Abstract
Purpose:
Interleukin-10 (IL-10) is a potent anti-inflammatory cytokine shown to inhibit scar formation in fetal wound healing. The role of IL-10 in adult tendon healing and scar formation, however, remains unknown. The objective of this study is to investigate the effect of IL-10 overexpression on the properties of adult healing tendon using a well established murine model of tendon injury and a lentiviral-mediated method of IL-10 overexpression.
Methods:
A murine model of patellar tendon injury was utilized and animals divided into 3 groups. Mice underwent bilateral patellar tendon injections with a lentiviral vector containing an IL-10 transgene (n=34) or no transgene (n=34). Control mice (n=34) received injections of sterile saline. All animals then underwent bilateral, central patellar tendon injuries 2 days post-injection and were sacrificed at 5, 10, 21, and 42 days post-injury. IL-10 content was analyzed by immunohistochemistry (n=4/group). Tendon healing was evaluated by histology (n=4/group) and biomechanical analysis (n=10/group).
Results:
Overexpression of IL-10 in patellar tendon was confirmed following injection of the lentiviral vector. IL-10 immunostaining was increased at day 10 in the IL-10 group relative to controls. Histologically, there was no significant difference in angular deviation between groups at day 21, but a trend toward decreased angular deviation in controls relative to empty vector was seen at day 42 (p≤0.1). Biomechanically, the IL-10 group showed significantly increased maximum stress at day 42 relative to controls (p≤0.05). Percent relaxation showed a trend toward an increase at day 10 (p≤0.1) and a significant increase at day 42 (p≤0.05) in the IL-10 group relative to controls.
Conclusions:
This study demonstrates successful gene transfer of IL-10 into adult murine patellar tendon using a lentiviral vector. While the effects of overexpression of IL-10 on adult tendon healing have not yet been fully elucidated, the current study may help to further clarify the mechanisms of tendon injury and repair.
Keywords: Interleukin-10, Tendon injury
Introduction
Tendon healing after injury and repair is often complicated by scar and adhesion formation that hinder restoration of function, a problem seen following injury and repair in both sheathed, flexor tendons and unsheathed, extensor tendons of the hand.1–10 While the initial formation of scar between tendon ends provides physical continuity at the site of disruption, proliferation of scar between the tendon and adjacent tissues is undesirable because these attachments can impede the gliding mechanism of the tendon, whether sheathed or not.11 The majority of research in the area of flexor and extensor tendon repair in the hand has focused on the development of improved suture repair techniques and the enhancement of postoperative rehabilitation protocols allowing early motion.4,5,12–23 These improved surgical and rehabilitation methods have led to better clinical outcomes, but scarring and adhesion formation remain significant complications.1,2 Even with the best surgical techniques and the optimal therapeutic protocols, results can be unpredictable.1–3,11
There is extensive experimental evidence that early and midgestational fetal tissue responds to injury in a fundamentally different way.11,24–28 Fetal wound healing has been shown to occur at a faster rate than adult healing and in the absence of scar formation. This scarless healing response has been observed in a number of fetal tissues, including tendon.11,25,29–32 Although the precise mechanisms of scarless fetal wound healing are not completely understood, the response has been attributed in part to the absence of a substantial inflammatory response. Studies in fetal wound healing suggest that the lack of inflammation is associated with alterations in the balance of pro- and anti-inflammatory cytokines in the fetal environment.33–38 Pro-inflammatory cytokines, such as interleukin-6 and interleukin-8, have been shown to be decreased in scarless fetal wound repair in dermal tissue,33,34 while anti-inflammatory cytokines have also been suggested to play a critical role.35,39,40 In particular, the presence of interleukin-10 (IL-10) appears to be necessary for scarless fetal wound repair to occur, and IL-10 overexpression in adult dermal tissue appears to be sufficient to produce a scarless healing response.35,39,40
While IL-10 appears to be important in scarless wound healing, the role of IL-10 in adult tendon healing and scar formation remains unknown. Therefore, the objective of this study is to investigate the effect of IL-10 overexpression on the properties of healing adult paratenon covered tendon. We hypothesized that IL-10 overexpression would lead to decreased inflammation and improved biomechanical and histological properties in adult healing tendon in comparison to the normal healing state. To test this hypothesis, we used a well established murine model of tendon injury41 and a lentiviral-mediated method of IL-10 gene delivery to create a state of IL-10 overexpression.
Materials and Methods
This study was approved by the Institutional Animal Care and Use Committee (IACUC) and utilized one hundred and twenty-six male, 10 week-old C57BL/6 mice (The Jackson Laboratory, Bar Harbor, ME). To complete the objectives of this study, a consistent and reproducible C57BL/6 murine model of patellar tendon injury was used to assess tendon healing41,42 and gene transfer with a lentiviral vector was used to create IL-10 overexpression.
Lentiviral Vector Preparation
A cDNA library was prepared from C57BL/6 bone marrow-derived dendritic cells cultured in the presence of LPS (1 μg/ml) using TRIzol Reagent and SuperScript (Invitrogen, Carlsbad, CA) per the manufacturer’s recommendations. The mouse IL-10 coding domain was amplified with the forward primer 5’-GCGGCCGCGGTACCATGCCTGGCTCAGCACTG-3’ and the reverse primer 5’-GCTAGCTTAGCTTTTCATTTTGATCATCATG-3’ using standard methods and confirmed by sequence analysis (GenBank Sequence accession number NM010548). The flanking NotI and NheI sites were inserted to simplify cloning into the HIV-1 based transfer plasmid. The CS-CG HIV-1 transfer plasmid, modified as previously described, was used to generate a self-inactivating lentiviral vector.43–46 This lentiviral vector allows expression of an eGFP reporter gene (Clontech Laboratories, Mountain View, California) or IL-10 and eGFP genes as a single transcript under the control of the human CMV promoter. For bicistronic gene expression, the internal ribosome entry sight (ires), identical to that of the encephalomyocarditis virus, was inserted between the IL-10 gene and the eGFP reporter. VSV-G protein pseudotyped viral particles were generated by transfection into a 293T cell line and titered as previously described47.
Gene Expression Pilot Study
A preliminary study was performed with the lentiviral vector to confirm incorporation of the IL-10 transgene into the patellar tendon and to determine the time point, post-injection of the lentiviral vector, when IL-10 gene expression peaked. The lentiviral vector expressing both the eGFP reporter gene and the IL-10 transgene was used and twenty-four C57BL/6 mice placed into 8 groups underwent injections. In twenty-one mice, 10μL of 1 × 1010 viral copies/mL titer of the lentiviral vector was injected into the patellar tendon of each hindlimb (right and left). Three mice each were then sacrificed at 1, 2, 3, 4, 5, 7, and 10 days post-injection. A final group of three mice served as negative controls and were sacrificed on the day of injection (day 0) after undergoing bilateral patellar tendon injections with sterile saline (10μl per side). In preparation for injections, mice were anesthetized with a mixture of isoflurane (5%) and oxygen (0.6%), weighed, and both hindlimbs shaved. During the procedures, anesthesia was delivered via a nose cone with the level of isoflurane reduced to 1% with the oxygen. For each hindlimb, a skin incision was made over the patellar tendon, the retinaculum was incised on both sides of the tendon, and a plastic coated blade was placed underneath the patellar tendon for support. In this position, the tendon was injected with the lentiviral vector or sterile saline using a syringe with 30-gauge needle placed in a longitudinal direction into the midsubstance of the tendon. Following injection, the skin wound was closed and the procedure repeated on the opposite hindlimb. Following the contralateral injection and skin closure, mice were allowed to resume normal cage activity until they were sacrificed. Mice were sacrificed by CO2 inhalation.
Following sacrifice, patellar tendons were dissected out and analyzed for eGFP and IL-10 gene expression using standard protocols for reverse transcription polymerase chain reaction (RT-PCR). The six experimentally injected tendons (two tendons in each of three animals) at each time point were combined for RNA extraction using TRIzol (Invitrogen, Carlsbad, CA) and reverse transcription using SuperScript II RT (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Semi-quantitative PCR was then performed with primers specific for the eGFP sequence within the IL-10 vector, the IL-10 sequence within the vector, endogenous murine IL-10, and β-actin. Gene expression was normalized to expression of the housekeeping gene, β-actin. Successful transfer of the IL-10 vector into the patellar tendon was confirmed, with peak expression of the eGFP reporter gene and IL-10 transgene at two days post-injection (see Results section).
Patellar Tendon Injury Experiments
Following the pilot study, one hundred and two C57BL/6 male mice divided into three treatment groups were utilized for the patellar tendon injury study: (1) a saline control group (n=34), which underwent patellar tendon injections with sterile saline; (2) an empty vector group (n=34), which underwent patellar tendon injections with the lentiviral vector expressing only the eGFP reporter gene; and (3) an IL-10 group (n=34), which underwent patellar tendon injections with the lentiviral vector expressing both the eGFP reporter gene and the IL-10 transgene. Control group mice received bilateral 10μl injections of sterile saline into their patellar tendons, empty vector group mice received bilateral 10μl injections of 1×1010 viral copies/ml titer of the empty vector (eGFP gene only), and IL-10 vector group mice received bilateral 10μl injections of 1×1010 viral copies/ml titer of the IL-10 vector (eGFP gene and IL-10 transgene) as described in the previous paragraphs.
All mice then underwent bilateral patellar tendon injuries 2 days post-injection, at the peak of eGFP and IL-10 gene expression. The tendon injury model used is one developed and established in our laboratory.41 Animals were prepared and anesthetized for surgery as described for the injection procedure, and the skin incision and exposure of the patellar tendon was made identically. With the patellar tendon supported by a plastic coated blade, a 0.75 mm diameter biopsy punch (Shoney Scientific, Waukesha, WI) was used to make a central defect (approximately 60% of tendon width) in the patellar tendon, creating a distinct and reproducible injury.41 Following bilateral injuries and skin closure, mice were allowed to resume normal cage activity until they were sacrificed. At 5, 10, 21, and 42 days following tendon injury, mice were sacrificed by CO2 inhalation. Four mice per treatment group were sacrificed at 5 days post-injury for immunohistochemical analysis. Ten mice per treatment group were sacrificed at 10, 21, and 42 days post-injury for immunohistochemical, histological, and biomechanical analysis. The post-injury time points for immunohistochemical, histological, and biomechanical analysis were chosen based on previous studies utilizing the patellar tendon injury model.41,42
Immunohistochemistry
For immunohistochemistry, the degree of immunostaining for IL-10 was evaluated at the patellar tendon injury site. Four patellar tendons per treatment group were analyzed at 5, 10, 21, and 42 days post-injury. Following sacrifice, patellar tendons were dissected free from the right or left hindlimb of each animal and processed with standard histological techniques (fixation, dehydration, clearing, infiltration). Specimens were subsequently embedded in paraffin blocks and serial, sagittal 7-μm sections were cut parallel to tendon fibers and collected on UltraStick slides (Gold Seal, Portsmouth, NH), three sections per slide. A rat anti-mouse IL-10 primary antibody (Endogen, Woburn, MA) was used to stain for IL-10 and followed standard methodology.41 One section per slide was reacted with the IL-10 primary antibody (1:200 dilution), with the other two sections serving as controls. One control section was reacted with rat IgG antibody (Jackson ImmunoResearch, West Grove, PA) to serve as an isotype control to detect non-specific background staining, and the other was treated with sterile saline to serve as a negative control. Following incubation with the primary antibody, isotype, or saline (60 minutes, room temperature), reactivity was detected and developed with the SuperPicTure Polymer Detection Kit (Zymed, San Francisco, CA) which utilizes a HRP polymer conjugate and diaminobenzidine tetrahydrochloride (DAB) chromogen. Slides were counterstained with hematoxylin.
A qualitative analysis of the sections was then performed by three independent, blinded graders to assess the degree of IL-10 immunostaining at the patellar tendon injury site, as previously described.41 IL-10 staining intensity was normalized by comparison of each tendon section treated with IL-10 antibody to the isotype control section on the same slide. Sections were subsequently graded for degree of IL-10 staining on a scale of 0–3, with 0 defined as undetectable, 1 low, 2 moderate, and 3 high relative to background staining. Standard images representing each grading level (0–3) were prepared and used to provide consistency across graders. An overall grade for each tissue section was determined by averaging the values from the three graders. Values were then averaged for each treatment group at each time point. A difference of ≥ 1 of the average grade between treatment groups at each post-injury time point was chosen as a meaningful result.
Histology
For histology, the angular deviation of the collagen fiber orientations at the injury site of patellar tendon specimens was determined. Angular deviation is a measure of collagen fiber distribution spread used to reflect overall collagen fiber organization and alignment. Well aligned, parallel fibers seen in uninjured tendon will have low angles of divergence between collagen fibers and low angular deviation, while poorly aligned fibers seen in disorganized scar tissue will have high angles of divergence between collagen fibers and high angular deviation.
Four patellar tendons per treatment group were analyzed at 21 and 42 days post-injury. Specimens were processed as described above and serial, sagittal, paraffin-embedded 7-μm sections were cut and stained with hematoxylin and eosin by standard protocol. Tissue sections were then analyzed using a quantitative polarized light microscopy method as described previously.41,42,48,49 Briefly, using a green bandpass filter (BP 546 nm), grayscale images of the tendon were taken at 10° increments with crossed analyzer and polarizer and simultaneously rotated through 90°. Subsequently, the filter was removed and images taken again at 10° increments while a λ compensator was rotated through 90° along with the crossed analyzer and polarizer. Custom-designed software was then used to determine collagen fiber orientations and the angular deviation of the collagen fiber orientations was calculated.
Biomechanical Analysis
For biomechanical testing, ten patellar tendons per treatment group were evaluated at 10, 21, and 42 days post-injury as previously described.41,42,50 Following sacrifice, patellar tendons were dissected free from the left hindlimb of each animal, leaving only the patella, patellar tendon, and tibia as one connected unit. The tendon was prepared as a standardized dumbbell-shaped specimen with Verhoeff stain lines placed on either end of the dumbbell to serve as a gauge section for optical strain analysis. Tendon width and thickness were quantified, and cross-sectional area calculated as the product of the two.41,42,51 The tibia was then embedded in polymethylmethacrylate in a custom-designed fixture and secured in place with a metal staple. The patella was held in place with a custom-designed cone-shaped wedge fixture and the potted tibial end was secured to a custom-designed base. Each tendon specimen then underwent a standard loading protocol while immersed in a physiologic 37°C saline bath; preloaded to 0.02 N at a rate of 0.1%/s (0.003 mm/s), preconditioned for 10 cycles from 0.02 to 0.04 N at a rate of 0.1%/s (0.003 mm/s), and held for 300 s. Immediately following this preconditioning, a stress-relaxation experiment was performed by elongating the tendon to a strain of 5% (0.15 mm) at a rate of 25%/s (0.75 mm/s), followed by a relaxation for 600 s. Finally, a ramp to failure was applied at a rate of 0.1%/s (0.003 mm/s).41,42 Local tissue strain was measured optically as described previously.11,41,42,50 From the ramp to failure test, maximum stress was determined and modulus was calculated using linear regression from the near-linear region of the stress-strain curve. The peak and equilibrium stresses were determined from the stress relaxation test and used to calculate a percent relaxation.
Statistical Analysis
The angular deviation and biomechanical data were averaged for each treatment group at each post-injury time point and a one-way analysis of variance (ANOVA) comparing treatments followed by Fisher’s post-hoc test were used to detect differences between the three treatment groups at each post-injury time point. Statistical significance was set at p≤0.05, with a trend set at p≤0.1.
Results
At the time of surgery and sacrifice, there were no visible differences among the three groups of mice and no differences in body mass. Eight patellar tendons (two specimens from the saline control group and six specimens from the empty vector group) were found to either be spontaneously ruptured at the time of dissection or damaged during testing and were excluded from the study.
Confirmation of IL-10 Overexpression
Successful transfer into patellar tendon of the lentiviral vector containing the eGFP reporter gene and the IL-10 transgene was confirmed in the gene expression pilot study, with parallel temporal expression of the eGFP and IL-10 PCR products. Expression of the eGFP reporter gene and IL-10 transgene were found to peak at two days post-injection of the vector, with the IL-10 transgene demonstrating more than six times greater expression in comparison to endogenous IL-10 at this time point (Figure 1).
Figure 1:
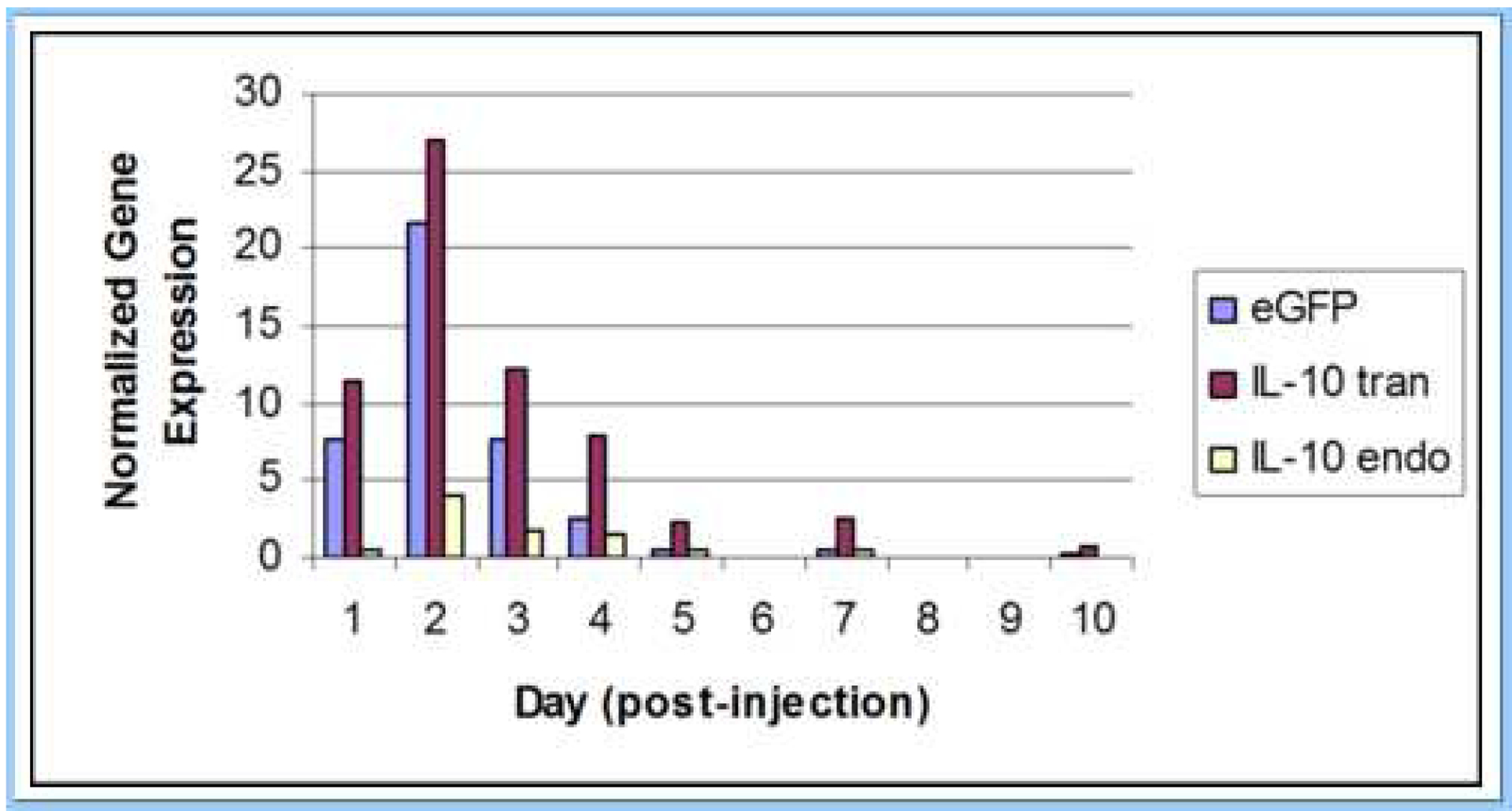
Expression of the eGFP reporter gene (eGFP), IL-10 transgene (IL-10 tran), and endogenous IL-10 (IL-10 endo) in adult mouse patellar tendon following injection of the lentiviral vector. Expression of the eGFP reporter gene and IL-10 transgene were found to peak at two days post-injection of the lentiviral vector. Gene expression was normalized to expression of the housekeeping gene, β-actin.
Immunohistochemistry
IL-10 immunostaining was increased at the patellar tendon injury site at day 10 in both the IL-10 and empty vector groups relative to saline controls (difference of ≥ 1 in the average staining grade between groups) (Figure 2). No other meaningful differences in IL-10 staining were found between the three treatment groups at any other time points, including no differences between the IL-10 and empty vector groups at all four post-injury time points. IL-10 staining peaked at day 10 in both the IL-10 and empty vector groups (Table 1).
Figure 2:
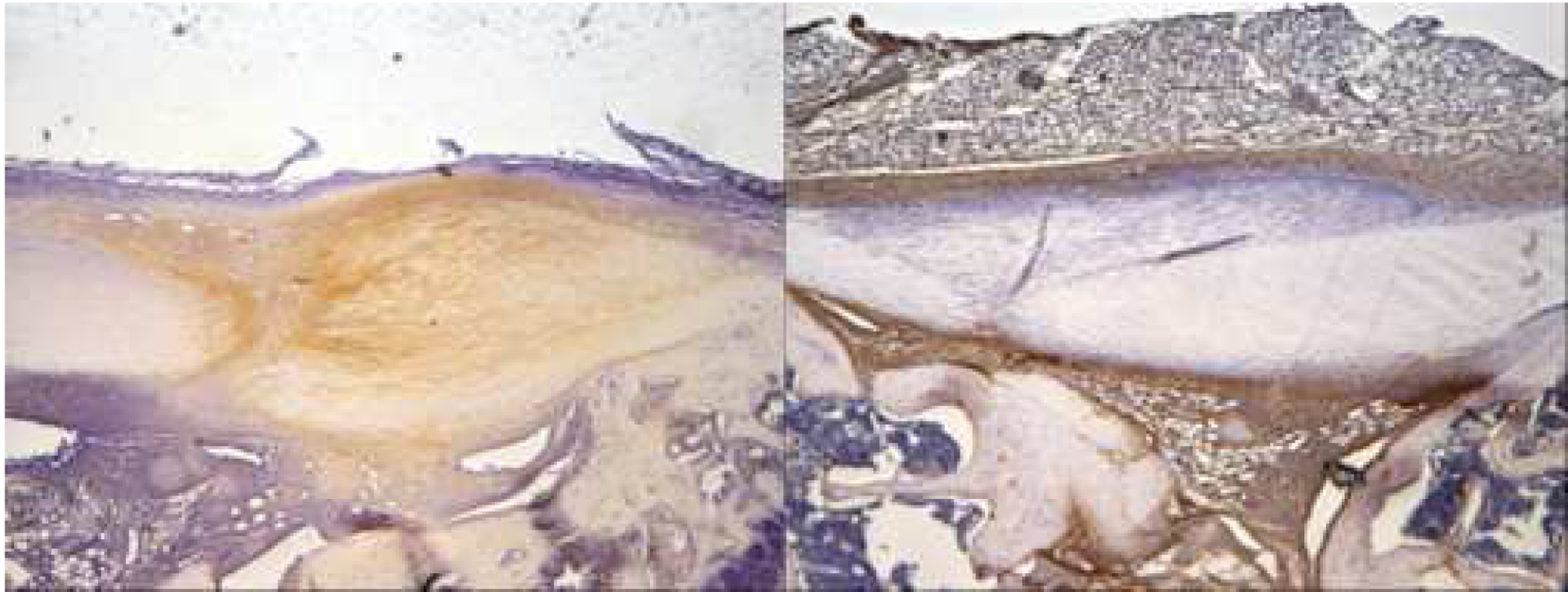
Representative immunohistochemistry slides from day 10 post-injury demonstrate increased IL-10 immunostaining (brown staining) at the patellar tendon injury site in the IL-10 group (left slide) relative to saline controls (right slide).
Table 1: IL-10 immunostaining grading at 5, 10, 21, and 42 days post-injury.
Staining for IL-10 at the patellar tendon injury site was increased at day 10 in both the IL-10 and empty vector groups relative to saline controls (highlighted in red). An increase or decrease in IL-10 staining across treatments was defined as a difference of ≥ 1 in the average staining grade between the groups.
| IL-10 Staining | Post-Injury Time Point (Days) | |||
|---|---|---|---|---|
| Treatment Group | 5 | 10 | 21 | 42 |
| Saline Control | 1.8 | 1.1 | 1.7 | 0.6 |
| Empty Vector | 1.9 | 2.1 | 1.9 | 1.4 |
| IL-10 | 2.3 | 2.6 | 1.1 | 1.3 |
Histology
There were no significant differences between the three treatment groups in collagen fiber organization as measured by angular deviation at day 21 post-injury. There was a trend (p≤0.1) towards decreased angular deviation at day 42 post-injury in the saline controls relative to the empty vector group (Table 2).
Table 2: Angular deviation at 21 and 42 days post-injury.
There were no significant differences between treatment groups at day 21. There was a trend (p≤0.1) towards decreased angular deviation at day 42 in the saline controls relative to the empty vector group, as denoted by the pound signs (#).
| Angular Deviation | Post-Injury Time Point (Days) | |
|---|---|---|
| Treatment Group | 21 | 42 |
| Saline Control | 7.6° ± 6.2° | 3.8° ± 1.1° # |
| Empty Vector | 9.9° ± 5.5° | 7.8° ± 4.8° # |
| IL-10 | 5.0° ± 0.8° | 4.8° ± 2.1° |
Biomechanical Analysis
A number of significant differences between the three treatment groups were noted on biomechanical testing. The empty vector group showed a trend (p≤0.1) toward increased maximum stress (MPa) at day 10 post-injury relative to the saline controls. Maximum stress was significantly increased (p≤0.05) in the IL-10 group at day 42 post-injury relative to the saline controls (Figure 3).
Figure 3:
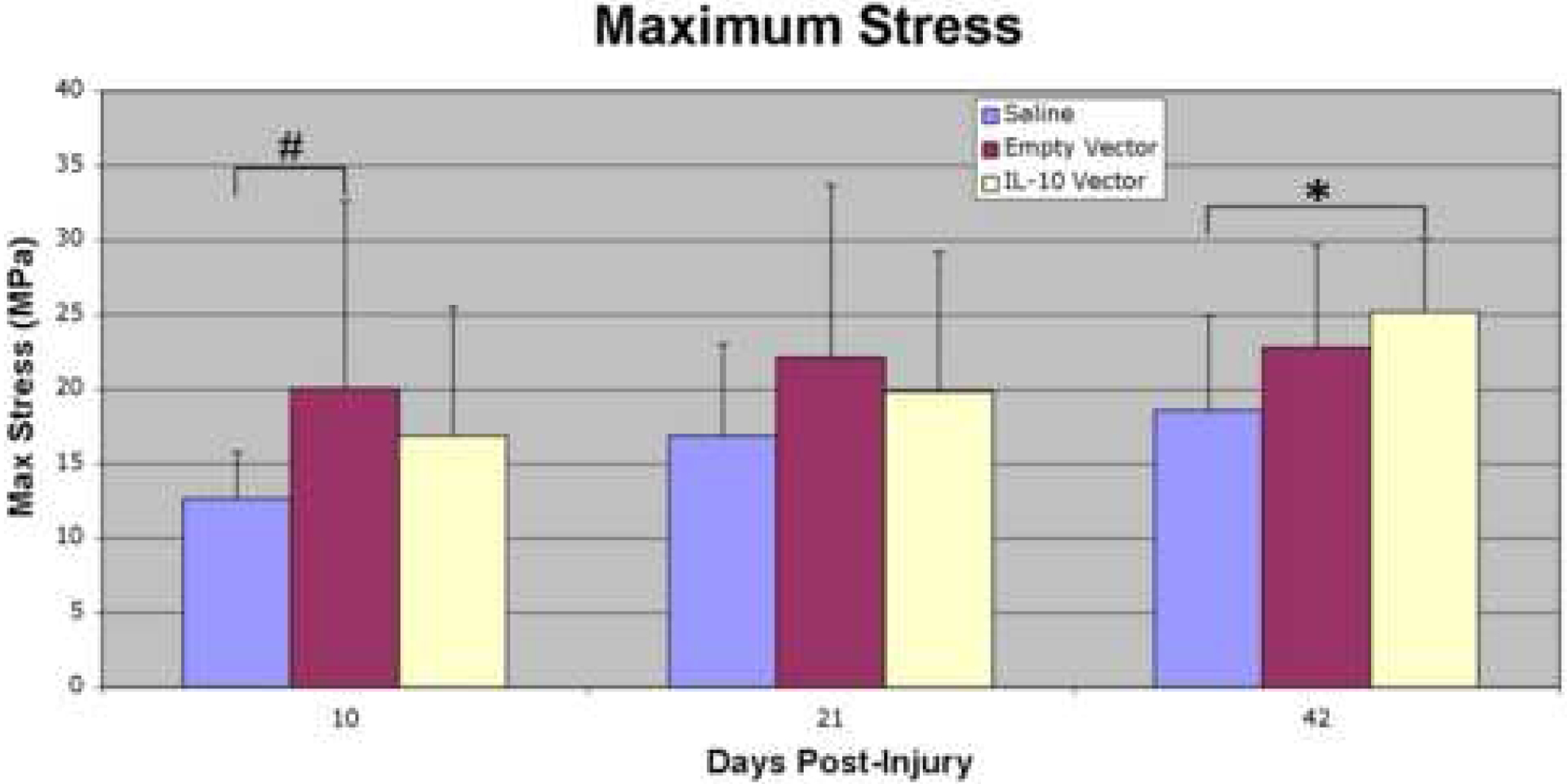
Maximum stress (MPa) at 10, 21, and 42 days post-injury. The empty vector group showed a trend toward increased maximum stress at day 10 post-injury relative to the saline controls, and maximum stress was significantly increased in the IL-10 group at day 42 post-injury relative to the saline controls. The asterisk (*) indicates a significant difference (p≤0.05) and the pound sign (#) denotes a trend (p≤0.1) when comparing between treatment groups within a post-injury time point.
Percent relaxation was significantly increased (p≤0.05) in the empty vector group relative to the saline controls at day 10 and day 42 post-injury. It showed a trend (p≤0.1) toward an increase at day 10 and a significant increase (p≤0.05) at day 42 post-injury in the IL-10 group relative to the saline controls (Figure 4).
Figure 4:
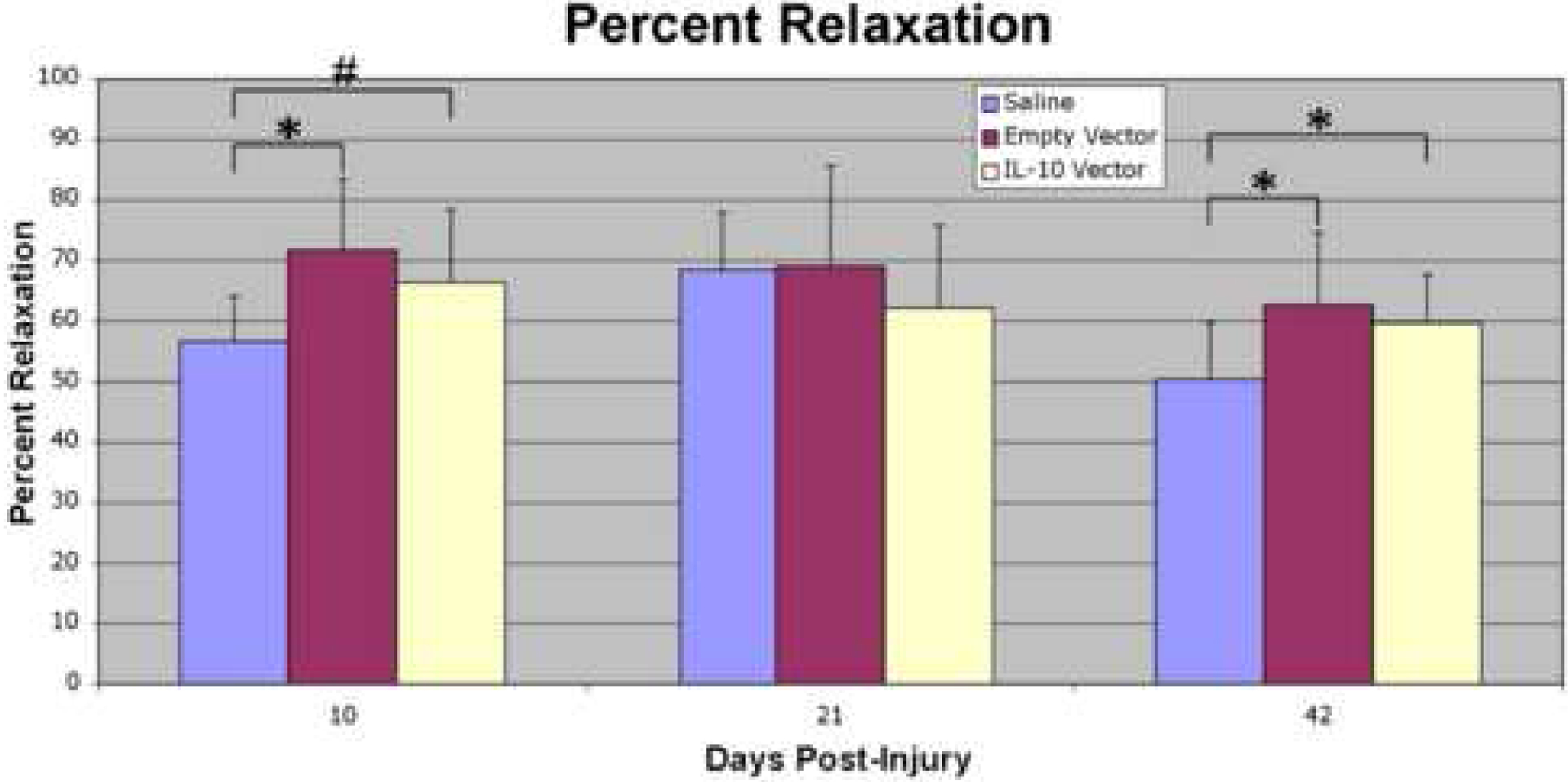
Percent relaxation (%) at 10, 21, and 42 days post-injury. Percent relaxation was significantly increased in the empty vector group relative to the saline controls at day 10 and 42 post-injury. It showed a trend toward an increase at day 10 and a significant increase at day 42 post-injury in the IL-10 group relative to the saline controls. The asterisk (*) indicates a significant difference (p≤0.05) and the pound sign (#) denotes a trend (p≤0.1) when comparing between treatment groups within a post-injury time point.
No significant differences or trends were found in modulus (MPa) between the three treatment groups at any of the post-injury time points (Figure 5).
Figure 5:
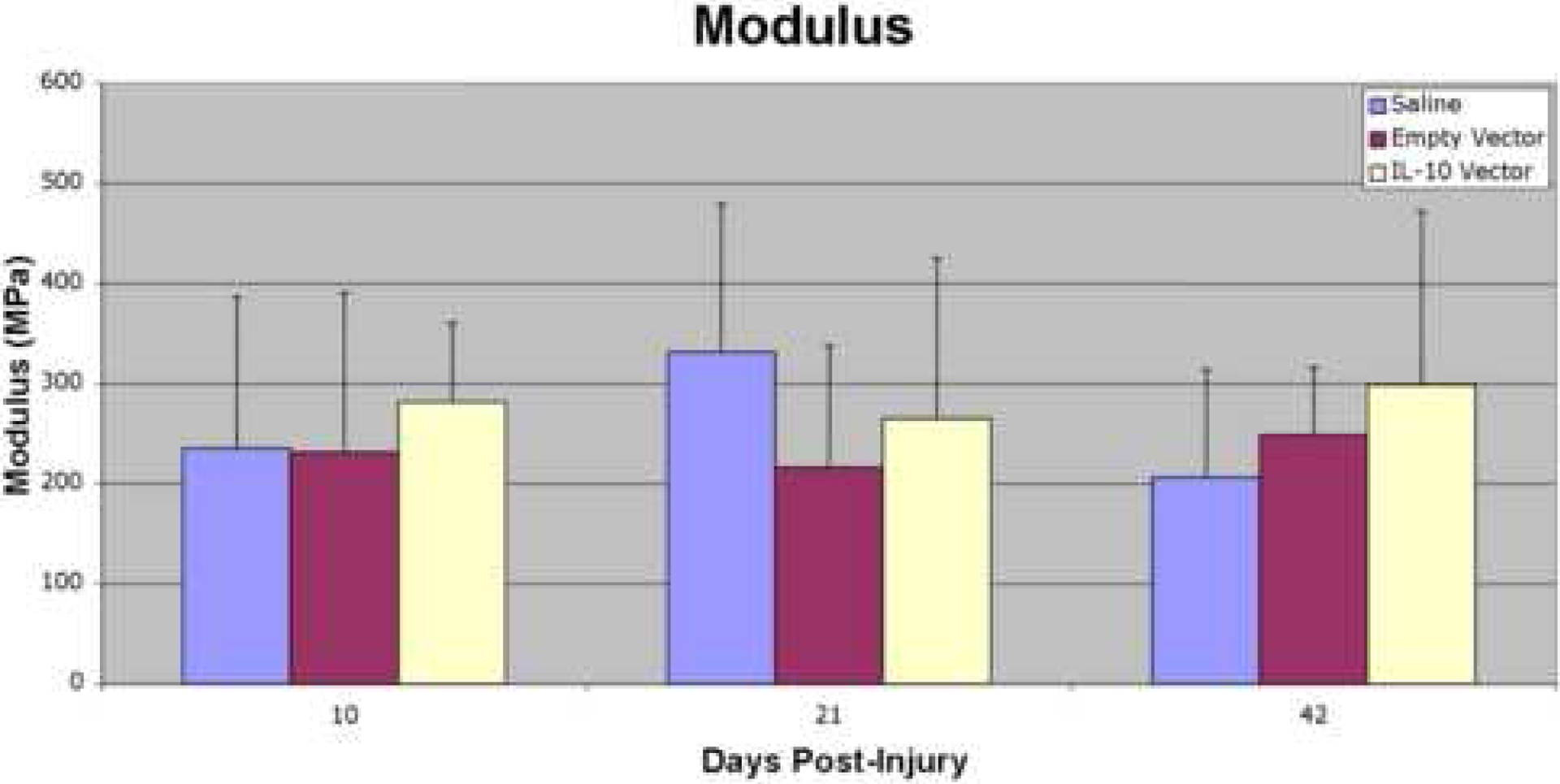
Modulus (MPa) at 10, 21, and 42 days post-injury. No significant differences or trends were found in modulus between the three treatment groups at any of the post-injury time points.
Discussion
Interleukin-10, originally termed cytokine inhibitory factor, is a potent anti-inflammatory cytokine known to be an important agent in the suppression of the inflammatory response.52 In addition to inhibiting the expression of pro-inflammatory cytokines, including IL-6 and IL-8, IL-10 has been shown to inhibit the migration of inflammatory cells to sites of injury by inhibiting the expression of specific chemokines, including macrophage inflammatory protein-1α (MIP-1α) and monocyte chemoattractant protein-1 (MCP-1).53–61 Evidence also suggests that IL-10 may play a specific anti-inflammatory role during pregnancy. The cytokine is known to be present in amniotic fluid and has been implicated in preventing immune and inflammatory responses to the fetus and fetal “foreign” antigens.39,62,63 The presence of IL-10 may be necessary for scarless fetal wound healing to occur, despite the potential availability of compensatory factors.35 Liechty et al35 demonstrated that while wounds in normal fetal skin grafts healed scarlessly with minimal inflammation and normal dermal reticular collagen, wounds in IL-10 deficient fetal skin grafts from IL-10 knockout mice healed with significant inflammation and scar formation. IL-10 has also been shown to have potentially powerful effects in adult wound healing. In two murine models of IL-10 delivery using gene therapy approaches, scarless healing responses were observed in adult skin that was intradermally injected prior to injury with viral vectors expressing the IL-10 transgene.39,40 While these scarless responses have been observed in dermal wound healing models, scarless healing has also been demonstrated in tendon. Using a sheep model of lateral extensor tendon injury, we have previously shown that fetal tendon heals in a scarless manner.11 While lateral extensor tendon injuries made in the limbs of adult sheep healed with significant scar formation that was adherent to surrounding structures, the same injuries made in fetal sheep healed with no signs of gross abnormality, scar, or adhesion formation. Histologically, the healed fetal tendons showed complete reconstitution of uninjured collagen architecture.11
With prior studies showing the potential role of IL-10 in scarless healing, as well as the potential to create a scarless healing response in tendon with a histologically normal appearance, the objective of this study was to investigate the effect of IL-10 overexpression on the properties of healing adult murine patellar tendon. We hypothesized that IL-10 overexpression would lead to decreased scar formation and improved biomechanical and histological properties and used a well established murine model of tendon injury and a lentiviral-mediated method of IL-10 overexpression to test this hypothesis. The animal model has previously been shown to create a distinct and reproducible tendon injury41 and in the current study we have demonstrated successful gene transfer and overexpression of IL-10 and eGFP transgenes in adult tendon through the use of a lentiviral vector. To our knowledge, this is the first study to report successful gene transfer into tendon using a lentiviral vector. The use of another retrovirus has been reported, but not through a direct in-vivo approach.64–67 The literature on the application of gene therapy in tendon healing and repair has been relatively scarce to date. The majority of studies have focused on an adenoviral-mediated method of gene delivery and while several have reported successful in-vivo delivery of a gene of interest (often a marker gene) into tendon,64–72 only a few studies have examined the effect of the altered gene expression on either injured or uninjured tendon.73–76 These studies has demonstrated mixed results; with some showing beneficial effects of increased gene expression of a growth factor of interest on tendon healing and repair,73,75 and others showing less clear findings.74,76
Overexpression of IL-10 in the current study led to several effects on tendon healing and repair. Following tendon injury, IL-10 treated patellar tendons showed improved properties relative to control tendons treated with saline. Maximum stress and percent relaxation were increased, particularly at 42 days post-injury. In addition, IL-10 immunostaining at the tendon injury site was increased in the IL-10 group relative to saline controls at 10 days post-injury. IL-10 immunostaining peaked at this 10 day time point in the IL-10 group, findings that seem to correlate with the PCR results in our preliminary gene expression study. Finally, a trend toward increased angular deviation (more collagen disorganization) in the empty vector group relative to saline controls was seen at day 42 post-injury (7.8° vs. 3.8°). Although not reaching significance or the level of a trend, angular deviation in the empty vector group was also higher than in saline controls at day 21 (9.9° vs. 7.6°) post-injury, and higher than in the IL-10 group at both day 21 (9.9° vs. 5.0°) and day 42 (7.8° vs. 4.8°) post-injury.
There are some limitations in the present study. A third treatment group was utilized to evaluate the effects of the lentiviral vector alone on tendon healing in our animal model. This empty vector group also showed improved tendon properties relative to the saline control group, including increased maximum stress and percent relaxation and increased IL-10 immunostaining at the injury site 10 days after wounding. While these findings were seen between each of the vector groups relative to the saline controls, no significant differences were found between the IL-10 group and the empty vector group when compared directly, although angular deviation showed some non-significant trends toward improvement in the IL-10 group relative to empty vector. This unexpected result potentially suggests that the differences seen in the two vector groups relative to the saline controls may represent an effect of injection of the vector itself, rather than IL-10. Injection of the lentiviral vector may cause a significant inflammatory response and increased scar formation following tendon injury, potentially obscuring the anti-inflammatory effects of IL-10. Indeed, an increased immune and inflammatory response has been observed in some studies of viral-mediated gene transfer, although adenoviral vectors have typically been more problematic in this regard than retroviral vectors such as lentivirus.65,66,72,77
The results of the current study are less dramatic than in prior dermal wound studies examining the effects of IL-10, where scarless responses were reported.39,40 The discrepancy in findings may relate to differences in tissue properties between tendon and dermis. While dermal tissue is highly cellular, tendon is relatively hypocellular, potentially impacting the amount of IL-10 gene transfer at the injury site and the magnitude of any anti-inflammatory or anti-fibrotic effects.66 Cellularity differences may also impact the degree of the inflammatory response potentially caused by injection of the viral vector, as this response has been reported to vary depending on the tissue that is investigated.65 In addition, the current tendon healing model involves a load-bearing environment, while dermal studies have not, potentially impacting the duration the lentiviral vector remains at the injury site and the degree of IL-10 gene transfer. Finally, it should be noted that this study was performed in unsheathed, paratenon covered tendons. These data should not be extrapolated or applied to the healing properties of sheathed tendons (synovial-lined flexor tendons in the hand).
In conclusion, the current study demonstrates successful gene transfer of IL-10 into adult tendon using a lentiviral vector. While the effects of overexpression of IL-10 on adult tendon healing have not yet been fully elucidated, the current study may help to further clarify the mechanisms of healing and repair. Additionally, other critical factors in scarless wound healing need to be investigated, such as hyaluronic acid38,78,79, as it is likely that multiple factors work in concert to produce a scarless response. Ultimately, understanding the complex process of tendon healing and the key factors involved may lead to future therapies to improve the outcomes following tendon injury and repair.
Acknowledgements:
This study was supported by grants from the American Society for Surgery of the Hand, the Orthopaedic Research and Education Foundation, and the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baratz ME, Schmidt CC, Hughes TB. Extensor Tendon Injuries. In: Green DP, Hotchkiss RN, Pederson WC, Wolfe SW, eds. Green’s Operative Hand Surgery. Vol. 1, 5th ed. Philadelphia: Elsevier Churchill Livingstone, 2005: 187–218. [Google Scholar]
- 2.Boyer MI. Flexor Tendon Injury: Acute Injuries. In: Green DP, Hotchkiss RN, Pederson WC, Wolfe SW, eds. Green’s Operative Hand Surgery. Vol. 1, 5th ed. Philadelphia: Elsevier Churchill Livingstone, 2005: 219–240. [Google Scholar]
- 3.Beredjiklian PK. Biologic aspects of flexor tendon laceration and repair. J Bone Joint Surg Am 2003; 85: 539–550. [DOI] [PubMed] [Google Scholar]
- 4.Bruner S, Wittemann M, Jester A, Blumenthal K, Germann G. Dynamic splinting after extensor tendon repair in zones V to VII. J Hand Surg [Br] 2003; 28: 224–227. [DOI] [PubMed] [Google Scholar]
- 5.Chow JA, Dovelle S, Thomes LJ, Ho PK, Saldana J. A comparison of results of extensor tendon repair followed by early controlled mobilisation versus static immobilisation. J Hand Surg [Br] 1989; 14: 18–20. [DOI] [PubMed] [Google Scholar]
- 6.Evans RB. Clinical application of controlled stress to the healing extensor tendon: a review of 112 cases. Phys Ther 1989; 69: 1041–1049. [DOI] [PubMed] [Google Scholar]
- 7.Evans RB, Thompson DE. The application of force to the healing tendon. J Hand Ther 1993; 6: 266–284. [DOI] [PubMed] [Google Scholar]
- 8.Gelberman RH, Manske PR. Factors influencing flexor tendon adhesions. Hand Clin 1985; 1: 35–42. [PubMed] [Google Scholar]
- 9.Mason ML, Allen HS. The rate of healing of tendons: an experimental study of tensile strength. Ann Surg 1941; 113: 424–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newport ML, Blair WF, Steyers CM Jr. Long-term results of extensor tendon repair. J Hand Surg [Am] 1990; 15: 961–966. [DOI] [PubMed] [Google Scholar]
- 11.Beredjiklian PK, Favata M, Cartmell JS, Flanagan CL, Crombleholme TM, Soslowsky LJ. Regenerative versus reparative healing in tendon: a study of biomechanical and histological properties in fetal sheep. Ann Biomed Eng 2003; 31: 1143–1152. [DOI] [PubMed] [Google Scholar]
- 12.Bulstrode NW, Burr N, Pratt AL, Grobbelaar AO. Extensor tendon rehabilitation a prospective trial comparing three rehabilitation regimes. J Hand Surg [Br] 2005; 30: 175–179. [DOI] [PubMed] [Google Scholar]
- 13.Chester DL, Beale S, Beveridge L, Nancarrow JD, Titley OG. A prospective, controlled, randomized trial comparing early active extension with passive extension using a dynamic splint in the rehabilitation of repaired extensor tendons. J Hand Surg [Br] 2002; 27: 283–288. [DOI] [PubMed] [Google Scholar]
- 14.Chow JA, Thomes LJ, Dovelle S, Milnor WH, Seyfer AE, Smith AC. A combined regimen of controlled motion following flexor tendon repair in “no man’s land”. Plast Reconstr Surg 1987; 79: 447–455. [DOI] [PubMed] [Google Scholar]
- 15.Crosby CA, Wehbe MA. Early protected motion after extensor tendon repair. J Hand Surg [Am] 1999; 24: 1061–1070. [DOI] [PubMed] [Google Scholar]
- 16.Evans RB. Immediate active short arc motion following extensor tendon repair. Hand Clin 1995; 11: 483–512. [PubMed] [Google Scholar]
- 17.Gelberman RH, Botte MJ, Spiegelman JJ, Akeson WH. The excursion and deformation of repaired flexor tendons treated with protected early motion. J Hand Surg [Am] 1986; 11: 106–110. [DOI] [PubMed] [Google Scholar]
- 18.Ip WY, Chow SP. Results of dynamic splintage following extensor tendon repair. J Hand Surg [Br] 1997; 22: 283–287. [DOI] [PubMed] [Google Scholar]
- 19.Kerr CD, Burczak JR. Dynamic traction after extensor tendon repair in zones 6, 7, and 8: a retrospective study. J Hand Surg [Br] 1989; 14: 21–22. [DOI] [PubMed] [Google Scholar]
- 20.Khandwala AR, Webb J, Harris SB, Foster AJ, Elliot D. A comparison of dynamic extension splinting and controlled active mobilization of complete divisions of extensor tendons in zones 5 and 6. J Hand Surg [Br] 2000; 25: 140–146. [DOI] [PubMed] [Google Scholar]
- 21.Kleinert HE, Kutz JE, Atasoy E, Stormo A. Primary repair of flexor tendons. Orthop Clin North Am 1973; 4: 865–876. [PubMed] [Google Scholar]
- 22.Slater RR Jr., Bynum DK. Simplified functional splinting after extensor tenorrhaphy. J Hand Surg [Am] 1997; 22: 445–451. [DOI] [PubMed] [Google Scholar]
- 23.Sylaidis P, Youatt M, Logan A. Early active mobilization for extensor tendon injuries. The Norwich regime. J Hand Surg [Br] 1997; 22: 594–596. [DOI] [PubMed] [Google Scholar]
- 24.Adzick NS, Longaker MT. Scarless fetal healing. Therapeutic implications. Ann Surg 1992; 215: 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin KY, Posnick JC, al-Qattan MM, Vajsar J, Becker LE. Fetal nerve healing: an experimental study. Plast Reconstr Surg 1994; 93: 1323–1333. [PubMed] [Google Scholar]
- 26.Lin RY, Sullivan KM, Argenta PA, Meuli M, Lorenz HP, Adzick NS. Exogenous transforming growth factor-beta amplifies its own expression and induces scar formation in a model of human fetal skin repair. Ann Surg 1995; 222: 146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lorenz HP, Lin RY, Longaker MT, Whitby DJ, Adzick NS. The fetal fibroblast: the effector cell of scarless fetal skin repair. Plast Reconstr Surg 1995; 96: 1251–1261. [DOI] [PubMed] [Google Scholar]
- 28.Rowlatt U Intrauterine wound healing in a 20 week human fetus. Virchows Arch A Pathol Anat Histol 1979; 381: 353–361. [DOI] [PubMed] [Google Scholar]
- 29.Namba RS, Meuli M, Sullivan KM, Le AX, Adzick NS. Spontaneous repair of superficial defects in articular cartilage in a fetal lamb model. J Bone Joint Surg Am 1998; 80: 4–10. [DOI] [PubMed] [Google Scholar]
- 30.Slate RK, Posnick JC, Wells MD, Goldstein JA, Keeley FW, Thorner PS. Fetal tibial bone healing in utero: the effects of miniplate fixation. Plast Reconstr Surg 1993; 92: 874–883. [PubMed] [Google Scholar]
- 31.Weinzweig J, Panter KE, Pantaloni M, Spangenberger A, Harper JS, Lui F, et al. The fetal cleft palate: I. Characterization of a congenital model. Plast Reconstr Surg 1999; 103: 419–428. [DOI] [PubMed] [Google Scholar]
- 32.Weinzweig J, Panter KE, Pantaloni M, Spangenberger A, Harper JS, Lui F, et al. The fetal cleft palate: II. Scarless healing after in utero repair of a congenital model. Plast Reconstr Surg 1999; 104: 1356–1364. [DOI] [PubMed] [Google Scholar]
- 33.Liechty KW, Adzick NS, Crombleholme TM. Diminished interleukin 6 (IL-6) production during scarless human fetal wound repair. Cytokine 2000; 12: 671–676. [DOI] [PubMed] [Google Scholar]
- 34.Liechty KW, Crombleholme TM, Cass DL, Martin B, Adzick NS. Diminished interleukin-8 (IL-8) production in the fetal wound healing response. J Surg Res 1998; 77: 80–84. [DOI] [PubMed] [Google Scholar]
- 35.Liechty KW, Kim HB, Adzick NS, Crombleholme TM. Fetal wound repair results in scar formation in interleukin-10-deficient mice in a syngeneic murine model of scarless fetal wound repair. J Pediatr Surg 2000; 35: 866–73. [DOI] [PubMed] [Google Scholar]
- 36.Adzick NS, Harrison MR, Glick PL, Beckstead JH, Villa RL, Scheuenstuhl H, Goodson WH 3rd. Comparison of fetal, newborn, and adult wound healing by histologic, enzyme-histochemical, and hydroxyproline determinations. J Pediatr Surg 1985; 20: 315–319. [DOI] [PubMed] [Google Scholar]
- 37.Krummel TM, Nelson JM, Diegelmann RF, Lindblad WJ, Salzberg AM, Greenfield LJ, Cohen IK. Fetal response to injury in the rabbit. J Pediatr Surg 1987; 22: 640–644. [DOI] [PubMed] [Google Scholar]
- 38.Longaker MT, Adzick NS, Hall JL, Stair SE, Crombleholme TM, Duncan BW, et al. Studies in fetal wound healing, VII. Fetal wound healing may be modulated by hyaluronic acid stimulating activity in amniotic fluid. J Pediatr Surg 1990; 25: 430–433. [DOI] [PubMed] [Google Scholar]
- 39.Gordon A, Kozin ED, Keswani SG, Vaikunth SS, Katz AB, Zoltick PW, et al. Permissive environment in postnatal wounds induced by adenoviral-mediated overexpression of the anti-inflammatory cytokine interleukin-10 prevents scar formation. Wound Repair Regen 2008; 16: 70–79. [DOI] [PubMed] [Google Scholar]
- 40.Peranteau WH, Zhang L, Muvarak N, Badillo AT, Radu A, Zoltick PW, et al. IL-10 overexpression decreases inflammatory mediators and promotes regenerative healing in an adult model of scar formation. J Invest Dermatol 2008; epub: 1–9. [DOI] [PubMed] [Google Scholar]
- 41.Lin TW, Cardenas L, Glaser DL, Soslowsky LJ. Tendon healing in interleukin-4 and interleukin-6 knockout mice. J Biomech 2006; 39: 61–69. [DOI] [PubMed] [Google Scholar]
- 42.Lin TW, Cardenas L, Soslowsky LJ. Tendon properties in interleukin-4 and interleukin-6 knockout mice. J Biomech 2005; 38: 99–105. [DOI] [PubMed] [Google Scholar]
- 43.Donello JE, Loeb JE, Hope TJ. Woodchuck hepatitis virus contains a tripartite posttranscriptional regulatory element. J Virol 1998; 72: 5085–5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miyoshi H, Blomer U, Takahashi M, Gage FH, Verma IM. Development of a self-inactivating lentivirus vector. J Virol 1998; 72: 8150–8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 1996; 272: 263–267. [DOI] [PubMed] [Google Scholar]
- 46.Zennou V, Serguera C, Sarkis C, Colin P, Perret E, Mallet J, Charneau P. The HIV-1 DNA flap stimulates HIV vector-mediated cell transduction in the brain. Nat Biotechnol 2001; 19: 446–450. [DOI] [PubMed] [Google Scholar]
- 47.Sena-Esteves M, Tebbets JC, Steffens S, Crombleholme T, Flake AW. Optimized large-scale production of high titer lentivirus vector pseudotypes. J Virol Methods 2004; 122: 131–139. [DOI] [PubMed] [Google Scholar]
- 48.Gimbel JA, Van Kleunen JP, Mehta S, Perry SM, Williams GR, Soslowsky LJ. Supraspinatus tendon organizational and mechanical properties in a chronic rotator cuff tear animal model. J Biomech 2004; 37: 739–749. [DOI] [PubMed] [Google Scholar]
- 49.Thomopoulos S, Williams GR, Gimbel JA, Favata M, Soslowsky LJ. Variation of biomechanical, structural, and compositional properties along the tendon to bone insertion site. J Orthop Res 2003; 21: 413–419. [DOI] [PubMed] [Google Scholar]
- 50.Soslowsky LJ, Thomopoulos S, Tun S, Flanagan CL, Keefer CC, Mastaw J, et al. Neer Award 1999. Overuse activity injures the supraspinatus tendon in an animal model: a histologic and biomechanical study. J Shoulder Elbow Surg 2000; 9: 79–84. [PubMed] [Google Scholar]
- 51.Soslowsky LJ, An CH, Johnston SP, Carpenter JE. Geometric and mechanical properties of the coracoacromial ligament and their relationship to rotator cuff disease. Clin Orthop Relat Res 1994; 304: 10–17. [PubMed] [Google Scholar]
- 52.Moore KW, O’Garra A, de Waal Malefyt R, Vieira P, Mosmann TR. Interleukin-10. Annu Rev Immunol 1993; 11: 165–190. [DOI] [PubMed] [Google Scholar]
- 53.Alam R, Kumar D, Anderson-Walters D, Forsythe PA. Macrophage inflammatory protein-1 alpha and monocyte chemoattractant peptide-1 elicit immediate and late cutaneous reactions and activate murine mast cells in vivo. J Immunol 1994; 152: 1298–1303. [PubMed] [Google Scholar]
- 54.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med 1991; 174: 1209–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.DiPietro LA, Burdick M, Low QE, Kunkel SL, Strieter RM. MIP-1alpha as a critical macrophage chemoattractant in murine wound repair. J Clin Invest 1998; 101: 1693–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.DiPietro LA, Polverini PJ, Rahbe SM, Kovacs EJ. Modulation of JE/MCP-1 expression in dermal wound repair. Am J Pathol 1995; 146: 868–875. [PMC free article] [PubMed] [Google Scholar]
- 57.Elgert KD, Alleva DG, Mullins DW. Tumor-induced immune dysfunction: the macrophage connection. J Leukoc Biol 1998; 64: 275–290. [DOI] [PubMed] [Google Scholar]
- 58.Engelhardt E, Toksoy A, Goebeler M, Debus S, Brocker EB, Gillitzer R. Chemokines IL-8, GROalpha, MCP-1, IP-10, and Mig are sequentially and differentially expressed during phase-specific infiltration of leukocyte subsets in human wound healing. Am J Pathol 1998; 153: 1849–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O’Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol 1991; 147: 3815–3822. [PubMed] [Google Scholar]
- 60.Fortunato SJ, Menon R, Lombardi SJ. The effect of transforming growth factor and interleukin-10 on interleukin-8 release by human amniochorion may regulate histologic chorioamnionitis. Am J Obstet Gynecol 1998; 179: 794–799. [DOI] [PubMed] [Google Scholar]
- 61.Fortunato SJ, Menon R, Swan KF, Lombardi SJ. Interleukin-10 inhibition of interleukin-6 in human amniochorionic membrane: transcriptional regulation. Am J Obstet Gynecol 1996; 175: 1057–1065. [DOI] [PubMed] [Google Scholar]
- 62.Cadet P, Rady PL, Tyring SK, Yandell RB, Hughes TK. Interleukin-10 messenger ribonucleic acid in human placenta: implications of a role for interleukin-10 in fetal allograft protection. Am J Obstet Gynecol 1995; 173: 25–29. [DOI] [PubMed] [Google Scholar]
- 63.Heyborne KD, McGregor JA, Henry G, Witkin SS, Abrams JS. Interleukin-10 in amniotic fluid at midtrimester: immune activation and suppression in relation to fetal growth. Am J Obstet Gynecol 1994; 171: 55–59. [DOI] [PubMed] [Google Scholar]
- 64.Gerich TG, Ghivizani S, Fu FH, Robbins PD, Evans CH. [Gene transfer into the patellar tendon of rabbits: a preliminary study of locoregional expression of growth factors]. Wien Klin Wochenschr 1997; 109: 384–389. [PubMed] [Google Scholar]
- 65.Gerich TG, Kang R, Fu FH, Robbins PD, Evans CH. Gene transfer to the rabbit patellar tendon: potential for genetic enhancement of tendon and ligament healing. Gene Ther 1996; 3: 1089–1093. [PubMed] [Google Scholar]
- 66.Gerich TG, Kang R, Fu FH, Robbins PD, Evans CH. Gene transfer to the patellar tendon. Knee Surg Sports Traumatol Arthrosc 1997; 5: 118–123. [DOI] [PubMed] [Google Scholar]
- 67.Gerich TG, Lobenhoffer HP, Fu FH, Robbins PD, Evans CH. [Virally mediated gene transfer in the patellar tendon. An experimental study in rabbits]. Unfallchirurg 1997; 100: 354–362. [DOI] [PubMed] [Google Scholar]
- 68.Dai Q, Manfield L, Wang Y, Murrell GA. Adenovirus-mediated gene transfer to healing tendon--enhanced efficiency using a gelatin sponge. J Orthop Res 2003; 21: 604–609. [DOI] [PubMed] [Google Scholar]
- 69.Lattermann C, Zelle BA, Whalen JD, Baltzer AW, Robbins PD, Niyibizi C, et al. Gene transfer to the tendon-bone insertion site. Knee Surg Sports Traumatol Arthrosc 2004; 12: 510–515. [DOI] [PubMed] [Google Scholar]
- 70.Lou J In vivo gene transfer into tendon by recombinant adenovirus. Clin Orthop Relat Res 2000; 379S: S252–S255. [DOI] [PubMed] [Google Scholar]
- 71.Lou J, Manske PR, Aoki M, Joyce ME. Adenovirus-mediated gene transfer into tendon and tendon sheath. J Orthop Res 1996; 14: 513–517. [DOI] [PubMed] [Google Scholar]
- 72.Mehta V, Kang Q, Luo J, He TC, Haydon RC, Mass DP. Characterization of adenovirus-mediated gene transfer in rabbit flexor tendons. J Hand Surg [Am] 2005; 30: 136–141. [DOI] [PubMed] [Google Scholar]
- 73.Bolt P, Clerk AN, Luu HH, Kang Q, Kummer JL, Deng ZL, et al. BMP-14 gene therapy increases tendon tensile strength in a rat model of Achilles tendon injury. J Bone Joint Surg Am 2007; 89: 1315–1320. [DOI] [PubMed] [Google Scholar]
- 74.Lou J, Kubota H, Hotokezaka S, Ludwig FJ, Manske PR. In vivo gene transfer and overexpression of focal adhesion kinase (pp125 FAK) mediated by recombinant adenovirus-induced tendon adhesion formation and epitenon cell change. J Orthop Res 1997; 15: 911–918. [DOI] [PubMed] [Google Scholar]
- 75.Lou J, Tu Y, Burns M, Silva MJ, Manske P. BMP-12 gene transfer augmentation of lacerated tendon repair. J Orthop Res 2001; 19: 1199–1202. [DOI] [PubMed] [Google Scholar]
- 76.Rickert M, Wang H, Wieloch P, Lorenz H, Steck E, Sabo D, et al. Adenovirus-mediated gene transfer of growth and differentiation factor-5 into tenocytes and the healing rat Achilles tendon. Connect Tissue Res 2005; 46: 175–183. [DOI] [PubMed] [Google Scholar]
- 77.Gerich TG, Fu FH, Robbins PD, Evans CH. Prospects for gene therapy in sports medicine. Knee Surg Sports Traumatol Arthrosc 1996; 4: 180–187. [DOI] [PubMed] [Google Scholar]
- 78.Longaker MT, Chiu ES, Adzick NS, Stern M, Harrison MR, Stern R. Studies in fetal wound healing. V. A prolonged presence of hyaluronic acid characterizes fetal wound fluid. Ann Surg 1991; 213: 292–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.West DC, Shaw DM, Lorenz P, Adzick NS, Longaker MT. Fibrotic healing of adult and late gestation fetal wounds correlates with increased hyaluronidase activity and removal of hyaluronan. Int J Biochem Cell Biol 1997; 29: 201–210. [DOI] [PubMed] [Google Scholar]


