Abstract
Background & aims
To investigate the association of nutritional risk at admission with the length of hospital stay (LOS) and mortality in older patients with COVID-19.
Methods
Retrospective monocentric study in an acute geriatric hospital. Data were collected after an extensive review of medical records and the nutritional risk was assessed according to the Nutritional Risk Screening (NRS). Univariate and multivariate (adjusted for age, sex and comorbidity burden) Cox proportional-hazard and linear regression models were used to investigate the association with the above-mentioned outcomes.
Results
Of a total of 245 patients (86.1 ± 6.4 yrs), 50.6% had a severe nutritional risk with an NRS≥5/7 at admission. Lower BMI, cognitive impairment and swallowing disorders were more prevalent in the patients with a higher NRS. A NRS≥5 was not associated with mortality but prolonged by more than 3 days the LOS among the 173 survivors (β 3.69; 0.71–6.67 95% CI; p = 0.016), with a discharge rate delayed by 1.8 times (HR 0.55; 0.37–0.83 95% CI; p = 0.101).
Conclusion
Among the survivors of COVID-19 in an acute geriatric hospital, a NRS ≥5 at admission was associated with a longer LOS, but not with mortality.
Keywords: COVID-19, Malnutrition, Nutritional risk screening, Mortality, LOS
1. Introduction
Malnutrition is a highly prevalent condition in the geriatric care, present in up to half of the older patients admitted to hospital [1] and associated with functional decline, mortality, prolonged length of stay (LOS) and rehospitalization rates [2].
With the emerging knowledge on Coronavirus Disease (COVID-19) in older populations, older patients have been identified as a high-risk group for SARS-CoV-2 infection, adverse outcomes and mortality [3]. Malnutrition has a bidirectional link with infections, as malnourished patients are at increased risk of infectious complications, while the acute illness can rapidly worsen nutritional status.
Poor nutritional status compromises the immune response and reduces the effectiveness of treatments leading to greater morbidity [4]. Importantly, recent studies in COVID-19 cohorts associated compromised nutritional status with the severity of respiratory symptoms, weight loss during hospitalization, persistent anorexia, all-cause mortality and transfer to intensive care wards [5]. Thus, adequate nutritional screening, with consequent malnutrition diagnosis and treatment have the potential to impact prognosis in COVID-19.
We hypothesize that the nutritional status has a prognostic significance in older patients hospitalized with COVID-19. Therefore, this study aimed to investigate the association of nutritional risk screening (NRS-2002) performed at hospital admission with the LOS and in-hospital mortality in a population of old patients.
2. Methods
2.1. Design, setting and population
This monocentric retrospective study analyzed patients hospitalized in the geriatric hospital of the Geneva University Hospitals. In this cohort, patients were ineligible to intensive care, according to a global geriatric assessment considering the severity of the disease, underlying comorbidities or to the patients' and their proxy's wishes. Patients with COVID-19 were hospitalized if they had one or more of these clinical features: a) a pneumonia with a severity assessed by the CURB-65 score ≥2, b) a new dependence on oxygen or increase of oxygen needs, c) a respiratory rate ≥20 breaths/minute, d) a decompensated chronic disease, e) a severely altered general state of health, f) a deteriorating clinical course.
We included the data from all patients who were hospitalized with a SARS-CoV-2 infection between March 13th, 2020 and May 17th, 2020 in the geriatric hospital of the Geneva University Hospitals and who had an NRS at admission. The diagnosis of COVID-19 was defined by a positive reverse transcriptase-polymerase chain reaction (RT-PCR) test for the SARS-CoV-2 on nasopharyngeal swabs. The project was authorized by Geneva's committee for research ethics (project ID: 2020-00819).
2.2. Data collection
Data regarding demographics, comorbidities, clinical symptoms, and laboratory values were retrospectively collected by a dedicated research nurse, and reflect information from the first 24 h of hospitalization.
Nutritional risk screening was performed at hospital admission by dieticians according to the NRS-2002. This score assesses the severity of undernutrition (0–3 points) and the severity of the acute disease (0–3 points), with a total score ranging from 0 to 7, as an additional point is added for patients aged 70 or older [6]. A total score of 3–7 points indicates a risk of malnutrition. In this study, we categorized the risk of malnutrition in three levels: NRS<3 corresponded to patients at no risk of malnutrition, while patients with a NRS≥3 were considered at risk and those with NRS≥5 at high-risk of malnutrition. The NRS was extracted from medical records or retrospectively calculated by a single research nurse based on information from the hospital admission. The Cumulative Illness Rating Scale-Geriatric (CIRS-G) was performed at admission, measuring a patient's comorbidity burden by taking into account chronic diseases as well as the severity of acute illnesses, with higher scores representing a higher overall disease burden. LOS was calculated according to the number of days of hospitalization in acute care, independently of the destination after discharge. In-hospital mortality and its rate were computed according to the documentation of the event and the time from hospital admission to the event.
2.3. Statistical analysis
The study population was categorized in three subgroups according to NRS (≤2, 3–4 and ≥5 points). The three subgroups were compared by Cuzick's nonparametric test for trend across ordered groups, and Kruskal–Wallis for continuous and ordinal variables. A statistically significant difference among the three groups was set at p values < 0.05.
The association of nutritional screening obtained by the NRS on in-hospital mortality and LOS was assessed by univariate (model 1) and multiple (model 2 and model 3) variable Cox proportional-hazard models, and linear regression models for LOS. In order to adjust for comorbidity burden, we used the CIRS-G. As they were 10.6% missing CIRS-G values, we imputed the missing ones with a regression model adjusted for inhospital mortality, age and sex. Results were expressed as hazard Ratio (HR) or β coefficient with the 95% Confidence Interval for Cox regression and linear models, respectively. Statistical analysis was performed using Stata software version 16.1 (Stata Corp., College Station, TX, USA).
3. Results
The study population consisted of 245 patients, with a mean age of 86.1 ± 6.4 and 42% of male patients. The distribution of NRS scores categorized 17.6% of patients at 0–2 points, 32.2% at 3–4 points and 50.2% with NRS ≥5, corresponding to a majority of patients with high nutritional risk at hospital admission. Lower BMI, the presence of cognitive impairment, as well as swallowing disorders previous to the acute event were more prevalent in patients with high nutritional risk with an increasing gradient across NRS categories (Table 1 ).
Table 1.
Characteristics of the study population according to the NRS score.
| Nutritional Risk Screening |
p value | Total |
|||
|---|---|---|---|---|---|
| 0–2 points |
3–4 points |
≥5 points |
|||
| N = 43 | N = 79 | N = 123 | N = 245 | ||
| Age y | 86.7 ± 6.2 | 86.6 ± 6.3 | 85.5 ± 6.5 | 0.449 | 86.1 ± 6.4 |
| Male sex | 17 (39.5%) | 31 (39.2%) | 55 (44.7%) | 0.426 | 103 (42.0%) |
| LOS d | 11.7 ± 6.0 | 12.8 ± 8.1 | 13.9 ± 7.8 | 0.055 | 13.2 ± 7.6 |
| Deceased | 9 (20.9%) | 20 (25.3%) | 42 (34.1%) | 0.063 | 71 (29.0%) |
| Number of medications | 6.8 ± 4.1 | 7.3 ± 4.5 | 7.6 ± 3.8 | 0.465 | 7.4 ± 4.1 |
| CURB-65 | 0.907 | ||||
| 1 | 9 (20.9%) | 17 (21.5%) | 27 (22.0%) | 53 (21.6%) | |
| 2 | 21 (48.8%) | 31 (39.2%) | 52 (42.3%) | 104 (42.4%) | |
| 3 | 11 (25.6%) | 29 (36.7%) | 40 (32.5%) | 80 (32.7%) | |
| 4 | 2 (4.7%) | 2 (2.5%) | 3 (2.4%) | 7 (2.9%) | |
| 5 | 0 (0.0%) | 0 (0.0%) | 1 (0.8%) | 1 (0.4%) | |
| BMI kg/m2 | <0.001 | ||||
| <20 | 1 (2.4%) | 5 (7.0%) | 49 (41.9%) | 55 (23.9%) | |
| 20–24.9 | 21 (50.0%) | 33 (46.5%) | 29 (24.8%) | 83 (36.1%) | |
| 25–29.9 | 9 (21.4%) | 17 (23.9%) | 27 (23.1%) | 53 (23.0%) | |
| 30+ | 11 (26.2%) | 16 (22.5%) | 12 (10.3%) | 39 (17.0%) | |
| CIRS-G | 17.6 ± 5.9 | 18.5 ± 6.6 | 19.7 ± 5.1 | 0.053 | 19.0 ± 5.8 |
| Hypertension | 32 (74.4%) | 56 (70.9%) | 85 (69.1%) | 0.534 | 173 (70.6%) |
| Diabetes under treatment | 11 (25.6%) | 10 (12.7%) | 36 (29.8%) | 0.096 | 57 (23.5%) |
| Heart Failure | 14 (33.3%) | 33 (42.3%) | 37 (31.9%) | 0.435 | 84 (35.6%) |
| Cognitive disorders | 18 (41.9%) | 36 (45.6%) | 71 (58.2%) | 0.028 | 125 (51.2%) |
| Swallowing disorders | 2 (4.7%) | 3 (3.8%) | 18 (14.8%) | <0.001 | 23 (9.5%) |
| COPD | 3 (7.0%) | 6 (7.6%) | 19 (15.6%) | 0.053 | 28 (11.5%) |
| Smoking | 0.497 | ||||
| No smoking | 28 (65.1%) | 56 (71.8%) | 80 (65.6%) | 164 (67.5%) | |
| Past | 14 (32.6%) | 18 (23.1%) | 34 (27.9%) | 66 (27.2%) | |
| Present | 1 (2.3%) | 4 (5.1%) | 8 (6.6%) | 13 (5.3%) | |
| Kidney disease | 12 (27.9%) | 26 (32.9%) | 28 (23.0%) | 0.241 | 66 (27.0%) |
| Liver disease | 3 (7.0%) | 8 (10.1%) | 12 (9.9%) | 0.682 | 23 (9.5%) |
| Parkinson's disease | 0 (0.0%) | 4 (5.1%) | 6 (5.0%) | 0.305 | 10 (4.1%) |
| Active neoplasia | 5 (11.6%) | 3 (3.8%) | 14 (11.6%) | 0.409 | 22 (9.1%) |
| Atrial Fibrillation | 6 (14.0%) | 21 (26.6%) | 31 (25.2%) | 0.301 | 58 (23.7%) |
| Dyslipidemia | 14 (32.6%) | 32 (40.5%) | 37 (30.1%) | 0.362 | 83 (33.9%) |
| C-Reactive Protein mg/l (n = 238) | 60.0 ± 54.1 | 66.2 ± 76.8 | 65.1 ± 66.7 | 0.931 | 64.6 ± 68.0 |
| Lymphocytes G/l (n = 235) | 1.6 ± 2.5 | 1.2 ± 0.9 | 1.1 ± 1.0 | 0.09 | 1.2 ± 1.4 |
| Albumin g/l (n = 168) | 38.0 ± 9.3 | 38.1 ± 16.4 | 35.1 ± 7.0 | 0.151 | 36.5 ± 11.3 |
Abbreviations: NRS = Nutritional Risk Screening; LOS = length of stay; BMI = body mass index; CIRS-G = Cumulative Illness Rating Scale-Geriatric; COPD = chronic obstructive pulmonary disease.
p values were calculated by Cuzick's nonparametric test for trend across ordered groups and Kruskal–Wallis test for continuous and ordinal variables.
While the overall in-hospital mortality was at 29%, there were no differences according to NRS categories at admission. Similarly, the Cox proportional-hazards models did not show NRS as a factor associated with in-hospital mortality in this population (Table 2 ).
Table 2.
Association of NRS with in-hospital mortality and length of stay in acute care.
| In-Hospital Mortalitya |
Length of Stayb |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | β | 95% CI | p value | |
| Model 1 | ||||||
| NRS score | ||||||
| <3 | Ref. | Ref. | ||||
| 3–4 | 1.18 | 0.54–2.59 | 0.682 | 1.74 | −1.39–4.88 | 0.275 |
| ≥5 | 1.35 | 0.66–2.79 | 0.413 | 3.69 | 0.71–6.67 | 0.016 |
| Model 2 | ||||||
| NRS score | ||||||
| <3 | Ref. | Ref. | ||||
| 3–4 | 1.16 | 0.53–2.55 | 0.713 | 1.78 | −1.38–4.94 | 0.268 |
| ≥5 | 1.19 | 0.58–2.47 | 0.640 | 3.78 | 0.75–6.81 | 0.015 |
| Age | 1.01 | 0.97–1.04 | 0.759 | 0.05 | −0.13–0.23 | 0.603 |
| Male sex | 2.49 | 1.52–4.08 | <0.001 | 0.29 | −2.06–2.65 | 0.805 |
| Model 3 | ||||||
| NRS score | ||||||
| <3 | Ref. | Ref. | ||||
| 3–4 | 1.08 | 0.49–2.38 | 0.852 | 1.56 | −1.58–4.69 | 0.328 |
| ≥5 | 1.04 | 0.49–2.16 | 0.926 | 3.22 | 0.19–6.26 | 0.037 |
| Age | 0.99 | 0.96–1.04 | 0.960 | 0.003 | −0.18–0.18 | 0.972 |
| Male sex | 2.42 | 1.47–3.99 | <0.001 | 0.12 | −2.21–2.45 | 0.919 |
| CIRS-G | 1.07 | 1.02–1.11 | 0.002 | 0.22 | 0.02–0.41 | 0.029 |
NRS: Nutritional Risk Screening; HR: Hazard Ratio; CI: Confidence Interval; CIRS-G: Cumulative Illness Rating Scale-Geriatric.
Cox proportional-hazards models (N = 245); Model 1: univariate; Model 2: adjusted for age and sex and Model 3: adjusted for age, sex and CIRS-G.
Linear regression models (N = 173; survivors only); Model 1: univariate; Model 2: adjusted for age and sex and Model 3: adjusted for age, sex and CIRS-G.
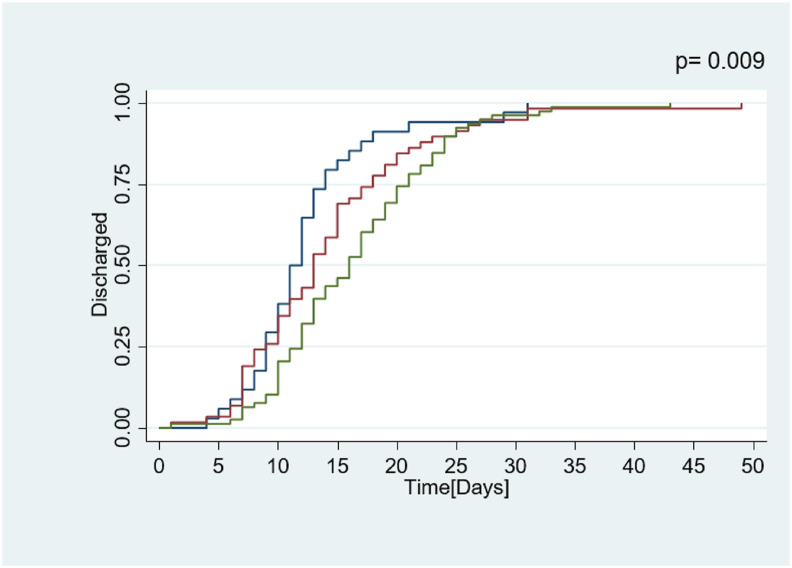
On the other hand, the NRS at admission was significantly associated with the LOS in the survivors (n = 173). In the linear regression models, we observed that patients with a NRS ≥5 had a LOS 3.69 days longer (0.71–6.67 95%CI; p = 0.016), which remained statistically significant after adjustment for age, sex and comorbidity burden (Table 2). Furthermore, NRS ≥5 was associated with a discharge rate 1.8 times slower according to Cox Proportional Models (HR 0.55; 0.37–0.83 95% CI; p = 0.101) as visualized in the Kaplan–Meier curves represented in Fig. 1 .
Fig. 1.
Kaplan–Meier curves of length of stay and discharge from acute geriatric wards among COVID-19 survivors according to nutritional risk at admission:  NRS 0–2;
NRS 0–2;  NRS 3–4 and
NRS 3–4 and  NRS ≥5. p value represents the difference between the three curves calculated by the log-rank test. NRS: Nutritional Risk screening (0–7 points).
NRS ≥5. p value represents the difference between the three curves calculated by the log-rank test. NRS: Nutritional Risk screening (0–7 points).
4. Discussion
This study showed that a high nutritional risk at hospital admission for COVID-19 was highly prevalent and associated with a prolonged LOS and a delayed discharge rate from acute geriatric care. Differently from our study which examined nutritional risk assessed by a screening tool, another recent study investigated malnutrition according to Global Initiative on Malnutrition (GLIM) criteria showed that this diagnosis was present in up to 50% of patients with COVID-19 from an Italian cohort [7,8]. They revealed a similar prevalence of malnutrition in hospitalized patients from that described in the historical non-COVID cohorts [1,9].
The relationship between nutritional risk and the LOS was reported in studies performed in other settings such as intensive care, acute and rehabilitation geriatric care [10]. Malnutrition in older people is frequently a proxy of comorbidity burden and the subsequent functional loss, which altogether are strong factors that prolong LOS in geriatric care. The enhanced activity of the immune system in the course of an infection, followed by increased metabolism rates and subsequent requirement of energy sources, highlights the importance of nutrition support for an optimal response against infections. Likewise, micronutrients and vitamins which have an immunomodulatory function, have been increasingly acknowledged as key-role elements in COVID-19 host response. Clinically, the impact of malnutrition on immunity translates to the severity of symptoms, the duration of the infection and the consequent LOS for hospitalized patients.
Moreover, malnourished old patients with COVID-19 are at increased risk of mortality, although our analysis did not reproduce this previously described association [5]. However, it is important to highlight that we investigated the association of nutritional risk performed by a screening tool instead of confirmed malnutrition diagnosis. We observed a trend towards a higher mortality with higher nutritional risk at screening, although it did not reach statistical significance. Furthermore, we assessed for in-hospital mortality, but nutritional screening and malnutrition diagnosis are also known to be associated with later outcomes, such as mortality at 12 months after hospital discharge, as well as institutionalization and re-hospitalization rates [2], which have not yet been assessed in this population. In a frail polymorbid population of very old patients, the effect of nutritional risk on mortality may be overpassed by those conditions, thus explaining the absence of a relationship with mortality in acute care. This study has several limitations. First, the retrospective design does not allow us to investigate causality. Second, some negative results may be the consequence of a lack of statistical power. Finally, these results reflect a specific population, thus hindering a general extrapolation to other scenarios. Further analysis combining a larger cohort with follow-up will build up the complementary information needed on the crucial role of nutritional screening, malnutrition assessment and support in the acute geriatric care of patients suffering of COVID-19.
5. Conclusion
In conclusion, a high nutritional risk at hospital admission was associated with a longer LOS among survivors of COVID-19 in the acute geriatric care. These results call up attention to the importance of early assessment of nutritional risk, and likely support, in the course of hospitalization of old patients.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
A.M. and L.G. were in charge of the conception, writing, and overall presentation of the manuscript. A.M., C.S. and F.H. performed the statistical analyses. C.S., C.G., G.G., D.Z. and L.G. verified the analytical methods. C.G., C.S., G.G., D.Z. and L.G participated giving input concerning formulations and provided expertise for the analysis of the results and their presentation. All authors were consulted to discuss the results and contributed to the final version of the manuscript.
Conflict of Interest
The authors have no conflict of interest to declare.
References
- 1.Cerri A.P., Bellelli G., Mazzone A., Pittella F., Landi F., Zambon A., et al. Sarcopenia and malnutrition in acutely ill hospitalized elderly: prevalence and outcomes. Clin Nutr. 2015;34:745–751. doi: 10.1016/j.clnu.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 2.O'Shea E., Trawley S., Manning E., Barrett A., Browne V., Timmons S. Malnutrition in hospitalised older adults: a multicentre observational study of prevalence, associations and outcomes. J Nutr Health Aging. 2017;21:830–836. doi: 10.1007/s12603-016-0831-x. [DOI] [PubMed] [Google Scholar]
- 3.Mendes A., Serratrice C., Herrmann F.R., Genton L., Périvier S., Scheffler M., et al. Predictors of in-hospital mortality in older patients with COVID-19: the COVIDAge study. J Am Med Dir Assoc. 2020;21:1546–1554. doi: 10.1016/j.jamda.2020.09.014. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zabetakis I., Lordan R., Norton C., Tsoupras A. COVID-19: the inflammation link and the role of nutrition in potential mitigation. Nutrients. 2020;12 doi: 10.3390/nu12051466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou J., Ma Y., Liu Y., Xiang Y., Tao C., Yu H., et al. A correlation analysis between the nutritional status and prognosis of COVID-19 patients. J Nutr Health Aging. 2020;25(1):84–93. doi: 10.1007/s12603-020-1457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kondrup J., Rasmussen H.H., Hamberg O., Stanga Z., Ad Hoc ESPEN Working Group Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;22:321–336. doi: 10.1016/s0261-5614(02)00214-5. [DOI] [PubMed] [Google Scholar]
- 7.Pironi L., Sasdelli A.S., Ravaioli F., Baracco B., Battaiola C., Bocedi G., et al. Malnutrition and nutritional therapy in patients with SARS-CoV-2 disease. Clin Nutr. 2020;40(3):1330–1337. doi: 10.1016/j.clnu.2020.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bedock D., Bel Lassen P., Mathian A., Moreau P., Couffignal J., Ciangura C., et al. Prevalence and severity of malnutrition in hospitalized COVID-19 patients. Clin Nutr ESPEN. 2020;40:214–219. doi: 10.1016/j.clnesp.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaiser M.J., Bauer J.M., Rämsch C., Uter W., Guigoz Y., Cederholm T., et al. Frequency of malnutrition in older adults: a multinational perspective using the mini nutritional assessment. J Am Geriatr Soc. 2010;58:1734–1738. doi: 10.1111/j.1532-5415.2010.03016.x. [DOI] [PubMed] [Google Scholar]
- 10.Cervantes-Pérez E., Cervantes-Guevara G., Martínez-Soto Holguín M.C., Cervantes-Pérez L.A., Cervantes-Pérez G., Cervantes-Cardona G.A., et al. Medical nutrition therapy in hospitalized patients with SARS-CoV-2 (COVID-19) infection in a non-critical care setting: knowledge in progress. Curr Nutr Rep. 2020;9:309–315. doi: 10.1007/s13668-020-00337-x. [DOI] [PMC free article] [PubMed] [Google Scholar]