Abstract
Opioid use disorder affects over 2 million Americans with an increasing number of deaths due to overdose from the synthetic opioid fentanyl and its analogs. The Food and Drug Administration–approved opioid receptor antagonist naloxone (e.g., Narcan) is used currently to treat overdose; however, a short duration of action limits its clinical utility. Methocinnamox (MCAM) is a long-lasting opioid receptor antagonist that may reverse and prevent the ventilatory-depressant effects of fentanyl. This study compared the ability of naloxone (0.0001–10 mg/kg) and MCAM (0.0001–10 mg/kg) to reverse and prevent ventilatory depression by fentanyl and compared the duration of action of MCAM intravenously and subcutaneously in two procedures: ventilation and warm-water tail withdrawal. In male Sprague-Dawley rats (N = 8), fentanyl (0.0032–0.178 mg/kg, i.v.) decreased minute volume in a dose- and time-dependent manner with a dose of 0.178 mg/kg decreasing VE to less than 40% of control. MCAM and naloxone reversed the ventilatory-depressant effects of 0.178 mg/kg fentanyl in a dose-related manner. The day after antagonist administration, MCAM but not naloxone attenuated the ventilatory-depressant effects of fentanyl. The duration of action of MCAM lasted up to 3 days and at least 2 weeks after intravenous and subcutaneous administration, respectively. MCAM attenuated the antinociceptive effects of fentanyl, with antagonism lasting up to 5 days and more than 2 weeks after intravenous and subcutaneous administration, respectively. Reversal and prolonged antagonism by MCAM might provide an effective treatment option for the opioid crisis, particularly toxicity from fentanyl and related highly potent analogs.
SIGNIFICANCE STATEMENT
This study demonstrates that like naloxone, methocinnamox (MCAM) reverses the ventilatory-depressant effects of fentanyl in a time- and dose-related manner. However, unlike naloxone, the duration of action of MCAM was greater than 2 weeks when administered subcutaneously and up to 5 days when administered intravenously. These data suggest that MCAM might be particularly useful for rescuing individuals from opioid overdose, including fentanyl overdose, as well as protecting against the reemergence of ventilatory depression (renarconization).
Introduction
The Centers for Disease Control and Prevention estimate that drug overdose deaths in the United States increased 4.6% in 2019 to nearly 71,000, including more than 50,000 deaths from opioids. The opioid epidemic appears to have worsened during the current pandemic, and the economic burden of the opioid epidemic in the United States is expected to exceed $500 billion in the near future (https://cdc.gov). Opioid overdose results from ventilatory depression through the activation of μ-opioid receptors within the central nervous system and neuromuscular components of the chest wall and diaphragm (Dahan et al., 2010). Fentanyl is a potent, high-efficacy synthetic opioid receptor agonist that suppresses ventilation, often leading to overdose and death (Henderson et al., 2014). There were 142,557 emergency department visits reported in 45 US states between July and September 2017 for patients of unintentional, nonfatal overdose who survived to be discharged from a medical facility (Vivolo-Kantor et al., 2018; Weiner et al., 2020). Of the 2000 opioid-related deaths that occurred in the US state of Massachusetts, one in five patients who were initially rescued from overdose and subsequently discharged died from an opioid overdose within a month of discharge. One in five patients who died from overdose in the 1st month did so in the first 2 days after discharge. These deaths occurred despite the availability, broad distribution, and use of the opioid receptor antagonist naloxone (Weiner et al., 2020).
Naloxone, the only Food and Drug Administration–approved medication available to treat opioid overdose, is administered by intramuscular, intravenous, and intranasal routes (Loimer et al., 1994). Recent reports suggest that naloxone may be less effective at reversing ventilatory depression caused by synthetic opioids (e.g., fentanyl) compared with its ability to reverse ventilatory depression by heroin (Schumann et al., 2008; Peterson et al., 2016; Somerville et al., 2017; Rzasa Lynn and Galinkin, 2018). Patients that overdose can require multiple doses of intranasal naloxone, and in extreme cases of overdose, naloxone is given by intravenous infusion to rapidly reverse and prevent ventilatory depression (Sutter et al., 2017; Bell et al., 2019). Despite the effectiveness of naloxone, its therapeutic utility is limited by a short duration action, which is about 1 hour in humans (Kaufman et al., 1981).
Methocinnamox (MCAM) is a long-lasting μ-opioid receptor antagonist (Lewis et al., 1988; Broadbear et al., 2000; Peckham et al., 2005). In nonhuman primates, doses of MCAM that antagonize self-administration of opioids do not affect responding maintained by cocaine or maintained by food or alter cardiovascular function, suggesting that MCAM might not have significant adverse effects (Wermeling, 2015; Maguire et al., 2019). The interaction of MCAM with μ-opioid receptors is selective and long-lasting with antagonist effects evident for days after acute administration (Broadbear et al., 2000; Gerak et al., 2019b; Maguire et al., 2019). Sustained antagonism of heroin-induced ventilatory depression by MCAM has been reported (Gerak et al., 2019a); however, the ability of MCAM to reverse and/or prevent fentanyl-induced ventilatory depression has not been reported.
Fentanyl decreases drive in ventilation and a mechanical resistance to breathing, which can lead to death by overdose (Burns et al., 2016); death can occur within minutes, potentially before remedial action can be taken, which is in part because of the high lipophilicity and rapid onset of fentanyl (Dahan et al., 2005; Gill et al., 2019). An effective, long-acting opioid receptor antagonist might be useful for reversing and providing sustained protection from fentanyl-induced ventilatory depression, thereby reducing the risk of postrescue renarconization (Sutter et al., 2017; Han et al., 2019; Moss and Carlo, 2019).
This study compared the ability of MCAM and naloxone to reverse and protect against the ventilatory-depressant effects of fentanyl in rats. Because the duration of action observed in this study when MCAM was administered intravenously was less than reported previously (Gerak et al., 2019b) for MCAM administered subcutaneously, antagonism by MCAM after intravenous and subcutaneous administration was compared directly. The duration of action of 10 mg/kg MCAM administered intravenously or subcutaneously was compared by measuring the ventilatory-depressant effects of fentanyl at various times after MCAM was administered. To compare directly with the previous study that reported a very long duration of action for subcutaneous MCAM in antagonizing antinociceptive effects of morphine, intravenous MCAM was also studied for its ability to antagonize the antinociceptive effects of fentanyl. This study extends an ongoing characterization of MCAM to ventilatory-depressant effects of another μ-opioid receptor agonist, fentanyl, including a direct comparison of different routes of administration.
Materials and Methods
Subjects
Sixteen adult male Sprague-Dawley rats (initially weighing 250–300 g) were purchased from Envigo Inc. (Livermore, CA). Rats were maintained under a 14-hour light/10-hour dark cycle with experiments conducted during the light cycle. Rats were housed individually in a colony room maintained at 25 ± 1°C. Subjects had free access to food and water in the home cage and were habituated to the experimental conditions and handled for 5–7 days prior to beginning the experiments. All animals used in these studies were maintained in accordance with the Institutional Animal Care and Use Committee at The University of Texas Health Science Center at San Antonio and the guidelines of the Committee on Care and Use of Laboratory Animal Resources, National Research Council [Department of Health, Education and Welfare, publication No. (National Institutes of Health) 85-23, revised 2011].
Drugs
Fentanyl hydrochloride (Drug Supply Program, National Institute on Drug Abuse, Rockville, MD) was dissolved in normal saline and administered intravenously in a volume of 0.5 ml/kg b.wt. MCAM hydrochloride (Syncom, Groningen, Netherlands) and naloxone hydrochloride (Drug Supply Program, National Institute on Drug Abuse, Rockville, MD) were dissolved in a vehicle of 10% w/v β-cyclodextrin in saline and administered intravenously or subcutaneously as described in Materials and Methods.
Surgery
Briefly, a chronic indwelling catheter (CNC-3H-30-6/6.5; Access Technologies, Skokie, IL) was implanted in the left femoral vein of rats anesthetized with 2% isoflurane as described previously (Minervini et al., 2019). The catheter was attached to a 22-G access port (VAB95BS; Instech Laboratories Inc.) anchored in a subcutaneous pocket. Immediately after surgery, rats received subcutaneous injections of 0.5 ml penicillin (300,000 U/ml) and 1.0 mg/kg meloxicam; rats were allowed 5 days to recover before resuming sessions. Catheters were flushed with 0.5 ml heparinized saline (100 U/ml) on nonexperimental days and with 0.5 ml of sterile saline before drug infusions. If a catheter failed, a new catheter was implanted in the right femoral or jugular vein.
Whole-Body Plethysmography
Prior to testing drugs, rats were habituated to the whole-body plethysmography chambers (Buxco Electronics, Inc., Wilmington, NC) located inside sound-attenuating enclosed cubicles. There was a continuous flow of fresh air (2.2 l/min) through the chambers, and experiments were conducted at room temperature (23–25°C). Fentanyl was delivered from 5- or 10-ml syringes mounted in a syringe pump (PHM-100; Med Associates, Fairfax, VT) that was connected to and controlled by a PC computer operating MED-PC IV software (Med Associates). Prior to and after each intravenous drug infusion, catheters were flushed with sterile saline. Ventilation was recorded by changes in chamber pressure as detected by a signal transducer and then amplified and digitized by FinePointe software (DSI, St. Paul, MN). Software calculated the ventilatory frequency (f), tidal volume (VT), and minute volume (VE = f × VT). The average ventilatory frequency (breaths per minute), tidal volume (milliliter per breath), and minute volume (milliliter per minute) were calculated for each rat.
Procedures
Fentanyl-Induced Ventilatory Depression.
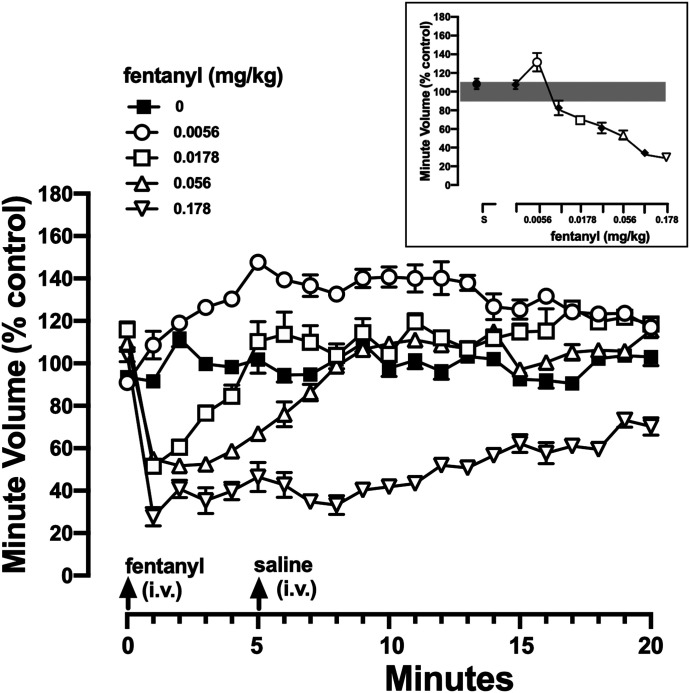
Ventilation was recorded for 20 minutes prior to (i.e., baseline) and 20 minutes after an acute intravenous infusion of fentanyl with different doses (0.0032–0.178 mg/kg) administered on different days in a randomized manner (Fig. 1). Doses were administered no more often than every 3rd day; saline was administered the day before drug tests to redetermine individual control values. A dose of 0.178 mg/kg fentanyl reliably decreased ventilation to at least 40% of control and was selected for subsequent reversal and prevention studies.
Fig. 1.
Effects of fentanyl on ventilation. Minute volume is plotted as a function of time after administration of intravenous vehicle or fentanyl (0.0056, 0.0178, 0.056, and 0.178 mg/kg). After a 20-minute baseline period (see Table 1), rats received an intravenous infusion of vehicle or fentanyl (arrow at “0” minutes) followed 5 minutes later by an intravenous infusion of saline (arrow at “5” minutes). Data are presented as a percentage of the average minute volume during the 20-minute baseline period calculated individually for each rat. The inset summarizes the average minute volume during the 5 minutes immediately after infusion of saline or fentanyl (0.0032–0.178 mg/kg), and filled symbols represent doses not shown in the main figure; the gray bar represents the 95% CI for control conditions (i.e., saline infusions at “0” and “5” minutes). Ordinate: minute volume plotted as percent of control and averaged among eight rats ± 1 S.E.M. Abscissae: time in minutes. CI, confidence interval.
Reversal and Prevention of Fentanyl-Induced Ventilatory Depression.
To test the ability of naloxone and MCAM to reverse the ventilatory-depressant effects of 0.178 mg/kg fentanyl, an intravenous infusion of naloxone (0.0001–10 mg/kg) or MCAM (0.0001–10 mg/kg) was administered 5 minutes after intravenous infusion of fentanyl with ventilation recorded for an additional 15 minutes. Dose-response curves were generated by taking the first 5-minute average of VE after antagonist or vehicle. To test the ability of MCAM and naloxone to prevent fentanyl-induced ventilatory depression after the initial reversal, rats were challenged with a single dose of fentanyl (0.178 mg/kg) 1 day after ventilatory depression was reversed with MCAM or naloxone; on those test days, rats received intravenous fentanyl and, 5 minutes later, intravenous vehicle. Fentanyl was tested 1 and 3 days after reversal with MCAM (0.1–10 mg/kg) and 1 day after reversal with naloxone (10 mg/kg) (i.e., when the effect of 0.178 mg/kg fentanyl was not different in rats treated with MCAM or naloxone compared with effects of that dose of fentanyl under control conditions).
Prevention of Fentanyl-Induced Ventilatory Depression by Intravenous and Subcutaneous Routes.
The duration of action of intravenous MCAM in the reversal experiment was less than the duration of action reported previously for subcutaneous MCAM in an antinociception study (Gerak et al., 2019b); consequently, intravenous and subcutaneous MCAM were compared for antagonism of the ventilatory-depressant effects of fentanyl. It is not practical to conduct a reversal experiment with subcutaneous MCAM because of the need to break the seal of the respiration chamber to administer the injection. At least 21 days separated tests with MCAM and subsequent antagonism tests with MCAM or naloxone. Thus, on different occasions MCAM was administered intravenously or subcutaneously 1 day prior to beginning tests with 0.178 mg/kg fentanyl i.v. The effect of fentanyl on ventilation was scheduled to be determined 1, 3, 6, 13, and 21 days after MCAM. Testing ended when ventilatory depression by this dose of fentanyl was not different from the effects of this dose under control conditions (i.e., no antagonist). After day 6, control ventilation (i.e., no fentanyl was administered) was assessed every 3 days.
Warm-Water Tail Withdrawal.
Two water baths (EW-14576-00; Cole-Parmer, Vernon Hills, IL) were maintained at constant temperatures (40 or 50°C) throughout the experiment, and a stopwatch was used to measure the latency for rats to remove their tails from water maintained at each temperature.
Procedure
Experiments began by determining baseline latencies for two temperatures with the lower portion of the tail placed in the water baths. The remainder of the session was divided into 30-minute cycles with the order of the presentation of the different temperatures randomized as described elsewhere (Gerak et al., 2019b). In some sessions, only vehicle was administered for all cycles. In other sessions, a fentanyl dose-effect curve was determined, with saline administered in the first cycle and fentanyl administered in subsequent cycles and with the cumulative dose (0.01, 0.032, and 0.1 mg/kg) increasing in one-half–log increments across cycles. Fentanyl dose-effect curves were redetermined 1, 5, and 21 days after rats received an acute intravenous infusion of 10 mg/kg MCAM i.v. (i.e., testing ended when tail-withdrawal latency with a cumulative dose of 0.1 mg/kg fentanyl was not different from the tail-withdrawal latency of that dose of fentanyl under control conditions). Vehicle tests were conducted periodically (every 3 days) to ensure stable baseline latencies and continued habituation to handling.
Data Analyses
Graphs were constructed, and analyses were conducted with GraphPad Prism version 8.4.1 for Mac (GraphPad Software, La Jolla, CA,). A power analysis was used (α = 0.05, 80% power) to determine the number of animals needed to detect a shift of at least 2 fold (G*Power version 3.1.9.6). These studies included only males and did not investigate possible sex difference. Significance was set at P < 0.05.
Plethysmography.
Data are expressed as the mean ± 1 S.E.M. for eight rats. Differences between treatment and control were evaluated using two-way ANOVA or multiple t tests followed by the Bonferroni-Dunn test.
Warm-Water Tail Withdrawal.
Tail-withdrawal latencies were converted to a percentage of the maximum possible effect (15 seconds) as follows: [(test latency − control latency)/(15 seconds − control latency)] × 100% and then averaged across eight rats; mean latencies (±1 S.E.M.) were plotted as function of fentanyl dose or time in days. Statistical significance was determined by ANOVA followed by Dunnett’s multiple comparisons, comparing the percentage of the maximum possible latency obtained after the largest cumulative dose of fentanyl (i.e., 0.1 mg/kg) for each route of administration at each time point after administration of MCAM.
Results
Baseline Ventilatory Parameters.
Baseline ventilation parameters (i.e., VT, f, and VE) determined prior to infusion of vehicle, naloxone, or MCAM are summarized in Table 1; under these conditions, there were no significant differences between the eight rats used for the reversal study and the eight rats used for the prevention study. Five minutes after an infusion of 10 mg/kg naloxone or 10 mg/kg MCAM (in the absence of fentanyl), VE was not significantly different from VE 5 minutes after administration of vehicle or saline (Table 2).
TABLE 1.
Baseline values for minute volume (VT), tidal volume (VE), and frequency (f) under control conditions
Data are shown as the mean ± 1 S.E.M. NLX, naloxone. N = 8 per study.
| Parameter | Reversal Study | Prevention Study | |||||
|---|---|---|---|---|---|---|---|
| Vehicle (i.v.) | NLX (i.v.) | MCAM (i.v.) | Vehicle (i.v.) | Vehicle (s.c.) | MCAM (i.v.) | MCAM (s.c.) | |
| Minute volume (ml/min) | 198 ± 10 | 216 ± 13 | 212 ± 17 | 202 ± 11 | 200 ± 31 | 198 ± 22 | 200 ± 34 |
| Tidal volume (ml) | 2.15 ± 0.10 | 2.22 ± 0.16 | 2.20 ± 0.18 | 2.20 ± 0.20 | 2.30 ± 0.10 | 2.36 ± 0.21 | 2.28 ± 0.15 |
| Frequency (breaths/min) | 135 ± 13 | 130 ± 10 | 128 ± 11 | 132 ± 10 | 122 ± 17 | 132 ± 9 | 134 ± 10 |
TABLE 2.
Ventilatory parameters for minute volume (VT) tidal volume (VE), and frequency (f) after intravenous infusion of fentanyl, vehicle, saline, naloxone, or MCAM
Data are shown as the mean ± 1 S.E.M.; fentanyl (0.178 mg/kg). NLX, naloxone. N = 8.
| Parameter | Fentanyl | Vehicle | Saline | NLXa | MCAMa |
|---|---|---|---|---|---|
| Minute volume (ml/min) | 74 ± 4 | 220 ± 10 | 210 ± 25 | 205 ± 10 | 220 ± 8 |
| Tidal volume (ml) | 1.03 ± 0.02 | 1.90 ± 0.14 | 1.98 ± 0.10 | 2.20 ± 0.2 | 2.5 ± 0.10 |
| Frequency (breaths/min) | 70 ± 4 | 135 ± 5 | 135 ± 2 | 140 ± 3 | 140 ± 5 |
Cumulative ventilatory values 5 min after infusion of 10 mg/kg NLX or 10 mg/kg MCAM.
Reversal of Fentanyl-Induced Ventilatory Depression by MCAM and Naloxone.
Fentanyl (0.0032–0.178 mg/kg) rapidly decreased VE in a dose- and time-related manner, with a dose of 0.178 decreasing VE to less than 40% of control within 1 minute of intravenous infusion. Decreased VE by 0.178 mg/kg fentanyl did not fully recover in the 20-minute observation period (Fig. 1). A dose of 0.0178 decreased VE to less than 60% of control within 1 minute of intravenous infusion, with ventilation recovering to control values by 5 minutes postinfusion (Fig. 1). Some doses of fentanyl increased VE; for example, a dose of 0.0056 mg/kg fentanyl increased VE to more than 130% of control within 5 minutes after intravenous administration (circles, Fig. 1 and inset, which summarizes all eight doses that were studied in a dose-response function).
Fentanyl (0.178 mg/kg)-induced decreases in VE were reversed in a dose- and time-related matter by MCAM and naloxone. Figure 2 shows the effects of smaller doses of each antagonist (0.0001, 0.001, and 0.01 mg/kg) in the lower panels and larger doses of each antagonist (0.1, 1.0, and 10.0 mg/kg) in the upper panels. The smallest effective doses of each antagonist (0.01 mg/kg, squares, lower panels, Fig. 2) restored VE to control values 2 (naloxone) and 4 (MCAM) minutes after intravenous infusion. Larger doses of each antagonist reversed fentanyl-induced decreases in VE more rapidly compared with the effects of smaller doses. For example, a dose of 0.1 mg/kg naloxone restored VE to control values within 1 to 2 minutes, and the same dose of MCAM restored VE to control values within 3 to 4 minutes (circles, upper panels, Fig. 2). Compared with smaller doses, reversal of decreased VE was even more rapid after administration of 1.0 or 10.0 mg/kg of naloxone or MCAM, although the rate of reversal did not appear to be different between these two largest doses for either antagonist (upper panels, Fig. 2). Larger doses of naloxone and MCAM (0.1–10 mg/kg) that rapidly reversed the ventilatory-depressant effects of fentanyl also increased VE above control values (Figs. 2 and 3; Table 3). Elevated VE was the result of marked increases in VT and f. For example, VT was 1.03–1.05 ml after vehicle and was increased to 2.80 ± 0.11 ml after 10 mg/kg MCAM and to 2.06 ± 0.80 ml after 10 mg/kg naloxone (Table 3). Similarly, f was 62–68 breaths per minute after vehicle and was increased to 164 ± 2 breaths per minute after 10 mg/kg MCAM and to 142 ± 2 breaths per minute after 10 mg/kg naloxone (Table 3). Increases in ventilation after the administration of large doses of antagonists in fentanyl-treated rats returned toward control values in a dose- and time-related manner [Fig. 2; insets show corresponding significant changes in area under for MCAM (e.g., 10 mg/kg: t = 30.26, 1.0 mg/kg: t = 11.47, 0.1 mg/kg: t = 10.94, 0.01 mg/kg: t = 9.0, 0.001 mg/kg: t = 4.89; P < 0.05) and naloxone (e.g., 10 mg/kg: t = 31.81, 1.0 mg/kg: t = 27.03, 0.1 mg/kg: t = 23.57, 0.01 mg/kg: t = 25.81, 0.001 mg/kg: t = 5.89; P < 0.05)].
Fig. 2.
Reversal of the ventilatory-depressant effects of fentanyl by MCAM and naloxone. Rats received an intravenous infusion of 0.178 mg/kg fentanyl at “0” minutes (arrows) and a second infusion of vehicle, (squares, both panels), MCAM (left panels), or naloxone (right panels) at “5” minutes. Upper panels include data for vehicle, 0.1, 1.0, and 10.0 mg/kg; lower panels include data for vehicle, 0.0001, 0.001, and 0.01 mg/kg. The gray bar represents the 95% CI for minute volume under control conditions (saline followed by vehicle). Insets represent the area under the curve (AUC) for MCAM or naloxone curves compared with vehicle curves. Asterisks indicate that AUC obtained after the administration of an antagonist was significantly different from AUC after vehicle, P < 0.05. Ordinates: minute volume plotted as percent of control and averaged among eight rats ± 1 S.E.M. Abscissae: time in minutes. CI, confidence interval.
Fig. 3.
Dose-response curves summarizing the same data shown in Fig. 2 for MCAM and naloxone in reversing ventilatory-depressant effects of 0.178 mg/kg fentanyl intravenously. Each data point is the mean minute volume for 5 minutes after infusion of vehicle (V), MCAM, or naloxone and averaged (±1 S.E.M.) among eight rats. Ordinate: minute volume presented as a percent of baseline. Abscissa: dose of MCAM or naloxone in mg/kg body weight intravenously. C represents a control session in which rats received only vehicle.
TABLE 3.
Reversal of fentanyl-induced ventilatory depression by MCAM and naloxone
Data are shown as the mean ± 1 S.E.M.; fentanyl (0.178 mg/kg) followed by naloxone or MCAM, N = 8.
| Parameter | Reversal with MCAM (mg/kg) | 1 Day after MCAM (mg/kg)a | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vehicle | 0.0001 | 0.001 | 0.01 | 0.1 | 1.0 | 10.0 | 0.1 | 1.0 | 10 | |
| Minute volume (ml/min) | 68 ± 4 | 64 ± 4 | 82 ± 17 | 92 ± 12 | 240 ± 8 | 340 ± 10 | 440 ± 10 | 66 ± 4 | 72 ± 3 | 210 ± 14 |
| Tidal volume (ml) | 1.05 ± 0.06 | 1.02 ± 0.1 | 1.70 ± 0.15 | 1.90 ± 0.28 | 2.5 ± 0.10 | 2.50 ± 0.8 | 2.80 ± 0.11 | 1.03 ± 0.06 | 1.08 ± 0.05 | 2.06 ± 0.8 |
| Frequency (breaths/min) | 62 ± 3 | 68 ± 2 | 138 ± 6 | 138 ± 10 | 140 ± 5 | 154 ± 2 | 164 ± 2 | 68 ± 3 | 64 ± 3 | 142 ± 2 |
| Reversal with Naloxone (mg/kg) | 1 Day after Naloxone (mg/kg)a | |||||||||
| Vehicle | 0.0001 | 0.001 | 0.01 | 0.1 | 1.0 | 10 | 0.1 | 1.0 | 10 | |
| Minute volume (ml/min) | 66 ± 6 | 68 ± 4 | 88 ± 6 | 290 ± 6 | 340 ± 10 | 440 ± 10 | 460 ± 23 | — | — | 66 ± 3 |
| Tidal volume (ml) | 1.03 ± 0.1 | 1.05 ± 0.06 | 1.90 ± 0.28 | 2.50 ± 0.10 | 2.70 ± 0.11 | 2.60 ± 0.12 | 2.72 ± 0.10 | — | — | 1.10 ± 0.08 |
| Frequency (breaths/min) | 60 ± 3 | 65 ± 3 | 138 ± 10 | 140 ± 5 | 160 ± 2 | 164 ± 2 | 162 ± 6 | — | — | 60 ± 8 |
Five minutes after fentanyl 1 day after reversal with MCAM or naloxone.
Duration of Action of Naloxone and MCAM.
To test the duration of action of naloxone and MCAM administered intravenously, the same rats that were studied in the reversal experiment described above received 0.178 mg/kg fentanyl 1 (naloxone and MCAM) and 3 (MCAM only) days after an acute injection of naloxone or MCAM (Fig. 4). One day after administration of 10 mg/kg naloxone, 0.178 mg/kg fentanyl decreased VE for the duration of the 20-minute observation period in a manner that was not different from vehicle-treated rats (compare diamonds and squares, right panel, Fig. 4). In contrast, 1 day after administration of 10 mg/kg MCAM, the same dose of fentanyl was not effective with VE remaining at or above control values for the duration of the 20-minute observation period (compare diamonds and squares, left panel, Fig. 4). One day after a 10-fold smaller dose of MCAM (1.0 mg/kg), 0.178 mg/kg fentanyl decreased VE to less than 40% of control, although that effect of fentanyl waned over the subsequent 20 minutes compared with fentanyl in vehicle-treated rats. One day after a still-smaller dose of MCAM (0.1 mg/kg), fentanyl decreased VE for the duration of the session in a manner that was not different from vehicle-treated rats (compare circles and squares, left panel, Fig. 4). Three days after administration, 10 mg/kg MCAM i.v. was no longer effective, with 0.178 mg/kg fentanyl decreasing VE to less than 40% of control (unpublished data).
Fig. 4.
Protection against the ventilatory-depressant effects of 0.178 mg/kg fentanyl by MCAM (left) and naloxone (right). The preceding day, rats received 0.178 mg/kg fentanyl intravenously followed by intravenous MCAM or naloxone. On the (next) test day shown in this figure, rats received an intravenous infusion of fentanyl at minute “0” and an infusion of vehicle intravenously at “5” minutes. The gray bar represents the 95% CI for minute volume under control conditions (saline followed by vehicle). Insets represent the area under the curve (AUC) for MCAM or naloxone curves compared with vehicle curves. Asterisks indicate that AUC obtained after the administration of an antagonist was significantly different from AUC after vehicle, P < 0.05. Ordinates: minute volume plotted as percent of control and averaged among eight rats ± 1 S.E.M. Abscissae: time in minutes. CI, confidence interval.
Prevention of Fentanyl-Induced Ventilatory Depression by 10 mg/kg MCAM Administered Intravenously and Subcutaneously.
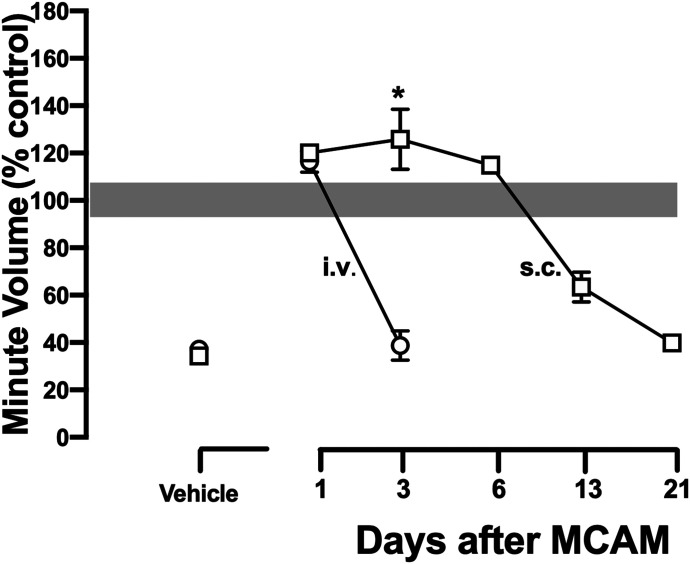
In the reversal study described above, 10 mg/kg MCAM was no longer effective 3 days after intravenous administration. In a previous study (Gerak et al., 2019b), 10 mg/kg MCAM was effective for several weeks after subcutaneous administration. To further examine possible differences in the effectiveness of MCAM by different routes of administration or whether reversal of the effects of fentanyl impacted the duration of action of a single infusion of MCAM, fentanyl-induced ventilatory depression was studied beginning 1 day and for up to 21 days after rats received 10 mg/kg MCAM intravenously or subcutaneously. One day after administration, both intravenous and subcutaneous MCAM (10 mg/kg) prevented the ventilatory-depressant effects of 0.178 mg/kg fentanyl (circles, both panels, Fig. 5; Table 4). Three days after administration, intravenous MCAM was no longer effective, whereas subcutaneous MCAM completely blocked the ventilatory-depressant effects of fentanyl (squares, both panels, Fig. 5; Table 4). For example, under control conditions, a dose of 0.178 mg/kg fentanyl decreased VE to 68 ± 4 ml/min; 3 days after 10 mg/kg MCAM intravenously, that dose of fentanyl decreased VE to 68 ± 3 ml/min, whereas 3 days after 10 mg/kg MCAM subcutaneously, that dose of fentanyl had no effect (VE = 284 ± 6 ml/min; Table 4). Figure 6 summarizes the effects of 0.178 mg/kg fentanyl on VE (VT and f are shown in Table 4) in rats treated with MCAM intravenously (circles) or subcutaneously (squares) and shows the significantly longer duration of action of MCAM when administered subcutaneously compared with that administered intravenously. Sensitivity to the ventilatory-depressant effects of fentanyl did not recover until 21 days after administration of 10 mg/kg MCAM subcutaneously. In vehicle-treated rats, the effects of fentanyl on ventilatory parameters were not different in repeated tests over the same time period (unpublished data).
Fig. 5.
Protection against the ventilatory-depressant effects of 0.178 mg/kg fentanyl 1 and 3 days after intravenous (left) or subcutaneous (right) administration of MCAM. In contrast with data shown in Fig. 4, rats did not receive fentanyl on the day MCAM was administered (i.e., protection only and no rescue). On the test days shown, rats received an intravenous infusion of fentanyl at minute “0” and an infusion of vehicle intravenously at “5” minutes. The gray bar represents the 95% CI for minute volume under control conditions (saline followed by vehicle). Insets represent the area under the curve (AUC) for intravenous or subcutaneous MCAM compared with vehicle curves. Asterisks indicate that AUC obtained after the administration of an antagonist was significantly different from AUC after vehicle, P < 0.05. Ordinates: minute volume plotted as percent of control and averaged among eight rats ± 1 S.E.M. Abscissae: time in minutes. CI, confidence interval.
TABLE 4.
Prevention of fentanyl-induced ventilatory depression by MCAM administered intravenously or subcutaneously
Data are shown as the mean ± 1 S.E.M.; fentanyl (0.178 mg/kg) was administered on days after 10 mg/kg MCAM, N = 8.
| Parameter | Prevention Study | Days after MCAM (i.v.) | ||||||
|---|---|---|---|---|---|---|---|---|
| Fentanyl | Vehicle | MCAM | 1 | 3 | 6 | 13 | 21 | |
| Minute volume (ml/min) | 68 ± 4 | 220 ± 8 | 218 ± 5 | 216 ± 21 | 68 ± 3 | — | — | — |
| Tidal volume (ml) | 1.05 ± 0.06 | 1.85 ± 0.12 | 1.90 ± 0.11 | 2.00 ± 0.10 | 1.02 ± 0.10 | — | — | — |
| Frequency (breaths/min) | 65 ± 3 | 140 ± 10 | 142 ± 2 | 142 ± 3 | 62 ± 7 | — | — | — |
| Days after MCAM (s.c.) | ||||||||
| 1 | 3 | 6 | 13 | 21 | ||||
| Minute volume (ml/min) | — | — | — | 280 ± 11 | 284 ± 6 | 298 ± 12 | 150 ± 10 | 64 ± 3 |
| Tidal volume (ml) | — | — | — | 1.90 ± 0.10 | 2.00 ± 0.05 | 1.86 ± 0.11 | 1.48 ± 0.15 | 1.08 ± 0.10 |
| Frequency (breaths/min) | — | — | — | 158 ± 7 | 152 ± 2 | 162 ± 4 | 134 ± 10 | 64 ± 1 |
Fig. 6.
Time-response curves summarizing the same data shown in Fig. 5 and Table 2 for MCAM administered intravenously and subcutaneously in protecting against the ventilatory-depressant effects of 0.178 mg/kg fentanyl intravenously. Each data point is the mean minute volume for 5 minutes after infusion of fentanyl averaged (±1 S.E.M.) among eight rats. Asterisk indicates duration of MCAM (i.e., day) that was different between intravenous and subcutaneous administration, P < 0.05. Ordinate: minute volume presented as a percent of baseline. Abscissa: days after administration of MCAM. Vehicle points show data for fentanyl in rats that did not receive MCAM.
Warm-Water Tail Withdrawal: Baseline Values.
Under control conditions (i.e., no drug), tail-withdrawal latencies were 15 (maximum possible effect) and 2.88 ± 0.12 seconds at 40 and 50°C water, respectively. Under control conditions, fentanyl dose dependently increased tail-withdrawal latencies to more than 90% of the maximum possible effect at a cumulative dose of 0.1 mg/kg (filled circles, both panels, Fig. 7). MCAM (10 mg/kg) alone did not increase tail-withdrawal latencies after intravenous or subcutaneous administration (unpublished data).
Fig. 7.
Effects of fentanyl on tail-withdrawal latency from warm water 1, 5, and 21 days after intravenous (left) or subcutaneous (right; replotted with permission from Gerak et al., 2019b) administration of 10 mg/kg MCAM. Inset summarizes data (maximum possible effect) for a cumulative dose of 0.1 mg/kg fentanyl under control conditions (C) and 1, 5, and 21 days after intravenous or subcutaneous administration of 10 mg/kg MCAM. Asterisks indicate tail-withdrawal latency that was different between MCAM administered intravenously or subcutaneously, P < 0.05. Ordinate: average latency (±1 S.E.M.) to remove tails from 50°C water expressed as a percentage of control and averaged among eight rats. Abscissae: dose of fentanyl administered intraperitoneally in mg/kg body weight. S represents the effects of saline.
Warm-Water Tail Withdrawal: Duration of Action of 10 mg/kg MCAM iIntravenously and Subcutaneously.
To test whether differences in the duration of action of MCAM by different routes of administration (i.e., ventilation studies described above) generalized to other procedures, intravenous MCAM was studied using the same tail-withdrawal procedure in which subcutaneous MCAM was shown to be effective for several weeks [right panel of Fig. 7 replotted with permission from Gerak et al. (2019b)]. A single dose of 10 mg/kg MCAM administered intravenously antagonized the effects of fentanyl on tail-withdrawal latency, as indicated by a downward shift in the dose-effect curve (left panel, Fig. 7). Whereas a dose of 0.1 mg/kg fentanyl produced a near maximum possible effect under control conditions, this dose produced less than 20% of the maximum 1 day and less than 50% of the maximum 5 days after intravenous MCAM (squares and triangles, respectively, left panel, Fig. 7). Sensitivity to fentanyl recovered to control values 21 days after a single injection of 10 mg/kg MCAM intravenously. In contrast, 21 days after subcutaneous administration of MCAM (inverted triangles, right panel, Fig. 7), 0.1 mg/kg fentanyl produced less than 20% of the maximum possible effect. The inset in the right panel of Fig. 7 comparing the effects of 0.1 mg/kg fentanyl 1, 5, and 21 days after intravenous (circles) or subcutaneous (squares) MCAM shows a progressive recovery in sensitivity to fentanyl over 21 days after intravenous MCAM but not after subcutaneous MCAM.
Discussion
The number of opioid overdose deaths in the United States remains alarmingly high and, by many estimates, has increased markedly during the COVID-19 pandemic. Although naloxone effectively reverses opioid overdose, a significant number of patients who are released from medical care after being rescued from an opioid overdose die within the next month, with one in five of those deaths occurring within 2 days (D’Onofrio et al., 2015; Weiner et al., 2020). Moreover, it is estimated that one in four opioid overdoses in the United States occurs in a private residence where the individual is often alone and unable to self-administer life-saving medication. A long-lasting, effective medication that could be administered when individuals who have been rescued from an opioid overdose are released from medical care could provide long-term protection and dramatically reduce the number of overdose deaths (Weiner et al., 2020). Despite the increasing availability of naloxone, its effectiveness is limited by its pharmacological properties. For example, because it is effective for approximately 1 hour, after rescue with naloxone, ventilatory depression often reemerges (i.e., renarconization), particularly for long-acting opioid receptor agonists (Dahan et al., 2010; Tomassoni et al., 2017). In addition, there has been speculation as to whether naloxone is less effective at reversing ventilatory depression caused by fentanyl and its ultrapotent analogs compared with its ability to reverse ventilatory depression caused by other opioids, such as heroin (Mayer et al., 2018; Rzasa Lynn and Galinkin, 2018; Gill et al., 2019; Hill et al., 2020).
MCAM is an opioid receptor antagonist with a long duration of action (Broadbear et al., 2000; Peckham et al., 2005; Gerak et al., 2019a,b; Maguire et al., 2019, 2020; Minervini et al., 2020). MCAM has been shown to antagonize several different effects of μ-opioid receptor agonists in mice, rats, and nonhuman primates, including the ventilatory-depressant effects of heroin in nonhuman primates. Although MCAM has been shown to reverse and prevent the ventilatory-depressant effects of heroin, it has not been assessed for its ability to reverse or prevent the ventilatory-depressant effects of fentanyl, which is a major contributor to the ongoing opioid epidemic. Results of the current study extend those of prior studies in several ways. First, this is the first study to evaluate MCAM for its ability to attenuate the ventilatory-depressant effects of a μ-opioid receptor agonist in rats; previous studies on MCAM and ventilation used nonhuman primates. Second, this study demonstrates that MCAM is as effective as naloxone in reversing the ventilatory-depressant effects of fentanyl; previous studies examined MCAM for its ability to antagonize ventilatory-depressant effects of heroin. Third, consistent with effects reported for other procedures and other species, an acute injection of MCAM antagonizes the ventilatory-depressant effects of fentanyl for several days. Fourth, the duration of antagonism by MCAM after intravenous administration is considerably less than its duration of antagonism after subcutaneous administration.
Ventilation was decreased to less than 40% of control within 1 minute after administration of 0.178 mg/kg fentanyl i.v. This rapid and severe depression of ventilation resembles what is observed in patients with human fentanyl overdose (Magosso et al., 2004; Prekupec et al., 2017; Han et al., 2019). After 5 minutes of significant ventilatory depression, MCAM and naloxone rapidly reversed ventilatory depression in a dose- and time-dependent manner. VE increased to 140%–280% of control within 1 minute after the administration of MCAM (0.1–10 mg/kg) or naloxone (0.01–10 mg/kg). This hyperventilation (i.e., ventilatory overshoot) was the result of marked increases in VT and f compared with control conditions. Other than occasional acute injections of fentanyl, rats in this study were not treated with opioid receptor agonists and, therefore, were not physically dependent on opioids. Nevertheless, hyperventilation and the accompanying behavioral excitation (not studied directly) are similar to what has been referred to as acute opioid dependence, as reflected by precipitation of withdrawal signs after acute administration of an opioid receptor agonist followed by an opioid receptor antagonist (Harris and Gewirtz, 2005; Walker and Sterious, 2005; Avetian et al., 2018).
Onset and duration of action are important properties of any rescue medication and might be related to the adverse cardiovascular events that occurred in some patients that received naloxone for opioid overdose (Dahan et al., 2010; Rzasa Lynn and Galinkin, 2018). In this study, naloxone had a slightly faster onset of action and was slightly more potent than MCAM. A more-significant difference between naloxone and MCAM was duration of action. One day after reversal of fentanyl-induced ventilatory depression by 10 mg/kg naloxone, 0.178 mg/kg fentanyl was fully effective, decreasing VE to 40% of control. In contrast, 1 day after reversal of fentanyl-induced ventilatory depression by 10 mg/kg MCAM, fentanyl had no effect on VE. The much-longer duration of protection provided by MCAM compared with naloxone might be especially relevant considering the high mortality rate for individuals that are rescued and subsequently overdose (e.g., within 2 days) after discharge from medical care.
A previous study (Gerak et al., 2019b) reported that 10 mg/kg MCAM administered subcutaneously antagonized the antinociceptive effects of opioid receptor agonists for several weeks in rats. When the same dose of MCAM was administered intravenously in the current study, its duration of action was less than 3 days. Thus, another goal of this study was to compare directly the duration of action of MCAM administered intravenously and subcutaneously. Consistent with the prior study on antagonism of antinociceptive effects of opioids, MCAM had a much-longer duration of antagonist action when administered subcutaneously compared with intravenous MCAM, which also has a long duration of action after subcutaneous administration in nonhuman primates, attenuating the ventilatory-depressant effects of heroin for up to a week (Gerak et al., 2019a). This difference in duration of action across routes of administration might be related to pharmacokinetic factors, including rate of metabolism, elimination, and the possible generation of metabolites (Jin et al., 2015).
To further examine the difference in duration of MCAM across routes of administration, a warm-water tail-withdrawal procedure was used to test the duration of action of intravenous MCAM (Gerak et al., 2019b tested subcutaneous MCAM and antinociception). The antagonist properties of intravenous MCAM were diminished 5 days after and were no longer evident 21 days after administration of 10 mg/kg. In contrast, subcutaneous MCAM continues to antagonize the antinociceptive effects of fentanyl for more than 21 days (Gerak et al., 2019b; Fig. 7 in this paper). Thus, marked differences in duration of action for MCAM administered subcutaneously or intravenously occur across different procedures [antagonism of ventilatory depression (the current study) and antagonism of antinociception (the current study and Gerak et al., 2019b)], further supporting the notion that these differences are related to pharmacokinetic factors. Compared with naloxone, MCAM has a much-longer duration of action regardless of route of administration. Nevertheless, route of administration would be an important consideration for using MCAM to treat overdose and/or opioid use disorder.
The number of overdose deaths caused by fentanyl and its analogs has soared over the past several years (Chevillard et al., 2009; Colon-Berezin et al., 2019; Spencer et al., 2019) despite the increasing availability of the life-saving medication naloxone. The two challenges confronting any new medication for opioid overdose are the rapid reversal of opioid-induced ventilatory depression and the subsequent protection from ventilatory depression (e.g., renarconization; Algera et al., 2019). A medication that satisfies both of these challenges could have a significant positive impact on the treatment of opioid overdose as well as opioid use disorder. Both MCAM and naloxone rapidly reverse the ventilatory-depressant effects of fentanyl in a dose- and time-dependent manner; however, under some conditions and in some patients, MCAM could have significant advantages over naloxone because a single injection of MCAM would be expected to prevent fentanyl-induced ventilatory depression for days or weeks. This study demonstrates that MCAM effectively reverses and protects against the ventilatory-depressant effects of fentanyl; this antagonist might be an additional useful medication for confronting the current opioid epidemic and the continuing high rate of overdose and death from fentanyl and its ultrapotent analogs.
Acknowledgments
The authors thank Lisa R Gerak and David R Maguire for their invaluable suggestions, feedback, and support.
Abbreviations
- f
ventilatory frequency
- MCAM
methocinnamox
- VE
minute volume
- VT
tidal volume
Authorship Contributions
Participated in research design: Jimenez, France.
Conducted experiments: Jimenez, Castaneda.
Contributed new reagents or analytic tools: France.
Performed data analysis: Jimenez.
Wrote or contributed to the writing of the manuscript: Jimenez, France.
Footnotes
This work was supported by National Institutes of Health National Institute on Drug Abuse [Grants R01-DA05018 and R01-DA048417] (to C.P.F.), the Welch Foundation [Grant AQ-0039] (to C.P.F.), and T32 Training Program [Grant T32DA031115] (to C.P.F.). All funding sources had no involvement beyond financial support of this study. The content is solely the responsibility of the authors and does not represent the official views of National Institutes of Health or National Institute on Drug Abuse.
Disclosure/Conflict of Interest: C.P.F. is coholder of a provisional US patent for MCAM
References
- Algera MH, Kamp J, van der Schrier R, van Velzen M, Niesters M, Aarts L, Dahan A, Olofsen E (2019) Opioid-induced respiratory depression in humans: a review of pharmacokinetic-pharmacodynamic modelling of reversal. Br J Anaesth 122:e168–e179. [DOI] [PubMed] [Google Scholar]
- Avetian GK, Fiuty P, Mazzella S, Koppa D, Heye V, Hebbar P (2018) Use of naloxone nasal spray 4 mg in the community setting: a survey of use by community organizations. Curr Med Res Opin 34:573–576. [DOI] [PubMed] [Google Scholar]
- Bell A, Bennett AS, Jones TS, Doe-Simkins M, Williams LD (2019) Amount of naloxone used to reverse opioid overdoses outside of medical practice in a city with increasing illicitly manufactured fentanyl in illicit drug supply. Subst Abus 40:52–55. [DOI] [PubMed] [Google Scholar]
- Broadbear JH, Sumpter TL, Burke TF, Husbands SM, Lewis JW, Woods JH, Traynor JR (2000) Methocinnamox is a potent, long-lasting, and selective antagonist of morphine-mediated antinociception in the mouse: comparison with clocinnamox, β-funaltrexamine, and β-chlornaltrexamine. J Pharmacol Exp Ther 294:933–940. [PubMed] [Google Scholar]
- Burns RM, Pacula RL, Bauhoff S, Gordon AJ, Hendrikson H, Leslie DL, Stein BD (2016) Policies related to opioid agonist therapy for opioid use disorders: the evolution of state policies from 2004 to 2013. Subst Abus 37:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevillard L, Mégarbane B, Risède P, Baud FJ (2009) Characteristics and comparative severity of respiratory response to toxic doses of fentanyl, methadone, morphine, and buprenorphine in rats. Toxicol Lett 191:327–340. [DOI] [PubMed] [Google Scholar]
- Colon-Berezin C, Nolan ML, Blachman-Forshay J, Paone D (2019) Overdose deaths involving fentanyl and fentanyl analogs - New York City, 2000-2017. MMWR Morb Mortal Wkly Rep 68:37–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan A, Aarts L, Smith TW (2010) Incidence, reversal, and prevention of opioid-induced respiratory depression. Anesthesiology 112:226–238. [DOI] [PubMed] [Google Scholar]
- Dahan A, Yassen A, Bijl H, Romberg R, Sarton E, Teppema L, Olofsen E, Danhof M (2005) Comparison of the respiratory effects of intravenous buprenorphine and fentanyl in humans and rats. Br J Anaesth 94:825–834. [DOI] [PubMed] [Google Scholar]
- D’Onofrio G, O’Connor PG, Pantalon MV, Chawarski MC, Busch SH, Owens PH, Bernstein SL, Fiellin DA (2015) Emergency department-initiated buprenorphine/naloxone treatment for opioid dependence: a randomized clinical trial. JAMA 313:1636–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerak LR, Maguire DR, Woods JH, Husbands SM, Disney A, France CP (2019a) Reversal and prevention of the respiratory-depressant effects of heroin by the novel μ-opioid receptor antagonist methocinnamox in rhesus monkeys. J Pharmacol Exp Ther 368:229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerak LR, Minervini V, Latham E, Ghodrati S, Lillis KV, Wooden J, Disney A, Husbands SM, France CP (2019b) Methocinnamox produces long-lasting antagonism of the behavioral effects of μ-opioid receptor agonists but not prolonged precipitated withdrawal in rats. J Pharmacol Exp Ther 371:507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill H, Kelly E, Henderson G (2019) How the complex pharmacology of the fentanyls contributes to their lethality. Addiction 114:1524–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Yan W, Zheng Y, Khan MZ, Yuan K, Lu L (2019) The rising crisis of illicit fentanyl use, overdose, and potential therapeutic strategies. Transl Psychiatry 9:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AC, Gewirtz JC (2005) Acute opioid dependence: characterizing the early adaptations underlying drug withdrawal. Psychopharmacology (Berl) 178:353–366. [DOI] [PubMed] [Google Scholar]
- Henderson F, May WJ, Gruber RB, Discala JF, Puskovic V, Young AP, Baby SM, Lewis SJ (2014) Role of central and peripheral opiate receptors in the effects of fentanyl on analgesia, ventilation and arterial blood-gas chemistry in conscious rats. Respir Physiol Neurobiol 191:95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R, Santhakumar R, Dewey W, Kelly E, Henderson G (2020) Fentanyl depression of respiration: comparison with heroin and morphine. Br J Pharmacol 177:254–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin JF, Zhu LL, Chen M, Xu HM, Wang HF, Feng XQ, Zhu XP, Zhou Q (2015) The optimal choice of medication administration route regarding intravenous, intramuscular, and subcutaneous injection. Patient Prefer Adherence 9:923–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman RD, Gabathuler ML, Bellville JW (1981) Potency, duration of action and pA2 in man of intravenous naloxone measured by reversal of morphine-depressed respiration. J Pharmacol Exp Ther 219:156–162. [PubMed] [Google Scholar]
- Lewis J, Smith C, McCarthy P, Walter D, Kobylecki R, Myers M, Haynes A, Lewis C, Waltham K (1988) New 14-aminomorphinones and codeinones. NIDA Res Monogr 90:136–143. [PubMed] [Google Scholar]
- Loimer N, Hofmann P, Chaudhry HR (1994) Nasal administration of naloxone is as effective as the intravenous route in opiate addicts. Int J Addict 29:819–827. [DOI] [PubMed] [Google Scholar]
- Magosso E, Ursino M, van Oostrom JH (2004) Opioid-induced respiratory depression: a mathematical model for fentanyl. IEEE Trans Biomed Eng 51:1115–1128. [DOI] [PubMed] [Google Scholar]
- Maguire DR, Gerak LR, Sanchez JJ, Javors MA, Disney A, Husbands SM, France CP (2020) Effects of acute and repeated treatment with methocinnamox, a mu opioid receptor antagonist, on fentanyl self-administration in rhesus monkeys. Neuropsychopharmacology 45:1986–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, Gerak LR, Woods JH, Husbands SM, Disney A, France CP (2019) Long-lasting effects of methocinnamox on opioid self-administration in rhesus monkeys. J Pharmacol Exp Ther 368:88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer S, Boyd J, Collins A, Kennedy MC, Fairbairn N, McNeil R (2018) Characterizing fentanyl-related overdoses and implications for overdose response: findings from a rapid ethnographic study in Vancouver, Canada. Drug Alcohol Depend 193:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minervini V, Disney A, Husbands SM, France CP (2020) Methocinnamox (MCAM) antagonizes the behavioral suppressant effects of morphine without impairing delayed matching-to-sample accuracy in rhesus monkeys. Psychopharmacology (Berl) 237:3057–3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minervini V, Osteicoechea DC, Casalez A, France CP (2019) Punishment and reinforcement by opioid receptor agonists in a choice procedure in rats. Behav Pharmacol 30:335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss RB, Carlo DJ (2019) Higher doses of naloxone are needed in the synthetic opiod era. Subst Abuse Treat Prev Policy 14:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckham EM, Barkley LM, Divin MF, Cicero TJ, Traynor JR (2005) Comparison of the antinociceptive effect of acute morphine in female and male Sprague-Dawley rats using the long-lasting mu-antagonist methocinnamox. Brain Res 1058:137–147. [DOI] [PubMed] [Google Scholar]
- Peterson AB, Gladden RM, Delcher C, Spies E, Garcia-Williams A, Wang Y, Halpin J, Zibbell J, McCarty CL, DeFiore-Hyrmer J, et al. (2016) Increases in fentanyl-related overdose deaths - Florida and Ohio, 2013-2015. MMWR Morb Mortal Wkly Rep 65:844–849. [DOI] [PubMed] [Google Scholar]
- Prekupec MP, Mansky PA, Baumann MH (2017) Misuse of novel synthetic opioids: a deadly new trend. J Addict Med 11:256–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzasa Lynn R, Galinkin JL (2018) Naloxone dosage for opioid reversal: current evidence and clinical implications. Ther Adv Drug Saf 9:63–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann H, Erickson T, Thompson TM, Zautcke JL, Denton JS (2008) Fentanyl epidemic in Chicago, Illinois and surrounding Cook County. Clin Toxicol (Phila) 46:501–506. [DOI] [PubMed] [Google Scholar]
- Somerville NJ, O’Donnell J, Gladden RM, Zibbell JE, Green TC, Younkin M, Ruiz S, Babakhanlou-Chase H, Chan M, Callis BP, et al. (2017) Characteristics of fentanyl overdose - Massachusetts, 2014-2016. MMWR Morb Mortal Wkly Rep 66:382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer MR, Warner M, Bastian BA, Trinidad JP, Hedegaard H (2019) Drug overdose deaths involving fentanyl, 2011-2016. Natl Vital Stat Rep 68:1–19. [PubMed] [Google Scholar]
- Sutter ME, Gerona RR, Davis MT, Roche BM, Colby DK, Chenoweth JA, Adams AJ, Owen KP, Ford JB, Black HB, et al. (2017) Fatal fentanyl: one pill can kill. Acad Emerg Med 24:106–113. [DOI] [PubMed] [Google Scholar]
- Tomassoni AJ, Hawk KF, Jubanyik K, Nogee DP, Durant T, Lynch KL, Patel R, Dinh D, Ulrich A, D’Onofrio G (2017) Multiple fentanyl overdoses - New Haven, Connecticut, June 23, 2016. MMWR Morb Mortal Wkly Rep 66:107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivolo-Kantor AM, Seth P, Gladden RM, Mattson CL, Baldwin GT, Kite-Powell A, Coletta MA (2018) Vital signs: trends in emergency department visits for suspected opioid overdoses - United States, July 2016-September 2017. MMWR Morb Mortal Wkly Rep 67:279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EA, Sterious SN (2005) Opioid antagonists differ according to negative intrinsic efficacy in a mouse model of acute dependence. Br J Pharmacol 145:975–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner SG, Baker O, Bernson D, Schuur JD (2020) One-year mortality of patients after emergency department treatment for nonfatal opioid overdose. Ann Emerg Med 75:13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wermeling DP (2015) Review of naloxone safety for opioid overdose: practical considerations for new technology and expanded public access. Ther Adv Drug Saf 6:20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]