Abstract
Short-chain fatty acids (SCFAs) are metabolites produced almost exclusively by the gut microbiota and are an essential mechanism by which gut microbes influence host physiology. Given that SCFAs induce vasodilation, we hypothesized that they might have additional cardiovascular effects. In this study, novel mechanisms of SCFA action were uncovered by examining the acute effects of SCFAs on cardiovascular physiology in vivo and ex vivo. Acute delivery of SCFAs in conscious radiotelemetry-implanted mice results in a simultaneous decrease in both mean arterial pressure and heart rate (HR). Inhibition of sympathetic tone by the selective β-1 adrenergic receptor antagonist atenolol blocks the acute drop in HR seen with acetate administration, yet the decrease in mean arterial pressure persists. Treatment with tyramine, an indirect sympathomimetic, also blocks the acetate-induced acute drop in HR. Langendorff preparations show that acetate lowers HR only after long-term exposure and at a smaller magnitude than seen in vivo. Pressure-volume loops after acetate injection show a decrease in load-independent measures of cardiac contractility. Isolated trabecular muscle preparations also show a reduction in force generation upon SCFA treatment, though only at supraphysiological concentrations. These experiments demonstrate a direct cardiac component of the SCFA cardiovascular response. These data show that acetate affects blood pressure and cardiac function through parallel mechanisms and establish a role for SCFAs in modulating sympathetic tone and cardiac contractility, further advancing our understanding of the role of SCFAs in blood pressure regulation.
SIGNIFICANCE STATEMENT
Acetate, a short-chain fatty acid, acutely lowers heart rate (HR) as well as mean arterial pressure in vivo in radiotelemetry-implanted mice. Acetate is acting in a sympatholytic manner on HR and exerts negative inotropic effects in vivo. This work has implications for potential short-chain fatty acid therapeutics as well as gut dysbiosis-related disease states.
Introduction
A primary mechanism by which the gut microbiota communicates with the host and influences host physiology is via gut microbial metabolites, including short-chain fatty acids (SCFAs) (Natarajan and Pluznick, 2014; Natarajan et al., 2016). Acetate is the most abundant SCFA present in the plasma, followed by propionate and butyrate (den Besten et al., 2013; Trompette et al., 2014). SCFAs are virtually undetectable in germ-free animals, suggesting that gut microbiota is responsible for the vast majority of circulating SCFAs (Perry et al., 2016). SCFAs modulate host immune responses and metabolism, as well as blood pressure regulation (den Besten et al., 2013; Pluznick et al., 2013; Trompette et al., 2014). SCFAs have been considered a beneficial byproduct of the gut microbiota, acting primarily to promote host health (Aguilar-Nascimento et al., 2006; Bindels et al., 2012; Andrade-Oliveira et al., 2015). A greater understanding of the physiologic processes regulated by SCFAs and the mechanisms by which SCFAs signal in host cells is key to determining how gut microbiota can influence host physiology.
SCFAs induce vasodilation in blood vessels ex vivo and hypotension in vivo (Keshaviah, 1982; Daugirdas and Nawab, 1987; Mortensen et al., 1990; Natarajan et al., 2016; Onyszkiewicz et al., 2019). Acute administration of SCFAs lowers blood pressure, and this effect is mediated in part by G protein–coupled receptors such as olfactory receptor 78 and G protein–coupled receptor 41 (GPR41) (Pluznick et al., 2013; Natarajan et al., 2016; Onyszkiewicz et al., 2019). Exogenous SCFA treatment has also been shown to have cardioprotective effects, as well as protective effects against acute kidney injury, inflammation, and hypertension (Andrade-Oliveira et al., 2015; Marques et al., 2017; Wang et al., 2017; Ganesh et al., 2018; Bartolomaeus et al., 2019). Although the hypotensive effect of SCFAs has been studied for decades (Bauer and Richards, 1928; Keshaviah, 1982; Pluznick et al., 2013; Sircana et al., 2019; Toral et al., 2019), these previous studies have primarily used anesthetized or restrained animals (such as tail cuff measurements), which can potentially confound results. For the first time, our studies examine acute effects of SCFA administration in unanesthetized, unrestrained mice, allowing us to acquire mean arterial pressure (MAP) and heart rate (HR) data from a more accurate physiologic baseline. In this study, we report that acetate acutely lowers MAP and HR by acting through parallel mechanisms on the vasculature, the heart, and the autonomic nervous system and that acetate has both direct and indirect effects on cardiac physiology. Looking forward, a better understanding of the physiologic responses elicited by SCFAs and other microbial metabolites will improve our knowledge of host-microbiota communication.
Materials and Methods
Ethical Approval.
All animal protocols and procedures were approved by the Johns Hopkins Animal Care and Use Committee (accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care).
Animals.
C57BL/6J mice were either purchased directly from Jackson Laboratories or were the progeny of Jackson-originated C57BL/6J breeding pairs bred at Johns Hopkins. Mice were housed in individually ventilated cages with a maximum of five adult mice per cage. For radiotelemetry studies, mice were housed separately in static cages placed on telemetry receiver platforms. Cages were autoclaved with corncob bedding, and animals were maintained on Teklad 2018SX, 18% protein diet. Animals were given water ad libitum using automatic watering systems or water bottles.
Radiotelemetry.
Male and female C57BL/6J mice, 11 to 12 weeks of age, were implanted with radiotelemetry devices (PA-C10; Data Science International) as described previously (Natarajan et al., 2016; Shubitowski et al., 2019) to measure blood pressure and HR. Radiotelemetry catheters were inserted into the right carotid artery and the body of the transmitter was implanted subcutaneously. The surgery was performed under 1% isoflurane anesthesia. Meloxicam (Ostilox; VetOne) was administered postoperatively as a nonsteroidal anti-inflammatory agent. Mice were given 7–14 days for recovery after surgery during which time mice were monitored for distress by recording weight, food/water intake, and behavior before any telemetry measurements were taken. If a mouse did not recover from surgery or the radiotelemetry waveform was abnormal, it was excluded from the study. Once radiotelemetry measurements were completed, mice were euthanized by CO2 exposure followed by cervical dislocation.
Intraperitoneal Injections.
SCFAs were delivered to radiotelemetry-implanted mice via intraperitoneal injections. SCFAs used for intraperitoneal injections included sodium acetate (S2889; Sigma), sodium propionate (P1880; Sigma), sodium butyrate (B5887), sodium d-lactate (71716; Sigma), and sodium l-lactate (7022; Sigma). Lactate solutions were an equimolar mix of d- and l-lactate hereafter referred to as lactate. Pharmacological inhibitors used were metoprolol (M5391; Sigma), atenolol (A7655; Sigma), atropine (A0132; Sigma), and tyramine (T2879; Sigma). Intraperitoneal injections were done between 11 AM and 3 PM. For each intraperitoneal injection, the subcutaneous telemetry transmitter was turned on using a magnet, and a baseline MAP was recorded for a minimum of 10 minutes. Mice were briefly removed from the radiotelemetry receiver to be weighed, after which mice were injected i.p. with 1 g/kg SCFA or a matched volume of saline (0.9 g NaCl 100 ml). The total volume injected was 5 µl/g body weight. For pretreatments with pharmacological inhibitors, mice were first injected i.p. with either 4 mg/kg metoprolol, 2 mg/kg atenolol, or 4 mg/kg atropine. After a further 10 minutes of recording, a second SCFA injection was performed. For tyramine administration mice were first injected i.p. with 100 mg/kg tyramine. After 30 minutes a second injection with acetate was performed. MAP, HR, systolic, diastolic, and pulse pressure were then recorded until blood pressure returned to baseline.
Plasma Renin Activity and SCFA Analysis.
Plasma renin concentration was measured in 10–12 week old C57BL/6J mice with a modified angiotensin I measurement kit (S-1188; Peninsula Laboratories). After collection, plasma was diluted 30-fold and incubated with excess porcine angiotensinogen (SCP0021; Sigma) for 20 minutes at 37°C in a buffer containing 50 mM sodium acetate (pH 6.5), 10 mM 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride (AEBSF), 10 mM EDTA (pH 8.0), 1 µM porcine angiotensinogen, and 10 mM 8-hydroxyquinoline. After incubation with angiotensinogen, the samples were analyzed according to the protocol provided with the kit. Plasma renin concentration was assayed by competitive binding of angiotensin I generated to its antibody. For plasma SCFA analysis, plasma was collected and sent for by stable isotope dilution gas chromatography–tandem mass spectrometry analysis at the Lerner Research Institutes Metabolomics Shared Laboratory Resource at the Cleveland Clinic. A subset of the samples was sent in duplicate to ensure that data were reproducible (the Center was blinded to the samples’ identity and did not know which were duplicates).
Langendorff Hearts.
Male C57BL/6J male mice, 10–12 weeks of age, were injected with 500 U of heparin to prevent clotting. Animals were then euthanized by cervical dislocation, and the hearts were harvested. After harvesting, the aorta was cannulated and perfused with Krebs-Henseleit (KH) buffer at 37°C, gassed at 95% O2 and 5% CO2, and buffered to pH 7.4. A balloon connected to a pressure transducer was inserted into the left ventricle and functional parameters were measured as previously described (Cortassa et al., 2020). The heart was not paced during this process. After stabilization baseline values were recorded for 10 minutes followed by infusion with either pH balanced saline vehicle solution (pH 9.4) or an acetate solution into the KH buffer. A stock solution of sodium acetate (1 M) was introduced into the perfusate and diluted to a working concentration of 10 mM. This was then followed by 60 minutes of washout with KH buffer. Left ventricle functional parameters were recorded, and a separate pressure transducer connected to the aortic cannula was recorded, and coronary perfusion pressure. For isoproterenol treatment, isoproterenol (I6504; Sigma) was perfused into the heart for 5-minute intervals at a 10 nM working concentration.
In Vivo Hemodynamics and Pressure-Volume Loops.
Cardiac function and arterial loading were assessed by pressure-volume (PV) analyses by the Johns Hopkins Small Animal Cardiovascular Phenotyping Core, using a miniature micromanometer/conductance catheter (Millar, Inc.). Briefly, male and female mice were anesthetized with 1% to 2% isoflurane, 750–1000 mg/kg urethane i.p., 5–10 mg/kg etomidate i.p., and 1 to 2 mg/kg morphine i.p.; animals were subjected to tracheostomy; and were ventilated with 6 to 7 μl/g tidal volume and 130 breaths per minute. Volume expansion (12.5% human albumin, 50–100 μl over 5 minutes) was provided through a 30-gauge cannula via the right external jugular vein. The left ventricular apex was exposed through an incision between the seventh and eighth ribs, and a 1.4-Fr PV catheter (SPR 839; Millar Instruments Inc.) was advanced through the apex to lie along the longitudinal axis. Absolute volume was calibrated, and PV data were measured at steady state and during a transient reduction of venous return by occluding the inferior vena cava with a 6-0 silk snare suture. The end-systolic PV relation slope was derived from the 10 to 15 successive cardiac cycles during the inferior vena cava occlusion. The arterial load was determined by peak systolic pressure, effective arterial elastance (ventricular end-systolic pressure/stroke volume), and systemic resistance.
Isolated Trabecular Muscles.
Mice (15+ weeks old, ∼30 g weight) were anesthetized with an intra-abdominal injection of pentobarbital (100 mg/kg). The heart was exposed by mid-sternotomy, rapidly excised, and placed in a dissection dish. The aorta was cannulated and the heart perfused in a retrograde fashion with dissecting KH solution equilibrated with 95% O2 and 5% CO2. The dissecting KH solution was composed of (in millimolar) NaCl 120, NaHCO3 20, KCl 5, MgCl 1.2, glucose 10, CaCl2 0.5, and 2,3-butanedione monoxime 20 [pH 7.35–7.45 at room temperature (21°C to 22°C)]. Trabecular muscles (in millimeter: 1·01 ± 0·22 long, 0·17 ± 0·07 wide, and 0·09 ± 0·02 thick; means ± S.D.) from the right ventricles of the heart were dissected and mounted between a force transducer and a motor arm, superfused with KH solution at a rate of ∼10 ml/min, and stimulated at 0.5 Hz.
Force was measured by a force transducer system (KG7; Scientific Instruments GmbH, Heidelberg, Germany) and expressed in milliNewtons per square millimeter of cross-sectional area. The muscles underwent isometric contractions with the resting muscle length set so that resting force was 15% of total force development (i.e., optimal muscle length which corresponds to a sarcomere length of 2.2–2.3 μm, as determined previously (Gao et al., 1998). This resting muscle length was maintained throughout the experiments.
Stock solutions (50 mM or 5 M) of sodium acetate and sodium propionate were added to the 50 ml KH buffer system such that the final concentration range was 0–200 mM. For sodium butyrate, the stock solution was 50 mM, and the final concentration was varied from 0 to 400 μM.
Statistical Analysis.
For radiotelemetry monitoring after intraperitoneal injections, data were recorded and exported from Dataquest A.R.T. Gold software (Data Sciences International). Data were collected as a 10-second average, exported into Microsoft Excel, and analyzed in Graphpad Prism 8. SCFA intraperitoneal injections were compared with their volume-matched saline control injections via multiple t test comparisons, followed by Holm-Sidak correction for multiple comparisons. This test was chosen due to missing values caused by removing the mouse from the telemetry receiver to administer injections. Significance was defined as a P value <0.05 versus saline intraperitoneal injection trace at the same time point adjusted for multiple comparisons. For maximum delta (Δ) and half-recovery time (t1/2) analysis after SCFA injection, MAP and HR were averaged every 30 seconds. The 5 minutes immediately prior to SCFA intraperitoneal injection were averaged and used as a baseline value. The Δ (positive or negative) was defined as the greatest difference between the baseline value and any point within 30 minutes postinjection. t1/2 (minutes) was defined as the time required to reach the baseline value plus or minus 1/2 of the Δ. Significant differences between treatments were defined by a one-way ANOVA followed by Dunnett’s test with a P value <0.05 versus baseline defined as significant after adjustment for multiple comparisons. In-text references to ΔMAP, ΔHR, and t1/2 are shown as means ± S.D. Box-and-whisker plots show all points, with each point corresponding to one experiment, minimum to maximum values. Some injections were repeated (on different days) in the same mouse; results from multiple days were averaged.
For Langendorff heart analysis, data were recorded in AcqKnowledge and exported into Microsoft Excel and Graphpad Prism 8 for analysis. During the experiment, timepoints were compared using two-way ANOVA with Sidak correction for multiple comparisons when comparing saline- and acetate-treated hearts, or one-way ANOVA with Geisser-Greenhouse correction and Tukey’s multiple comparisons test for isoproterenol-treated hearts. Adjusted P values <0.05 were defined as significant. In-text values are shown as means ± S.D.
For PV loop inferior vena cava (IVC) occlusion, the analysis was performed using LabChart 8 (ADInstruments) with the PV loop analysis add-on. After pressure-volume calibration, 10–15 successive cardiac cycles during IVC occlusion were selected for analysis. From these cycles was derived the end-systolic PV relation slope, also known as end-systolic elastance (Ees), preload recruitable stroke work (PRSW) relationship, and maximum rate of pressure change (dP/dtmax) versus end-diastolic volume plot. IVC occlusions were performed 10 minutes after acetate or volume/pH-matched saline intraperitoneal injection. For steady-state parameters, 10–15 cycles were analyzed and averaged immediately before intraperitoneal injection (basal) and 10 minutes after intraperitoneal injection (saline and acetate). Baseline and intraperitoneal injection values were compared using a mixed-effects analysis with Geisser-Greenhouse correction, and Tukey’s multiple comparisons test. IVC occlusion values were omitted for a particular time point if the PV loop displayed an end-systolic “spike” due to catheter entrapment or other artifacts of PV loop measurement. Adjusted P values <0.05 were defined as significant.
Results
Intraperitoneal Injection of Acetate in Radiotelemetry-Implanted Mice.
It has been previously reported that intravenous administration of SCFAs causes acute hypotension in mice (Pluznick et al., 2013), but this prior study used anesthetized mice and HR was not monitored. Here, radiotelemetry with intraperitoneal delivery of SCFAs allowed us to monitor both blood pressure and HR responses to individual SCFAs in conscious, unrestrained, unanesthetized animals. Intraperitoneal injection (a mode of delivery that has been used to modulate acetate levels in vivo (Kimura et al., 2011; Ciarlo et al., 2016)) was used because it allows for the delivery of acetate without anesthesia. To determine the effect of acetate on cardiovascular function, a 1 g/kg i.p. dose of acetate (Kimura et al., 2011; Ciarlo et al., 2016; Shubitowski et al., 2019) was delivered in male and female radiotelemetry C57BL/6J mice. As a control, mice were given volume-matched saline i.p. injections (5 µl/g) to account for stress and handling effects on MAP and HR (Fig. 1). MAP was significantly depressed from minutes 12–32 after 1 g/kg acetate (Fig. 1A). Saline injection caused a transient increase in MAP, likely due to a stress response. After acetate intraperitoneal injection, MAP decreased an average maximum (ΔMAP) of −52.2 ± 12.1 mmHg (Fig. 1B), a 44% drop from baseline, and had a t1/2 of 22.3 ± 5.4 minutes (Fig. 1C). No differences were observed between male and female mice. Systolic, diastolic, and pulse pressure were also recorded (Supplemental Fig. 1).
Fig. 1.
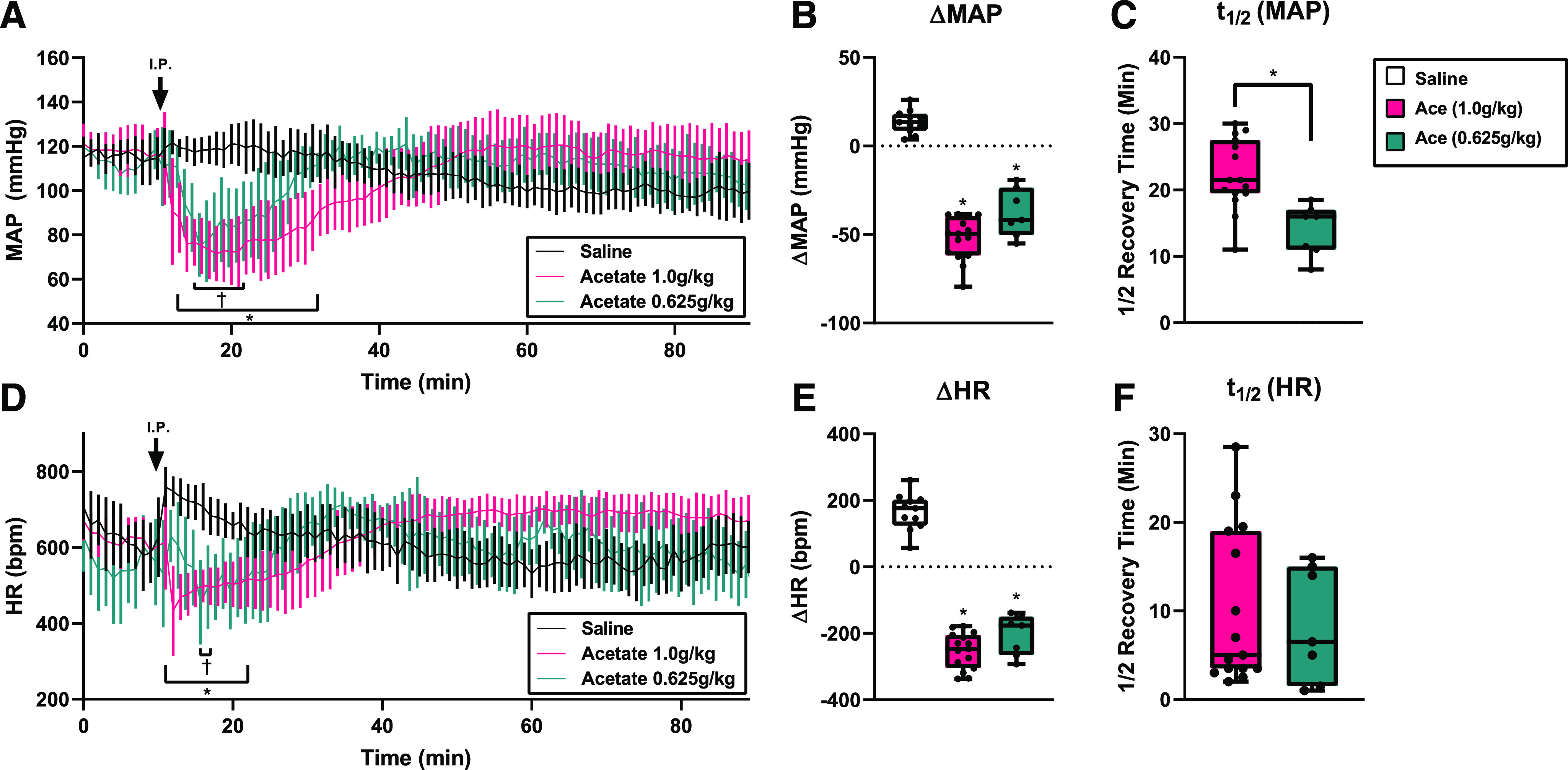
MAP and HR after acetate intraperitoneal injection in radiotelemetry-implanted mice. (A) Conscious mice implanted with radiotelemetry transmitters were given an i.p. injection (arrow) of 1.0, 0.625 g/kg sodium acetate or a volume‐matched saline control. MAP was significantly lower for 1.0 g/kg (*) and the 0.625 g/kg (†) acetate group for the ranges indicated (*/†P < 0.05, multiple t tests with Holm-Sidak correction; error bars indicate S.D.). (B) Change in MAP (ΔMAP) after i.p. injection of saline, 1.0 g/kg acetate, or 0.625 g/kg. (*P < 0.0001 vs. saline by one-way ANOVA). (C) MAP t1/2 after intraperitoneal injection. *P = 0.0015 vs. acetate 1.0 g/kg by Student’s t test. (D) HR after acetate intraperitoneal injection was significantly lower for 1.0 g/kg (*) and the 0.625 g/kg (†) acetate injection for the ranges indicated (*/†P < 0.05, multiple t tests with Holm-Sidak correction; error bars indicate S.D.). (E) Change in HR (ΔHR) after intraperitoneal injection (*P < 0.0001 vs. saline by one-way ANOVA). (F) HR t1/2 after intraperitoneal delivery. For all intraperitoneal injection experiments, n = 15, acetate 1.0 g/kg; n = 6, acetate 0.625 g/kg; n = 11, saline.
A lower dose of acetate (0.625 g/kg) yielded a decrease in MAP that was similar in magnitude to that of the higher 1 g/kg dose (−37.7 ± 13.5 mmHg, Fig. 1B), but the t1/2 (14.0 ± 3.8 minutes, Fig. 1C) was significantly shorter. MAP was significantly depressed from minutes 14–21 as compared with saline injection controls (Fig. 1A).
Based on the literature (Daugirdas and Nawab, 1987; Mortensen et al., 1990; Natarajan et al., 2016), we had hypothesized that the drop in MAP was driven by vasodilation, and expected to observe a reflex tachycardia. However, after 1 g/kg acetate i.p. injection, HR decreased significantly from minutes 11–22 (Fig. 1, D–F), with a maximum change from baseline (ΔHR) of −252.8 ± 54.3 bpm. A lower dose of 0.625 g/kg acetate decreased HR significantly from minute 15 to 16, with a maximum change from baseline of −205.3 ± 60.9 bpm. In contrast, saline transiently increased HR. These data suggest that intraperitoneal administration of acetate can acutely depress HR simultaneously with MAP in vivo.
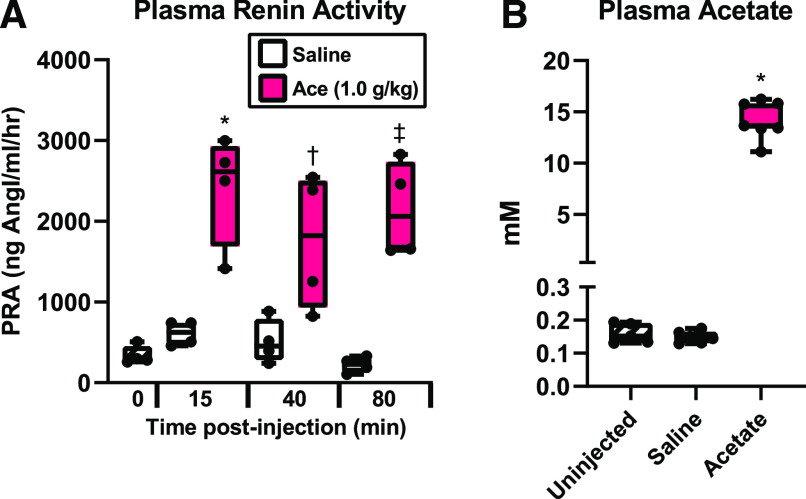
Acute hypotension increases plasma renin (Khambatta et al., 1979; Bertolino et al., 1994), and acetate induces renin release in the kidney (Pluznick et al., 2013). To assess if acetate intraperitoneal injection increases plasma renin, renin activity was measured at multiple time points after 1 g/kg acetate i.p. injection in a separate cohort of mice. Intraperitoneal injection of acetate significantly increased plasma renin activity (vs. saline control) at all time points, indicating that acetate intraperitoneal injection induces sustained high levels of plasma renin (Fig. 2A).
Fig. 2.
Plasma renin and acetate levels after acetate injection. (A) Plasma renin activity (PRA) after acetate or saline intraperitoneal injection. Plasma was harvested from separate groups of mice 15, 40, or 80 minutes after injection of either acetate (1.0 g/kg) or saline control. (n = 4 for each time point and injection; *P = 0.0006; †P = 0.023; ‡P = 0.0003 acetate vs. corresponding saline time point by one-way ANOVA). (B) Plasma acetate concentration measured by gas chromatography–mass spectrometry in uninjected mice or 15 minutes after saline or 1.0 g/kg acetate i.p. injection (n = 5 baseline, n = 6 saline, n = 7 acetate; *P < 0.0001, one-way ANOVA).
We previously found that plasma acetate peaks at ∼15 minutes after injection of 1 g/kg acetate (Shubitowski et al., 2019), but this previous study examined relative changes acetate over baseline. To quantitatively determine plasma acetate in millimolar at 15 minutes after intraperitoneal injection, plasma acetate was analyzed by gas chromatography–mass spectrometry (Fig. 2B). Acetate concentrations in uninjected mice were 0.16 ± 0.030 mM, which was unchanged 15 minutes after saline i.p. injection (0.15 ± 0.018 mM). A 1 g/kg acetate i.p. injection increased plasma acetate to 14.9 ± 1.8 mM. Baseline plasma acetate levels in mice generally range from 0.1 to 0.6 mM (Ge et al., 2008; Perry et al., 2016; Tumanov et al., 2016; Nishitsuji et al., 2017), although venous levels are typically much higher (∼4 to 5 mM (Bugaut, 1987), and arterial levels have been reported as high as ∼2 mM range (Bugaut, 1987).
HR Effects of Acetate are Inhibited by β Blockers and Sympathomimetics In Vivo.
The rapid drop in heart rate led us to hypothesize that acetate acts on the autonomic nervous system in either a vagotonic or a sympatholytic manner. To test this, male mice were injected intraperitoneally with atropine (muscarinic acetylcholinergic antagonist) followed by acetate, as well as atenolol (selective β-1 adrenergic receptor antagonist) followed by acetate (Fig. 3). Atropine alone increased HR after injection, as would be expected due to parasympathetic inhibition (ΔHR 172.3 ± 72.2 bpm, Fig. 3D), and atenolol alone decreased HR (Fig. 3D, average decrease of −124.6 ± 53.3 bpm), consistent with inhibition of sympathetic tone. Ten minutes after injection, a subset of mice was given a second i.p. injection of 1 mg/kg acetate. As a control, acetate alone was injected.
Fig. 3.
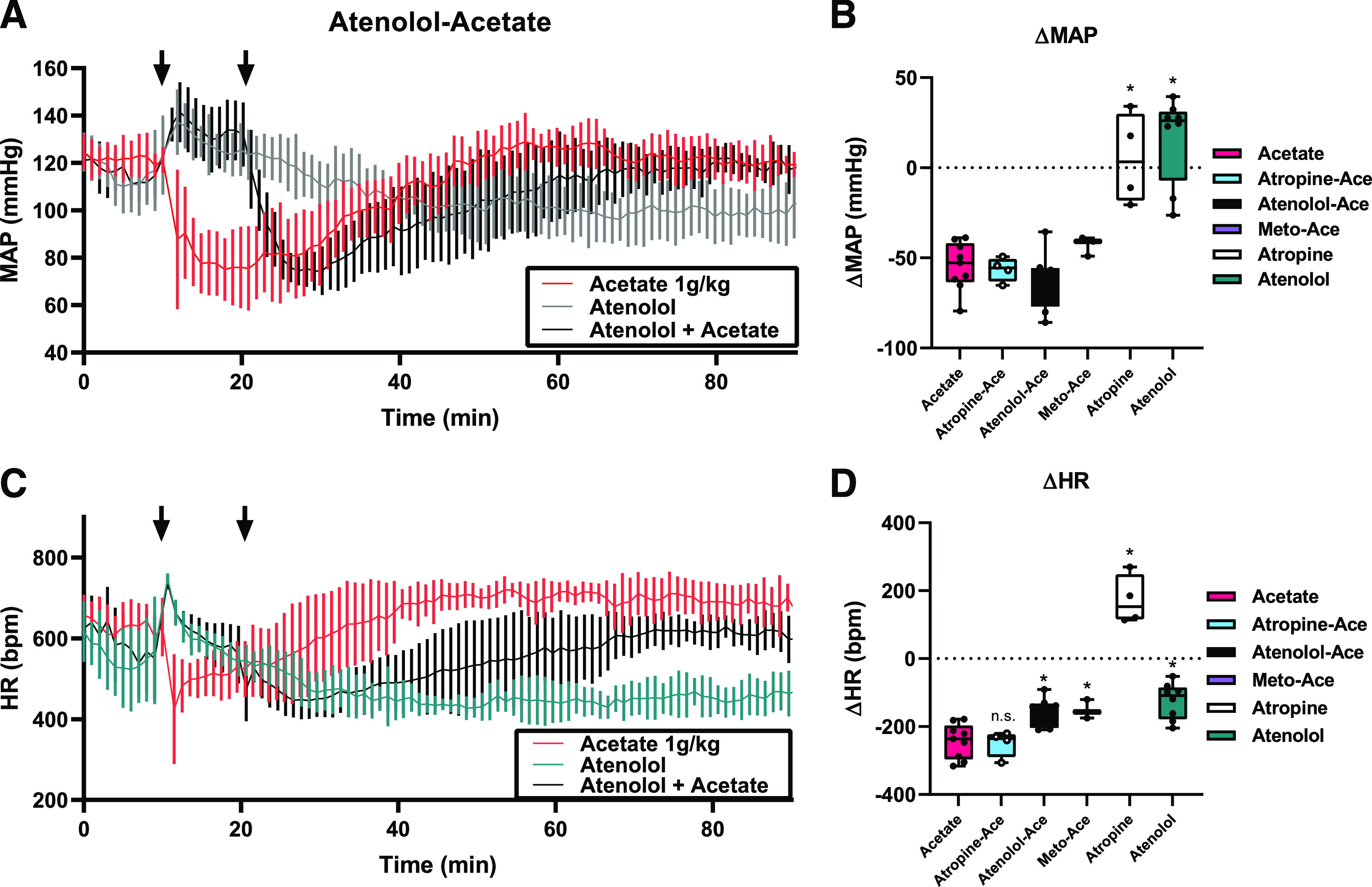
β blockers inhibit the effect of acetate on HR. Radiotelemetry mice were treated, on different days, with either acetate alone (a subset of the mice from Fig. 1) or a pharmacological compound, with or without acetate. In Fig. 3A, mice were given an i.p. injection (first arrow) with either atenolol (2 mg/kg) or acetate (1.0 g/kg). After a further 10 minutes, a subset of the atenolol-treated mice was given an i.p. injection of acetate (1.0 g/kg, second arrow). On separate days, mice were treated with muscarinic antagonist atropine (4 mg/kg) or alternative β blocker metoprolol (4 mg/kg) (B and D). Traces for MAP are shown in (A), and HR is shown in (C). For all intraperitoneal injection experiments n = 9 acetate; n = 8 atenolol, atenolol-acetate; n = 4 atropine-acetate, atropine; n = 3 metoprolol-acetate; error bars = S.D. Atropine-ace, atenolol-ace, and metoprolol(meto)-ace indicate values for acetate (ace) responses after 10 minutes of pretreatment with the indicated compound. ΔMAP is quantified in (B) (P = 0.99 atropine-acetate, P = 0.80 atenolol-acetate, P = 0.82 metoprolol-acetate, P < 0.0001 atropine alone and atenolol alone, vs. acetate by one-way ANOVA), and ΔHR is quantified in (D) (n.s - not significant P = 0.999 atropine-acetate, *P = 0.007 atenolol-acetate, P = 0.041 metoprolol-acetate; P < 0.0001 atropine alone and P = 0.0002 atenolol alone, vs. acetate alone by one-way ANOVA).
The MAP and HR changes observed after pretreatment with atropine were similar to acetate alone, suggesting that acetate’s effect is not due to an increase in vagal tone (Fig. 3, B and D). Full hemodynamic traces for atropine and atropine/acetate injections are shown in Supplemental Fig. 2A. However, atenolol did impact the acetate response. As seen previously in Fig. 1, acetate alone decreased both MAP and HR (first arrow in red traces in Fig. 2, max Δ −54.5 ± 13.3 mmHg and −242.7.4 ± 50.8 bpm, respectively). Systolic, diastolic, and pulse pressures were also recorded after atenolol and atenolol/acetate injection (Supplemental Fig. 2B). When mice were pretreated with atenolol (first arrow) and then given acetate (second arrow, black trace, Fig. 3, A and C), MAP decreased similarly, but the drop in HR was significantly attenuated (ΔMAP −63.3 ± 15.6 mmHg and ΔHR −158.3 ± 41.6 bpm, respectively), suggesting that HR but not MAP is affected by changes in sympathetic tone. To confirm this, an alternative β blocker, metoprolol, was injected in a small cohort of mice (Fig. 3, B and D). Metoprolol-treated mice had less acetate-induced HR depression than acetate alone (P = 0.041 ΔHR −150.5 ± 27.8 bpm). Full hemodynamic traces for metoprolol and metoprolol/acetate injection are shown in Supplemental Fig. 2C. ΔMAP after acetate was not significantly different between atropine-, atenolol-, or metoprolol-pretreated mice.
To further demonstrate the role of sympathetic tone in the acetate-mediated response, male mice were injected intraperitoneally with tyramine, an indirect sympathomimetic which induces catecholamine release (Fig. 4). Because we hypothesized that the acetate-induced drop in HR (but not MAP) is sympatholytic, we predicted that pretreatment with tyramine would attenuate the effect of acetate on HR, but would not alter the effect of acetate on MAP. As a sympathomimetic, tyramine increased MAP (Fig. 4A), which initially caused a transient decrease in HR (Fig. 4B), though HR quickly recovered and became elevated above baseline (mean increase of 111.6 bpm 30 minutes after tyramine intraperitoneal injection alone). Acetate alone decreased both MAP and HR (first arrow in red traces in Fig. 4, max Δ −56.9 ± 15.9 mmHg and −239.8 ± 58.6 bpm, respectively). When mice were treated with 1 mg/kg acetate (second arrow) 30 minutes after tyramine pretreatment (first arrow), the decrease in MAP was not altered (Fig. 4B, ΔMAP −47.8 ± 14.1 mmHg), but the drop in HR was significantly attenuated (Fig. 4D, ΔHR –101.7 ± 24.7 bpm). Systolic, diastolic, and pulse pressures were also recorded after tyramine and tyramine/acetate injection (Supplemental Fig. 3). These data imply that the effect of acetate on HR is primarily sympatholytic.
Fig. 4.
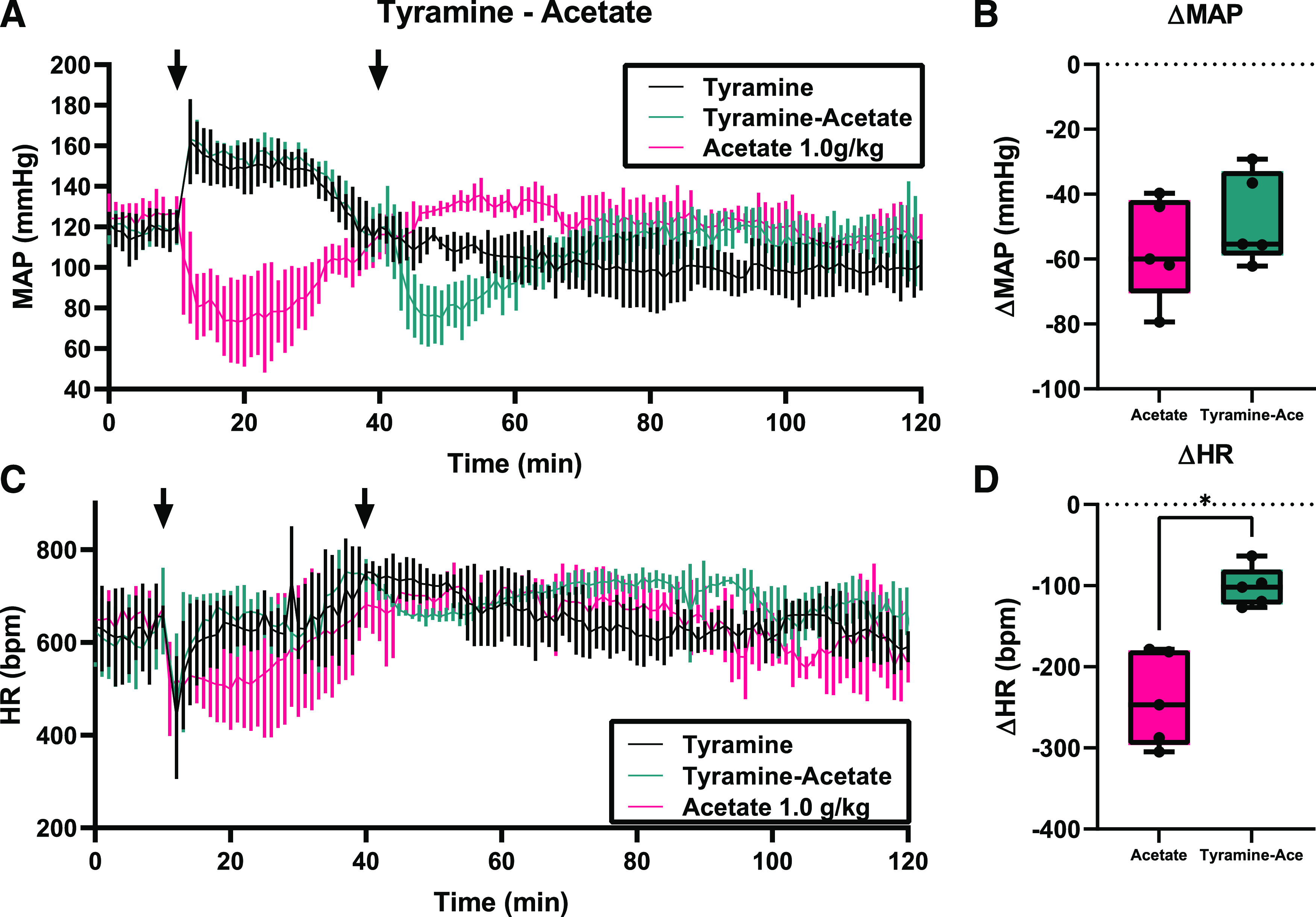
Tyramine inhibits the effect of acetate on HR. Radiotelemetry mice were treated, on different days, with either acetate alone (a subset of mice from Fig. 1) or, with tyramine with or without acetate. In Fig. 4A, mice were given an i.p. injection (first arrow) with either tyramine (100 mg/kg) or acetate (1.0 g/kg). After 30 minutes, a subset of tyramine-treated mice was given an i.p. injection of acetate (1.0 g/kg, second arrow). Traces for MAP are shown in (A), and HR is shown in (C). n = 5 for tyramine, acetate, and tyramine-acetate; error bars indicate S.D. ΔMAP after acetate is quantified in (B) (P = 0.09 atropine-acetate vs. acetate alone by paired t test), and ΔHR after acetate is quantified in (D) (*P = 0.01 tyramine-acetate vs. acetate alone by paired t test).
Direct Acetate Effects in Ex Vivo Langendorff Hearts.
To determine if acetate can act directly on the heart to reduce HR, ex vivo Langendorff hearts from male mice were used (Fig. 5). Constant perfusate pressure was maintained, and acetate was administered in the perfusate with a working concentration of 10 mM. This dose was chosen because it is similar to the peak dose seen with the acetate intraperitoneal injections (Fig. 1), is at the high end of the physiologic range, and it corresponds to a concentration we have used previously for intravenous acetate administration (Pluznick et al., 2013). Hearts were not paced during these experiments. After 10 minutes of acetate infusion, HR was not significantly reduced compared with saline (314 ± 26 bpm acetate vs. 341 ± 57 bpm saline). HR was only significantly depressed after 30 minutes of continuous acetate infusion (275 ± 21 bpm acetate vs. 354 ± 39 bpm saline, a 22% decrease) (Fig. 5A); in contrast, peak decrease in HR is seen in vivo (intraperitoneal injections) after 2 to 3 minutes. Developed pressure (Pdev) (Fig. 5B), dP/dtmax, minimum rate of pressure change (dP/dtmin), and rate-pressure product (RPP) were not significantly impacted during acetate infusion compared with saline (Table 1). These data suggest that acetate only has a direct effect on HR after extended exposure and likely does not contribute to the significant HR reduction seen in vivo.
Fig. 5.
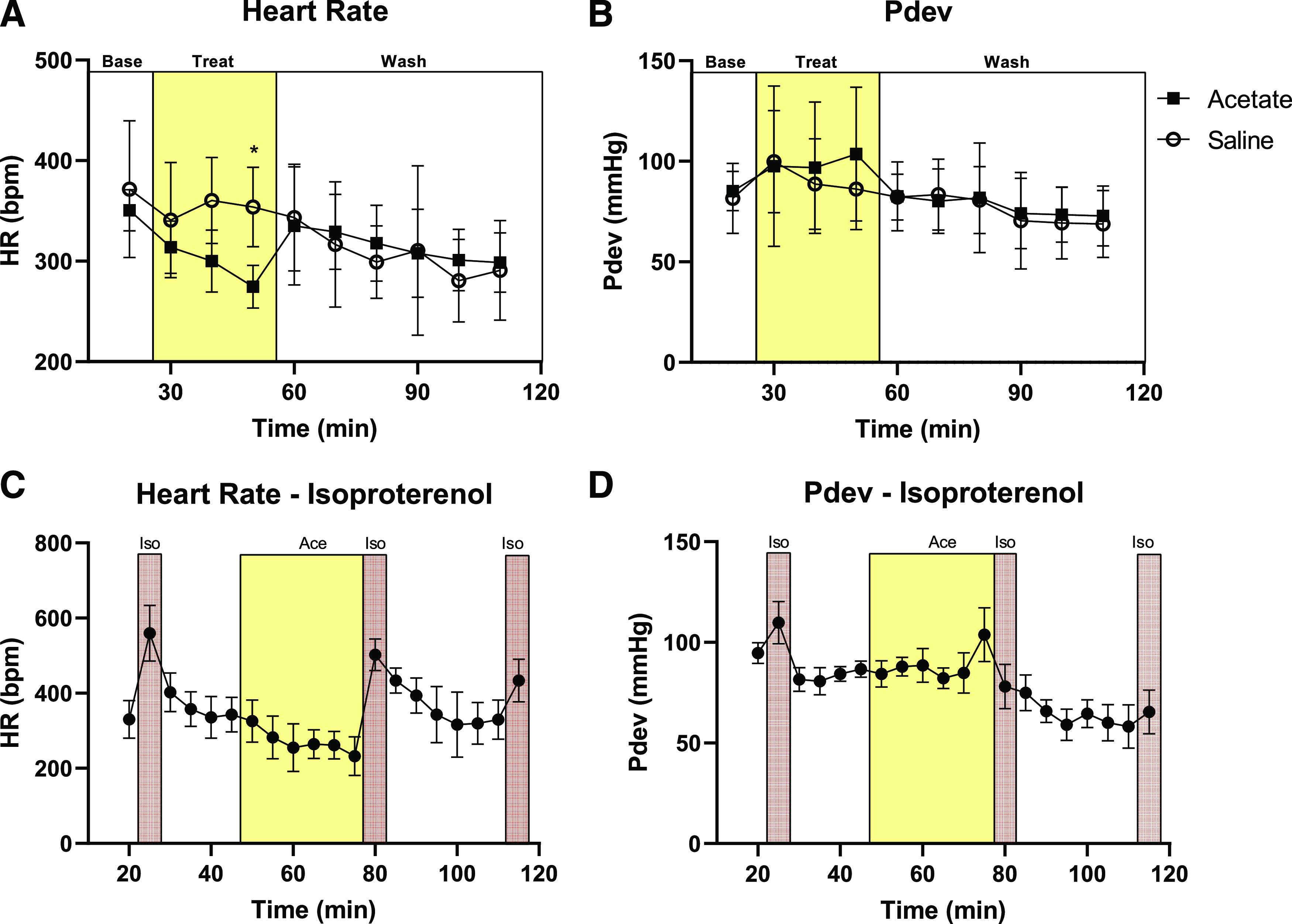
Acetate effects on Langendorff hearts. After baseline measurement, hearts were perfused with either pH normalized saline vehicle or acetate. The treatment period is shown by the yellow box, with acetate at 10 mM working concentration for 30 minutes. This was followed by 60 minutes of washout. Values for HR (A) and Pdev (B) were averaged over 10-minute time intervals (n = 8 saline, 5 acetate, *P = 0.0068 vs. saline at the same time point, compared by two-way ANOVA; bars indicate S.D.). (C) After baseline measurement, hearts were perfused with 10 nM isoproterenol (Iso) for 5 minutes, followed by 20 minutes of washout. Hearts were then perfused with acetate at 10 mM working concentration (Ace, yellow box) for 35 minutes. Ten nanomolar isoproterenol was infused along with acetate for the last 5 minutes (Iso, orange box). This was followed by 30 minutes of washout and a final 5-minute treatment of isoproterenol. Values for HR (C) and Pdev (D) were averaged over 5-minute time intervals (n = 5 isoproterenol, 7 acetate; bars indicate S.D.).
TABLE 1.
Langendorff heart parameters during acetate infusion
| Heart parameter | HR | Pdev | dP/dtmax | dP/dtmin | RPP |
|---|---|---|---|---|---|
| bpm | mmHg | mmHg/s | mmHg/s | HR × Pdev | |
| Baseline | |||||
| Saline | 371 ± 68 | 82 ± 18 | 3463 ± 806 | −2548 ± 693 | 29688 ± 2418 |
| Acetate | 351 ± 20 | 85 ± 10 | 3272 ± 850 | −2111 ± 614 | 30023 ± 2132 |
| 30-min infusion | |||||
| Saline | 354 ± 40 | 86 ± 20 | 3892 ± 1030 | −2244 ± 501 | 30083 ± 2177 |
| Acetate | 275 ± 21* | 104 ± 33 | 4217 ± 1821 | −2087 ± 777 | 28147 ± 4377 |
Isolated ex vivo Langendorff heart hemodynamic data were recorded from the left ventricle at baseline, after 30 minutes of saline infusion, and after 30 minutes of acetate infusion (10 mM working concentration). n = 8 saline, n = 5 acetate. Values are means ± S.D.
P = 0.0068.
β Adrenergic Receptors are Not Inhibited by Acetate Ex Vivo.
Given that acetate’s effect on HR is sympatholytic, we hypothesized that the Langendorff preparations did not show an immediate HR drop due to a lack of sympathetic tone. To address this, ex vivo Langendorff hearts from male mice were treated with the β agonist isoproterenol (ISO) either alone, or after treatment with acetate (Fig. 5, C and D). ISO markedly increased HR in the Langendorff hearts, as expected, increasing HR from 331 ± 50 to 560 ± 74 bpm (Fig. 5C). After 30 minutes of acetate infusion, ISO was again added, and HR changed from 232 ± 51 to 503 ± 42 bpm. After a subsequent 30-minute washout period, a final ISO treatment was imposed, which increased the HR to 434 ± 57 bpm. There were no significant differences in HR between the three different ISO treatments. These data suggest that any direct acetate effects on the heart are β receptor independent and that acetate acts upstream of the β receptor to induce a decrease in HR in vivo.
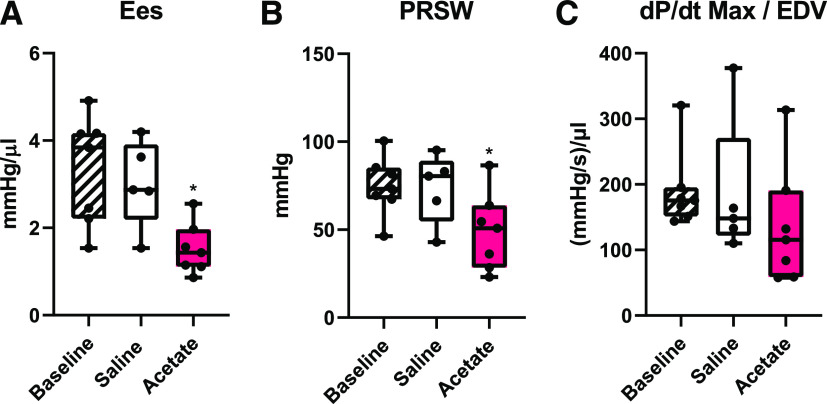
Acetate Intraperitoneal Injection Reduces Cardiac Inotropy as Revealed by Pressure-Volume Analysis.
To determine if acetate may also be altering cardiac contractility, we performed PV loops on male mice in conjunction with acetate intraperitoneal injection. Of note, our telemetry experiments have the advantage of recording data from conscious animals; however, cardiac contractility cannot be assayed in vivo without anesthesia. Despite the use of anesthesia, the PV loop approach is the only one able to parse out potential primary effects imparted by acetate on the heart, such as changes in inotropy or lusitropy, as opposed to alterations in cardiac performance due to variations in preload or afterload. Therefore these data lend valuable context to our conscious telemetry readings.
PV loops were recorded on a closed chest cavity that allowed us to replicate the route (i.p.) and dose (1.0 g/kg) of acetate injection from our telemetry studies. IVC occlusion during baseline measurements and after saline or acetate intraperitoneal injection, allowed us to analyze load-independent measures of cardiac contractility (Fig. 6). After acetate intraperitoneal injection, the slope of the end-systolic PV relation was reduced, indicating a decrease in Ees (Fig. 6A). PRSW (Fig. 6B) was also significantly reduced (P = 0.0038 vs. baseline). Similarly, dP/dtmax/EDV (Fig. 6C) trended downward but was not significantly decreased. Other cardiac parameters were measured during the PV loops (summarized in Table 2); of note, although the dose and route of SCFA delivery were identical to that of the telemetry experiments, anesthesia blunted changes in HR and systolic pressure. These data show that acetate affects the heart in part by lowering parameters of cardiac contractility.
Fig. 6.
Load-independent measures of cardiac contractility after acetate intraperitoneal injection. In PV loop experiments, IVC occlusion was done under basal conditions or 10 minutes after intraperitoneal injection with either saline or acetate to measure load-independent cardiac contractility. A significant reduction in Ees [P = 0.042 vs. baseline in (A)] and PRSW [P = 0.0038 vs. baseline in (B)] is seen with acetate treatment, and a nonsignificant downward trend is observed for dP/dtmax/EDV [P = 0.1092 vs. baseline in (C)] (n = 5 saline, 7 acetate). * compared with baseline by one-way ANOVA.
TABLE 2.
Steady-state PV loop parameters after acetate intraperitoneal injection
| Hemodynamic parameter | Baseline (n = 7) | Saline (n = 5) | Acetate (n = 7) |
|---|---|---|---|
| HR (bpm) | 447 ± 32 | 475 ± 46 | 484 ± 37 |
| Pmax (mmHg) | 85 ± 6 | 93 ± 8 | 89 ± 14 |
| Pes (mmHg) | 77 ± 4 | 82 ± 5 | 80 ± 13 |
| Ped (mmHg) | 6 ± 3 | 8 ± 3 | 8 ± 3 |
| Ves (µl) | 19 ± 4 | 17 ± 6 | 32 ± 15 |
| Ved (µl) | 39 ± 3 | 43 ± 8 | 60 ± 19 |
| SW (mmHg*µl) | 1901 ± 282 | 2481 ± 260* | 2689 ± 977 |
| SV (µl) | 26 ± 3 | 32 ± 3 | 36 ± 10 |
| CO (µl/min) | 11736 ± 1293 | 15122 ± 2108 | 17444 ± 5323 |
| EF (%) | 65 ± 7 | 72 ± 10 | 59 ± 12 |
| dP/dt max (mmHg/s) | 6821 ± 1176 | 8289 ± 1426 | 8336 ± 2016 |
| dP/dt min (mmHg/s) | −6271 ± 1128 | −7776 ± 868* | −8317 ± 2572 |
| Tau (ms) | 9 ± 1 | 8 ± 1 | 8 ± 1 |
Pressure volume loop hemodynamic data were measured from left ventricular apex at baseline, 10 min after i.p. injection of saline, and 10 min after i.p. injection with 1.0 g/kg sodium acetate. Measurements were done at a steady state. n = 5 saline, n = 7 acetate. CO, cardiac output; EF, ejection fraction; Ped, end-diastolic pressure; Pes, end-systolic pressure; Pmax, maximum pressure; SV, stroke volume; SW, stroke work; tau, isovolumetric relaxation constant; Ved, end-diastolic volume; Ves, end-systolic volume. Values are means ± S.D.
P < 0.05 vs. basal.
Propionate and Butyrate Depress MAP and HR Acutely In Vivo.
To determine if other SCFAs have similar MAP and HR effects, we delivered a 1 g/kg i.p. dose of propionate, butyrate, and lactate in male and female C57BL/6J male mice (Fig. 7). Propionate and butyrate (both SCFAs) also significantly depressed MAP (ΔMAP of −40.3 ± 3.2 and −37.3 ± 15.5 mmHg, respectively) (Fig. 7A), but lactate (a non-SCFA control) did not change MAP compared with saline (Fig. 7B). Propionate and butyrate also depressed HR and showed a ΔHR of −188.6 ± 69.3 bpm and −177.0 ± 71.0 bpm, respectively (Fig. 7, D and E). Systolic, diastolic, and pulse pressures were also recorded (Supplemental Fig. 4). Lactate did not significantly decrease HR compared with saline controls. Heart rate variability made it more challenging to precisely pinpoint the HR t1/2; however, the HR consistently recovered before the MAP (as seen by t1/2 values) (Fig. 7F), implying that HR recovery contributes to MAP recovery. There were no sex differences observed in acetate, propionate, butyrate, or lactate responses. These data demonstrate that propionate and butyrate have depressive effects on MAP and HR similar to acetate and may be working through similar mechanisms.
Fig. 7.
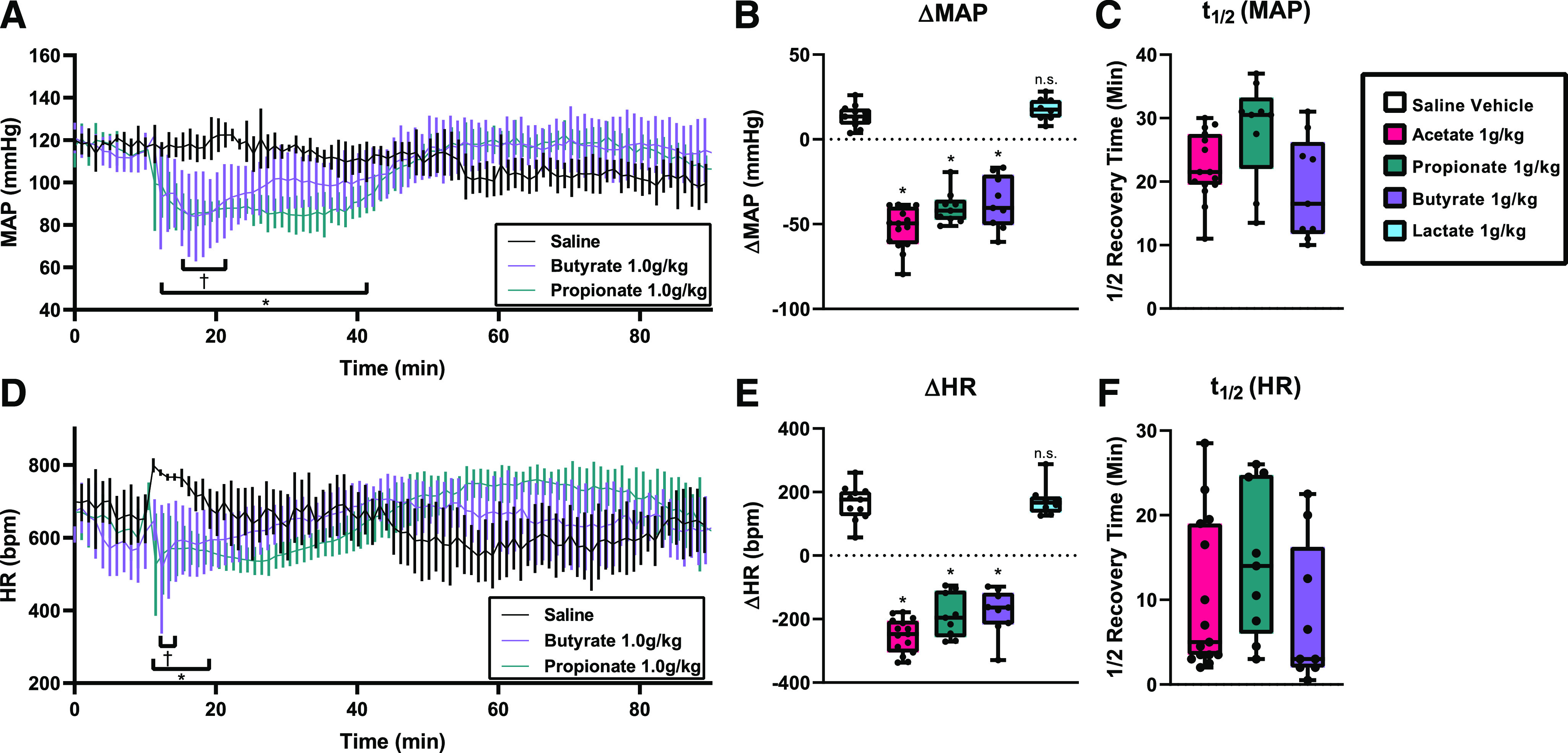
MAP and HR after propionate and butyrate intraperitoneal injection in radiotelemetry-implanted mice. (A) Conscious mice implanted with radiotelemetry transmitters were given an i.p. injection (arrow) of 1.0 g/kg sodium propionate, sodium butyrate, or a volume‐matched saline control. MAP was significantly lower for sodium propionate (*) and sodium butyrate (†) acetate group for the ranges indicated (*/†P < 0.05, multiple t tests with Holm-Sidak correction; error bars indicate S.D.). (B) Change in MAP (ΔMAP) after intraperitoneal injection of acetate, propionate, butyrate, or lactate (n = 8–11, *P < 0.0001 vs. saline (replotted from Fig. 1), n.s. - not significant P = 0.883 vs. saline by one-way ANOVA). (C) MAP t1/2 after intraperitoneal delivery. (D) HR after intraperitoneal injection was significantly lower for sodium propionate (*) and sodium butyrate (†) injection for the ranges indicated (*/†P < 0.05, multiple t tests with Holm-Sidak correction; error bars indicate S.D.). (E) Change in HR (ΔHR) after intraperitoneal injection (*P < 0.0001 vs. saline, n.s. - not significant P = 0.994 vs. saline by one-way ANOVA). (F) HR t1/2 after intraperitoneal delivery. For all intraperitoneal injection experiments n = 11 saline, n= 15 acetate (re-plotted from Figure 1 for comparison), n = 7 butyrate, propionate, n = 6 lactate.
SCFAs Reduce Force Generation in Isolated Trabecular Muscles.
To further assess the direct effects of SCFA administration on isolated cardiac muscles ex vivo, force generation was measured in trabeculae isolated from the murine right ventricle. After exposure to increasing doses of acetate (Fig. 8A), propionate (Fig. 8B), and butyrate (Fig. 8C), the force generated by isometric contraction decreased, reaching significance at the higher concentrations of acetate and butyrate. These data suggest that SCFAs at the doses used here can act directly on cardiac cells to reduce contractility.
Fig. 8.
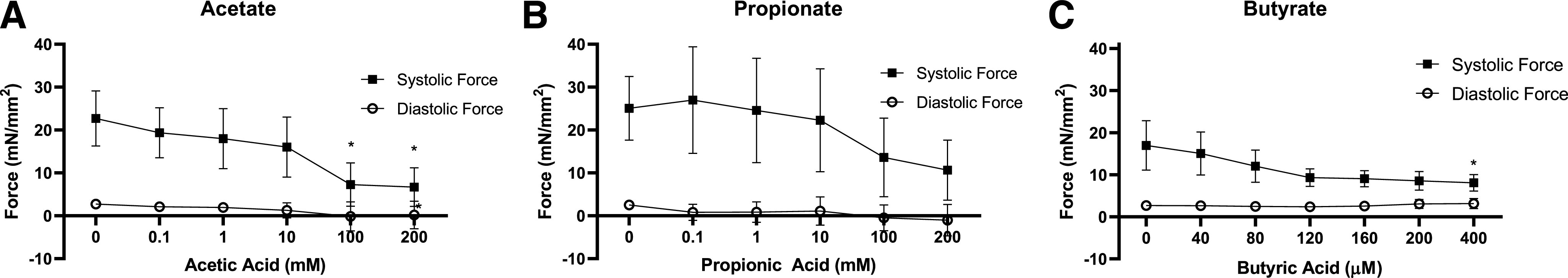
Force generation in isolated trabecular muscle cells. Mouse trabecular muscles isolated from the right ventricles underwent isometric contractions during exposure to increasing concentrations of sodium acetate (A), sodium propionate (B), and sodium butyrate (C). Force generation was significantly decreased after acetic acid treatment at 100 and 200 mM (P = 0.0001 vs. 0 mM) and butyric acid treatment at 400 µM (P = 0.0426 vs. 0 µM). Propionic acid decreased force generation, but at borderline significance at 200 mM (P = 0.05 vs. 0 mM). Force generation values (n = 6 acetate, n = 7 propionate, n = 3 butyrate; bars indicate S.D.).
Discussion
To better understand the role of SCFAs in whole animal physiology, we used radiotelemetry to continuously measure MAP and HR after intraperitoneal delivery of SCFAs to conscious mice. These experiments revealed an unexpected effect of SCFAs on HR, and subsequent experiments using pharmacological inhibitors and classic physiologic techniques further elucidated SCFA effects on cardiac function.
SCFA Effects on MAP.
SCFAs vasodilate blood vessels ex vivo (Mortensen et al., 1990; Nutting et al., 1991; Pluznick et al., 2013; Natarajan et al., 2016), and intravenous delivery of propionate to anesthetized animals decreases MAP (Pluznick et al., 2013). Our data show that acetate, as well as propionate and butyrate, acutely reduce both MAP and HR. Although it was previously hypothesized that the drop in MAP is a consequence of SCFA-mediated vasodilation (Pluznick et al., 2013; Natarajan et al., 2016), hypotension driven by vasodilation would typically trigger a reflex tachycardia.
SCFA Effects on HR.
The short time scale of the HR drop in vivo suggests that the decline in HR is autonomically mediated. Pretreatment of mice with β blockers atenolol or metoprolol attenuated any further acetate-induced reduction in heart rate, indicating that acetate is acting in a sympatholytic manner on the heart. It may appear counterintuitive that blocking sympathetic tone attenuates a sympatholytic effect. However, if the presence of a β blocker has already dampened sympathetic tone, then an abrupt decrease in sympathetic tone due to SCFAs would not yield a dramatic effect.
Additionally, pretreatment with indirectly acting sympathomimetic tyramine also attenuated the drop in heart rate. This suggests that catecholamine release is a key component of the acetate response. Acetate could be acting to inhibit neurotransmitter release, which is preempted by sympathomimetic treatment. Neither β adrenergic blockers nor sympathomimetics prevented the drop in MAP, demonstrating that these effects occur through distinct mechanisms. Direct vasodilation by SCFAs, as seen previously (Mortensen et al., 1990; Natarajan et al., 2016) could explain this drop in MAP.
SCFAs are known to interact with vagal efferent nerves (Lal et al., 2001) and cholinergic nerves, where SCFA uptake results in increased acetylcholine synthesis (O’Regan, 1983; Carroll, 1997). Although an increase in acetylcholine release could trigger a hypotensive response via muscarinic receptors, the acetate response’s lack of atropine effects suggests that this is not the case. Other studies have also noted the atropine resistance of SCFA-mediated hypotensive effects (Kirkendol et al., 1978; Onyszkiewicz et al., 2019). Neurotransmitter exocytosis can be inhibited in presynaptic terminals via G protein–coupled receptor activation and Gβ subunit release (Blackmer et al., 2001; Gerachshenko et al., 2009). If SCFA-binding G protein–coupled receptors are present on enteric neurons, the hypotension we observe could be mediated by similar mechanisms. We hypothesize that when SCFAs are intraperitoneally injected, they interact with the enteric nervous system to rapidly drop sympathetic tone and HR. This effect is attenuated if the sympathetic tone is already low or absent, or is already increased via sympathomimetics.
The initial decrease in HR after acetate delivery is in agreement with the recent publication by Onyszkiewicz et al. (2019) who observed a simultaneous reduction in HR and MAP when butyric acid was delivered into the colon. Notably, they saw a drop in HR when butyric acid was delivered intracolonically, but not when it was given intravenously. Given that the HR effect is seen when SCFAs are given intraperitoneally or intracolonically, but is not seen when SCFAs are given intravenously, the effect may be driven by local SCFA action in the mesentery, as opposed to the actions of circulating SCFAs. Onyszkiewicz et al. (2019) also showed that subdiaphragmatic vagotomy attenuated butyrate effects on HR, demonstrating autonomic mediation. Differences in the cardiovascular responses to SCFAs are also affected by the presence of anesthesia, as was observed in our PV loop experiments. We hypothesize that the increase in HR observed at later timepoints is a delayed tachycardia as SCFA levels return to baseline, and HR increases to support MAP.
MAP and HR recovery is also supported by renin-angiotensin-aldosterone system activation. Renin activity is elevated 15 minutes after acetate intraperitoneal injection, when MAP is still depressed but is beginning to recover, and remains elevated for an extended period after MAP recovers. Similar increases in plasma renin levels after sodium nitroprusside–induced hypotension validate the hypothesis that the renin-angiotensin-aldosterone system is likely supporting the longer-term recovery of MAP (Miller et al., 1977; Khambatta et al., 1979). Plasma renin levels can be significantly elevated as soon as 5 minutes after acute hypotension (Bertolino et al., 1994), and SCFAs can induce renin release (Pluznick et al., 2013). Studies of acetate infusion in humans have also shown a delayed tachycardia response, as discussed below.
In support of our hypothesis that autonomically mediated SCFA effects are responsible for the drop in HR, treatment of Langendorff hearts with acetate did not yield a drop in HR in the same timescale observed in vivo. Although Langendorff hearts are sensitive to β adrenergic stimulation by isoproterenol due to the presence of sympathetic terminals, they are not in contact with external sympathetic ganglia. Other studies analyzing isolated rat cardiac tissue showed decreases in contractility and isometric tension after 10 minutes of acetate exposure, but no change in HR, which is in agreement with our ex vivo data (Daugirdas et al., 1988). The difference in the HR response in telemetry versus Langendorff experiments implies that the drop in HR is primarily dependent on external stimulation.
SCFA Effects on Cardiac Contractility.
Pressure-volume loop IVC occlusion demonstrated a significant decrease in load-independent cardiac contractility after acetate treatment, suggesting that acetate has a negative inotropic effect. Systolic force generation in isolated trabecular muscle was also decreased. Given that the SCFA receptor GPR41 is expressed in cardiac tissue (Kimura et al., 2011), acetate may be activating GPR41.
SCFAs: Physiologic and Pathophysiological Relevance.
In our study, intraperitoneal injection of acetate increased plasma acetate levels to ∼15 mM 15 minutes after injection, a time point at which MAP is still depressed, but HR is beginning to recover. Plasma acetate ranges from 100 to 600 µM (Ge et al., 2008; Perry et al., 2016; Tumanov et al., 2016; Nishitsuji et al., 2017) but has been reported as high as 2 mM range (Bugaut, 1987); venous levels are typically higher (∼4 to 5 mM; Bugaut, 1987). The SCFA doses in our study are relevant for two reasons. First, these doses are pharmacologically relevant: SCFAs have been proposed to have therapeutic effects (Lührs et al., 2002; Cox et al., 2009; Needell et al., 2017; Ganesh et al., 2018), and acetate has also been reported to induce hypotension and cardiac instability in the context of hemodialysis (Aizawa et al., 1977; Kirkendol et al., 1978; Vreman et al., 1980; Keshaviah, 1982; Vincent et al., 1982; Velez et al., 1984; Hakim et al., 1985; Noris et al., 1998). Thus it is important to understand the full consequences of dosing with exogenous SCFAs. Second, these doses are pathophysiologically relevant as SCFA levels in the colon are orders of magnitude higher: ∼100 mM in humans and mice (Bugaut, 1987; Cummings et al., 1987; Garner et al., 2009; Tahara et al., 2018; Onyszkiewicz et al., 2019). Thus, we hypothesize that plasma SCFA concentrations could be significantly increased in the context of a leaky gut or intestinal barrier injury. Studying exogenous SCFAs in healthy animals allows us to examine the effects of increased SCFAs themselves (in the absence of other pathology). To our knowledge, plasma SCFA levels have not been measured in sepsis; however, one of the hallmarks of sepsis is persistent hypotension requiring the use of vasopressors to maintain normal MAP (Hotchkiss et al., 2016), and sepsis can also jeopardize myocardial function (Merx and Weber, 2007).
Model of SCFA Action.
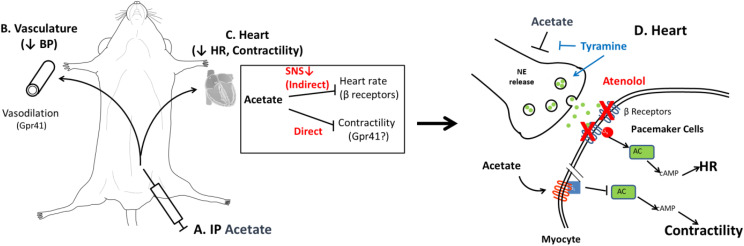
We propose that SCFAs act on both the vasculature and the heart. SCFAs are known to cause endothelium-dependent vasodilation, likely mediated in part by Gpr41 (Fig. 9B) (Pluznick et al., 2013; Onyszkiewicz et al., 2019). Our data suggest two independent effects of SCFAs on cardiac tissue (Fig. 9C). Indirectly, SCFAs act acutely on the autonomic nervous system to decrease sympathetic tone and reduce HR; this effect is attenuated by blocking cardiac β receptors and sympathetic tone to the heart or by pretreatment with a sympathomimetic to release catecholamines (Fig. 9C) indirectly. We propose (Fig. 9D) that SCFAs act upstream of the sympathetic nerve terminal to block the release of noradrenaline to β receptors on the heart, inhibiting the sympathetic signaling pathway that acts through Gs. Second, we hypothesize that acetate is acting directly on the heart to decrease cardiac contractility. Given our Langendorff data in isoproterenol-treated hearts, acetate may be acting on myocytes. We hypothesize that acetate is binding to a currently unknown cardiac G protein–coupled receptor, which, if Gi coupled, could result in the modest contractility defect observed. SCFA-sensing G protein–coupled receptors have been characterized in the heart and have been shown to have a direct impact on cardiac function (Jovancevic et al., 2017).
Fig. 9.
Working model of acetate effects on whole-animal physiology. Intraperitoneal injection of acetate or other SCFAs (A) causes endothelium-dependent vasodilation of the vasculature, lowering blood pressure (BP) (B). SCFAs have both direct and indirect actions on the heart (C). Indirectly, SCFAs work on the sympathetic nervous system (SNS) to decrease sympathetic tone, which results in a drop in HR; β blockers such as atenolol can attenuate this effect. (D) We hypothesize that SCFAs act upstream of the sympathetic nerve terminal to block the release of norepinephrine (NE release) to β receptors on the heart, inhibiting the canonical sympathetic signaling pathway that acts through Gs. This effect is attenuated by treatment with sympathomimetics such as tyramine. We hypothesize that acetate is acting directly on the heart in a different cell type by binding to a currently unknown G protein–coupled receptor.
We report here that acetate depresses MAP, HR, and cardiac contractility. This study further expands our knowledge of the physiologic processes impacted by microbial metabolites.
Acknowledgments
We are grateful to Jochen Steppan (Dept of Anesthesiology and Critical Care Medicine, Johns Hopkins) for his assistance, expertise, and advice. We are also grateful to Tyler Shubitowski for his assistance with the initial experiments. We would also like to thank the Johns Hopkins Small Animal Cardiovascular Phenotyping Core. We’d also like to thank Stan Hazen, Chris Strauch, and Belinda Willard at the Cleveland Clinic. Finally, we would like to thank the members of the Pluznick Laboratory for helpful discussions.
Abbreviations
- dP/dtmax
maximum rate of pressure change
- dP/dtmin
minimum rate of pressure change EDV End-Diastolic Volume
- Ees
end-systolic elastance
- GPR41
G protein–coupled receptor 41
- HR
heart rate
- ISO
isoproterenol
- IVC
inferior vena cava
- KH
Krebs-Henseleit
- MAP
mean arterial pressure
- PRSW
preload recruitable stroke work
- Pdev
developed pressure
- PV
pressure-volume
- RPP
rate-pressure product
- SCFA
short-chain fatty acid
- t1/2
half-recovery time
Authorship Contributions:
Participated in research design: Poll, Xu, Sanchez, Zaidman, Lester, Berkowitz, Paolocci, Gao, Pluznick.
Conducted experiments: Poll, Xu, Jun, Sanchez, He, Zaidman, Pluznick.
Contributed new reagents or analytic tools: Poll, Jun, Paolocci, Gao, Pluznick.
Performed data analysis: Poll, Xu, Zaidman, Lester, Berkowitz, Paolocci, Gao, Pluznick.
Wrote or contributed to the writing of the manuscript: Poll, Zaidman, Jun, He, Lester, Berkowitz, Paolocci, Gao, Pluznick.
Footnotes
This work was supported by National Institutes of Health National Heart Lung and Blood Institute [F31HL144061, R01HL128512] and National Institute for Diabetes and Digestive and Kidney Diseases [R01DK107726].
This work was published in a modified form as part of a doctoral dissertation under embargo for 4 years: Poll BG (2020) Exploring Microbiome-Host Interactions: Elucidating the Effects of Short-Chain Fatty Acids and Olfactory Receptor 78 on Hemodynamics and Renal Function. Doctoral dissertation, Johns Hopkins University School of Medicine, Baltimore, MD. This work has also been presented at the following meetings: Poll BG, Lester L, Jun S, Berkowitz D, Paolocci N, and Pluznick JL (2020) Acute administration of gut microbial metabolites reduce blood pressure and cardiac contractility (Abstract). FASEB J 34(S1); Poll BG, Steppan J, Lester L, Jun S, Berkowitz D, Paolocci N, and Pluznick JL (2019) A short chain fatty acid produced by the gut microbiota plays a role in blood pressure regulation and cardiac contractility, in Proceedings of the APS/ASN Conference: Control of Renal Function in Health and Disease; 2019 June 23–27; Charlottesville, VA; and Poll BG, Steppan J, Lester L, Berkowitz D, and Pluznick J (2019) A short-chain fatty acid produced by the gut microbiota plays a role in blood pressure regulation and cardiac contractility (Abstract). FASEB J 33(S1):569.19.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Aguilar-Nascimento JE, Salomão AB, Nochi RJ Jr, Nascimento M, Neves JdeS (2006) Intraluminal injection of short chain fatty acids diminishes intestinal mucosa injury in experimental ischemia-reperfusion. Acta Cir Bras 21:21–25. [DOI] [PubMed] [Google Scholar]
- Aizawa Y, Ohmori T, Imai K, Nara Y, Matsuoka M, Hirasawa Y (1977) Depressant action of acetate upon the human cardiovascular system. Clin Nephrol 8:477–480. [PubMed] [Google Scholar]
- Andrade-Oliveira V, Amano MT, Correa-Costa M, Castoldi A, Felizardo RJ, de Almeida DC, Bassi EJ, Moraes-Vieira PM, Hiyane MI, Rodas AC, et al. (2015) Gut bacteria products prevent AKI induced by ischemia-reperfusion. J Am Soc Nephrol 26:1877–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomaeus H, Balogh A, Yakoub M, Homann S, Markó L, Höges S, Tsvetkov D, Krannich A, Wundersitz S, Avery EG, et al. (2019) Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation 139:1407–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer W, Richards DW (1928) A vasodilator action of acetates. J Physiol 66:371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino S, Julien C, Medeiros IA, Vincent M, Barrès C (1994) Pressure-dependent renin release and arterial pressure maintenance in conscious rats. Am J Physiol 266:R1032–R1037. [DOI] [PubMed] [Google Scholar]
- Bindels LB, Porporato P, Dewulf EM, Verrax J, Neyrinck AM, Martin JC, Scott KP, Buc Calderon P, Feron O, Muccioli GG, et al. (2012) Gut microbiota-derived propionate reduces cancer cell proliferation in the liver. Br J Cancer 107:1337–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmer T, Larsen EC, Takahashi M, Martin TFJ, Alford S, Hamm HE (2001) G protein betagamma subunit-mediated presynaptic inhibition: regulation of exocytotic fusion downstream of Ca2+ entry. Science 292:293–297. [DOI] [PubMed] [Google Scholar]
- Bugaut M (1987) Occurrence, absorption and metabolism of short chain fatty acids in the digestive tract of mammals. Comp Biochem Physiol B 86:439–472. [DOI] [PubMed] [Google Scholar]
- Carroll PT (1997) Evidence to suggest that extracellular acetate is accumulated by rat hippocampal cholinergic nerve terminals for acetylcholine formation and release. Brain Res 753:47–55. [DOI] [PubMed] [Google Scholar]
- Ciarlo E, Heinonen T, Herderschee J, Fenwick C, Mombelli M, Le Roy D, Roger T (2016) Impact of the microbial derived short chain fatty acid propionate on host susceptibility to bacterial and fungal infections in vivo. Sci Rep 6:37944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortassa S, Caceres V, Tocchetti CG, Bernier M, de Cabo R, Paolocci N, Sollott SJ, Aon MA (2020) Metabolic remodelling of glucose, fatty acid and redox pathways in the heart of type 2 diabetic mice. J Physiol 598:1393–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox MA, Jackson J, Stanton M, Rojas-Triana A, Bober L, Laverty M, Yang X, Zhu F, Liu J, Wang S, et al. (2009) Short-chain fatty acids act as antiinflammatory mediators by regulating prostaglandin E(2) and cytokines. World J Gastroenterol 15:5549–5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT (1987) Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28:1221–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugirdas JT, Nawab ZM (1987) Acetate relaxation of isolated vascular smooth muscle. Kidney Int 32:39–46. [DOI] [PubMed] [Google Scholar]
- Daugirdas JT, Wang X, Nutting C, Swanson V, Agrawal A (1988) Depressant effect of acetate in isolated cardiac tissue. Cardiovasc Res 22:566–570. [DOI] [PubMed] [Google Scholar]
- den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM (2013) The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res 54:2325–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesh BP, Nelson JW, Eskew JR, Ganesan A, Ajami NJ, Petrosino JF, Bryan RM Jr, Durgan DJ (2018) Prebiotics, probiotics, and acetate supplementation prevent hypertension in a model of obstructive sleep apnea. Hypertension 72:1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao WD, Perez NG, Marban E (1998) Calcium cycling and contractile activation in intact mouse cardiac muscle. J Physiol 507:175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner CD, Antonopoulos DA, Wagner B, Duhamel GE, Keresztes I, Ross DA, Young VB, Altier C (2009) Perturbation of the small intestine microbial ecology by streptomycin alters pathology in a Salmonella enterica serovar typhimurium murine model of infection. Infect Immun 77:2691–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge H, Li X, Weiszmann J, Wang P, Baribault H, Chen J-L, Tian H, Li Y (2008) Activation of G protein-coupled receptor 43 in adipocytes leads to inhibition of lipolysis and suppression of plasma free fatty acids. Endocrinology 149:4519–4526. [DOI] [PubMed] [Google Scholar]
- Gerachshenko T, Schwartz E, Bleckert A, Photowala H, Seymour A, Alford S (2009) Presynaptic G-protein-coupled receptors dynamically modify vesicle fusion, synaptic cleft glutamate concentrations, and motor behavior. J Neurosci 29:10221–10233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakim RM, Pontzer MA, Tilton D, Lazarus JM, Gottlieb MN (1985) Effects of acetate and bicarbonate dialysate in stable chronic dialysis patients. Kidney Int 28:535–540. [DOI] [PubMed] [Google Scholar]
- Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent JL (2016) Sepsis and septic shock. Nat Rev Dis Primers 2:16045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovancevic N, Dendorfer A, Matzkies M, Kovarova M, Heckmann JC, Osterloh M, Boehm M, Weber L, Nguemo F, Semmler J, et al. (2017) Medium-chain fatty acids modulate myocardial function via a cardiac odorant receptor. Basic Res Cardiol 112:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshaviah PR (1982) The role of acetate in the etiology of symptomatic hypotension. Artif Organs 6:378–387. [DOI] [PubMed] [Google Scholar]
- Khambatta HJ, Stone JG, Khan E (1979) Hypertension during anesthesia on discontinuation of sodium nitroprusside-induced hypotension. Anesthesiology 51:127–130. [DOI] [PubMed] [Google Scholar]
- Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi S, Kobayashi M, Hirasawa A, Tsujimoto G (2011) Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc Natl Acad Sci USA 108:8030–8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkendol RL, Pearson JE, Bower JD, Holbert RD (1978) Myocardial depressant effects of sodium acetate. Cardiovasc Res 12:127–136. [DOI] [PubMed] [Google Scholar]
- Lal S, Kirkup AJ, Brunsden AM, Thompson DG, Grundy D (2001) Vagal afferent responses to fatty acids of different chain length in the rat. Am J Physiol Gastrointest Liver Physiol 281:G907–G915. [DOI] [PubMed] [Google Scholar]
- Lührs H, Gerke T, Müller JG, Melcher R, Schauber J, Boxberge F, Scheppach W, Menzel T (2002) Butyrate inhibits NF-kappaB activation in lamina propria macrophages of patients with ulcerative colitis. Scand J Gastroenterol 37:458–466. [DOI] [PubMed] [Google Scholar]
- Marques FZ, Nelson E, Chu PY, Horlock D, Fiedler A, Ziemann M, Tan JK, Kuruppu S, Rajapakse NW, El-Osta A, et al. (2017) High-Fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation 135:964–977. [DOI] [PubMed] [Google Scholar]
- Merx MW, Weber C (2007) Sepsis and the heart. Circulation 116:793–802. [DOI] [PubMed] [Google Scholar]
- Miller ED Jr, Ackerly JA, Vaughan ED Jr, Peach MJ, Epstein RM (1977) The renin-angiotensin system during controlled hypotension with sodium nitroprusside. Anesthesiology 47:257–262. [PubMed] [Google Scholar]
- Mortensen FV, Nielsen H, Mulvany MJ, Hessov I (1990) Short chain fatty acids dilate isolated human colonic resistance arteries. Gut 31:1391–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan N, Hori D, Flavahan S, Steppan J, Flavahan NA, Berkowitz DE, Pluznick JL (2016) Microbial short chain fatty acid metabolites lower blood pressure via endothelial G-protein coupled receptor 41. Physiological genomics 48:826–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan N, Pluznick JL (2014) From microbe to man: the role of microbial short chain fatty acid metabolites in host cell biology. Am J Physiol Cell Physiol 307:C979–C985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needell JC, Ir D, Robertson CE, Kroehl ME, Frank DN, Zipris D (2017) Maternal treatment with short-chain fatty acids modulates the intestinal microbiota and immunity and ameliorates type 1 diabetes in the offspring. PLoS One 12:e0183786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitsuji K, Xiao J, Nagatomo R, Umemoto H, Morimoto Y, Akatsu H, Inoue K, Tsuneyama K (2017) Analysis of the gut microbiome and plasma short-chain fatty acid profiles in a spontaneous mouse model of metabolic syndrome. Sci Rep 7:15876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noris M, Todeschini M, Casiraghi F, Roccatello D, Martina G, Minetti L, Imberti B, Gaspari F, Atti M, Remuzzi G (1998) Effect of acetate, bicarbonate dialysis, and acetate-free biofiltration on nitric oxide synthesis: implications for dialysis hypotension. Am J Kidney Dis 32:115–124. [DOI] [PubMed] [Google Scholar]
- Nutting CW, Islam S, Daugirdas JT (1991) Vasorelaxant effects of short chain fatty acid salts in rat caudal artery. Am J Physiol 261:H561–H567. [DOI] [PubMed] [Google Scholar]
- O’Regan S (1983) Uptake of acetate and propionate by isolated nerve endings from the electric organ of Torpedo marmorata and their incorporation into choline esters. J Neurochem 41:1596–1601. [DOI] [PubMed] [Google Scholar]
- Onyszkiewicz M, Gawrys-Kopczynska M, Konopelski P, Aleksandrowicz M, Sawicka A, Koźniewska E, Samborowska E, Ufnal M (2019) Butyric acid, a gut bacteria metabolite, lowers arterial blood pressure via colon-vagus nerve signaling and GPR41/43 receptors. Pflugers Arch 471:1441–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RJ, Peng L, Barry NA, Cline GW, Zhang D, Cardone RL, Petersen KF, Kibbey RG, Goodman AL, Shulman GI (2016) Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature 534:213–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan LX, Rey F, Wang T, et al. (2013) Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci USA 110:4410–4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shubitowski TB, Poll BG, Natarajan N, Pluznick JL (2019) Short-chain fatty acid delivery: assessing exogenous administration of the microbiome metabolite acetate in mice. Physiol Rep 7:e14005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sircana A, De Michieli F, Parente R, Framarin L, Leone N, Berrutti M, Paschetta E, Bongiovanni D, Musso G (2019) Gut microbiota, hypertension and chronic kidney disease: recent advances. Pharmacol Res 144:390–408. [DOI] [PubMed] [Google Scholar]
- Tahara Y, Yamazaki M, Sukigara H, Motohashi H, Sasaki H, Miyakawa H, Haraguchi A, Ikeda Y, Fukuda S, Shibata S (2018) Gut microbiota-derived short chain fatty acids induce circadian clock entrainment in mouse peripheral tissue. Sci Rep 8:1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toral M, Robles-Vera I, de la Visitación N, Romero M, Yang T, Sánchez M, Gómez-Guzmán M, Jiménez R, Raizada MK, Duarte J (2019) Critical role of the interaction gut microbiota - sympathetic nervous system in the regulation of blood pressure. Front Physiol 10:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL, et al. (2014) Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 20:159–166. [DOI] [PubMed] [Google Scholar]
- Tumanov S, Bulusu V, Gottlieb E, Kamphorst JJ (2016) A rapid method for quantifying free and bound acetate based on alkylation and GC-MS analysis. Cancer Metab 4:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velez RL, Woodard TD, Henrich WL (1984) Acetate and bicarbonate hemodialysis in patients with and without autonomic dysfunction. Kidney Int 26:59–65. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Vanherweghem JL, Degaute JP, Berré J, Dufaye P, Kahn RJ (1982) Acetate-induced myocardial depression during hemodialysis for acute renal failure. Kidney Int 22:653–657. [DOI] [PubMed] [Google Scholar]
- Vreman HJ, Assomull VM, Kaiser BA, Blaschke TF, Weiner MW (1980) Acetate metabolism and acid-base homeostasis during hemodialysis: influence of dialyzer efficiency and rate of acetate metabolism. Kidney Int Suppl 10:S62–S74. [PubMed] [Google Scholar]
- Wang L, Zhu Q, Lu A, Liu X, Zhang L, Xu C, Liu X, Li H, Yang T (2017) Sodium butyrate suppresses angiotensin II-induced hypertension by inhibition of renal (pro)renin receptor and intrarenal renin-angiotensin system. J Hypertens 35:1899–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]