities of the council by its experienced specialists all over the country. Center Morgue Department (Istanbul) of CFM is well-developed and well-equipped facility having a special autopsy room that meets the standards recommended by the Centers for Disease, Control, and Prevention (CDC) for autopsies of COVID-19-positive patients [7]. Until establishment of “Airborne Infection Isolation Rooms” for autopsy facilities in other 5 provinces of the country, all COVID-19-positive autopsy cases were transferred to Istanbul for performing autopsies.
We aimed to share our autopsy findings of COVID-19-positive cases and algorithms developed for safely handling of suspicious bodies during this pandemic.
Materials and methods
This study was carried out with the approval of the Council of Forensic Medicine Scientific Committee and special permission of Ministry of Health.
Case selection and data collection
Autopsy records of Istanbul Morgue Department of the Council of Forensic Medicine during the COVID-19 pandemic (between February 2020 and June 2020) were retrospectively analyzed.
The algorithms of the Department for proper handling of bodies during pandemic were also evaluated together with the sampling indications for COVID-19 in suspicious deaths.
In PCR-positive cases, indications for autopsy or determination of cause of death without autopsy were discussed. In addition, macroscopic and microscopic findings and toxicological results obtained in cases with positive PCR test were evaluated.
Autopsy procedure and sample collection
During the COVID-19 pandemic, all autopsies were performed with a minimally invasive autopsy approach, and tracheal swab and lung swab samples were collected in addition to nasopharyngeal swab samples collected prior to autopsy. Histopathological samples were taken at autopsy, fixed in 10% formalin solution, and examined under light microscope after hematoxylin and eosin staining. Immunohistochemical investigations could not be performed due to lack of the supply of antibodies in the markets at the early stages of the pandemic. In addition, body fluid samples (blood, intraocular fluid, bile, and urine), liver and kidney tissue samples, and stomach contents were collected accordingly in suitable bodies and toxicological examination was performed.
In accordance with the law, all forensic autopsies were carried out by opening three cavities of the body. Unlike the standard autopsy technique, in minimally invasive autopsy technique, no organ or system was eviscerated except for the central nervous system and the heart, although it varies according to the case. Noneviscerated organs were evaluated by an in situ technique.
In cases considered as “possible COVID-19,” a deep nasopharyngeal swab sample was collected prior to autopsy. During the autopsy, the tracheal swab sample was collected as shown in Fig. 1. Postmortem approach algorithm is shown in Fig. 2.
Fig. 1.
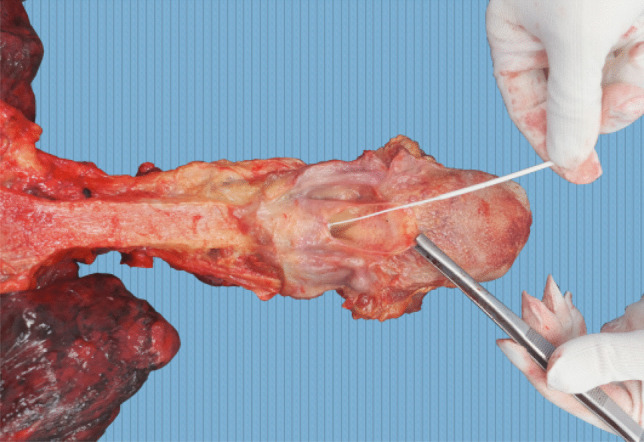
Tracheal swab collection
Fig. 2.
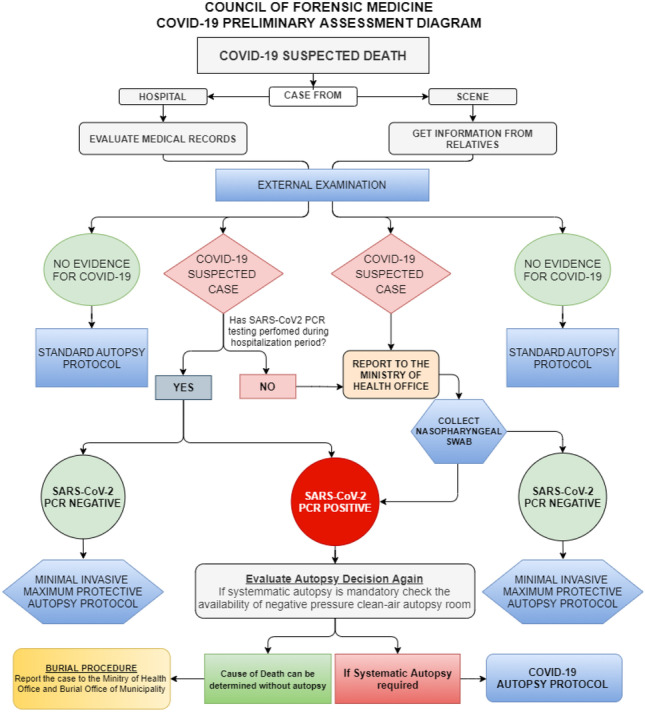
COVID-19 preliminary assessment diagram of Council of Forensic Medicine
SARS-CoV-2 RNA real-time PCR
In order to determine SARS-CoV-2 in nasopharyngeal/tracheal swab samples and paraffin embedded lung tissue samples, real-time RT-PCR method, which is a nucleic acid amplification method that detects viral RNA, was applied. Nucleic acids were extracted on the QIASymphony (Qiagen/Germany) device using the “QIAsymphony DSP Virus/Pathogen midi” kit. “RealStar® SARS-CoV-2 RT-PCR Kit RUO (Altona Diagnostics, Hamburg, Germany)” was used by RT-PCR method and amplified in Rotor Gene (Qiagen/Germany) device in accordance with the manufacturer’s recommendations.
In addition, nucleic acids were extracted from all paraffin-embedded lung tissues fixed with formalin. Three or four 10-mm-thick sections of each block were cut by a microtome. The xylene and ethyl alcohol deparaffinization procedure was applied to paraffin embedded lung tissues. It was then incubated for 24 h by adding 20 mL of proteinase K and 600 mL of ATL buffer solution [8].
Autopsy environment
Autopsy room of Istanbul Morgue Department has negative pressure ventilation system with Hygienic Air Handling Units, performing 15 air changes per hour. Air in the autopsy room exhausted outside through a high efficiency particulate aerosol (HEPA) filter and also clean air flow supplied through a HEPA filter. Negative-pressure autopsy rooms were designed in accordance with the criteria defined by CDC as airborne infection isolation rooms (AIIRs) [7].
Results
In Turkey, the first case was reported on March 10, 2020, and the first COVID-19-related death occurred on March 15, 2020. The 2019-nCoV-RNA real-time PCR test was first started on February 22, 2020 in the Istanbul Morgue Department and continued to be applied as a routine examination on selected cases.
Between February 22, 2020 and June 9, 2020, 345 of 1336 autopsy cases were tested for COVID-19. A total of 348 cases were tested, three of which being tested on lung tissue samples collected at autopsy. In 26 (7.5%) cases, 2019-nCoV-RNA real-time PCR test was found positive. Limited autopsy procedure was performed in 7 cases with positive test results, and in the remaining 19 cases, the cause of death was determined by a detailed external examination together with the data obtained from death scene examination and medical records.
Male-to-female ratio was found 3.3:1 among the PCR test–positive cases. Most common age group was 41–70 age group. Mean age was 60.0 ± 13.6 among PCR-positive cases. Demographic features are shown in Table 1.
Table 1.
Demographic features of cases
| SARS-CoV-2-RNA real-time PCR | Positive | Negative | Total | |
|---|---|---|---|---|
| Sex | ||||
| Male | 20 | 254 | 274 | |
| Female | 6 | 68 | 74 | |
| Total | 26 | 322 | 348 | |
| Age groups | ||||
| Infant | 0 | 6 | 6 | |
| 11–20 years | 0 | 8 | 8 | |
| 21–30 years | 0 | 28 | 28 | |
| 31–40 years | 1 | 47 | 48 | |
| 41–50 years | 6 | 65 | 71 | |
| 51–60 years | 6 | 76 | 82 | |
| 61–70 years | 6 | 50 | 56 | |
| 71–80 years | 5 | 28 | 33 | |
| 81–90 years | 1 | 12 | 13 | |
| 91–100 years | 1 | 2 | 3 | |
| Mean age | ||||
| Male | 58.2 ± 10.2 | 51.1 ± 17.3 | ||
| Female | 67.6 ± 23.1 | 49.3 ± 20.2 | ||
| All | 60.0 ± 13.6 | 50.7 ± 17.9 | ||
Cause of death was determined as viral pneumonia (COVID-19) in 14 cases, blunt trauma in 4 cases (three fall from height and one road transportation injury), and hanging in one case among the cases in which cause of death was determined without autopsy (Table 2). In all autopsied cases, cause of death was determined to be viral pneumonia (COVID-19) (Table 3).
Table 2.
Cases in which the causes of death were determined without autopsy
| Gender Age |
Clinical findings | Co-morbidity | Previous COVID-19 diagnosis | Incident | PCR test at hospital after death | Cause of death |
|---|---|---|---|---|---|---|
|
Male 59 |
Cough, dyspnea, vomiting | Hepatitis B | No | Felt sick and taken to the hospital | Yes, positive | Viral pneumonia (COVID-19) |
|
Female 84 |
Weakness and dyspnea 18 days ago | No known chronic disease | Positive | Felt sick and died at home | No | Viral pneumonia (COVID-19) |
|
Male 58 |
No | No | No | Car crash | No | Blunt trauma |
|
Male 45 |
Cough | Hypertension | No | Felt sick and taken to the hospital | No | Viral pneumonia (COVID-19) |
|
Male 62 |
Not known | No known chronic disease | No | Felt sick and taken to the hospital | No | Viral pneumonia (COVID-19) |
|
Male 61 |
Not known | No known chronic disease | No | Fall from height | No | Blunt trauma |
|
Male 53 |
Not known | Diabetes | No | Found dead at home | No | Viral pneumonia (COVID-19) |
|
Female 40 |
Chest pain | No known chronic disease | No | Felt sick and died at home | Yes, positive | Viral pneumonia (COVID-19) |
|
Male 74 |
Not known | No known chronic disease (mental retardation) | No | Found dead at home (decomposition) | No | Viral pneumonia (COVID-19) |
|
Male 71 |
Not known | Dementia | No | Found dead at nursing home | No | Viral pneumonia (COVID-19) |
|
Male 45 |
Fever, cough | No known chronic disease | No | Found dead at home | No | Viral pneumonia (COVID-19) |
|
Male 71 |
Weakness, cough | Chronic Bronchitis | No | Found dead at home | No | Viral pneumonia (COVID-19) |
|
Male 49 |
Fever | Chronic Pulmonary disease | No | Fall from height | No | Blunt trauma |
|
Male 64 |
No | No known chronic disease | Positive | Hanging | No | Hanging |
|
Male 73 |
CT findings | Chronic heart and liver diseases, diabetes | Test negative | Felt sick and taken to the hospital | No | Viral pneumonia (COVID-19) |
|
Female 72 |
Flu-like symptoms | Hypertension, bedridden | No | Felt sick and died at home | Yes, positive | Viral pneumonia (COVID-19) |
|
Male 43 |
Fever, cough, dyspnea | Not known | No | Felt sick and died at home | No | Viral pneumonia (COVID-19) |
|
Female 94 |
CT findings | Bedridden | Not known | Felt sick and died at home | No | Viral pneumonia (COVID-19) |
|
Male 55 |
Hospitalized for COVID-19 | Not known | Yes | Fall from height | No | Blunt trauma |
Table 3.
PCR test–positive autopsied cases
| No. | Gender Age |
Clinical findings | Co-morbidity | PCR during hospitalization | COVID-19 diagnosis | Incident | Duration of the disease | Cause of death |
|---|---|---|---|---|---|---|---|---|
| 1 |
Male 68 |
No data | Hypertension, Diabetes | No | No | Felt sick and taken to the hospital, death in the hospital | No data | Viral infection (COVID-19) |
| 2 |
Male 63 |
Hypotension, X-ray findings | Hypertension | Yes, positive | Yes | Prisoner—died in hospital | Death occurred 4 days after the onset of symptoms | Viral infection (COVID-19) |
| 3 |
Male 67 |
CT findings | Rheumatic disease | Yes, negative | No | Found dead | No data | Viral infection (COVID-19) |
| 4 |
Female 48 |
Dyspnea, weakness | No data | No | No | Felt sick and taken to the hospital, death in the hospital | Death occurred 7 days after the onset of symptoms | Viral infection (COVID-19) |
| 5 |
Male 43 |
ICU admission—dead at ICU | Nonspecific pulmonary diseases | Yes, positive | Yes | Prisoner—died in hospital | Death occurred 11 days after the onset of symptoms | Viral infection (COVID-19) |
| 6 |
Male 53 |
Pulmonary symptoms (lung cancer also) | Lung cancer | Yes, positive | Yes | Malpractice claim—lung cancer false operation claim | Death occurred 5 days after the onset of symptoms | Viral infection (COVID-19) |
| 7 |
Male 53 |
ICU patient due to renal failure—uremic encephalopathy | Renal failure (hemodialysis patient) | Yes, negative (3 days ago) | No | Died in airplane (coming to Turkey for renal transplantation) | No data | Viral infection (COVID-19) |
In one case, since the PCR test was negative in the nasopharyngeal swab taken during the examination of the body at the time of investigation by prosecutor prior to the arrival of the body to the autopsy unit, no other PCR test was performed before the autopsy. However, due to the suspicious appearance in the lungs at autopsy, the PCR test was performed on paraffin embedded lung tissue and was found positive. In all other cases, positive results were obtained in PCR tests performed before autopsy, and autopsies were initiated by taking precautions against known risks. All test results are shown in Table 4.
Table 4.
2019-nCoV-RNA real-time PCR tests after death
| No. | PCR test before prosecutor examination | PCR test before autopsy | PCR test during autopsy | PCR test to paraffin embedded tissue |
|---|---|---|---|---|
| 1 | Yes, negative | No | No | Yes, positive |
| 2 | Yes, positive | Yes, positive | Yes, positive | Yes, positive |
| 3 | No | Yes, positive | Yes, positive | Yes, negative |
| 4 | No | Yes, positive | Yes, positive | Yes, positive |
| 5 | No | Yes, positive | Yes, positive | Yes, positive |
| 6 | No | Yes, positive | Yes, positive | No |
| 7 | No | Yes, positive | Yes, positive | No |
At autopsy, pleural adhesions were observed except for two cases. There was an accumulation of sticky gelatinous fluid in the chest cavity, plastered on the visceral pleura in two of the cases (Fig. 3). In all cases, the lungs filled the chest cavity and were swollen and tight. Two cases had unilateral and five cases had bilateral firm lungs. There was no sign of major thromboembolism in the pulmonary truncus. In all cases, areas of consolidation of varying degrees were noted in lung sections.
Fig. 3.
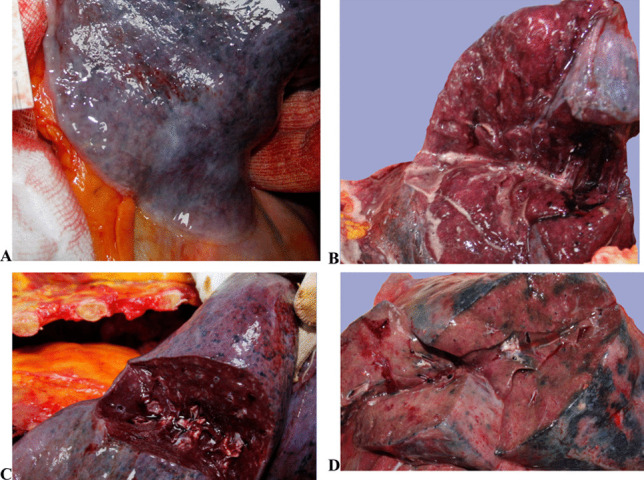
In situ macroscopical lung findings. A Sticky gelatinous fluid on the contours of the lungs. B Excessive edema. C Dilated vessels and consolidation on the cut surfaces. D Consolidation, red hepatization
At autopsy, samples were taken from the lungs, one from each lobe, and embedded in paraffin blocks. Routine histopathological examination on hematoxylin and eosin staining procedure was performed and examined under a light microscope. Regardless of the duration of the disease, patchy or diffuse interstitial lymphocytic infiltration of viral pneumonia and diffuse alveolar epithelial damage (DAD) in various stages was detected in lung tissues of all 7 cases. Prominent hyaline membrane formation, type II pneumocyte hyperplasia, fibrinous exudate, or fibrinous plaques in the alveoli were seen as the components of DAD (Fig. 4). Fibrin thrombi were seen in the vascular lumens in three of the cases. Two cases were found to have squamous metaplasia in lung samples.
Fig. 4.
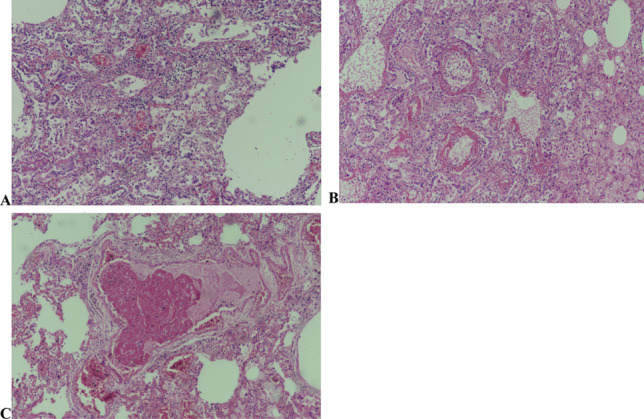
A Type II pneumocyte hyperplasia with reactive cytologic atypia. B Alveoli wall covered with hyaline membranes and type II pneumocytes. C Fibrin thrombi in vessel lumens
Symptoms of acute phase DAD were detected in patients with short disease duration, while in the patient with a disease duration of 11 days, findings of organized phase DAD and presence of intra-alveolar necrotic material due to viral cytopathic effect were detected (Fig. 5). The findings were consistent with acute lung injury in the acute phase depending on the duration of the disease. Autopsy findings are summarized in Tables 5 and 6.
Fig. 5.
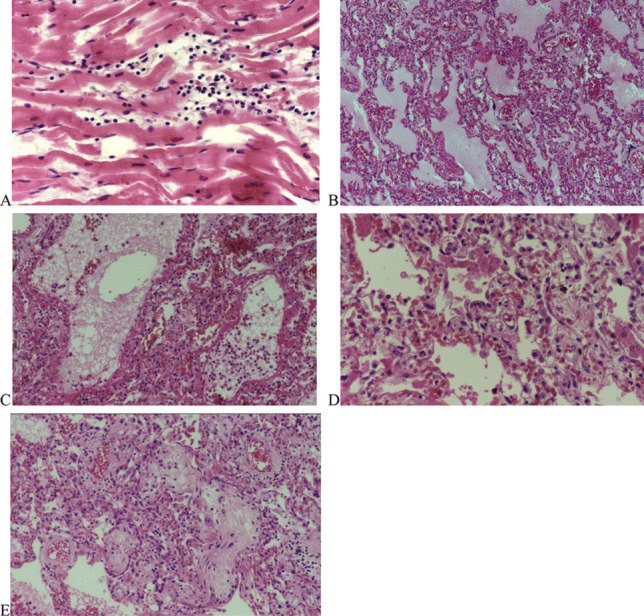
Case #2. A Focal lymphocytic infiltration of myocardium, without myocyte necrosis (H&E, × 400). B DAD with hyaline membrane formation, paucicellular interstitial lymphocytic infiltration, edema and congestion, no microvascular thrombosis (H&E, × 200). C Granulocytic infiltration of focal bronchopneumonia associated with DAD (H&E, × 400). D Prominent proliferation of type II pneumocytes, subacute phase of DAD (H&E, × 400). E Fibrotic plugs in airspaces, subacute phase of DAD (H&E, × 400)
Table 5.
Gross autopsy findings
| No. | Gender Age |
Height/weight (BMI) | Thorax cavity | Pleural effusion | Heart weight | Pulmonary truncus | Lung consistency | Lung surfaces | Lung slices | Other |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
Male 68 |
179 cm/130 kg (40.6) | Pleural adhesions | No | 490 g | No thrombi | Bilateral firm |
Anthracotic pigmentation Fibrous pleural thickening |
Edema Consolidation |
No specific finding |
| 2 |
Male 63 |
179 cm/94 kg (29.3) | No finding | No | 512 g | No thrombi |
Firm left lung |
Subpleural petechiae Covered with gelatinous sticky fluid |
Edema Consolidation Vessel dilatation |
Polycystic kidneys |
| 3 |
Male 67 |
167 cm/80 kg (28.7) | Pleural adhesions | Small amount Serous fluid | 357 g | No thrombi | Bilateral firm | Anthracotic pigmentation |
Edema Consolidation |
Sub-acute subdural hematoma |
| 4 |
Female 48 |
158 cm/73 kg (29.2) | Pleural adhesions | No | 374 g | No thrombi |
Firm right lung |
Anthracotic pigmentation |
Edema Consolidation |
Trachea filled with foamy edema fluid |
| 5 |
Male 43 |
175 cm/93 kg (30.4) | Left side pleural adhesions | 480 mL serous fluid at left side | 300 g | No thrombi | Bilateral firm | Covered with gelatinous sticky fluid |
Edema Consolidation |
Trachea mucosa covered with mucoid infectious fluid |
| 6 |
Male 53 |
180 cm/98 kg (30.2) | Pleural adhesions | No | 483 g | No thrombi | Bilateral firm | Excessive pleural adhesions |
Edema Consolidation (Hepatization) |
Operation scars on the right lung Space occupying lesion on right lung (filled with hematoma) Submucosal hemorrhages on trachea |
| 7 |
Male 53 |
179 cm/84 kg (26.2) | No finding | 400 mL purulent effusion at right side | 338 g | No thrombi | Bilateral firm | Fibrous pleural thickening |
Edema Consolidation |
Trachea mucosa covered with foamy mucoid infectious fluid |
BMI body mass index
Table 6.
Histopathologic findings (H&E staining)
| No. | Gender Age |
Heart | Pulmonary | Other | |||||
|---|---|---|---|---|---|---|---|---|---|
| DAD | Hyaline membranes | Type II pneumocyte hyperplasia | Pneumonia | Thrombi | Other | ||||
| 1 |
Male 68 |
Moderate perivascular and interstitial fibrosis | + | + | + | Lobular | Fibrin thrombi in large and medium calibrated vessels | Squamous metaplasia |
Liver: moderate steatosis Kidney: chronic pyelonephritis CNS: hyperemia |
| 2 |
Male 63 |
Mild perivascular fibrosis Hypertrophy Focal lymphocytic infiltration without myocytes necrosis |
+ | + | + | Interstitial | - |
Focal bronchopneumonia excessive edema Fibrotic plugs in airspaces |
None |
| 3 |
Male 67 |
Focal myocardial scarring Perivascular and interstitial fibrosis Hypertrophy |
+ | + | + | Interstitial | - |
Excessive edema, Patchy sparse inflammation |
Liver: grade II steatosis Duramater: organizing hematoma (1 to 3 months) |
| 4 |
Female 48 |
Edema, congestion | + | + | + | Interstitial | Fibrin thrombi in pulmonary vessels | Edema | Thyroid: nodular hyperplasia |
| 5 |
Male 43 |
Focal scarring on papillary muscle | + | + | + | Interstitial | Fibrin thrombi in pulmonary vessels |
Squamous metaplasia Viral cytopathic effect (necrotic material in alveoli) |
Liver: mild steatosis Olfactory tract: severe corpora amylacea Deposits |
| 6 |
Male 53 |
Mild perivascular fibrosis | + | + | + | Interstitial | - | Pleuritis with purulent fibrin | Liver: mild steatosis |
| 7 |
Male 53 |
Granulation tissue Myocardial scarring |
+ | + | + | Lobular | - | - |
Liver: Von Meyenburg complex (biliary microhamartoma) Kidney: chronic pyelonephritis, nodular glomerulosclerosis |
DAD diffuse alveolar damage, CNS central nervous system
Discussion
COVID-19 is a viral acute respiratory infection reported to affect many systems in the body [9]. After the epidemic was declared as a pandemic, academic studies were published by many centers around the world and information was shared. Publications sharing the clinical hypotheses and treatment recommendations regarding the pathogenesis of the disease, diagnostic criteria of the disease from a propedeutics perspective, personal protective measures in terms of biosafety, and findings of autopsy in terms of public health protective measures and forensic medicine have taken their places in the literature.
Although the necessity of autopsy for the diagnosis of SARS-CoV 2 is discussed by some authors [10], in autopsy studies, useful information was presented in revealing the pathological findings of the disease and discussing the pathophysiology of the disease. In the commentary they wrote, Pomara et al. defended the lack of autopsy data of the disease and the necessity of postmortem sampling and autopsy in revealing the pathophysiology [11]. Moreover, as Edler et al. stated, in order to keep the statistical data of COVID-19 deaths accurate, appropriate cases should be autopsied [12].
In autopsy cases and clinical case series, bilateral ground-glass appearance, and alveolar opacities on thoracic computed tomography, typical ARDS findings such as diffuse alveolar damage, pulmonary edema, formation of hyaline membranes, type II pneumocyte hyperplasia, lymphadenopathy, pleural effusion, and microthrombi in small vessels are frequently reported respiratory system findings in histopathological examination. In contrast to SARS-CoV infection, computed tomography has also detected pleural effusion, lymphadenopathy, and cystic changes [9, 13–18].
In the study of Wichmann et al., postmortem computerized tomography was performed to the cases and demonstrated reticular infiltrations and bilateral consolidating infiltrates [19]. In the review of Lai et al., it was reported that bilateral lung involvement was prominent in terms of findings detected by imaging methods in SARS-CoV-2 pneumonia, and the most common finding in computed tomography examination was the ground-glass appearance [20]. Computed tomography findings were not directly evaluated as specific for COVID-19 or as diagnostic alone [16]. It has been shown that cardiomegaly, epicarditis/pericarditis, and myocarditis are prominent findings in the cardiovascular system, which is the other system frequently involved. Clinical findings of cardiovascular system involvement have also been reported as acute myocardial damage, arrhythmias, ST segment changes, and troponin elevation. In addition to the lung and heart, liver, brainstem, and kidney involvements have also been reported [9].
General views on pathogenesis are that COVID-19 infection targets the respiratory system, causing pneumonia and cardiovascular complications. Although clinical findings include fever, dry cough, rhinorrhea, sore throat, weakness, headache, loss of smell, hypoxemia, dyspnea, hemoptysis, diarrhea, lymphopenia, and increase in plasma proinflammatory cytokines [9, 15, 18, 21, 22], acute respiratory distress syndrome, cardiac pathologies, and thromboembolism have been blamed for the severe clinical course [9, 15]. In the COVID-19 case series presented with central nervous system complications (stroke) published by Avula et al., they reported that hypercoagulability and microthrombi formation caused by the disease may be responsible for the pathogenesis. In addition, it has been discussed that the virus directly invades the central nervous system and that hypoxia may also be effective [23]. Although the name of the COVID-19 epidemic was defined as SARS-CoV-2 (severe acute respiratory syndrome), as in previous coronavirus outbreaks, it was argued that the virus did not only affect the respiratory system but also could have central nervous system involvement. Nervous system findings such as headache, dizziness, confusion, disturbances in consciousness, ataxia, acute cerebrovascular disease, and loss of smell have been reported. Seizures have also been reported in some publications. Nervous system involvement is defined as a poor prognosis criterion for COVID-19 [24, 25].
Thromboembolism is frequently emphasized in studies discussing the pathogenesis in various clinical presentations [13, 23, 26]. Lodigiani et al. reported that thromboembolic complications developed in 7.7% of their cases in their study in which they investigated the frequency of venous thromboembolism in COVID-19 patients hospitalized in a university hospital in Italy [27]. Increased d-dimer level and fibrin degradation products shown in deceased cases indicate coagulation activity resulting in thromboembolism [9, 26]. In addition to the increase in coagulation activity, it is argued that virus-induced endotheliitis in endothelial cells may also play a role in thrombotic events in organs [9] which are probably shown as one of the main targets of the virus [26].
Similar to SARS-CoV, ACE2 (angiotensin converting enzyme) receptors in the lung have been defined as functional receptors for SARS-CoV-2 [2, 28]. SARS-CoV-2 virus binds directly to the ACE2 receptor, which is a cellular receptor, and it is thought to enter the cell via this receptor [13, 15, 24]. ACE2 receptor is an ectoenzyme attached to the plasma membrane and is found in many tissues such as the lower respiratory tract, heart, kidney, and gastrointestinal tract [2]. It is thought that it acts as ACE2 as a result of binding to the receptor, causing an increase in angiotensin II protein and the release of reactive oxygen derivatives accordingly. It has been reported that hypoxia and complement activity were caused by the inability to restrict blood flow to damaged vessels in the lung, and the influence of coagulation pathways plays an important role in the pathogenesis [13]. It is claimed that hypercoagulopathy and changes in coagulation pathways are associated with inflammatory response [26, 29].
In the autopsies of Istanbul, the cases were evaluated by the sampling criteria recommended by the Ministry of Health and modified by the Morgue Department in terms of death cases. Only three cases had a positive PCR test during hospitalization. This has shown the importance of correctly determining the sampling criteria. The SARS-CoV-2-RNA real-time PCR test was performed on nasopharyngeal swabs obtained at the Morgue Department before autopsy in two cases who had suspicious CT findings during the hospitalization period but were reported to be negative for the PCR test, and positive results were obtained. It is thought that many factors, from sampling methods to test methods, as well as the ability of the test to detect positive cases, may play a role. While evaluating the indications for sampling, as well as the standard criteria of the Department, anamnesis questioning the clinical findings, suspicious contacts, and symptoms is of great importance.
Macroscopically and microscopically lung pathologies were prominent in Istanbul autopsies. Regardless of the duration of the disease, it was observed that there was a viscous firmness in the lungs in all cases, and the lungs were covered with sticky gelatinous material in two cases. Consolidation was observed in lung sections in all cases.
COVID-19 has often been defined as a systemic infection involving the pulmonary and cardiovascular system. It has been reported that inflammatory mechanisms and immune system play a role in this involvement [9, 30]. Cytokine storm syndrome with immune system irregularities is thought to exacerbate interstitial pneumonia resulting in diffuse alveolar damage and cause viral sepsis with hypercoagulability, thus creating a vicious cycle [31].
Pleurisy, pericarditis, diffuse or patchy lung consolidation, congestion, and pulmonary edema have been reported among the gross lung findings. It has been reported that there may be an increase in weight in the lungs or that the weight may be within normal limits [18, 19, 32–34]. Hypercoagulability, pulmonary thrombotic microangiopathy, deep vein thrombosis, and pulmonary thromboemboli, which have been reported as having important role in pathogenesis, were the most frequently reported findings in COVID-19 related deaths. While pulmonary intravascular coagulation occurs due to the inflammatory response, it has also been reported that dilatation develops in the right heart cavities due to pulmonary hypertension arising from infected alveolar ACE2 cells [29]. Fibrotic phase is rarely observed in cases with short disease periods in terms of pulmonary findings [33].
Barton et al. reported patchy and sparse interstitial inflammation of the lung in one of the Oklahoma cases. A similar patchy sparse inflammation was detected in one of Istanbul’s COVID-19 autopsies (Fig. 6c). In the same case, a negative result was obtained from the PCR test performed on paraffin embedded lung tissue. Although bronchopneumonia is accepted as a finding of secondary infection, which is one of the criteria for poor prognosis, a focal bronchopneumonia focus was found in one of the Istanbul cases similarly. As in the New Orleans cases reported by Fox et al., no finding indicating secondary pulmonary infection was found in the other 6 cases in Istanbul [34].
Fig. 6.
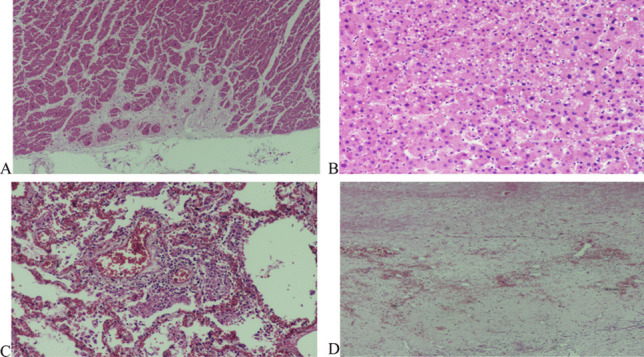
Case #3. A Focal area of myocardial scar (H&E, × 200). B Steatosis of Hepatocytes (H&E, × 200). C Patchy lymphocytic interstitial infiltration and fibrinous exudate in the alveoli. This image was taken from one of the few areas where inflammation was obvious. In other areas, infiltration was sparse or absent (H&E, × 400). D Organizing subdural hemorrhage (H&E, × 200)
Considering the pathological findings of Istanbul autopsies, although the time from the onset of symptoms to death is variable, findings indicating that the cases are in different stages of ARDS have been identified. Although diffuse alveolar damage was detected in all cases, type II pneumocyte hyperplasia was accompanied by squamous metaplasia in only two cases. There was no information about the duration of the disease in one of these two cases; the other died after 11 days of the disease. Also, in this case, the presence of necrotic material in the alveoli, which indicates viral cytopathic effect, was evaluated as a sign of the subacute phase of ARDS. However, in a case known as the disease period of 4 days, fibrotic plugs were observed, indicating subacute diffuse alveolar damage period. Although it is thought that the presence of bronchopneumonia, which is considered as a secondary infection in the same case, may be effective in the emergence of this finding, it is thought that the staging of lung findings may be influenced by other personal risk factors, regardless of the duration of the disease.
When evaluated in terms of thromboembolism, which is an important criterion in poor prognosis and mortality, no thrombus was observed in the pulmonary truncus and main pulmonary vessels in Istanbul autopsies, but fibrin thrombi or fibrinous deposits were observed in histopathological examination. There was no evidence of major pulmonary thromboses like the cases reported by Grimes et al. [35]. Fox et al. similarly reported that there was no thrombus in the main pulmonary vessels in their cases [34]. Edler et al. reported that 40% of the cases had deep vein thrombosis and 9 cases had pulmonary thromboembolism in their studies on 80 cases autopsied in Germany with positive COVID-19 tests. Considering that the vast majority of these cases were hospitalized or cared for in a nursing home, it was thought that immobilization during the illness could also be effective in the formation of thromboembolism [12]. Pulmonary microthromboembolism was not also detected in any of the autopsy cases of Istanbul. This finding has shown that in COVID-19 cases of Istanbul, thromboembolism involving small vessels is more prominent than microthrombus or large pulmonary vessel thromboembolism.
Like the lungs, it has been observed that the heart is one of the organs involved in COVID-19 to varying degrees. Common cardiac findings have been reported to be cardiomegaly and right ventricular enlargement [18, 34]. It has been argued that the presence of lymphocytes together with degenerated myocytes in rare areas may be a sign of the early stages of viral myocarditis [19, 34]. Although no findings suggestive of myocarditis were detected in any of the Istanbul cases, focal lymphocytic infiltration in the myocardium without myocardial necrosis was detected in the patient with bronchopneumonia. In six of the Istanbul COVID-19 autopsies, nonspecific chronic ischemia findings accompanied by varying degrees of coronary artery stenosis in the heart were detected. These cardiac findings indicate an underlying chronic heart disease which is one of the poor prognosis criteria rather than the direct effect of viral pneumonia.
As a conclusion, similar with the other published studies, pulmonary histopathological findings include edema, proteinous exudate, vascular congestion, inflammatory deposits accompanied by fibrinoid material, multinuclear giant cells, interstitial edema, reactive alveolar epithelial hyperplasia, fibroblastic proliferation, type II pneumocyte proliferation, and varying spectrum from acute respiratory distress syndrome (ARDS) to diffuse alveolar membrane damage [9, 13–15, 34, 36, 37]. It has been reported that there is diffuse alveolar damage with exudates especially in the case whose ground-glass appearance was described in radiological examination [32]. Viral particles in the pneumocyte cytoplasm were demonstrated in electron microscopy studies [33, 37].
Among the reported cases, it has been argued that the detection of pathology suggesting cardiac Kawasaki disease with inflammatory involvement in the vessels and especially in the coronary arteries is not caused by viral alveolitis but due to the immune system response [29, 38].
In COVID-19-related deaths, it has been reported that the presence of some chronic diseases besides COVID-19 pneumonia is a poor prognosis criterion and increases mortality. These are stated as chronic lung diseases, coronary artery diseases, arterial hypertension, hepatitis C, renal failure, malignancies, and obesity [12, 13, 18, 19, 22]. In addition, pregnancy, one of the clinical groups that increase the susceptibility to thromboembolism, is stated to be in the risky group. In Istanbul cases, one patient has hypertension and diabetes, one has pulmonary cancer, one another has renal failure requiring hemodialysis, and one has a history of undiagnosed nonspecific lung disease. Medical histories of the cases whose cause of death was determined as viral infection (COVID-19) without autopsy revealed that one of these cases had hepatitis B, one had diabetes, two had hypertension and one had chronic bronchitis, one had diabetes with chronic heart and liver diseases, and two of them were bedridden patients. One patient had dementia and one had mental retardation. These cases were considered to be in the high-risk group for COVID-19 deaths. In other cases, no other chronic disease was identified that would put it in the risk group, except chronic cardiovascular disease and hypertension.
The age of 60 is cited as an important determinant for mortality. In the early stages of the pandemic, approximately 80% of COVID-19-related deaths were reported to be seen in cases over 60 years of age, and in later periods, this age limit was updated to 65 years and over [39]. It has also been reported that the period from the onset of symptoms to death is shorter over the age of 70 [15]. The risk of serious disease picture increases with age in addition to mortality [18, 39]. In the study in which Northern Italy COVID-19 autopsies were reported, the male to female ratio of cases was 6.6:1; the average age was reported to be 69 [33]. Considering all cases with positive PCR test results in the Istanbul Morgue Department, the most frequent case group was 41–70 age group and the average age was 58.2 ± 10.2 for males and 67.6 ± 23.1 for females (Table 1).
In conclusion, in autopsies of COVID-19 PCR-positive cases, pulmonary findings were prominent, and the main pathology was pneumonia; diffuse alveolar epithelial damage in the lung, type II pneumocyte hyperplasia, and hyaline membrane formation were found to be present in all cases regardless of the duration of the disease. Chronic ischemic cardiovascular disease findings in cases, lung cancer in one case, and chronic renal failure requiring hemodialysis in one case indicate that the cases were in the risk group in terms of COVID-19 mortality.
Declarations
Ethics approval
The study was approved by the Ministry of Health of Turkey and of Forensic Medicine Scientific Committee.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Murat Nihat Arslan, Email: mnarslan@yahoo.com.
Yalçın Büyük, Email: doctorbuyuk@gmail.com.
Nihan Ziyade, Email: nihanziyade@gmail.com.
Neval Elgörmüş, Email: neyelgormus@yahoo.com.
Gözde Şirin, Email: gorucug@yahoo.com.
İsmail Çoban, Email: doktorcoban@gmail.com.
Muhammed Emin Gökşen, Email: emingoksen@gmail.com.
Taner Daş, Email: tanerdas@hotmail.com.
Arzu Akçay, Email: arzuakcay12@gmail.com.
References
- 1.Yin Y, Wunderink RG. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. 2018;23(2):130–137. doi: 10.1111/resp.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petrosillo N, Viceconte G, Ergonul O et al (2020) COVID-19, SARS and MERS: are they closely related? Clin Microbio Infect [DOI] [PMC free article] [PubMed]
- 3.De Groot RJ, Baker S, Baric RS, Enjuanes L, Gorbalenya AE, Holmes KV, Perlman S, Poon LLM, Rottier P, Talbot PJ, Woo PCY, Ziebuhr J. Family Coronaviridae. In: King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ, editors. Virus taxonomy. Ninth Report of the International Committee on Taxonomy of Viruses. Academic Press, International Union of Microbiological Societies Virology Division: Elsevier; 2012. pp. 806–817. [Google Scholar]
- 4.Pillaiyar T, Meenakshisundaram S, Manickam M. Recent discovery and development of inhibitors targeting coronaviruses. Drug discovery today. 2020;25(4):668–688. doi: 10.1016/j.drudis.2020.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahase E. Coronavirus: covid-19 has killed more people than SARS and MERS combined, despite lower case fatality rate. BMJ. 2020;368:m641. doi: 10.1136/bmj.m641. [DOI] [PubMed] [Google Scholar]
- 6.Gorbalenya AE, Baker SC, Baric RS et al (2020) The species severe acute respiratory syndrome related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol 5:536-544 [DOI] [PMC free article] [PubMed]
- 7.CDC - COVID-19 (2020) Guidance postmortem specimens. Collection and submission of postmortem specimens from deceased persons with known or suspected COVID-19. Cent Dis Control Prev
- 8.Chan P, Chan D, To K, Yu MY, Cheung J, Cheng AF. Evaluation of extraction methods from paraffin wax embedded tissues for PCR amplification of human and viral DNA. Journal of clinical pathology. 2001;54:401–403. doi: 10.1136/jcp.54.5.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buja LM, Wolf DA, Zhao B, Akkanti B, McDonald M, Lelenwa L, Reilly N, Ottaviani G, Elghetany MT, Trujillo DO, Aisenberg GM, Madjid M, Kar B. The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID-19): Report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities. Cardiovascular Pathology. 2020;48:107233. doi: 10.1016/j.carpath.2020.107233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sapino A, Facchetti F, Bonoldi E et al (2020) The autopsy debate during the COVID-19 emergency: the Italian experience. Virchows Archiv:1 [DOI] [PMC free article] [PubMed]
- 11.Pomara C, Li Volti G, Cappello F. COVID-19 deaths: are we sure it is pneumonia? Please, autopsy, autopsy, autopsy! Journal of Clinical Medicine. 2020;9(5):1259. doi: 10.3390/jcm9051259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edler C, Schröder AS, Aepfelbacher M et al (2020) Dying with SARS-CoV-2 infection—an autopsy study of the first consecutive 80 cases in Hamburg, Germany. Int J Legal Med 1 [DOI] [PMC free article] [PubMed]
- 13.Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, Baxter-Stoltzfus A, Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Translational Research. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carbone M, Green JB, Bucci EM, Lednicky JA. Coronaviruses: facts, myths, and hypotheses. J Thorac Oncol. 2020;15(5):675–678. doi: 10.1016/j.jtho.2020.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rothan HA, Byrareddy SN (2020) The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun 102433 [DOI] [PMC free article] [PubMed]
- 16.Shi H, Han X, Jiang N et al (2020) Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis [DOI] [PMC free article] [PubMed]
- 17.Sessa F, Bertozzi G, Cipolloni L et al (2020) Clinical-forensic autopsy findings to defeat COVID-19 disease: a literature review. J Clin Med 9(7):2026 [DOI] [PMC free article] [PubMed]
- 18.Menter T, Haslbauer JD, Nienhold R, Savic S, Hopfer H, Deigendesch N, Frank S, Turek D, Willi N, Pargger H. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77(2):198–209. doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wichmann D, Sperhake JP, Lütgehetmann M et al (2020) Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med [DOI] [PMC free article] [PubMed]
- 20.Lai CC, Shih TP, Ko W-C et al (2020) Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and corona virus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents 105924 [DOI] [PMC free article] [PubMed]
- 21.Tian S, Hu N, Lou J et al (2020) Characteristics of COVID-19 infection in Beijing. J Infect [DOI] [PMC free article] [PubMed]
- 22.Schaller T, Hirschbühl K, Burkhardt K, Braun G, Trepel M, Märkl B, Claus R. Postmortem examination of patients with COVID-19. JAMA. 2020;323(24):2518–2520. doi: 10.1001/jama.2020.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Avula A, Nalleballe K, Narula N, Sapozhnikov S, Dandu V, Toom S, Glaser A, Elsayegh D. COVID-19 presenting as stroke. Brain, Behavior, and Immunity. 2020;87:115–119. doi: 10.1016/j.bbi.2020.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mao L, Wang M, Chen S et al (2020) Neurological manifestations of hospitalized patients with COVID-19 in Wuhan, China: a retrospective case series study. https://www.medrxiv.org/content/. 10.1101/2020.02.22.20026500v1. Accessed 29 June 2020
- 25.Asadi-Pooya AA, Simani L. Central nervous system manifestations of COVID-19: a systematic review. Journal of the Neurological Sciences. 2020;413:116832. doi: 10.1016/j.jns.2020.116832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benhamou D, Keita H, Ducloy-Bouthors AS. Coagulation changes and thromboembolic risk in Covid-19 obstetric patients. Anaesthesia Critical Care & Pain Medicine. 2020 doi: 10.1016/j.accpm.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lodigiani C, Iapichino G, Carenzo L et al (2020) Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan Italy Thrombosis research [DOI] [PMC free article] [PubMed]
- 28.Maiese A, Manetti AC, La Russa R et al (2020) Autopsy findings in COVID-19-related deaths: a literature review. Foren Sci Med Pathol 1-18 [DOI] [PMC free article] [PubMed]
- 29.McGonagle D, Plein S, O'Donnell JS et al (2020) Increased cardiovascular mortality in African Americans with COVID-19. Lancet Respir Med [DOI] [PMC free article] [PubMed]
- 30.Falasca L, Nardacci R, Colombo D, Lalle E, Di Caro A, Nicastri E, Antinori A, Petrosillo N, Marchioni L, Biava G. Postmortem findings in Italian patients with COVID-19: a descriptive full autopsy study of cases with and without comorbidities. J Infect Dis. 2020;222(11):1807–1815. doi: 10.1093/infdis/jiaa578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maiese A, Passaro G, Matteis AD et al (2020) Thromboinflammatory response in SARS-CoV-2 sepsis. 88(2):78-80 [DOI] [PubMed]
- 32.Hanley B, Lucas SB, Youd E, Swift B, Osborn M. Autopsy in suspected COVID-19 cases. Journal of clinical pathology. 2020;73(5):239–242. doi: 10.1136/jclinpath-2020-206522. [DOI] [PubMed] [Google Scholar]
- 33.Carsana L, Sonzogni A, Nasr A et al (2020) Pulmonary post-mortem findings in a large series of COVID-19 cases from Northern Italy. medRxiv:2020.2004.2019.20054262. 10.1101/2020.04.19.20054262 [DOI] [PMC free article] [PubMed]
- 34.Fox SE, Akmatbekov A, Harbert JL, Li G, Quincy Brown J, Vander Heide RS. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. The Lancet Respiratory Medicine. 2020 doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grimes Z, Bryce C, Sordillo EM, Gordon RE, Reidy J, AE PM, Fowkes M, Fatal pulmonary thromboembolism in SARS-CoV-2-infection. Cardiovascular pathology: the official journal of the Society for Cardiovascular Pathology. 2020;48:107227–107227. doi: 10.1016/j.carpath.2020.107227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tian S, Hu W, Niu L, Liu H, Xu H, Xiao S-Y. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. Journal of Thoracic Oncology. 2020;15(5):700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yao X, Li T, He Z et al (2020) A pathological report of three COVID-19 cases by minimally invasive autopsies. Zhonghua bing li xue za zhi. Chinese J Pathol 49:E009-E009 [DOI] [PubMed]
- 38.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. The Lancet. 2020;395(10237):1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.CDC COVID-19 Response Team (2020) Severe outcomes among patients with coronavirus disease 2019 COVID-19)—United States, February 12–March 16, 2020. . MMWR Morb Mortal Wkly Rep. 2020;69(12):343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]


