Abstract
Viral respiratory infections are a common cause of severe disease, especially in infants, people who are immunocompromised, and in the elderly. Neutrophils, an important innate immune cell, infiltrate the lungs rapidly after an inflammatory insult. The most well-characterized effector mechanisms by which neutrophils contribute to host defense are largely extracellular and the involvement of neutrophils in protection from numerous bacterial and fungal infections is well established. However, the role of neutrophils in responses to viruses, which replicate intracellularly, has been less studied. It remains unclear whether and, by which underlying immunological mechanisms, neutrophils contribute to viral control or confer protection against an intracellular pathogen. Furthermore, neutrophils need to be tightly regulated to avoid bystander damage to host tissues. This is especially relevant in the lung where damage to delicate alveolar structures can compromise gas exchange with life-threatening consequences. It is inherently less clear how neutrophils can contribute to host immunity to viruses without causing immunopathology and/or exacerbating disease severity. In this review, we summarize and discuss the current understanding of how neutrophils in the lung direct immune responses to viruses, control viral replication and spread, and cause pathology during respiratory viral infections.
Introduction
Neutrophils, the most abundant cell type in the blood in humans, are a fundamental component of the innate immune response. It is estimated that each day 1 billion neutrophils are produced per kilogram of body weight and that this can increase to 10 billion during an infection.1 At steady state, developing neutrophils reside in the bone marrow, while mature neutrophils are released into the circulation and rapidly recruited into affected tissues in response to infection or injury. Neutrophils are short-lived, although their precise life span is debated.2,3 As the most abundant and short-lived cell in the circulation, neutrophil turnover must be tightly regulated during both homeostasis and disease.
The role of neutrophils in host immunity is well described during bacterial and fungal infections. However, neutrophils are also detected in the lungs and/or bronchoalveolar lavage (BAL) of mice, rats, and humans after infection with respiratory viruses including human metapneumovirus (HMPV),4–6 human respiratory syncytial virus (HRSV; herein referred to as RSV),7–11 coronavirus,12–17 rhinovirus,18–22 measles,23 pneumonia virus of mice (PVM;24) mouse adenovirus type I,25 adenovirus 7,26 mouse cytomegalovirus (MCMV;27), and influenza A virus (IAV; reviewed in ref. 28). During such viral infections, where the pathogen replicates intracellularly, it is less clear whether neutrophil recruitment and activation benefit the host by contributing to host defense or whether their presence is a bystander effect of local inflammation and contributes to tissue damage and disease.29,30
Neutrophils in human respiratory viral disease
Neutrophils are present in the lungs during acute respiratory distress syndrome, which can be induced by many different pathogens including many viruses (see table in ref. 28), as well as trauma and autoimmunity. Many studies suggest that neutrophil recruitment to the lungs is associated with disease severity during viral infections. For example, in infants with severe RSV-induced bronchiolitis, neutrophils can make up > 90% of the cellular composition of the BAL10,11 and therefore neutrophils have been implicated as drivers of disease pathogenesis.10,11,31,32 Also, in both rhinovirus and hMPV-infected children as well as in severe cases of influenza and SARS-CoV-2 infection, lung neutrophils and their markers have been observed to be elevated.16,33–37 Furthermore, whole-blood transcriptomic analyses have shown that genes related to neutrophil function and activation were among the overexpressed genes in infants hospitalized with RSV,38 in severely ill patients hospitalized during the 2009 IAV pandemic,39 and on the 1st day of hospitalization in patients that will require intensive care during SARS-CoV-2 infection.40 As neutrophil elevation is so commonly observed clinically during severe respiratory viral infections it is easy to speculate that their recruitment to the lung and further activation can enhance tissue pathology and contribute to disease. However, studying the causality of neutrophils in the human lung is challenging and therefore more detailed investigations into the function and role of neutrophils in viral respiratory disease have been performed in animal models. Many animal models of respiratory viral infections replicate the notable recruitment of neutrophils to the lung, and neutrophils are abundant in the airways and lungs of mice, calves, and ferrets during infections with RSV, IAV, coronaviruses, HMPV, and PMV.4,7,9,17,24,41–44 Here we discuss both the beneficial and detrimental effects of neutrophils during respiratory viral infections mostly from animal studies but also from human observations where data are available.
Neutrophil recruitment
In the lungs of both mice and humans, a population of neutrophils resides in the pulmonary vasculature and perivascular space at steady state.45,46 It is thought that these neutrophils are retained in the lung actively by upregulation of the chemokine receptor CXCR4, which binds CXCL12, a ligand expressed by a subset of lung endothelial cells.47 The role of these resident lung neutrophils is not well understood, but it has been suggested that they localize in the pulmonary vasculature in order to be able to mount a rapid response to pulmonary pathogens.45 Nonetheless, a defining feature of neutrophils is their ability to infiltrate tissues early and rapidly during infections or sterile injury. For neutrophil recruitment to occur, pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) must trigger their respective receptors to initiate the production of pro-inflammatory mediators and neutrophil chemoattractants.48,49 Major neutrophil chemoattractants include interleukin-8 (IL-8; only in humans), CXCL1 (KC), CXCL2 (MIP2-α), CXCL5, complement component 5a (C5a), N-formylmethionine-leucyl-phenylalanine (fMLP), platelet activating factor, and leukotriene B4 (LTB4).48,50–52 During inflammation, neutrophil transmigration to the affected tissue occurs in a step-wise process known as the leukocyte adhesion cascade.3,53 Many neutrophil chemoattractants are produced in the lungs and airways during viral infections, for example, CXCL1, CXCL2 and IL-17,7,17,25,28,50,54–57 resulting in neutrophil infiltration into the lungs of mice and ferrets.7,8,41,58–60 During RSV infection in mice this infiltration to the lungs is transient as neutrophils peak at 18 h postinfection (p.i.) and are almost absent by 36 h p.i.7–9,61 This is different during severe mouse IAV infection, in which neutrophils chemoattractants such as CXCL1 and neutrophils remain in the lungs for a longer time and are still detectable at day 12 p.i., especially when a highly pathogenic IAV strain is used.62–66 This would suggest that a sustained neutrophil recruitment and/or presence in the lungs is associated with more severe disease (Fig. 1).
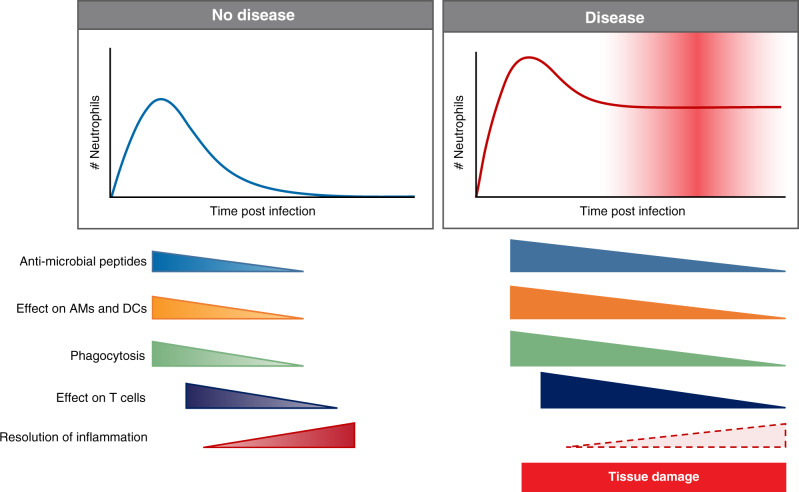
Fig. 1. Neutrophils in disease.
During a respiratory viral infection, neutrophils are recruited to and activated in the lung. In non-symptomatic or mild disease, neutrophil numbers peak early during infection and neutrophils exert their effector functions and aid in tissue repair and resolution of inflammation. In a severe infection, more neutrophils are recruited over a longer period. This results in more tissue damage and a delay or block in resolution of inflammation and tissue repair.
PAMPs from the virus, or from the infection process, are recognized by pattern recognition receptors (PRRs), through which signaling is crucial to initiate the inflammatory response.67–71 During RSV infection, neutrophil recruitment to the lung is dependent on MyD8872,73 or MyD88/TRIF7,74 signaling, which occurs downstream of a class of PRRs known as the toll-like receptors (TLRs), as well as cytokine receptors of the IL-1R family. Mavs−/− mice (unable to signal via cytosolic PRRs of the RIG-I-like family, which detect PAMPs predominantly associated with RNA viruses) do recruit neutrophils to the lung early after RSV infection, albeit fewer than wild-type mice.7 MyD88 signaling is also essential for neutrophil recruitment to the lung during IAV infection64,75,76 and during infection with mouse-adapted SARS-CoV.77 The role of TLR3 in recruitment of neutrophils to the lung during IAV infection is somewhat controversial as IAV-infected Tlr3−/− mice have shown reduced,64 increased,78 or similar76 neutrophil infiltration compared to wild-type mice. As different viral strains and different time points were used this will be important to study in detail in future studies as the magnitude and timing of the neutrophil responses might be dependent on which PRRs that are used for induction of the neutrophil attractants. Viral proteins can also contribute to the regulation of neutrophil recruitment. For example, during HMPV infection the attachment glycoprotein is involved in neutrophil infiltration.79
The key cell types in which PRR signaling takes place to induce production of neutrophil chemoattractants, or cytokines that can induce chemoattractants, appear to vary between pathogens. Signaling in non-hematopoietic cells was shown to be required for neutrophil recruitment during IAV infection.64 Furthermore, during RSV infection non-epithelial cells (ATII cells), non-endothelial, lung stromal cells were shown to be important for Cxcl1 induction in a MyD88/TRIF signaling dependent manner.7 The precise source of neutrophil chemoattractants during viral infection is an important research avenue for future directed therapies to reduce neutrophilic inflammation.
During RSV infection of both mouse and man, there are two temporally distinct waves of neutrophil-attracting chemokines. The first wave of chemokines is produced early after infection and then a later induction occurs concurrently with the peak of disease symptoms.56,80 Interestingly, neutrophil infiltration also occurs in two distinct waves during IAV infection of ferrets.60 External factors that regulate neutrophil-attracting chemokines are not yet well understood but it is not thought that the circadian rhythm influences neutrophil infiltration to the lung during IAV infection.81 In RSV-infected infants, neutrophils are the predominant cell type in the BAL.10 However, this is at a later stage of infection when children are admitted to the hospital with symptoms. When studied early after RSV infection in the neonatal mouse model relatively few neutrophils infiltrate the lungs.82,83 Furthermore, Cxcl1 is not induced in lungs of neonatal mice after RSV infection.84 However, Cxcl1, CXCL2, and neutrophils are detected in the lung tissue and airways of neonatal mice after IAV infection.84,85 If the relatively reduced neutrophil recruitment to the lower airways in the early stages of RSV infection of neonatal mice is also a phenomena of RSV infection in infants is not yet clear. Sampling infants prior to symptoms is extremely challenging and therefore rarely done except for perhaps in birth cohort studies, which can include longitudinal nasal sampling, virus detection, and symptom scoring. Therefore, little is known about the early stages of disease caused by respiratory viral infection in children so far and more studies are needed. Overall, neutrophils commonly infiltrate the lungs of both humans and animals during all respiratory viral infections but interestingly both the magnitude and timing of neutrophil infiltration is dependent on the type of infection studied.
Neutrophil priming and activation
Activation is essential for neutrophils to exert their full antimicrobial functions and contribute to host defense (Fig. 2). Historically, neutrophils were considered as unsophisticated responder cells, yet it is increasingly evident that the role of neutrophils in inflammation is more complex than has previously been appreciated. Neutrophils can respond differentially to harmful stimuli,86,87 interact with other arms of the immune response,88 and can also have roles in wound healing and resolution of inflammation.89 Neutrophil activation is a process that occurs over time, starting already during recruitment.90 However, in order to become fully activated to degranulate and be capable of undergoing oxidative burst, neutrophils must undergo a further series of priming steps (Fig. 2). As neutrophil activation can be highly destructive and cause local damage to host tissues, driving immunopathology,91 this multistep priming process for activation acts as a mechanism to safeguard against these potentially damaging effector functions.
Fig. 2. Multiple ways to activate a neutrophil.
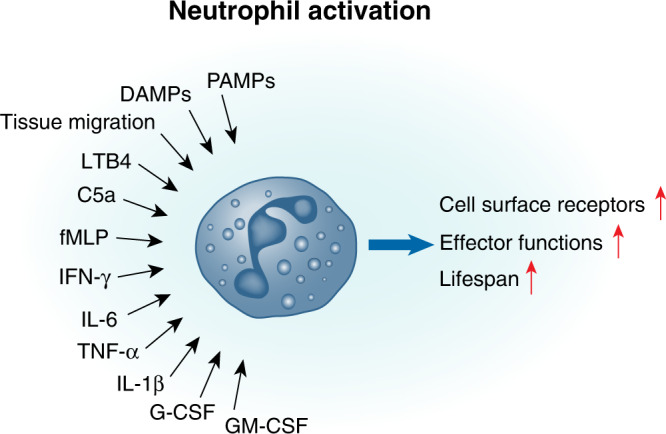
Neutrophils receive many different signals from the inflammatory environment that can lead to cell activation and elicit effector functions. These activating signals include PAMPs, DAMPs, the process of migration, neutrophil chemoattractants, and cytokines.
Factors that activate neutrophils
Once in the tissue, an inflammatory tissue micro-environment containing host-derived compounds such as TNF-α, IL-1β, IFN-γ, and GM-CSF can drive neutrophil activation as well as amplify neutrophil recruitment to the site of infection7,92–95 (Fig. 2). Compounds released during tissue damage can also trigger activation; heme, released abundantly from damaged cells such as red blood cells, was shown in vitro to potently trigger oxidative burst in human neutrophils in a dose dependent manner.92 In addition, neutrophils express a broad repertoire of PRRs, including TLRs (TLR1/2, TLR2/6, TLR4, TLR5, TLR7–TLR9), RIG-I-like receptors (RIG-I, MDA-5), C-type lectin receptors, and NOD-like receptors, all of which can directly bind PAMPs.96 Neutrophil chemoattractants such as LTB4, C5a, and fMLP can also activate neutrophils.90 Binding of these factors to their respective receptors (often G-protein-coupled receptors) triggers a downstream signaling cascade often via the MAPK/ERK pathway,95,97 which then induces neutrophil effector functions such as oxidative burst and degranulation (see details below). The concentration of host chemokines can also impact the effect these mediators have on neutrophil activation status; for example, IL-8 induces shedding of L-selectin and upregulation of certain integrins at low concentrations, while at higher concentrations it can induce the oxidative burst.53,90 Binding of IL-1β can directly induce reactive oxygen species (ROS) production in human neutrophils in a MAPK-dependent manner, while stimulation with GM-CSF can activate neutrophils in an ERK-dependent manner.98 Notably, stimulation of human neutrophils with both IL-1β and GM-CSF has an additive effect, resulting in activation of both MAPK and ERK pathways, and demonstrating how activation can be enhanced by the presence of multiple stimuli.98 PAMPs can also act synergistically on neutrophils; for example, lipopolysaccharide (LPS) can induce assembly of the cellular machinery required for oxidative burst on the membrane of neutrophils, while recognition of fMLP provides the final signal to drive the production of ROS.99,100
Multiple inflammatory signals have been demonstrated to increase neutrophil life span including IFN-γ, GM-CSF, G-CSF, IL-6, and PAMPs such as LPS (Fig. 2).101–103 It has also been suggested that RSV-induced neutrophil activation delays apoptosis in vitro,104 but whether this would be beneficial or detrimental to disease outcome during infection in vivo remains unclear. In vitro studies of virus-induced activation of neutrophils should be carefully considered as the inflammatory mediators produced by the cell line in which the virus is propagated could result in neutrophil activation. For example, a study using RSV “washed” of pro-inflammatory mediators produced by epithelial cells shows that this RSV preparation stimulates neutrophils in vitro significantly less compared to neutrophils stimulated with “unwashed” RSV,105 suggesting that RSV particles on their own do not stimulate neutrophils. Overall, the activation of neutrophils will be very dependent on the inflammatory environment they encounter once entering the lungs (Fig. 2) and it is possible that a more severe infection results in a different, larger, and prolonged presence of neutrophil activation signals that then drives an excessive neutrophil effector response (Fig. 1) contributing to disease severity.
Cell surface receptor upregulation
Several cell surface receptors change on neutrophils after recruitment and activation. For example, CD64 (high affinity FcγRI), CD11b, and CD69 can be upregulated and CD62L and CD182 downregulated on neutrophils after activation.59,102,106–108 CD69 has, in mice, been demonstrated in mice to be upregulated on BAL neutrophils specifically in response to IAV,59 however, this was not observed during RSV infection.7 Furthermore, on blood neutrophils from IAV-infected humans, CD11b was upregulated,109 while CD64 expression was upregulated in one study110 and downregulated in another study.109 Also, during rhinovirus infection of chronic obstructive pulmondary disease (COPD) patients, sputum neutrophils upregulated CD11b, CD63, and CD66.21 In mice, CD64 was the only marker specifically upregulated on lung neutrophils in response to RSV infection.7 Triggering of CD64 drives an intracellular signaling cascade, which has been suggested to drive actin polymerization and facilitate phagocytosis.111 Increased phagocytosis by neutrophils could have a role in clearing up debris from dying cells in the lung, as has been reported in other inflammatory contexts.112 Furthermore, Fc receptors such as CD64 can bind opsonized pathogens and immune complexes.113 Therefore, it is possible that later during a primary infection or during a reinfection when virus-specific IgG are present, activated neutrophils can increase phagocytosis of IgG-bound viral particles and have a more pronounced role in viral clearance. This has so far not been studied in detail and will be an important avenue for future studies.
The magnitude and the combination of specific mediators in the inflammatory environment will drive neutrophil activation and determine the extent and type of their effector programs initiated (see below). Overall, the data so far suggest that neutrophils are differentially activated depending on the specific respiratory virus in question and support recent literature that neutrophils can tailor their response during activation to specific pathogens by reacting to a certain mix of activating signals (Fig. 2).87
Neutrophil effector functions in the antiviral response
Activated neutrophils have many different functions (Fig. 3). In recent years, experimental evidence has suggested that there may be various subtypes of neutrophils with different roles in infection, cancer, and autoimmunity.114 These subtypes have been defined based on size (N1 and N2 neutrophils)115,116 or density (low-density neutrophils (LDNs) and high-density neutrophils).117,118 Furthermore, both immunosuppressive and pro-inflammatory LDNs have been described.117,118 Immunosuppressive LDNs have been found to suppress T-cell proliferation and IFN-γ production and may have a more immature phenotype in some situations.117 However, it is not yet known whether these subtypes have differing functions in tissue damage or host defense against respiratory pathogens. It is also unclear if the phenotypic differences represent true developmentally distinct subtypes or whether these differences can be attributed to differences in the priming, maturation and/or activation status of neutrophils. In addition, data on human lung neutrophils are very limited but potential differences between mouse and human neutrophils in the context of subtypes need to be further investigated.
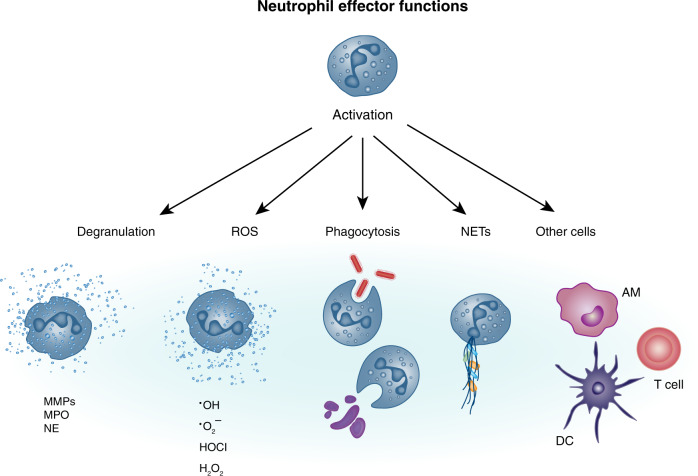
Fig. 3. Neutrophils exhibit a plethora of effector functions.
Neutrophil activation can trigger degranulation whereby neutrophils secrete mediators and proteolytic enzymes such as MMPs, MPO, and NE, which are prestored in cytoplasmic granules. Neutrophils can also mediate pathogen clearance by producing ROS, which can either occur intracellularly in the phagolysosome to kill internalized microbes or extracellularly, to combat larger pathogens. Phagocytosis of pathogens can limit microbial spread, while phagocytosis of cellular debris and apoptotic material can contribute to the resolution of inflammation. Neutrophils can also restrict microbial spread by secreting their chromatin as NETs, trapping pathogens. Finally, it is increasingly appreciated that neutrophils can have direct and indirect interactions with other cells such as alveolar macrophages (AM), dendritic cells (DC), and T cells, which can contribute to both innate and adaptive immunity.
Degranulation
Neutrophil granules contain > 1200 unique proteins prestored in membrane-bound vesicles in the cytoplasm.119 These include proteolytic enzymes, antimicrobial proteins, components of the NADPH oxidase as well as membrane-bound receptors for endothelial adhesion molecules, extracellular matrix proteins, and soluble mediators of inflammation.120 Degranulation, the secretion of neutrophil granules, is a critical effector function of neutrophils initiated early during neutrophil recruitment. Neutrophils contain three types of granules, primary (azurophil), secondary (specific), tertiary (gelatinase) granules, and also secretory vesicles.121,122 Degranulation occurs in a sequential manner; first to be released are the secretory vesicles, while azurophilic granules are last, requiring the most potent activation signals for their release.121 Neutrophil granule contents have widespread effector functions. Gelatinases and collagenases such as MMP-8 and MMP-9 (stored in secondary and tertiary granules) aid neutrophils in their migration through the extracellular matrix.64,123 As they degrade extracellular matrix, this results in release of proline–glycine–prolines, which act as further neutrophil chemoattractants.124 MMP-9 has also been shown to be important for viral clearance during RSV infection of mice.125 Other proteins are thought to be directly antimicrobial, such as neutrophil elastase (NE) and myeloperoxidase (MPO) (stored in primary granules), which can either act proteolytically or by catalyzing ROS production.126 NE has been detected in the serum and airways of infants hospitalized with RSV infection.127,128 The detection of NE suggests that neutrophils recruited to the lung during RSV infection are fully activated. However, the relationship between this and the putative role of neutrophils in disease protection versus pathogenesis is not clear. Antimicrobial peptides including the cathelicidins (e.g., LL-37) and the alpha-defensins are stored in neutrophil granules.90,121 Cathelicidins are activated upon cleavage by proteinase 3129 and can be directly antimicrobial, or contribute to host defense by inducing or modulating chemokine and cytokine production.130 Alpha-defensins have antimicrobial activity against a wide range of bacteria, fungi, and enveloped viruses; the mechanism of action is thought to be via disruption of the plasma membrane by pore formation or by covering the pathogen.131 For example, human cathelicidin LL-37 (produced by both neutrophils and epithelial cells) can inhibit IAV and RSV infection132–134 and human neutrophil peptides, released from neutrophils, can inhibit IAV infectivity by increasing IAV uptake by neutrophils via a mechanism probably involving viral aggregation.135,136 Interestingly, airway secretions from RSV-infected children contain more neutrophil-mediated antibacterial activity compared to RSV-negative controls.137 The extent of neutrophil degranulation must be carefully balanced against the potential harm caused by the pathogen as the dysregulated release of proteolytic enzymes by neutrophils can degrade the extracellular matrix, contributing to immunopathology91 (see more below).
ROS
Oxidative burst via the production of ROS is a powerful tool to eliminate pathogens. In neutrophils this is largely mediated by the NADPH oxidase enzyme complex (reviewed elsewhere138,139) ROS are thought to be harmful to pathogens in multiple ways; directly by causing damage to the pathogen as well as indirectly by inducing autophagy, inhibiting mTOR kinase to trigger an antiviral response, promoting NETosis (see below) and by promoting cell death of infected cells that act as pathogen reservoirs.140 Using different physical forms of the fungal pathogen Candida albicans, it was recently demonstrated that ROS localization can act as a mechanism for neutrophils to sense microbe size and that this influences the ensuing neutrophil response.86,87 Spores of “small” C. albicans induced intracellular ROS production in phagosomes, while “large” C. albicans hyphae induced extracellular ROS. The induction of intracellular ROS in response to “small” pathogens suppressed IL-1β production and restricted the recruitment of more neutrophils to the site of infection, while extracellular ROS in response to a “large” pathogen had the opposite effect on IL-1β production and neutrophil recruitment.86,87 These findings suggest that the induction of ROS, in addition to the well-known role in pathogen removal, also has an important role in directing the ensuing neutrophil response. Oxidative burst has been reported during IAV, PVM, and RSV infection in mice24,105,141 and RSV have also been shown to cause oxidative stress in epithelial cells.142 It is therefore interesting to speculate that ROS levels during virus infection could be limiting the neutrophil response.
Phagocytosis
As professional phagocytes, neutrophils contribute to host defense by clearing up pathogens, dead cells, and other debris during inflammation.3,143 Pathogens opsonized with complement and antibody can be engulfed following interactions with their respective receptors on neutrophils, the complement receptors and FcγRs, such as CD64113 (discussed above). For example, surfactant protein D can opsonize RSV and IAV for neutrophil phagocytosis that then results in ROS production.144,145 Following phagocytosis, preformed granules in the cytoplasm of the neutrophil containing hydrolytic enzymes and NADPH oxidase will fuse with the phagosome.143 The pathogen can either be destroyed enzymatically or by ROS in the mature phagosome. Also, neutrophils can phagocytose infected, apoptotic cells, which are important for resolution of inflammation146 (see more below).
Neutrophil extracellular traps (NETs)
A more recently described effector function of neutrophils is their ability to secrete NETs147–151 (Fig. 3). NETs are large extracellular structures of modified, decondensed chromatin and DNA decorated with the protein contents of neutrophil granules.150 NETs are induced during infections with bacteria, fungi, parasites, and viruses.150 Multiple factors can trigger neutrophils to secrete NETs and several molecular pathways regulate their secretion.150 The main way NETs are induced is via a form of cell death termed NETosis, which is distinct from apoptosis and necrosis, and dependent on ROS production by NADPH oxidase.152 Alternatively, NETs can be induced in a manner that does not kill the neutrophil in a process termed non-lytic NETosis.153,154 This is independent of ROS production and occurs by exocytosis of vesicles filled with nuclear DNA.154 Interestingly, this leaves behind both neutrophil cytoplasts with both diffuse, intracellular cytoplasmic DNA and completely anucleated cytoplasts, neutrophil “ghosts”155 (more about these below). The induction of NETs is thought to be especially beneficial in host defense against larger pathogens, which cannot be removed by phagocytosis.86,87,150 However, during viral infections it is possible that damaged cells trigger NETosis instead of the virus itself, or that different triggers in addition to pathogen size regulate whether neutrophils undergo NETosis. Patients with chronic granulomatous disease have a defect in expressing fully functional NADPH oxidase and therefore have impaired ROS and NET production.156 These patients develop frequent and/or severe bacterial and fungal infections but are not particularly susceptible to viral pathogens.29,156 However, NETs in both lung tissue and serum of severe COVID-19 patients have been detected14,35,157,158 and patients with severe H1N1 and H7N9 IAV infection showed high concentrations of NETs in their serum.159 In addition, in mouse in vivo studies, NETs were detected in areas of tissue injury in the lung during infection with a mouse-adapted IAV strain (PR8) or Sendai virus160–163 as well as during rhinovirus infection in an allergic asthma mouse model.164 As NETs can trap pathogens, these structures could be beneficial for the host. Indeed, histones have been shown to neutralize H3N2 and H1N1 IAV165 and NETs have been suggested to capture RSV particles in vitro.43 It is also possible that NETs are used to plug holes from dying epithelial cells in the barrier but this is still controversial and yet to be formally proven. However, it has been shown that peptidylarginine deiminase 4-mediated NET formation is not required for the host response and survival during IAV infection166 but several studies suggest that excessive neutrophil influx during IAV infection results in the release of toxic NETs and granule enzymes, which are associated with pulmonary pathology.160,167,168
During PVM infection, very few NETs are detected,24 but there is evidence that RSV infection can trigger neutrophils to excrete NETs.24,42,43,169–171 Neutrophils isolated from the airways of patients with RSV-induced bronchiolitis and cultured ex vivo expelled strands of DNA, as detected by staining with the DNA stains Hoechst and SYTOX.170 Furthermore, formation of NETs in lung tissue sections from RSV-infected calves was detected by staining for citrullinated histone H3, a more specific way to detect the presence of NETs than DNA stains.24,43 In mice, the two major studies that have tried to visualize NETs in vivo during RSV infection are limited by the use of a DNA stain, which detects both NETs and extracellular DNA.169,171 Therefore, it cannot be excluded that these studies are also detecting cell death in the lung in response to RSV infection. Nonetheless, these studies showed that RSV could induce NET structures in the lung and, more specifically, RSV F protein alone was possibly sufficient to induce MPO-coated NETs in vivo.169,172 The role of NETs during viral infection is debated and whether NETs can benefit the host in defense from viruses, which are largely intracellular, or if NETs contribute to tissue damage is still being evaluated.30
Activation and regulation of other cells
Neutrophils are thought to be a source of cytokines and chemokines during infections173 and they can modulate the function of other immune cells174 (Fig. 3). For example, lung neutrophils constitutively express pro-IL-1β.175 Recently it has been shown that neutrophils regulate the production of IL-1β by alveolar macrophages (AMs) during infection with IAV.58 However, during RSV infection, no differences in IFN-α, IL-1β, or IL-6 levels in BAL were detected after neutrophil depletion,176 suggesting neutrophils do not modulate the cytokine production by AMs during RSV infection. Furthermore, TNF-α levels were lower after neutrophil depletion during RSV infection in one study177 but not in another study.176 The reasons for the disparity between these studies may be due to the mouse or viral strains used or the time point studied. Overall, cytokine/chemokine responses during neutrophil depletion are not massively altered58,176,177 so it is important to carefully establish which cytokines/chemokines neutrophils produce during specific infections and their contribution to the overall inflammatory environment. Furthermore, neutrophils have been shown to inhibit lung inflammation by reducing the accumulation of γδ T cells during HMPV infection4 and they could also inhibit inflammation via NE cleaving TLRs on macrophages and co-receptors on T cells.174 It is very likely that neutrophils and/or the mediators they secrete regulate the activation of other cell types in the lung and future studies will be required to further uncover the impact of this in the overall antiviral response.
Viral replication and clearance
Whether neutrophils can have direct antiviral effects during respiratory viral infections is debated and has been explored using animal models. Interestingly, after antibody mediated neutrophil depletion in mice there is overall very limited effect, if any, on the viral load during RSV, IAV, PVM, or HMPV infection (Table 1). However, in a study where neutrophils were depleted in rats during rat coronavirus infection, an increase in viral load was found.13 Also, in MCMV infection neutrophil depletion resulted in increased viral load in the lung and this study suggested that neutrophils use TRAIL for viral control.27
Table 1.
The effect of neutrophil depletion in mice on viral load, weight loss and lung pathology during multiple respiratory viral infections.
| Virus | Viral load | Weight loss/clinical score | Pathology and cell infiltration | Viral strain | Species and strain | Refs. |
|---|---|---|---|---|---|---|
| IAV | – | – | ? | X31 | Mouse; BALB/c | 58 |
| IAV | ↑ | ↑ | ↑ | X31 | Mouse; C57BL/6 | 44,65 |
| IAV | ↑ | ↑ | ? | PR8 | Mouse; C57BL/6 | 65 |
| IAV | – | – | – | PR8 | Mouse; BALB/c | 160 |
| IAV | – | ↑ | ? | PR8 | Mouse; C57BL/6 | 66 |
| IAV | – | ↑ | ? | PR8 | Aged mice; C57BL/6 | 66 |
| PVM | – | – | – | J3666 | Mouse; BALB/c | 24 |
| PVM | – | – | – | J3666 | Mouse; C57BL/6 | 24 |
| RSV | – | ? | ? | 2-20 | Mouse; BALB/c | 177 |
| RSV | – | – | ? | A2 | Mouse; C57BL/6 | 176 |
| HMPV | – | ↑ | ↑ | CAN97-83 | Mouse; BALB/c | 4 |
| MCMV | ↑ | ↑ | ? | Smith strain | Mouse; C57BL6 | 27 |
| rCoV | ↑ | ↑ | ↓ | Sialodacryoadenitis virus | Rat; Fisher 344 | 13 |
– no difference; ↑increase; ? not evaluated.
It has been suggested that the massive neutrophil recruitment to the lung early during infection might provide cellular sites for viral replication.178 One study did indeed detect RSV mRNA transcripts in neutrophils isolated from infants with RSV-induced bronchiolitis.178 However, it remains to be established whether any viruses have the ability to replicate productively in neutrophils in vivo. Overall, the findings that neutrophil depletion early during infection does not generally alter lung viral loads in vivo (Table 1) suggest that they are not important host cells for viral replication nor are they major players in the control of viral replication or spread of most respiratory viruses.
Links to adaptive immunity and disease severity
Neutrophils influencing T and B cells
In recent years, it has become increasingly well appreciated that neutrophils can play a role in directing several aspects of adaptive immunity.179,180 It has been suggested that neutrophils can interact directly with dendritic cells (DCs) to enhance their antigen presentation ability,181,182 as well as indirectly, by secreting granule contents that then act on DCs183,184 (Fig. 3). In some contexts, neutrophils can act as antigen presenting cells (APCs) themselves185–187 and monocytes recruited to the trachea during IAV infection can engulf apoptotic neutrophils and serve as important APCs for restimulating T cells in the tissue.188 Furthermore, during IAV infection, the early influx of lung neutrophils has been shown to influence the later recruitment of antiviral CD8+ T cells,189,190 for example, by leaving behind trails of the T cell-attracting chemokine CXCL12.189 Conversely, it has also been suggested that CD11b+ neutrophils suppress T cells and limit T cell-mediated lung pathology during IAV infection.191 However, although neutrophil depletion during IAV infection disrupted CD8+ T cell recruitment to the lungs192 and trachea188 and also the functionality of these cells,188 it did not affect the formation of a functional resident T cell memory population nor did it affect susceptibility to lethal heterosubtypic IAV challenge.192 In contrast, during RSV infection, neutrophil depletion did not change the CD4+, CD8+, or RSV-specific CD8+ primary (day 8 p.i.) or memory T cells responses observed in the lung.176 Therefore, it is possible that neutrophils may have dual roles in balancing T cell immunity versus T cell-driven tissue damage during respiratory viral infections.
Neutrophils have also been suggested to contribute to the enhancement of B cell responses in some inflammatory contexts, thereby contributing to the development of antibody responses.180,193,194 This is in part due to the production of the cytokines BAFF and APRIL, which are regulators of B cell survival and activation.195 However, whether this contributes to the functionality of the antibody response is not clear. During IAV infection, it was shown that human neutrophils do not bind or internalize IAV immune complexes.196 However, in a mouse study, passively transferred serum from IAV-infected mice protected against infection and this protective effect was blocked by depleting recipient mice with an anti-GR-1 antibody, which is partially specific for neutrophils.197 Together, these data suggest that neutrophils can impact the induction of adaptive immune responses and/or the effect of such responses but the mechanisms used might differ in different viral infections and in primary versus memory responses to a specific virus infection.
Neutrophils in tissue damage and disease severity
Neutrophil recruitment alone does not appear enough to cause substantial tissue damage as artificial attraction of neutrophils into the lung using recombinant CXCL1 does not increase weight loss during RSV infection,176 a key measure of pathology and disease severity in this model.198,199 Therefore, neutrophils have to receive further signals from the virus-induced lung inflammatory environment28,69,200 to become fully activated and perform their effector functions.7 Many of the neutrophil effector mediators can, at high concentrations, damage tissues. For example, NE digests the extracellular matrix201 but can also drive mucus production, which can aid in pathogen clearance but also contribute to disease as mucus plugs can block the airways.202 NET release together with mucus production can also be detrimental by increasing tissue damage and impairing lung function.43,172 Neutrophil depletion studies during IAV infection provide conflicting evidence on whether the net effect of neutrophil recruitment and activation is to drive disease or to contribute to host defense.44,58,65,66,160,161,189,190 This is in part due to virus strain-specific differences as the number of neutrophils infiltrating the lungs during IAV infection is highly strain dependent.65,175,203 The mouse-adapted PR8 IAV strain induces more severe disease, in part thought to be mediated by neutrophils, while infection of mice with other IAV strains, causing milder disease, does not appear to induce neutrophil-driven pathology to the same extent (Fig. 1).65 Interestingly, age also seems to influence disease severity during respiratory infections as excessive neutrophil levels were found during SARS-CoV-2 infection204 and during IAV infection of aged mice.66
Neutrophils and resolution of inflammation
Neutrophils can aid in disease resolution and wound healing (as reviewed extensively in205,206). They can also contribute to resolution of inflammation by clearing up virus-infected cells by phagocytosis.146 In addition, apoptotic neutrophils taken up by macrophages via efferocytosis signal to macrophages to switch to a more anti-inflammatory phenotype.205,207 Neutrophils can also contribute to epithelial cell proliferation, important for keeping the barrier intact,112 and they can secrete pro-resolution products such as annexin A1.205 However, during respiratory viral infections, the contribution of these possible functions to disease resolution is not well understood, and it has been shown that human neutrophils can increase the epithelial cell damage during in vitro RSV infection.208 As neutrophils have been shown to deplete local O2, which increases resolution of acute colonic inflammation,209 it is possible that this is also the case in the airways. Although it has also been shown that hypoxia augments neutrophil degranulation and killing of airway epithelial cells210 and therefore could potentially cause more lung tissue damage. An interesting aspect of neutrophil depletion experiments during respiratory viral infections in mice is that several of the models showed increased weight loss or clinical scores when neutrophils were lacking in the early phases of infection (Table 1). This could suggest that neutrophils have an important role in the resolution phase as resolution of any inflammatory responses in the lungs is important for restoring steady state. Future detailed studies will inform on how neutrophils contribute to these processes and if and when they can potentially be manipulated to increase this function.
Neutrophils in co-infections and viral exacerbations of asthma
The role of neutrophils in co-infections
Co-infections with several viruses can occur, but the neutrophilic response in nasopharyngeal aspirate samples from children is not altered in the presence of several simultaneous virus infections.33 A secondary bacterial infection is a common feature after severe respiratory viral infections, and is often the cause of death.211–213 For example, the 1918 “Spanish flu” pandemic is thought to have reached the devastating death toll due to secondary bacterial infections.214 Several studies have shown a defect in subsequent neutrophil function after a respiratory viral infection. For example, IAV-induced neutrophil dysfunction contributed to increased susceptibility to a secondary Streptococcus pneumoniae infection215 and neutrophil depletion during S. pneumoniae/IAV co-infection increased mortality.216 Also, the production of neutrophil chemokines (CXCL1 and CXCL2) was impaired during S. pneumoniae infection of mice which had had prior IAV or HMPV infection.217,218 Interestingly, neutrophils in S. pneumoniae or S. pneumoniae/IAV co-infected mice did not show a functional difference in ROS, NET, or cytokine production,216 while human neutrophils simultaneously incubated with IAV and S. pneumoniae showed increased survival and respiratory burst activity.219 In IAV followed by Pseudomonas aeruginosa co-infection, neutrophils showed impaired bacterial killing and this was attributed to insufficient G-CSF production.220 In addition, increased MMP production during IAV infection was suggested to increase lung damage during P. aeruginosa co-infection.221 Furthermore, blood neutrophils exposed to aspirate fluid from children with viral/bacterial co-infections showed decreased respiratory burst and killing activity against Haemophilus influenzae and Staphylococcus aureus compared to those transmigrated into the aspirate fluid from children without bacterial co-infection.222 Suppressive neutrophils (CD16hiCD62Llo) were also found in blood and BAL from RSV-infected infants with a bacterial co-infection.223
In contrast, in a mouse model of IAV-dengue virus co-infection, where both viruses were detected in the lung and mice developed pneumonia, neutrophils did not contribute to the enhanced disease.224 However, in a IAV-Aspergillus fumigatus co-infection model, mice got more severe disease and there were fewer neutrophils recruited in the superinfected mice.225 Overall, the data so far suggest that a viral infection inhibits the neutrophil response and render the host susceptible to a subsequent bacterial or fungal infection. Interestingly, some of our recent data suggest that a signature of activated neutrophils in the nose prior to RSV challenge correlates with the development of symptomatic infection in human volunteers.226 Furthermore, neutrophils recruited to the lungs of mice prior to RSV infection also increase disease severity as measured by weight loss.226 These findings suggest that neutrophils present in the lungs and airways, potentially attracted by a prior infection, can increase disease susceptibility and/or severity after a respiratory viral infection.
The role of neutrophils in viral exacerbations of asthma
Viral infections commonly cause asthma exacerbations227–229 and infections in early life are associated with wheeze and asthma development in later life.230 For example, severe RSV infection is associated with later asthma development and asthma is, in turn, associated with increased susceptibility to severe RSV disease.231 The role of neutrophil recruitment in virus-induced exacerbations has not been fully elucidated, but it was recently shown that NETs and neutrophil enucleated cytoplasts may contribute to pathological neutrophilic inflammation in asthma.164,232 Furthermore, the presence of NETs and neutrophil cytoplasts in asthmatics positively correlated with higher levels of IL-17, an important mediator of neutrophil recruitment.232 In a recent study, it was further shown that CXCR4hi neutrophils are prone to induce NETs and that these NETs increase the uptake of house dust mite by inflammatory DCs, which results in an increase in the susceptibility to allergic asthma.162 Also, a dysregulation of TLR7/8 signaling in neutrophils may play a role in viral-induced asthma exacerbations.233 For future studies it will be interesting to elucidate how neutrophils contribute to the virus-induced exacerbations in different asthma endotypes. For example, in a more neutrophil biased asthma it is possible that a virus infection, driving an increased neutrophil response, will increase disease severity.
Summary and future research
Although neutrophils are a major effector immune cell recruited to the lungs during respiratory viral infections, their role is likely more complex than has previously been appreciated. An outstanding key question is whether neutrophils have an active role in the antiviral immune response or whether they are bystander cells recruited to the lungs and airways by virus-induced inflammation. In this review, we have summarized the current understanding of the immunological mechanisms regulating neutrophil recruitment, priming, and activation and their role during infection with different respiratory viruses. It is clear from mouse models using antibody-mediated neutrophil depletion that the effector functions of neutrophils do not have a major role in limiting viral replication or spread. However, their effect on tissue damage in the lung versus their ability to contribute to the resolution of inflammation is still unresolved and likely varies from one infection to another.
Balancing of antiviral responses in the lung is critical to manage the efficient clearance of the virus, while limiting tissue damage and avoiding compromising the lungs’ ability to perform gas exchange. In the ongoing SARS-CoV-2 pandemic as well as during severe RSV and IAV infections, dysregulated immune responses are contributing to disease severity. We therefore have to carefully consider all arms of the immune system in order to understand the underlying cause of this prolonged and heightened inflammation. The magnitude of the neutrophilic response (both in terms of the number and their activation) will determine the tissue damage that they cause. The timing of the recruitment, activation, and potentially regulation of life span in neutrophils are also key to severity of disease as both an activated neutrophilic signature prior to infection and sustained neutrophilic response are associated with disease severity in RSV, IAV, and SARS-CoV-2 infection (Fig. 1). In addition, neutrophils appear to have an underappreciated role in directing other components of both the innate and adaptive immune system. Furthermore, the presence of neutrophils might have important functions in protecting the lungs and, rather than executing a strong antiviral effect, protect the virus-infected lung from an increased exposure of commensal bacteria and fungi due to the breach in the epithelial barrier. These aspects will be crucial to study in future in vivo models.
Depleting or removing neutrophils will be a very difficult therapeutic approach as it opens up for bacterial and fungal infections to take hold and perhaps also decrease resolution of inflammation. Furthermore, it is possible that both positive and negative effects for the host response are removed in the models of disease discussed in this review and that therefore more specific knockout models, where neutrophil recruitment or effector functions are altered, will be important to fully elucidate the complex role of neutrophils during viral infections. This could also allow targeting of neutrophil function and could be a valid, future therapeutic strategy.
The neutrophilic response seems to differ between types of viral infections. We suspect that neutrophils likely receive distinct signals during their recruitment, priming, and activation in the lung, which can result in the differences observed in their effector functions. Very little is known about the combination of signals, both intrinsic and extrinsic, that drive distinct neutrophil effector functions and this will be an important research avenue to pursue in the future. It is possible that we can learn from viral infections where neutrophils can have a beneficial role, to understand how to modulate neutrophil function in a positive manner during infections with RSV, IAV, and SARS-CoV-2 where neutrophils are abundantly recruited but do not appear to benefit the host. Furthermore, the transcriptional profiles and cell surface markers of possible neutrophil subtypes and their precise role in the lung of both mice and humans during respiratory infections will be important to fully understand to further answer these questions. Overall, more research into the delicate balance of beneficial and detrimental effects of neutrophils during respiratory viral infections (Fig. 4) is crucial for our understanding of the biology of these cells and our understanding of the potential possibilities, which exist to manipulate their function in targeted therapies.
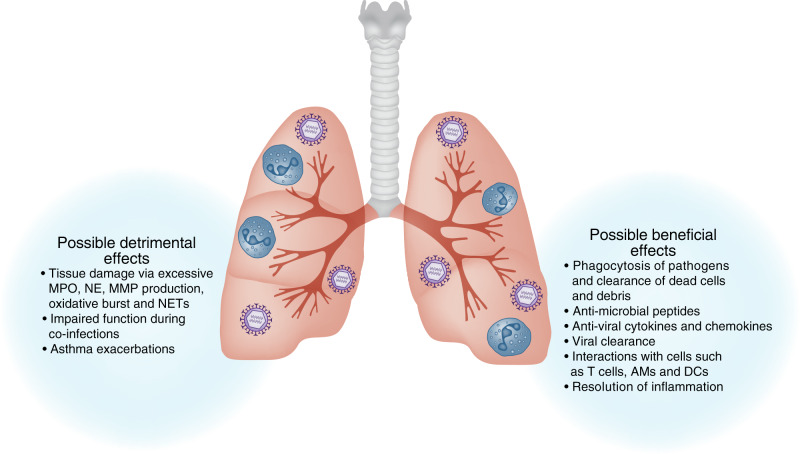
Fig. 4. Possible beneficial and detrimental effects of neutrophils during respiratory viral infections.
Neutrophils infiltrating the lungs during viral infections can aid the ongoing antiviral response by contributing to the production of antiviral cytokines and chemokines, as well as producing antimicrobial peptides. As phagocytes, neutrophils can clear pathogens and debris. These functions can aid in viral control/clearance as well as resolution of inflammation. An excess of activated neutrophils can contribute to lung tissue damage by excessive production of MPO, NE, MMPs, oxidative burst, and NETs, exacerbating disease severity.
Acknowledgements
We want to thank the Wellcome Trust (109058/Z/15/Z), CRUK (A27217), the Rosetrees Trust, and the Stoneygate Trust (M370-F1) for support. We also thank Caetano Reis e Sousa, Augusto Varese, Ana Farias, and Christina Michalaki for critically reading the review.
Author contributions
C.J. and F.C.M.K. devised the theme and scope of the review, wrote the paper, and designed the figures for preparation by the publisher. Some of the content of this paper is adapted from the Ph.D. thesis by F.C.M.K., “Neutrophils in Respiratory Syncytial Virus Infection” Imperial College London.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ley K, et al. Neutrophils: new insights and open questions. Sci. Immunol. 2018;3:eaat4579. doi: 10.1126/sciimmunol.aat4579. [DOI] [PubMed] [Google Scholar]
- 2.Summers C, et al. Neutrophil kinetics in health and disease. Trends Immunol. 2010;31:318–324. doi: 10.1016/j.it.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 4.Cheemarla NR, Baños-Lara MDR, Naidu S, Guerrero-Plata A. Neutrophils regulate the lung inflammatory response via γδ T cell infiltration in an experimental mouse model of human metapneumovirus infection. J. Leukoc. Biol. 2017;101:1383–1392. doi: 10.1189/jlb.4A1216-519RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soto JA, et al. Human metapneumovirus: mechanisms and molecular targets used by the virus to avoid the immune system. Front. Immunol. 2018;9:2466. doi: 10.3389/fimmu.2018.02466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darniot M, Petrella T, Aho S, Pothier P, Manoha C. Immune response and alteration of pulmonary function after primary human metapneumovirus (hMPV) infection of BALB/c mice. Vaccine. 2005;23:4473–4480. doi: 10.1016/j.vaccine.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 7.Kirsebom FCM, Kausar F, Nuriev R, Makris S, Johansson C. Neutrophil recruitment and activation are differentially dependent on MyD88/TRIF and MAVS signaling during RSV infection. Mucosal Immunol. 2019;12:1244–1255. doi: 10.1038/s41385-019-0190-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goritzka M, et al. Interferon-α/β receptor signaling amplifies early pro-inflammatory cytokine production in the lung during respiratory syncytial virus infection. J. Virol. 2014;88:6128–6136. doi: 10.1128/JVI.00333-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goritzka M, et al. Alveolar macrophage-derived type I interferons orchestrate innate immunity to RSV through recruitment of antiviral monocytes. J. Exp. Med. 2015;212:699–714. doi: 10.1084/jem.20140825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McNamara PS, Ritson P, Selby A, Hart CA, Smyth RL. Bronchoalveolar lavage cellularity in infants with severe respiratory syncytial virus bronchiolitis. Arch. Dis. Child. 2003;88:922–926. doi: 10.1136/adc.88.10.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Everard ML, et al. Analysis of cells obtained by bronchial lavage of infants with respiratory syncytial virus infection. Arch. Dis. Child. 1994;71:428–432. doi: 10.1136/adc.71.5.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Totura AL, et al. Toll-like receptor 3 signaling via TRIF contributes to a protective innate immune response to severe acute respiratory syndrome coronavirus infection. MBio. 2015;6:e00638-15. doi: 10.1128/mBio.00638-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haick AK, Rzepka JP, Brandon E, Balemba OB, Miura TA. Neutrophils are needed for an effective immune response against pulmonary rat coronavirus infection, but also contribute to pathology. J. Gen. Virol. 2014;95:578–590. doi: 10.1099/vir.0.061986-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Middleton EA, et al. Neutrophil extracellular traps (NETs) contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136:1169–1179. doi: 10.1182/blood.2020007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Channappanavar R, et al. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J. Immunol. 2017;198:4046–4053. doi: 10.4049/jimmunol.1601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou Z, et al. Heightened innate immune responses in the respiratory tract of COVID-19 patients. Cell Host Microbe. 2020;27:883–890. doi: 10.1016/j.chom.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winkler ES, et al. SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function. Nat. Immunol. 2020;181:1–21. doi: 10.1038/s41590-020-0778-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagarkar DR, et al. CXCR2 is required for neutrophilic airway inflammation and hyperresponsiveness in a mouse model of human rhinovirus infection. J. Immunol. 2009;183:6698–6707. doi: 10.4049/jimmunol.0900298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berman R, et al. MUC18 regulates lung rhinovirus infection and inflammation. PLoS ONE. 2016;11:e0163927–13. doi: 10.1371/journal.pone.0163927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winther B. Rhinovirus infections in the upper airway. Proc. Am. Thorac. Soc. 2011;8:79–89. doi: 10.1513/pats.201006-039RN. [DOI] [PubMed] [Google Scholar]
- 21.Mallia P, Message SD, Contoli M. Neutrophil adhesion molecules in experimental rhinovirus infection in COPD. Respir. Res. 2013;14:1–9. doi: 10.1186/1465-9921-14-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jarjour NN, et al. The effect of an experimental rhinovirus 16 infection on bronchial lavage neutrophils. JACI. 2000;105:1169–1177. doi: 10.1067/mai.2000.106376. [DOI] [PubMed] [Google Scholar]
- 23.Young Yull K, et al. Bronchoalveolar cellularity and interleukin-8 levels in measles bronchiolitis obliterans. Chest. 2015;131:1454–1460. doi: 10.1378/chest.06-0188. [DOI] [PubMed] [Google Scholar]
- 24.Cortjens B, Lutter R, Boon L, Bem RA, van Woensel JBM. Pneumovirus-induced lung disease in mice is independent of neutrophil-driven inflammation. PLoS ONE. 2016;11:e0168779. doi: 10.1371/journal.pone.0168779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCarthy MK, Zhu L, Procario MC, Weinberg JB. IL-17 contributes to neutrophil recruitment but not to control of viral replication during acute mouse adenovirus type 1 respiratory infection. Virology. 2014;456–457:259–267. doi: 10.1016/j.virol.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu W, et al. Human lung innate immune cytokine response to adenovirus type 7. J. Gen. Virol. 2010;91:1155–1163. doi: 10.1099/vir.0.017905-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stacey MA, et al. Neutrophils recruited by IL-22 in peripheral tissues function as TRAIL-dependent antiviral effectors against MCMV. Cell Host Microbe. 2014;15:471–483. doi: 10.1016/j.chom.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camp JV, Jonsson CB. A role for neutrophils in viral respiratory disease. Front. Immunol. 2017;8:11–17. doi: 10.3389/fimmu.2017.00550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galani IE, Andreakos E. Neutrophils in viral infections: current concepts and caveats. J. Leukoc. Biol. 2015;98:557–564. doi: 10.1189/jlb.4VMR1114-555R. [DOI] [PubMed] [Google Scholar]
- 30.Schönrich G, Raftery MJ. Neutrophil extracellular traps go viral. Front. Immunol. 2016;7:526–527. doi: 10.3389/fimmu.2016.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marguet C, et al. Neutrophil but not eosinophil inflammation is related to the severity of a first acute epidemic bronchiolitis in young infants. Pediatr. Allergy Immunol. 2008;19:157–165. doi: 10.1111/j.1399-3038.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- 32.Lukens MV, et al. A systemic neutrophil response precedes robust CD8(+) T-cell activation during natural respiratory syncytial virus infection in infants. J. Virol. 2010;84:2374–2383. doi: 10.1128/JVI.01807-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cavallaro EC, Liang K-K, Lawrence MD, Forsyth KD, Dixon D-L. Neutrophil infiltration and activation in bronchiolitic airways are independent of viral etiology. Pediatr. Pulmonol. 2017;52:238–246. doi: 10.1002/ppul.23514. [DOI] [PubMed] [Google Scholar]
- 34.Tang BM, et al. Neutrophils-related host factors associated with severe disease and fatality in patients with influenza infection. Nat. Commun. 2019;10:1–13. doi: 10.1038/s41467-019-11249-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zuo Y, et al. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5:e138999. doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiong Y, et al. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg. Microbes Infect. 2020;9:761–770. doi: 10.1080/22221751.2020.1747363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong P, et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584:463–469. doi: 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mejias A, et al. Whole blood gene expression profiles to assess pathogenesis and disease severity in infants with respiratory syncytial virus infection. PLoS Med. 2013;10:e1001549. doi: 10.1371/journal.pmed.1001549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dunning J, et al. Progression of whole-blood transcriptional signatures from interferon-induced to neutrophil-associated patterns in severe influenza. Nat. Immunol. 2018;19:625–635. doi: 10.1038/s41590-018-0111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meizlish ML, et al. A neutrophil activation signature predicts critical illness and mortality in COVID-19. Blood Adv. 2021;5:1164–1177. doi: 10.1182/bloodadvances.2020003568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van den Brand JMA, et al. Comparison of temporal and spatial dynamics of seasonal H3N2, pandemic H1N1 and highly pathogenic avian influenza H5N1 virus infections in ferrets. PLoS ONE. 2012;7:e42343-21. doi: 10.1371/journal.pone.0042343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hägglund S, et al. Proteome analysis of bronchoalveolar lavage from calves infected with bovine respiratory syncytial virus—insights in pathogenesis and perspectives for new treatments. PLoS ONE. 2017;12:e0186594-19. doi: 10.1371/journal.pone.0186594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cortjens B, et al. Neutrophil extracellular traps cause airway obstruction during respiratory syncytial virus disease. J. Pathol. 2015;238:401–411. doi: 10.1002/path.4660. [DOI] [PubMed] [Google Scholar]
- 44.Tate MD, et al. Neutrophils ameliorate lung injury and the development of severe disease during influenza infection. J. Immunol. 2009;183:7441–7450. doi: 10.4049/jimmunol.0902497. [DOI] [PubMed] [Google Scholar]
- 45.Nicolás-Ávila JÁ, Adrover JM, Hidalgo A. Neutrophils in homeostasis, immunity, and cancer. Immunity. 2017;46:15–28. doi: 10.1016/j.immuni.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 46.Kreisel D, et al. In vivo two-photon imaging reveals monocyte-dependent neutrophil extravasation during pulmonary inflammation. Proc. Natl Acad. Sci. USA. 2010;107:18073–18078. doi: 10.1073/pnas.1008737107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Devi S, et al. Neutrophil mobilization via plerixafor-mediated CXCR4 inhibition arises from lung demargination and blockade of neutrophil homing to the bone marrow. J. Exp. Med. 2013;210:2321–2336. doi: 10.1084/jem.20130056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams MR, Azcutia V, Newton G, Alcaide P, Luscinskas FW. Emerging mechanisms of neutrophil recruitment across endothelium. Trends Immunol. 2011;32:461–469. doi: 10.1016/j.it.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pittman K, Kubes P. Damage-associated molecular patterns control neutrophil recruitment. J. Innate Immun. 2013;5:315–323. doi: 10.1159/000347132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nuriev R, Johansson C. Chemokine regulation of inflammation during respiratory syncytial virus infection. F1000Res. 2019;8:1–11. doi: 10.12688/f1000research.20061.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Petri B, Sanz M-J. Neutrophil chemotaxis. Cell Tissue Res. 2018;371:425–436. doi: 10.1007/s00441-017-2776-8. [DOI] [PubMed] [Google Scholar]
- 52.Sadik CD, Kim ND, Luster AD. Neutrophils cascading their way to inflammation. Trends Immunol. 2011;32:452–460. doi: 10.1016/j.it.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ley K. Integration of inflammatory signals by rolling neutrophils. Immunol. Rev. 2002;186:8–18. doi: 10.1034/j.1600-065x.2002.18602.x. [DOI] [PubMed] [Google Scholar]
- 54.Stoppelenburg AJ, et al. Local IL-17A potentiates early neutrophil recruitment to the respiratory tract during severe RSV infection. PLoS ONE. 2013;8:e78461. doi: 10.1371/journal.pone.0078461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li H, et al. Internal genes of a highly pathogenic H5N1 influenza virus determine high viral replication in myeloid cells and severe outcome of infection in mice. PLoS Pathog. 2018;14:e1006821. doi: 10.1371/journal.ppat.1006821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Noah TL, Becker S. Chemokines in nasal secretions of normal adults experimentally infected with respiratory syncytial virus. Clin. Immunol. 2000;97:43–49. doi: 10.1006/clim.2000.4914. [DOI] [PubMed] [Google Scholar]
- 57.Booth JL, Coggeshall KM, Gordon BE, Metcalf JP. Adenovirus type 7 induces interleukin-8 in a lung slice model and requires activation of Erk. J. Virol. 2004;78:4156–4164. doi: 10.1128/JVI.78.8.4156-4164.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peiró T, et al. Neutrophils drive alveolar macrophage IL-1β release during respiratory viral infection. Thorax. 2017;73:546–556. doi: 10.1136/thoraxjnl-2017-210010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tate MD, Brooks AG, Reading PC. The role of neutrophils in the upper and lower respiratory tract during influenza virus infection of mice. Respir. Res. 2008;9:57. doi: 10.1186/1465-9921-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Camp JV, et al. Lower respiratory tract infection of the ferret by 2009 H1N1 pandemic influenza a virus triggers biphasic, systemic, and local recruitment of neutrophils. J. Virol. 2015;89:8733–8748. doi: 10.1128/JVI.00817-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Durant LR, et al. DNGR-1 is dispensable for CD8(+) T-cell priming during respiratory syncytial virus infection. Eur. J. Immunol. 2014;44:2340–2348. doi: 10.1002/eji.201444454. [DOI] [PubMed] [Google Scholar]
- 62.Perrone LA, Plowden JK, García-Sastre A, Katz JM, Tumpey TM. H5N1 and 1918 pandemic influenza virus infection results in early and excessive infiltration of macrophages and neutrophils in the lungs of mice. PLoS Pathog. 2008;4:e1000115–11. doi: 10.1371/journal.ppat.1000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rudd JM, et al. Neutrophils induce a novel chemokine receptors repertoire during influenza pneumonia. Front. Cell. Infect. Microbiol. 2019;9:1393–12. doi: 10.3389/fcimb.2019.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bradley LM, Douglass MF, Chatterjee D, Akira S, Baaten BJG. Matrix metalloprotease 9 mediates neutrophil migration into the airways in response to influenza virus-induced toll-like receptor signaling. PLoS Pathog. 2012;8:e1002641–12. doi: 10.1371/journal.ppat.1002641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tate MD, et al. The role of neutrophils during mild and severe influenza virus infections of mice. PLoS ONE. 2011;6:e17618. doi: 10.1371/journal.pone.0017618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kulkarni U, et al. Excessive neutrophil levels in the lung underlie the age-associated increase in influenza mortality. Mucosal Immunol. 2019;12:545–554. doi: 10.1038/s41385-018-0115-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on toll-like receptors. Nat. Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 68.Chow KT, Gale M, Jr, Loo Y-M. RIG-I and other RNA sensors in antiviral immunity. Annu. Rev. Immunol. 2018;36:667–694. doi: 10.1146/annurev-immunol-042617-053309. [DOI] [PubMed] [Google Scholar]
- 69.Openshaw PJM, Chiu C, Culley FJ, Johansson C. Protective and harmful immunity to RSV infection. Annu. Rev. Immunol. 2017;35:501–532. doi: 10.1146/annurev-immunol-051116-052206. [DOI] [PubMed] [Google Scholar]
- 70.Iwasaki A, Pillai PS. Innate immunity to influenza virus infection. Nat. Rev. Immunol. 2014;14:315–328. doi: 10.1038/nri3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Johansson C. Respiratory syncytial virus infection: an innate perspective. F1000Res. 2016;5:2898. doi: 10.12688/f1000research.9637.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rudd BD, et al. MyD88-mediated instructive signals in dendritic cells regulate pulmonary immune responses during respiratory virus infection. J. Immunol. 2007;178:5820–5827. doi: 10.4049/jimmunol.178.9.5820. [DOI] [PubMed] [Google Scholar]
- 73.Murawski MR, et al. Respiratory syncytial virus activates innate immunity through toll-like receptor 2. J. Virol. 2009;83:1492–1500. doi: 10.1128/JVI.00671-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goritzka M, Pereira C, Makris S, Durant LR, Johansson C. T cell responses are elicited against respiratory syncytial virus in the absence of signalling through TLRs, RLRs and IL-1R/IL-18R. Sci. Rep. 2015;5:18533. doi: 10.1038/srep18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seo S-U, et al. MyD88 signaling is indispensable for primary influenza A virus infection but dispensable for secondary infection. J. Virol. 2010;84:12713–12722. doi: 10.1128/JVI.01675-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leung YHC, et al. Highly pathogenic avian influenza A H5N1 and pandemic H1N1 virus infections have different phenotypes in toll-like receptor 3 knockout mice. J. Gen. Virol. 2014;95:1870–1879. doi: 10.1099/vir.0.066258-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sheahan T, et al. MyD88 is required for protection from lethal infection with a mouse-adapted SARS-CoV. PLoS Pathog. 2008;4:e1000240–12. doi: 10.1371/journal.ppat.1000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Le Goffic R, et al. Detrimental contribution of the toll-like receptor (TLR)3 to influenza A virus-induced acute pneumonia. PLoS Pathog. 2006;2:e53. doi: 10.1371/journal.ppat.0020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cheemarla NR, Guerrero-Plata A. Human metapneumovirus attachment protein contributes to neutrophil recruitment into the airways of infected mice. Viruses. 2017;9:310. doi: 10.3390/v9100310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miller AL, Bowlin TL, Lukacs NW. Respiratory syncytial virus-induced chemokine production: linking viral replication to chemokine production in vitro and in vivo. J. Infect. Dis. 2004;189:1419–1430. doi: 10.1086/382958. [DOI] [PubMed] [Google Scholar]
- 81.Sengupta S, et al. Circadian control of lung inflammation in influenza infection. Nat. Commun. 2019;10:4107. doi: 10.1038/s41467-019-11400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.You D, Siefker DT, Shrestha B, Saravia J, Cormier SA. Building a better neonatal mouse model to understand infant respiratory syncytial virus disease. Respir. Res. 2015;16:91. doi: 10.1186/s12931-015-0244-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Das S, et al. Respiratory syncytial virus infection of newborn CX3CR1-deficent mice induces a pathogenic pulmonary innate immune response. JCI Insight. 2017;2:14–16. doi: 10.1172/jci.insight.94605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Makris S, Johansson C. R848 or influenza virus can induce potent innate immune responses in the lungs of neonatal mice. Mucosal Immunol. 2020;10:5–10. doi: 10.1038/s41385-020-0314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lines JL, Hoskins S, Hollifield M, Cauley LS, Garvy BA. The migration of T cells in response to influenza virus is altered in neonatal mice. J. Immunol. 2010;185:2980–2988. doi: 10.4049/jimmunol.0903075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Warnatsch A, et al. Reactive oxygen species localization programs inflammation to clear microbes of different size. Immunity. 2017;46:421–432. doi: 10.1016/j.immuni.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Branzk N, et al. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat. Immunol. 2014;15:1017–1025. doi: 10.1038/ni.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mócsai A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J. Exp. Med. 2013;210:1283–1299. doi: 10.1084/jem.20122220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Castanheira FVS, Kubes P. Neutrophils and NETs in modulating acute and chronic inflammation. Blood. 2019;133:2178–2185. doi: 10.1182/blood-2018-11-844530. [DOI] [PubMed] [Google Scholar]
- 90.Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu. Rev. Immunol. 2012;30:459–489. doi: 10.1146/annurev-immunol-020711-074942. [DOI] [PubMed] [Google Scholar]
- 91.Bardoel BW, Kenny EF, Sollberger G, Zychlinsky A. The balancing act of neutrophils. Cell Host Microbe. 2014;15:526–536. doi: 10.1016/j.chom.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 92.Graça-Souza AV, Arruda MAB, de Freitas MS, Barja-Fidalgo C, Oliveira PL. Neutrophil activation by heme: implications for inflammatory processes. Blood. 2002;99:4160–4165. doi: 10.1182/blood.v99.11.4160. [DOI] [PubMed] [Google Scholar]
- 93.Condliffe AM, Kitchen E, Chilvers ER. Neutrophil priming: pathophysiological consequences and underlying mechanisms. Clin. Sci. 1998;94:461–471. doi: 10.1042/cs0940461. [DOI] [PubMed] [Google Scholar]
- 94.Buckle AM, Hogg N. The effect of IFN-gamma and colony-stimulating factors on the expression of neutrophil cell membrane receptors. J. Immunol. 1989;143:2295–2301. [PubMed] [Google Scholar]
- 95.Kato T, Kitagawa S. Regulation of neutrophil functions by proinflammatory cytokines. Int. J. Hematol. 2006;84:205–209. doi: 10.1532/IJH97.06141. [DOI] [PubMed] [Google Scholar]
- 96.Thomas CJ, Schroder K. Pattern recognition receptor function in neutrophils. Trends Immunol. 2013;34:317–328. doi: 10.1016/j.it.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 97.Selvatici R, Falzarano S, Mollica A, Spisani S. Signal transduction pathways triggered by selective formylpeptide analogues in human neutrophils. Eur. J. Pharmacol. 2006;534:1–11. doi: 10.1016/j.ejphar.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 98.Suzuki K, et al. Selective activation of p38 mitogen-activated protein kinase cascade in human neutrophils stimulated by IL-1β. J. Immunol. 2001;167:5940–5947. doi: 10.4049/jimmunol.167.10.5940. [DOI] [PubMed] [Google Scholar]
- 99.Guthrie LA, McPhail LC, Henson PM, Johnston RJ. Priming of neutrophils for enhanced release of oxygen metabolites by bacterial lipopolysaccharide. J. Exp. Med. 1984;160:1656–1671. doi: 10.1084/jem.160.6.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.El-Benna J, Dang PM-C, Gougerot-Pocidalo M-A. Priming of the neutrophil NADPH oxidase activation: role of p47phox phosphorylation and NOX2 mobilization to the plasma membrane. Semin. Immunopathol. 2008;30:279–289. doi: 10.1007/s00281-008-0118-3. [DOI] [PubMed] [Google Scholar]
- 101.Colotta F, Re F, Polentarutti N, Sozzani S, Mantovani A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood. 1992;80:2012–2020. [PubMed] [Google Scholar]
- 102.Constantini C, et al. Neutrophil activation and survival are modulated by interaction with NK cells. Int. Immunol. 2010;22:827–838. doi: 10.1093/intimm/dxq434. [DOI] [PubMed] [Google Scholar]
- 103.Dienz O, et al. Essential role of IL-6 in protection against H1N1 influenza virus by promoting neutrophil survival in the lung. Mucosal Immunol. 2012;5:258–266. doi: 10.1038/mi.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lindemans CA, et al. Respiratory syncytial virus inhibits granulocyte apoptosis through a phosphatidylinositol 3-kinase and NF-kappaB-dependent mechanism. J. Immunol. 2006;176:5529–5537. doi: 10.4049/jimmunol.176.9.5529. [DOI] [PubMed] [Google Scholar]
- 105.Bataki EL, Evans GS, Everard ML. Respiratory syncytial virus and neutrophil activation. Clin. Exp. Immunol. 2005;140:470–477. doi: 10.1111/j.1365-2249.2005.02780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cid J, Aguinaco R, Sanchez R, Garcia-Pardo G, Llorente A. Neutrophil CD64 expression as marker of bacterial infection: a systematic review and meta-analysis. J. Infect. 2010;60:313–319. doi: 10.1016/j.jinf.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 107.Fortunati E, Kazemier KM, Grutters JC, Koenderman L, Van den Bosch VJMM. Human neutrophils switch to an activated phenotype after homing to the lung irrespective of inflammatory disease. Clin. Exp. Immunol. 2009;155:559–566. doi: 10.1111/j.1365-2249.2008.03791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hoffmann JJML. Neutrophil CD64: a diagnostic marker for infection and sepsis. Clin. Chem. Lab. Med. 2009;47:671–14. doi: 10.1515/CCLM.2009.224. [DOI] [PubMed] [Google Scholar]
- 109.Pauksens K, Fjaertoft G, Douhan-Håkansson L, Venge P. Neutrophil and monocyte receptor expression in uncomplicated and complicated influenza A infection with pneumonia. Scand. J. Infect. Dis. 2009;40:326–337. doi: 10.1080/00365540701646287. [DOI] [PubMed] [Google Scholar]
- 110.Fjaertoft G, Pauksen K, Håkansson L, Xu S, Venge P. Cell surface expression of FcγRI (CD64) on neutrophils and monocytes in patients with influenza A, with and without complications. Scand. J. Infect. Dis. 2009;37:882–889. doi: 10.1080/00365540500348929. [DOI] [PubMed] [Google Scholar]
- 111.Melendez AJ, Tay HK. Phagocytosis: a repertoire of receptors and Ca2+ as a key second messenger. Biosci. Rep. 2008;28:287–298. doi: 10.1042/BSR20080082. [DOI] [PubMed] [Google Scholar]
- 112.Wang J. Neutrophils in tissue injury and repair. Cell Tissue Res. 2018;371:1–9. doi: 10.1007/s00441-017-2785-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang Y, Jönsson F. Expression, role, and regulation of neutrophil Fcγ receptors. Front. Immunol. 2019;10:1958. doi: 10.3389/fimmu.2019.01958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ng LG, Ostuni R, Hidalgo A. Heterogeneity of neutrophils. Nat. Rev. Immunol. 2019;19:255–265. doi: 10.1038/s41577-019-0141-8. [DOI] [PubMed] [Google Scholar]
- 115.Arnardottir HH, Freysdottir J, Hardardottir I. Two circulating neutrophil populations in acute inflammation in mice. Inflamm. Res. 2012;61:931–939. doi: 10.1007/s00011-012-0484-0. [DOI] [PubMed] [Google Scholar]
- 116.Peñaloza HF, Salazar-Echegarai FJ, Bueno SM. Interleukin 10 modulation of neutrophil subsets infiltrating lungs during Streptococcus pneumoniae infection. Biochem. Biophys. Rep. 2018;13:12–16. doi: 10.1016/j.bbrep.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hong C-W. Current understanding in neutrophil differentiation and heterogeneity. Immune Netw. 2017;17:298–299. doi: 10.4110/in.2017.17.5.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Carmona-Rivera C, Kaplan MJ. Low-density granulocytes: a distinct class of neutrophils in systemic autoimmunity. Semin. Immunopathol. 2013;35:455–463. doi: 10.1007/s00281-013-0375-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rørvig S, Østergaard O, Heegaard NHH, Borregaard N. Proteome profiling of human neutrophil granule subsets, secretory vesicles, and cell membrane: correlation with transcriptome profiling of neutrophil precursors. J. Leukoc. Biol. 2013;94:711–721. doi: 10.1189/jlb.1212619. [DOI] [PubMed] [Google Scholar]
- 120.Faurschou M, Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 2003;5:1317–1327. doi: 10.1016/j.micinf.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 121.Cowland JB, Borregaard N. Granulopoiesis and granules of human neutrophils. Immunol. Rev. 2016;273:11–28. doi: 10.1111/imr.12440. [DOI] [PubMed] [Google Scholar]
- 122.Pham CTN. Neutrophil serine proteases: specific regulators of inflammation. Nat. Rev. Immunol. 2006;6:541–550. doi: 10.1038/nri1841. [DOI] [PubMed] [Google Scholar]
- 123.Keck T, Balcom JH, IV, Castillo CFD, Antoniu BA, Warshaw AL. Matrix metalloproteinase-9 promotes neutrophil migration and alveolar capillary leakage in pancreatitis-associated lung injury in the rat. Gastroenterology. 2002;122:188–201. doi: 10.1053/gast.2002.30348. [DOI] [PubMed] [Google Scholar]
- 124.Kruger P, et al. Neutrophils: Between host defence, immune modulation, and tissue injury. PLoS Pathog. 2015;11:e1004651. doi: 10.1371/journal.ppat.1004651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dabo AJ, Cummins N, Eden E, Geraghty P. Matrix metalloproteinase 9 exerts antiviral activity against respiratory syncytial virus. PLoS ONE. 2015;10:e0135970–18. doi: 10.1371/journal.pone.0135970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Borregaard N. Development of neutrophil granule diversity. Ann. N. Y. Acad. Sci. 2006;15:62–68. doi: 10.1111/j.1749-6632.1997.tb46237.x. [DOI] [PubMed] [Google Scholar]
- 127.Emboriadou M, et al. Human neutrophil elastase in RSV bronchiolitis. Ann. Clin. Lab. Sci. 2007;37:79–84. [PubMed] [Google Scholar]
- 128.Abu-Harb M, et al. IL-8 and neutrophil elastase levels in the respiratory tract of infants with RSV bronchiolitis. Eur. Respir. J. 1999;14:139–143. doi: 10.1034/j.1399-3003.1999.14a23.x. [DOI] [PubMed] [Google Scholar]
- 129.Sørensen OE, et al. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood. 2001;97:3951–3959. doi: 10.1182/blood.v97.12.3951. [DOI] [PubMed] [Google Scholar]
- 130.Kościuczuk EM, et al. Cathelicidins: family of antimicrobial peptides. A review. Mol. Biol. Rep. 2012;39:10957–10970. doi: 10.1007/s11033-012-1997-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wimley WC, Selsted ME, White SH. Interactions between human defensins and lipid bilayers: evidence for formation of multimeric pores. Protein Sci. 2002;3:1362–1373. doi: 10.1002/pro.5560030902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tripathi S, et al. The human cathelicidin LL-37 inhibits influenza A viruses through a mechanism distinct from that of surfactant protein D or defensins. J. Gen. Virol. 2012;94:40–49. doi: 10.1099/vir.0.045013-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Currie SM, et al. Cathelicidins have direct antiviral activity against respiratory syncytial virus in vitro and protective function in vivo in mice and humans. J. Immunol. 2016;196:2699–2710. doi: 10.4049/jimmunol.1502478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Currie SM, et al. The human cathelicidin LL-37 has antiviral activity against respiratory syncytial virus. PLoS ONE. 2013;8:e73659. doi: 10.1371/journal.pone.0073659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tecle T, White MR, Gantz D, Crouch EC, Hartshorn KL. Human neutrophil defensins increase neutrophil uptake of influenza A virus and bacteria and modify virus-induced respiratory burst responses. J. Immunol. 2007;178:8046–8052. doi: 10.4049/jimmunol.178.12.8046. [DOI] [PubMed] [Google Scholar]
- 136.Doss M, et al. Interactions of α-, β-, and θ-Defensins with influenza A virus and surfactant protein D. J. Immunol. 2009;182:7878–7887. doi: 10.4049/jimmunol.0804049. [DOI] [PubMed] [Google Scholar]
- 137.Sande CJ, et al. Airway response to respiratory syncytial virus has incidental antibacterial effects. Nat. Commun. 2019;10:1–11. doi: 10.1038/s41467-019-10222-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nguyen GT, Green ER, Mecsas J. Neutrophils to the ROScue: mechanisms of NADPH oxidase activation and bacterial resistance. Front. Cell Infect. Microbiol. 2017;7:339–24. doi: 10.3389/fcimb.2017.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Winterbourn CC, Kettle AJ, Hampton MB. Reactive oxygen species and neutrophil function. Annu. Rev. Biochem. 2016;85:765–792. doi: 10.1146/annurev-biochem-060815-014442. [DOI] [PubMed] [Google Scholar]
- 140.Paiva CN, Bozza MT. Are reactive oxygen species always detrimental to pathogens? Antioxid. Redox Signal. 2014;20:1000–1037. doi: 10.1089/ars.2013.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Akiakie T, et al. Pathogenesis of influenza virus‐induced pneumonia: involvement of both nitric oxide and oxygen radicals. Proc. Natl Acad. Sci. USA. 1996;93:2448–2453. doi: 10.1073/pnas.93.6.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hosakote YM, Liu T, Castro SM, Garofalo RP, Casola A. Respiratory syncytial virus induces oxidative stress by modulating antioxidant enzymes. Am. J. Respir. Cell Mol. Biol. 2009;41:348–357. doi: 10.1165/rcmb.2008-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mayadas TN, Cullere X, Lowell CA. The multifaceted functions of neutrophils. Annu. Rev. Pathol. 2014;9:181–218. doi: 10.1146/annurev-pathol-020712-164023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.LeVine AM, et al. Surfactant protein-d enhances phagocytosis and pulmonary clearance of respiratory syncytial virus. Am. J. Respir. Cell Mol. Biol. 2004;31:193–199. doi: 10.1165/rcmb.2003-0107OC. [DOI] [PubMed] [Google Scholar]
- 145.Hartshorn KL, White MR, Tecle T, Holmskov U, Crouch EC. Innate defense against influenza A virus: activity of human neutrophil defensins and interactions of defensins with surfactant protein D. J. Immunol. 2006;176:6962–6972. doi: 10.4049/jimmunol.176.11.6962. [DOI] [PubMed] [Google Scholar]
- 146.Hashimoto Y, Moki T, Takizawa T, Shiratsuchi A, Nakanishi Y. Evidence for phagocytosis of influenza virus-infected, apoptotic cells by neutrophils and macrophages in mice. J. Immunol. 2007;178:2448–2457. doi: 10.4049/jimmunol.178.4.2448. [DOI] [PubMed] [Google Scholar]
- 147.Papayannopoulos V. Sweet NETs, bitter wounds. Immunity. 2015;43:223–225. doi: 10.1016/j.immuni.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 148.Brinkmann V, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 149.Branzk N, Papayannopoulos V. Molecular mechanisms regulating NETosis in infection and disease. Semin. Immunopathol. 2013;35:513–530. doi: 10.1007/s00281-013-0384-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018;18:134–147. doi: 10.1038/nri.2017.105. [DOI] [PubMed] [Google Scholar]
- 151.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 2010;191:677–691. doi: 10.1083/jcb.201006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fuchs TA, et al. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yipp BG, et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat. Med. 2019;18:1386–1393. doi: 10.1038/nm.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pilsczek FH, et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J. Immunol. 2010;185:7413–7425. doi: 10.4049/jimmunol.1000675. [DOI] [PubMed] [Google Scholar]
- 155.Wills-Karp M. Neutrophil ghosts worsen asthma. Sci. Immunol. 2018;3:eaau0112. doi: 10.1126/sciimmunol.aau0112. [DOI] [PubMed] [Google Scholar]
- 156.Anjani G, et al. Recent advances in chronic granulomatous disease. Genes Dis. 2019;7:84–92. doi: 10.1016/j.gendis.2019.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Radermecker C, et al. Neutrophil extracellular traps infiltrate the lung airway, interstitial, and vascular compartments in severe COVID-19. J. Exp. Med. 2020;217:120–13. doi: 10.1084/jem.20201012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Veras FP, et al. SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology. J. Exp. Med. 2020;217:551–15. doi: 10.1084/jem.20201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhu L, et al. High level of neutrophil extracellular traps correlates with poor prognosis of severe influenza A infection. J. Infect. Dis. 2018;217:428–437. doi: 10.1093/infdis/jix475. [DOI] [PubMed] [Google Scholar]
- 160.Narasaraju T, et al. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am. J. Pathol. 2011;179:199–210. doi: 10.1016/j.ajpath.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pillai PS, et al. Mx1 reveals innate pathways to antiviral resistance and lethal influenza disease. Science. 2016;352:463–466. doi: 10.1126/science.aaf3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Radermecker C, et al. Locally instructed CXCR4hi neutrophils trigger environment-driven allergic asthma through the release of neutrophil extracellular traps. Nat. Immunol. 2019;20:1444–1455. doi: 10.1038/s41590-019-0496-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Akk A, Springer LE, Pham CTN. Neutrophil extracellular traps enhance early inflammatory response in sendai virus-induced asthma phenotype. Front. Immunol. 2016;7:325. doi: 10.3389/fimmu.2016.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Toussaint M, et al. Host DNA released by NETosis promotes rhinovirus-induced type-2 allergic asthma exacerbation. Nat. Med. 2017;23:681–691. doi: 10.1038/nm.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hoeksema M, et al. Arginine-rich histones have strong antiviral activity for influenza A viruses. Innate Immun. 2015;21:736–745. doi: 10.1177/1753425915593794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hemmers S, Teijaro JR, Arandjelovic S, Mowen KA. PAD4-mediated neutrophil extracellular trap formation is not required for immunity against influenza infection. PLoS ONE. 2011;6:e22043–10. doi: 10.1371/journal.pone.0022043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rojas-Quintero J, et al. Matrix metalloproteinase-9 deficiency protects mice from severe influenza A viral infection. JCI Insight. 2018;3:593–21. doi: 10.1172/jci.insight.99022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ashar HK, et al. The role of extracellular histones in influenza virus pathogenesis. Am. J. Pathol. 2018;188:135–148. doi: 10.1016/j.ajpath.2017.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Funchal GA, et al. Respiratory syncytial virus fusion protein promotes TLR-4-dependent neutrophil extracellular trap formation by human neutrophils. PLoS ONE. 2015;10:e0124082. doi: 10.1371/journal.pone.0124082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Geerdink RJ, et al. LAIR-1 limits neutrophil extracellular trap formation in viral bronchiolitis. J. Allergy Clin. Immunol. 2017;141:811–814. doi: 10.1016/j.jaci.2017.08.031. [DOI] [PubMed] [Google Scholar]
- 171.Muraro SXFP, et al. Respiratory syncytial virus induces the classical ROS-dependent NETosis through PAD-4 and necroptosis pathways activation. Sci. Rep. 2018;8:14166. doi: 10.1038/s41598-018-32576-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Porto BN, Stein RT. Neutrophil extracellular traps in pulmonary diseases: too much of a good thing? Front. Immunol. 2016;7:173–13. doi: 10.3389/fimmu.2016.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cassatella MA, Östberg NK, Tamassia N, Soehnlein O. Biological roles of neutrophil-derived granule proteins and cytokines. Trends Immunol. 2019;40:648–664. doi: 10.1016/j.it.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 174.Giacalone VD, Margaroli C, Mall MA, Tirouvanziam R. Neutrophil adaptations upon recruitment to the lung: new concepts and implications for homeostasis and disease. Int J. Mol. Sci. 2020;21:851–21. doi: 10.3390/ijms21030851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Brandes M, Klauschen F, Kuchen S, Germain RN. A systems analysis identifies a feedforward inflammatory circuit leading to lethal influenza infection. Cell. 2013;154:197–212. doi: 10.1016/j.cell.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kirsebom F, Michalaki C, Agueda-Oyarzabal M, Johansson C. Neutrophils do not impact viral load or the peak of disease severity during RSV infection. Sci. Rep. 2020;24:1110. doi: 10.1038/s41598-020-57969-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Stokes KL, et al. The respiratory syncytial virus fusion protein and neutrophils mediate the airway mucin response to pathogenic respiratory syncytial virus infection. J. Virol. 2013;87:10070–10082. doi: 10.1128/JVI.01347-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Halfhide CP, et al. Respiratory syncytial virus binds and undergoes transcription in neutrophils from the blood and airways of infants with severe bronchiolitis. J. Infect. Dis. 2011;204:451–458. doi: 10.1093/infdis/jir280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Minns D, Smith KJ, Findlay EG. Orchestration of adaptive T cell responses by neutrophil granule contents. Mediators Inflamm. 2019;2019:1–15. doi: 10.1155/2019/8968943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Leliefeld PHC, Koenderman L, Pillay J. How neutrophils shape adaptive immune responses. Front. Immunol. 2015;6:471. doi: 10.3389/fimmu.2015.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Schuster S, Hurrell B, Tacchini-Cottier F. Crosstalk between neutrophils and dendritic cells: a context-dependent process. J. Leukoc. Biol. 2013;94:671–675. doi: 10.1189/jlb.1012540. [DOI] [PubMed] [Google Scholar]
- 182.van Gisbergen KPJM, Sanchez-Hernandez M, Geijtenbeek TBH, van Kooyk Y. Neutrophils mediate immune modulation of dendritic cells through glycosylation-dependent interactions between Mac-1 and DC-SIGN. J. Exp. Med. 2005;201:1281–1292. doi: 10.1084/jem.20041276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bennouna S, Denkers EY. Microbial antigen triggers rapid mobilization of TNF-alpha to the surface of mouse neutrophils transforming them into inducers of high-level dendritic cell TNF-alpha production. J. Immunol. 2005;174:4845–4851. doi: 10.4049/jimmunol.174.8.4845. [DOI] [PubMed] [Google Scholar]
- 184.Charmoy M, et al. Neutrophil-derived CCL3 is essential for the rapid recruitment of dendritic cells to the site of leishmania major inoculation in resistant mice. PLoS Pathog. 2010;6:e1000755–12. doi: 10.1371/journal.ppat.1000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Potter NS, Harding CV. Neutrophils process exogenous bacteria via an alternate class I MHC processing pathway for presentation of peptides to T lymphocytes. J. Immunol. 2001;167:2538–2546. doi: 10.4049/jimmunol.167.5.2538. [DOI] [PubMed] [Google Scholar]
- 186.Culshaw S, Millington OR, Brewer JM, McInnes IB. Murine neutrophils present class II restricted antigen. Immunol. Lett. 2008;118:49–54. doi: 10.1016/j.imlet.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hufford MM, et al. Influenza-infected neutrophils within the infected lungs act as antigen presenting cells for anti-viral CD8+ T cells. PLoS ONE. 2012;7:e46581-10. doi: 10.1371/journal.pone.0046581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lim K, et al. In situ neutrophil efferocytosis shapes T cell immunity to influenza infection. Nat. Immunol. 2020;21:1046–1057. doi: 10.1038/s41590-020-0746-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lim K, et al. Neutrophil trails guide influenza-specific CD8+ T cells in the airways. Science. 2015;349:aaa4352. doi: 10.1126/science.aaa4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Tate MD, Brooks AG, Reading PC, Mintern JD. Neutrophils sustain effective CD8(+) T-cell responses in the respiratory tract following influenza infection. Immunol. Cell Biol. 2012;90:197–205. doi: 10.1038/icb.2011.26. [DOI] [PubMed] [Google Scholar]
- 191.Tak T, et al. Neutrophil-mediated suppression of influenza-induced pathology requires CD11b/CD18 (MAC-1) Am. J. Respir. Cell Mol. Biol. 2018;58:492–499. doi: 10.1165/rcmb.2017-0021OC. [DOI] [PubMed] [Google Scholar]
- 192.Reilly EC, Lambert-Emo K, Topham DJ. The effects of acute neutrophil depletion on resolution of acute influenza infection, establishment of tissue resident memory (TRM), and heterosubtypic immunity. PLoS ONE. 2016;11:e0164247. doi: 10.1371/journal.pone.0164247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Cerutti A, Puga I, Magri G. The B cell helper side of neutrophils. J. Leukoc. Biol. 2013;94:677–682. doi: 10.1189/jlb.1112596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Costa S, Bevilacqua D, Cassatella MA, Scapini P. Recent advances on the crosstalk between neutrophils and B or T lymphocytes. Immunology. 2018;156:23–32. doi: 10.1111/imm.13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Puga I, et al. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat. Immunol. 2012;13:170–180. doi: 10.1038/ni.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ratcliffe DR, Michl J, Cramer EB. Neutrophils do not bind to or phagocytize human immune complexes formed with influenza virus. Blood. 1993;82:1639–1646. [PubMed] [Google Scholar]
- 197.Fujisawa H. Neutrophils play an essential role in cooperation with antibody in both protection against and recovery from pulmonary infection with influenza virus in mice. J. Virol. 2008;82:2772–2783. doi: 10.1128/JVI.01210-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Stokes KL, et al. Differential pathogenesis of respiratory syncytial virus clinical isolates in BALB/c mice. J. Virol. 2011;85:5782–5793. doi: 10.1128/JVI.01693-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Taylor G. Animal models of respiratory syncytial virus infection. Vaccine. 2017;35:469–480. doi: 10.1016/j.vaccine.2016.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Makris S, Paulsen M, Johansson C, Type I. Interferons as regulators of lung inflammation. Front. Immunol. 2017;8:259. doi: 10.3389/fimmu.2017.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Jasper AE, McIver WJ, Sapey E, Walton GM. Understanding the role of neutrophils in chronic inflammatory airway disease. F1000Res. 2019;8:557–17. doi: 10.12688/f1000research.18411.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Gehrig S, et al. Lack of neutrophil elastase reduces inflammation, mucus hypersecretion, and emphysema, but not mucus obstruction, in mice with cystic fibrosis-like lung disease. Am. J. Respir. Crit. Care Med. 2014;189:1082–1092. doi: 10.1164/rccm.201311-1932OC. [DOI] [PubMed] [Google Scholar]
- 203.Ascough S, Paterson S, Chiu C. Induction and subversion of human protective immunity: contrasting influenza and respiratory syncytial virus. Front. Immunol. 2018;9:117. doi: 10.3389/fimmu.2018.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sun S-H, et al. A mouse model of SARS-CoV-2 infection and pathogenesis. Cell Host Microbe. 2020;28:124–133.e4. doi: 10.1016/j.chom.2020.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Jones HR, Robb CT, Perretti M, Rossi AG. The role of neutrophils in inflammation resolution. Semin. Immunol. 2016;28:137–145. doi: 10.1016/j.smim.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 206.Peiseler M, Kubes P. More friend than foe: the emerging role of neutrophils in tissue repair. J. Clin. Investig. 2019;129:2629–2639. doi: 10.1172/JCI124616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Marwick JA, et al. Neutrophils induce macrophage anti-inflammatory reprogramming by suppressing NF-kB activation. Cell Death Dis. 2018;9:1–13. doi: 10.1038/s41419-018-0710-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Deng Y, et al. Neutrophil-airway epithelial interactions result in increased epithelial damage and viral clearance during respiratory syncytial virus infection. J. Virol. 2020;94:946. doi: 10.1128/JVI.02161-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Campbell EL, et al. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity. 2014;40:66–77. doi: 10.1016/j.immuni.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hoenderdos K, et al. Hypoxia upregulates neutrophil degranulation and potential for tissue injury. Thorax. 2016;71:1030–1038. doi: 10.1136/thoraxjnl-2015-207604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Thorburn K, Harigopal S, Reddy V, Taylor N, van Saene HKF. High incidence of pulmonary bacterial co-infection in children with severe respiratory syncytial virus (RSV) bronchiolitis. Thorax. 2006;61:611–615. doi: 10.1136/thx.2005.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hall CB, Powell KR, Schnabel KC, Gala CL, Pincus PH. Risk of secondary bacterial infection in infants hospitalized with respiratory syncytial viral infection. J. Pediatr. 1988;113:266–271. [PubMed] [Google Scholar]
- 213.Hendaus M, Jomha F, Alhammadi A. Virus-induced secondary bacterial infection: a concise review. Thera. Clin. Risk Manag. 2015;11:1265–1271. doi: 10.2147/TCRM.S87789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J. Infect. Dis. 2008;198:962–970. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.McNamee LA, Harmsen AG. Both influenza-induced neutrophil dysfunction and neutrophil-independent mechanisms contribute to increased susceptibility to a secondary Streptococcus pneumoniae infection. Infect. Immun. 2006;74:6707–6721. doi: 10.1128/IAI.00789-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ellis GT, et al. TRAIL + monocytes and monocyte-related cells cause lung damage and thereby increase susceptibility to influenza—Streptococcus pneumoniae coinfection. EMBO Rep. 2015;16:1203–1218. doi: 10.15252/embr.201540473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Shahangian A, et al. Type I IFNs mediate development of postinfluenza bacterial pneumonia in mice. JCI. 2009;119:1910–1920. doi: 10.1172/JCI35412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kukavica-Ibrulj I, et al. Infection with human metapneumovirus predisposes mice to severe pneumococcal pneumonia. J. Virol. 2009;83:1341–1349. doi: 10.1128/JVI.01123-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Engelich G, White M, Hartshorn KL. Neutrophil survival is markedly reduced by incubation with influenza virus and Streptococcus pneumoniae: role of respiratory burst. J. Leukoc. Biol. 2001;69:50–56. [PubMed] [Google Scholar]
- 220.Ishikawa H, et al. Influenza virus infection causes neutrophil dysfunction through reduced G-CSF production and an increased risk of secondary bacteria infection in the lung. Virology. 2016;499:23–29. doi: 10.1016/j.virol.2016.08.025. [DOI] [PubMed] [Google Scholar]
- 221.Villeret B, et al. Influenza a virus pre-infection exacerbates Pseudomonas aeruginosa-mediated lung damage through increased MMP-9 expression, decreased elafin production and tissue resilience. Front. Immunol. 2020;11:117. doi: 10.3389/fimmu.2020.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Grunwell JR, et al. Neutrophil dysfunction in the airways of children with acute respiratory failure due to lower respiratory tract viral and bacterial coinfections. Sci. Rep. 2019;9:1–13. doi: 10.1038/s41598-019-39726-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Cortjens B, et al. Neutrophil subset responses in infants with severe viral respiratory infection. Clin. Immunol. 2017;176:100–106. doi: 10.1016/j.clim.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 224.Schmid MA, et al. Influenza and dengue virus co-infection impairs monocyte recruitment to the lung, increases dengue virus titers, and exacerbates pneumonia. Eur. J. Immunol. 2017;47:527–539. doi: 10.1002/eji.201646675. [DOI] [PubMed] [Google Scholar]
- 225.Tobin JM, et al. Influenza suppresses neutrophil recruitment to the lung and exacerbates secondary invasive pulmonary aspergillosis. J. Immunol. 2020;205:480–488. doi: 10.4049/jimmunol.2000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Habibi MS, et al. Neutrophilic inflammation in the respiratory mucosa predisposes to RSV infection. Science. 2020;370:eaba9301. doi: 10.1126/science.aba9301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.J Jackson D, Johnston SL. The role of viruses in acute exacerbations of asthma. JACI. 2010;125:1178–1187. doi: 10.1016/j.jaci.2010.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Busse WW, Lemanske RF, Gern JE. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet. 2010;376:826–834. doi: 10.1016/S0140-6736(10)61380-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Ray A, Kolls JK. Neutrophilic inflammation in asthma and association with disease severity. Trends Immunol. 2017;38:942–954. doi: 10.1016/j.it.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Jartti T, Gern JE. Role of viral infections in the development and exacerbation of asthma in children. JACI. 2017;140:895–906. doi: 10.1016/j.jaci.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Graff Stensballe L, et al. The causal direction in the association between respiratory syncytial virus hospitalization and asthma. JACI. 2009;123:131–137. doi: 10.1016/j.jaci.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 232.Krishnamoorthy N, et al. Neutrophil cytoplasts induce TH17 differentiation and skew inflammation toward neutrophilia in severe asthma. Sci. Immunol. 2018;3:eaao4747. doi: 10.1126/sciimmunol.aao4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Tang FSM, et al. Differential neutrophil activation in viral infections: enhanced TLR-7/8-mediated CXCL8 release in asthma. Respirology. 2015;21:172–179. doi: 10.1111/resp.12657. [DOI] [PMC free article] [PubMed] [Google Scholar]