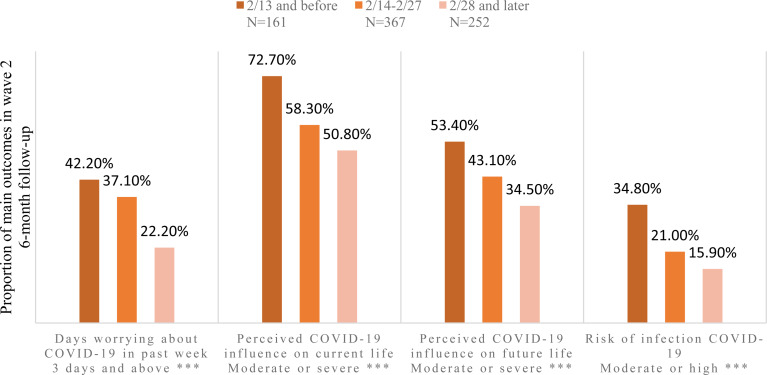Abstract
Aims
The coronavirus disease 2019 (COVID-19) pandemic represents an unprecedented threat to mental health. Herein, we assessed the impact of COVID-19 on subthreshold depressive symptoms and identified potential mitigating factors.
Methods
Participants were from Depression Cohort in China (ChiCTR registry number 1900022145). Adults (n = 1722) with subthreshold depressive symptoms were enrolled between March and October 2019 in a 6-month, community-based interventional study that aimed to prevent clinical depression using psychoeducation. A total of 1506 participants completed the study in Shenzhen, China: 726 participants, who completed the study between March 2019 and January 2020 (i.e. before COVID-19), comprised the ‘wave 1’ group; 780 participants, who were enrolled before COVID-19 and completed the 6-month endpoint assessment during COVID-19, comprised ‘wave 2’. Symptoms of depression, anxiety and insomnia were assessed at baseline and endpoint (i.e. 6-month follow-up) using the Patient Health Questionnaire-9 (PHQ-9), Generalised Anxiety Disorder-7 (GAD-7) and Insomnia Severity Index (ISI), respectively. Measures of resilience and regular exercise were assessed at baseline. We compared the mental health outcomes between wave 1 and wave 2 groups. We additionally investigated how mental health outcomes changed across disparate stages of the COVID-19 pandemic in China, i.e. peak (7–13 February), post-peak (14–27 February), remission plateau (28 February−present).
Results
COVID-19 increased the risk for three mental outcomes: (1) depression (odds ratio [OR] = 1.30, 95% confidence interval [CI]: 1.04–1.62); (2) anxiety (OR = 1.47, 95% CI: 1.16–1.88) and (3) insomnia (OR = 1.37, 95% CI: 1.07–1.77). The highest proportion of probable depression and anxiety was observed post-peak, with 52.9% and 41.4%, respectively. Greater baseline resilience scores had a protective effect on the three main outcomes (depression: OR = 0.26, 95% CI: 0.19–0.37; anxiety: OR = 1.22, 95% CI: 0.14–0.33 and insomnia: OR = 0.18, 95% CI: 0.11–0.28). Furthermore, regular physical activity mitigated the risk for depression (OR = 0.79, 95% CI: 0.79–0.99).
Conclusions
The COVID-19 pandemic exerted a highly significant and negative impact on symptoms of depression, anxiety and insomnia. Mental health outcomes fluctuated as a function of the duration of the pandemic and were alleviated to some extent with the observed decline in community-based transmission. Augmenting resiliency and regular exercise provide an opportunity to mitigate the risk for mental health symptoms during this severe public health crisis.
Key words: COVID-19, subthreshold depressive symptoms
Introduction
The coronavirus disease 2019 (COVID-19) pandemic poses an unprecedented mental health threat globally as a consequence of the fear of contraction, as well as government reaction to containing community spread (e.g. economic shut down, unemployment). Replicated studies from around the world have reported on the increased prevalence of mental disorders as a result of COVID-19, including depression, anxiety and insomnia (i.e. prevalence of 10.6%−50.7%, 10.4%−44.7% and 33.9%−36.1%, respectively) (Lai et al., 2020; Liu et al., 2020; Potloc Study, 2020; Wang et al., 2020b; Zhang et al., 2020). Furthermore, as a consequence of heightened anxiety due to the pandemic, along with the economic shock and stress related to quarantine, an increase in suicide is expected in Canada, the United States and possibly other countries (McIntyre and Lee, 2020a, 2020b).
Subthreshold depressive symptoms (i.e. not meeting minimum diagnostic threshold for a major depressive episode) are an important risk indicator for incident major depressive disorder (MDD). Persons with subthreshold depressive symptoms are approximately twice as likely to be diagnosed with MDD relative to those without (Rodríguez et al., 2012; Lee et al., 2019). In the general population, the absolute risk of conversion to MDD from subthreshold depressive symptoms (excluded lifetime MDD) ranged from 0.012 to 0.096 per 100 person years (Cuijpers and Smit, 2004). A 13-year prospective study in communities indicated the mean age of first depressive episode in people with subthreshold depressive symptoms was 34 years, which was similar with the age onset in MDD (Chen et al., 2000). It has been estimated that approximately 2.9%−9.9% of adults in primary care and 1.4%−17.2% of adults in community settings manifest subthreshold depressive symptoms (Lee et al., 2019).
Recent studies have raised concerns about populations that are more vulnerable to the detrimental mental health effects of the COVID-19 pandemic. For example, people with mental health conditions may be more substantially influenced by the emotional distress brought on by the COVID-19 pandemic, resulting in relapses or worsening of pre-existing mental health condition(s) compared with the general population (Yao et al., 2020). In addition, psychological response may change with fluctuations in the epidemic and clinical knowledge improvement. For example, a related longitudinal study investigating psychological adaptions during the severe acute respiratory syndrome (SARS) outbreak suggested that increasing knowledge and understanding of SARS could improve mental health outcomes (Su et al., 2007).
The primary aim of this longitudinal study is to evaluate the impact of COVID-19 in a subthreshold depressive symptom population. The secondary aim of this study is to explore potential predictors of mental health improvement to recommend feasible intervention under acute stress.
Methods
Study design and participants
Data in this study were derived from an ongoing longitudinal, population-based study for the early identification, treatment, prevention and management of depression and subthreshold depression (Depression Cohort in China [DCC] study, ChiCTR registry number 1900022145). Individuals were identified via a standardised community-based screening protocol for the detection of depression in two communities, 21 primary care centres, one general hospital and one specialised mental health hospital in Shenzhen, China. Participants aged 18–64 years meeting criteria for subthreshold depressive symptoms were enrolled between March 2019 and October 2019. Subthreshold depressive symptoms were operationalised as having a Patient Health Questionnaire-9 (PHQ-9) total score of ⩾ 5 without current or history of MDD. The follow-up period was 6 months. Exclusion criteria were: (1) a diagnosis of MDD, severe psychiatric disorders (i.e. schizophrenia, bipolar disorder, schizoaffective mental disorder, paranoid mental disorder, mental disorder caused by epilepsy, mental retardation) and/or alcohol or drug addiction disorder, (2) pregnant or perinatal women, (3) not being fluent in mandarin and (4) not having a plan to leave Shenzhen within 6 months. Psychiatric diagnoses were confirmed by trained psychiatrists using the Mini International Neuropsychiatric Interview (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria). The study protocol was approved by the Ethical Review Boards of all the participating centres, and all the participants gave written informed consent.
A total of 3715 people were screened, of which 2645 (71.2%) were from 21 primary care centres, 468 (12.6%) were from two communities, 368 (9.9%) were from a general hospital and 234 (6.3%) people were from a specialised mental health hospital. A total of 1722 participants were enrolled in the study between March 2019 and October 2019. Of the 1722 participants, 1506 (87.5%) participants completed the 6-month study; 216 participants were lost during follow-up (i.e. withdrew from study, n = 71; did not complete questionnaires, n = 29; unable to contact, n = 116). Of the 1506 participants with endpoint data, participants who completed the baseline and 6-month follow-up before the COVID-19 outbreak were classified into the ‘wave 1’ group (i.e. from March 2019 to January 2020) and participants who completed baseline before the COVID-19 outbreak but completed the 6-month follow-up during the COVID-19 outbreak were classified into ‘wave 2’ group (i.e. from August 2019 to April 2020).
BRIDGES integrate care
The DCC study used a Building Bridges to Integrate Care (BRIDGES) model, which used the Toronto-based BRIDGES model as a reference (Bhattacharyya et al., 2016), and linked primary care centres, specialist hospitals and community care in accordance to the health system in Shenzhen. In this healthcare model, psychiatrists from specialist hospitals trained general practitioners (GPs) in primary care centres to identify, and provided treatment and education programmes for participants with subthreshold depressive symptoms. Project managers, who were public health doctors, from specialist hospitals supervised and ensured the quality administration of the intervention provided by the GPs. Both wave 1 cohort and wave 2 cohort received the same usual care, including (1) delivering project introduction brochures; (2) sending a short message service (SMS) monthly that provide mental health education (i.e. mental health stereotypes, depression treatments and stress arrangement); (3) telephone-based mental health consult every 6 months (i.e. mental health condition communication and updated information of psychiatric evaluation in study); (4) referral and face-to-face psychiatric evaluation for participants with PHQ-9 > 9 or for those with an active request to see a doctor.
Outcome and covariates
Depression and anxiety symptoms in the past 2 weeks were assessed using the Patient Health Questionnaire-9 (PHQ-9, Cronbach's α = 0.89) and Generalised Anxiety Disorder-7 (GAD-7, Cronbach's α = 0.89), respectively (Kurt Kroenke and Williams, 2001; Löwe et al., 2008). The severity of depression and anxiety was divided into minimal, mild, moderate and severe based on a score of 0–4, 5–9, 10–14 (10–13 for anxiety) and 15–27 (14–21 for anxiety), respectively (Kurt Kroenke and Williams, 2001; Löwe et al., 2008). Participants with a score of PHQ-9 or GAD-7 ⩾ 5 were considered as probable depression or anxiety, respectively. Insomnia symptoms were assessed using the Insomnia Severity Index (ISI, Cronbach's α = 0.92), where participants were classified based on their score (i.e. group of none, subthreshold, moderate and severe of score 0–7, 8–14, 15–21 and 22–28, respectively) (Bastien et al., 2001; Gagnon et al., 2013). Participants with ISI > 7 was considered to have suspected insomnia.
To explore the fluctuations of the three main outcomes, as well as the COVID-19-related behaviours and perceived impacts during the outbreak, we separated wave 2 follow-up time into three intervals, which included the height of the pandemic (i.e. 7−13 February 2020 when the daily incidence of new COVID-19 cases climb to the peak and rigorous lockdown measures were implemented), the peak of the COVID-19 crisis (i.e. 14−27 February) and the remission plateau (i.e. 28 February−23 April). These three time intervals were defined as peak, post-peak and remission plateau, respectively. Before the COVID-19 outbreak, the questionnaires were conducted face-to-face or online. During the COVID-19 outbreak, all of our questionnaires were conducted online.
We also assessed somatic symptoms, resilience and demographic characteristics (only at baseline) to explore the risk factors related to the main outcomes. The mean score for the seven pain-related items (items 2, 3, 9, 14, 19, 27 and 28) in the 28-item Somatic Symptoms Inventory (SSI, Cronbach's α = 0.80) was used to assess painful and non-painful somatic symptoms. Participants with mean scores for each item <2.2 in SSI were considered to be without pain (Goldstein et al., 2004). Resilience was assessed using the Connor-Davidson Resilience Scale (CD-RISC, Cronbach's α = 0.91) (Yu and Zhang, 2007). As the cut-point score in the general population, we considered the mean score of CD-RISC below 80 as poor resilience (Connor and Davidson, 2003).
Demographic characteristics were measured using self-report questionnaires, which included basic information, health status and behaviours. COVID-19-related behaviours and perceived impacts were also measured in wave 2 at the 6-month follow-up during the outbreak, including the number of days worrying about COVID-19 in the past week (i.e. 0–2 days, 3 days and above), perceived COVID-19 influence on current life (i.e. none or mild, moderate or severe), perceived COVID-19 influence on future life (i.e. none or mild, moderate or severe) and perceived risk of infection COVID-19 (i.e. none or low, moderate or high).
Statistical analysis
We estimated the proportion of depression, anxiety and insomnia symptoms at baseline and 6-month follow-up using the longitudinal surveys. For interpretative purposes, all outcomes and baseline characteristics were measured as categorical variables. In the univariate analysis, we identified the change between baseline and 6-month follow-up of severity in three main outcomes using the chi-squared test, as well as comparing the difference between wave 1 and 2 at baseline and follow-up. To explore the impact of COVID-19 and other potential factors on outcomes, we included all participants (n = 1506) in the binary logistic regression analysis to estimate their associations with probable depression, anxiety and suspected insomnia during the 6-month follow-up by adjusting for baseline severity of depression, anxiety and insomnia categories, respectively, presenting results as odds ratios. To examine the relationship between main outcomes in different time intervals of the COVID-19 outbreak and COVID-19-related behaviours, we selected participants in wave 2 and applied the chi-square trend test. All tests were two-tailed, with a significance level of p-values < 0.05. Statistical analysis was performed on SPSS Statistic 25.0 (Property of IBM Corp.).
Results
Baseline characteristics
In our recruitment process, there were 36 people who were excluded due to the presence of a severe psychiatric illness in two waves (14 and 22 in wave 1 and 2, respectively). Of the 1506 participants, 726 participants completed the study in wave 1 and 780 participants completed the study in wave 2 (Table 1). Differences in baseline characteristics between participants who completed versus those who did not complete the study were not significant. Participants in wave 1 were older and more likely to be married when compared to participants in wave 2. Other baseline demographic characteristics were well balanced and similar between participants within the two waves. Furthermore, wave 2 participants had higher rates of chronic disease and smoking behaviour, as well as lower rate of exercise habit at baseline when compared to wave 1. There were no statistically significant differences in measures of depression, anxiety and insomnia at baseline between wave 1 and 2 (Table 2).
Table 1.
Baseline demographic information. All participants were enrolled in the 6-month study between March and October 2019
| All participants, N = 1506 | Wave 1 group, N = 726 | Wave 2 group, N = 780 | p-values | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age group, N (%) | 0.008 | |||
| 18–39 | 897 (59.6) | 408 (56.2) | 489 (62.7) | |
| 40–59 | 540 (35.9) | 275 (37.9) | 265 (34.0) | |
| ⩾60 | 69 (4.6) | 43 (5.9) | 26 (3.3) | |
| Gender, N (%) | 0.089 | |||
| Male | 560 (37.2) | 254 (35.0) | 306 (39.2) | |
| Female | 946 (62.8) | 472 (65.0) | 474 (60.8) | |
| Education, N (%) | 0.900 | |||
| Below undergraduate | 442 (61.7) | 451 (62.1) | 487 (62.4) | |
| Undergraduate and above | 274 (38.3) | 275 (37.5) | 293 (37.6) | |
| Occupation, N (%) | 0.055 | |||
| Unemployed | 133 (18.9) | 162 (22.3) | 143 (18.3) | |
| Employed | 583 (81.4) | 564 (77.7) | 637 (81.7) | |
| Marital status, N (%) | 0.025 | |||
| Unmarried | 370 (24.6) | 156 (21.5) | 214 (27.4) | |
| Married | 1085 (72.0) | 546 (75.2) | 539 (69.1) | |
| Divorced or widowed | 51 (3.4) | 24 (3.3) | 27 (3.5) | |
| Birth place, N (%) | 0.204 | |||
| Hubei | 159 (10.6) | 79 (10.9) | 80 (10.3) | |
| Local | 121 (8.0) | 49 (6.7) | 72 (9.2) | |
| Non-local | 1226 (81.4) | 598 (82.4) | 628 (80.5) | |
| Years lived in Shenzhen, N (%) | 0.709 | |||
| 5 years and below | 440 (29.2) | 205 (28.2) | 235 (30.1) | |
| 5–10 years | 251 (16.7) | 124 (17.1) | 127 (16.3) | |
| 10 years and above | 815 (54.1) | 397 (54.7) | 418 (53.6) | |
| Living status, N (%) | 0.292 | |||
| Single | 154 (10.2) | 75 (10.3) | 79 (10.1) | |
| With relatives | 1174(78.0) | 575 (79.2) | 599 (76.8) | |
| With non-relatives | 178 (11.8) | 76 (10.5) | 102 (13.1) | |
| Health status and behaviours | ||||
| Chronic diseases, N (%) | <0.001 | |||
| No | 521 (72.8) | 579 (79.8) | 540 (69.2) | |
| Yes | 195 (27.2) | 147 (20.2) | 240 (30.8) | |
| Insomnia drug, N (%) | 0.901 | |||
| No | 646 (90.2) | 652 (89.8) | 702 (90.0) | |
| Yes | 70 (9.8) | 74 (10.2) | 78 (10.0) | |
| Referral and see psychologist, N (%) | 0.586 | |||
| No | 1306 (86.7) | 626 (86.2) | 680 (87.2) | |
| Yes | 200 (13.3) | 100 (13.8) | 100 (12.8) | |
| Exercise habit per week (at least 1 time and ⩾30 min), N (%) | 0.031 | |||
| No | 854 (56.7) | 391 (53.9) | 463 (59.4) | |
| Yes | 652 (43.3) | 335 (46.1) | 317 (40.6) | |
| Frequency for smoke in a month, N (%) | 0.021 | |||
| 2 days and below | 1322 (87.8) | 652 (89.8) | 670 (85.9) | |
| 3 days and above | 184 (12.2) | 74 (10.2) | 110 (14.1) | |
| Frequency for alcohol consumption in a month, N (%) | 0.728 | |||
| 2 days and below | 1254 (83.3) | 692 (82.9) | 652 (83.6) | |
| 3 days and above | 252 (16.7) | 124 (17.1) | 128 (16.4) | |
| Body-mass index, N (%) | 0.525 | |||
| Normal (18.5–23.9) | 945 (62.7) | 463 (63.8) | 482 (61.8) | |
| Underweight | 153 (10.2) | 76 (10.5) | 77 (9.9) | |
| Overweight | 408 (27.1) | 187 (25.8) | 221 (28.3) | |
Abbreviation: wave 1: participants completed the baseline and 6-month follow-up before the COVID-19 outbreak (i.e. from March 2019 to January 2020); wave 2: participants completed the baseline before COVID-19 outbreak but 6-month follow-up during the COVID-19 outbreak (i.e. from August 2019 to April 2020).
Table 2.
Changes in depression, anxiety and insomnia symptom severity scores from baseline to endpoint
| Baseline, N (%) | 6-month follow-up, N (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| All | Wave 1 group | Wave 2 group | p-valuesa | All | Wave 1 group | Wave 2 group | p-valuesb | |
| N = 1506 | N = 726 | N = 780 | N = 1506 | N = 726 | N = 780 | |||
| PHQ-9 | 0.101 | <0.001 | ||||||
| Minimal | NA | NA | NA | 843 (56.0) | 436 (60.1) | 407 (52.2) | ||
| Mild | 1094 (72.6) | 543 (74.8) | 551 (70.6) | 491 (32.6) | 233 (32.1) | 258 (33.1) | ||
| Moderate | 299 (19.9) | 138 (19.0) | 161 (20.6) | 116 (7.7) | 38 (5.2) | 78 (10.0) | ||
| Severe | 113 (7.5) | 45 (6.2) | 68 (8.7) | 56 (3.7) | 19 (2.6) | 37 (4.7) | ||
| GAD-7 | 0.199 | 0.003 | ||||||
| Minimal | 744 (49.4) | 347 (47.8) | 397 (50.9) | 1023 (67.9) | 523 (72.0) | 500 (64.1) | ||
| Mild | 524 (34.8) | 271 (37.3) | 253 (32.4) | 387 (25.7) | 166 (22.9) | 221 (28.3) | ||
| Moderate | 157 (10.4) | 74 (10.2) | 83 (10.6) | 71 (4.7) | 24 (3.3) | 47 (6.0) | ||
| Severe | 81 (5.4) | 34 (4.7) | 47 (6.0) | 25 (1.7) | 13 (1.8) | 12 (1.5) | ||
| ISI | 0.981 | 0.022 | ||||||
| None | 756 (50.2) | 362 (49.9) | 394 (50.5) | 1037 (68.9) | 523 (72.0) | 514 (65.9) | ||
| Subthreshold | 495 (32.9) | 241 (33.2) | 254 (32.6) | 342 (22.7) | 154 (21.2) | 188 (24.1) | ||
| Moderate | 193 (12.8) | 92 (12.7) | 101 (12.9) | 96 (6.4) | 40 (5.5) | 56 (7.2) | ||
| Severe | 62 (4.1) | 31 (4.3) | 31 (4.0) | 31 (2.1) | 9 (1.2) | 22 (2.8) | ||
| SSI pain-related items | 0.468 | 0.192 | ||||||
| Without pain (mean <2.2) | 1351 (89.7) | 647 (89.1) | 704 (90.3) | 1386 (92.0) | 675 (93.0) | 711 (91.2) | ||
| With pain, N (%) | 155 (10.3) | 79 (10.9) | 76 (9.7) | 120 (8.0) | 51 (7.0) | 69 (8.8) | ||
| CD-RISC group | 0.213 | |||||||
| Weak resilience (⩽80) | 1253 (83.2) | 595 (82.0) | 658 (84.4) | NA | NA | NA | - | |
| Better resilience | 253 (16.8) | 131 (18.0) | 122 (15.6) | NA | NA | NA | - | |
Abbreviations: PHQ-9, Patient Health Questionnaire-9; GAD-7, Generalised Anxiety Disorder-7; ISI, Insomnia Severity Index; SSI, Somatic Symptoms Inventory; CD-RISC, Connor-Davidson Resilience Scale. Wave 1, participants completed the baseline and 6-month follow-up before the COVID-19 outbreak (i.e. from March 2019 to January 2020); Wave 2, participants completed the baseline before COVID-19 outbreak but 6-month follow-up during the COVID-19 outbreak (i.e. from August 2019 to April 2020).
Outcomes were additionally compared between participants who completed the study before the COVID-19 pandemic (i.e. March 2019 to January 2020) and during the COVID-19 pandemic (i.e. August 2019 to April 2020)
Baseline comparison of wave 1 and 2.
6-month follow-up comparison of wave 1 and 2.
Comparison of main outcomes between baseline and 6-month follow-up
The unadjusted percentages of depression severity, anxiety and insomnia at baseline and 6-month follow-up were compared across all and separated waves in Table 2. There was 56% of participants with probable depression at baseline improved into the minimal group at 6 months. The prevalence rate of probable anxiety and suspected insomnia also increased by approximately 20% in both the minimal and none group, respectively. Additionally, the mean (s.d.) score of depression, anxiety and insomnia at the 6-month follow-up had a significant decrease compared with baseline, which were 4.7 (4.2) v. 8.1 (4.0), 3.5 (3.6) v. 5.6 (4.4) and 5.9 (5.5) v. 8.6 (6.2), respectively. However, in terms of the magnitude of remission, wave 2 showed a dramatic magnitude of remission compared to wave 1. Focusing on the severe group, depression accounted for the highest proportion among three main outcomes with 4.7% in wave 2 follow-up, compared with 2.6% in wave 1, followed by insomnia (2.8% and 1.2%, respectively). However, anxiety had a lower prevalence rate in the severe group of wave 2, as it accounted for a higher rate of 6.0% in the moderate group, compared with 3.3% in wave 1. The severity of the mild group took up largest proportion in depression, anxiety and insomnia in wave 2, which were 33.1%, 28.3% and 24.1%, respectively.
COVID-19 and other indicators related to the main outcomes in 6-month follow-up
According to the severity change of mood scales, we examined whether the baseline characteristics had a favourable or unfavourable effect on depression, anxiety and insomnia. We defined 6-month outcomes into probable depression, probable anxiety and suspected insomnia. In Table 3, after adjusting for all indicators, participants who completed 6-month follow-up during COVID-19 outbreak was a risk factor for probable depression (OR = 1.30, 95% CI: 1.04, 1.62), anxiety (OR = 1.47, 95% CI: 1.16, 1.88) and suspected insomnia (OR = 1.37, 95% CI: 1.07, 1.77). Baseline severity of depression, anxiety and insomnia also showed a strong dose−response gradient with probable depression, anxiety and suspected insomnia, respectively. For somatic symptoms and mood resilience, SSI was not associated with all three main outcomes, whereas participants with better resilience (CD-RISC score >80) had a stronger beneficial effect on them.
Table 3.
Moderators of probable depression, anxiety and insomnia
| OR (95% CI) | |||
|---|---|---|---|
| PHQ-9 | GAD-7 | ISI | |
| Follow-up time point | |||
| Before outbreak (wave 1 group) | Ref. | Ref. | Ref. |
| During outbreak (wave 2 group) | 1.30 (1.04, 1.62) * | 1.47 (1.16, 1.88) ** | 1.37 (1.07, 1.77) * |
| Age | |||
| 18–39 | Ref. | Ref. | Ref. |
| 40–59 | 0.85 (0.63, 1.13) | 0.86 (0.63, 1.18) | 1.19 (0.86, 1.67) |
| ⩾60 | 1.02 (0.56, 1.86) | 1.24 (0.65, 2.37) | 1.60 (0.82, 3.11) |
| Gender | |||
| Male | Ref. | Ref. | Ref. |
| Female | 1.25 (0.96, 1.64) | 1.30 (0.97, 1.75) | 0.92 (0.68, 1.25) |
| Education | |||
| Below undergraduate | Ref. | Ref. | Ref. |
| Undergraduate and above | 1.06 (0.83, 1.35) | 0.87 (0.67, 1.14) | 1.28 (0.97, 1.69) |
| Occupation | |||
| Unemployed | Ref. | Ref. | Ref. |
| employed | 1.08 (0.79, 1.48) | 1.20 (0.85, 1.68) | 0.90 (0.64, 1.27) |
| Marital status | |||
| Unmarried | Ref. | Ref. | Ref. |
| Married | 0.61 (0.43, 0.87) ** | 0.57 (0.40, 0.84) ** | 0.57 (0.39, 0.85)** |
| Divorced or widowed | 1.13 (0.58, 2.22) | 1.26 (0.63, 2.51) | 1.05 (0.51, 2.16) |
| Years live in Shenzhen | |||
| 5 years and below | Ref. | Ref. | Ref. |
| 5–10 years | 0.97 (0.68, 1.38) | 1.33 (0.91, 1.94) | 1.25 (0.84, 1.87) |
| 10 years and above | 1.11 (0.83, 1.49) | 1.30 (0.94, 1.78) | 1.42 (1.02, 1.99) * |
| Living status | |||
| Single | Ref. | Ref. | Ref. |
| With relatives | 0.85 (0.56, 1.28) | 1.17 (0.75, 1.83) | 1.08 (0.68, 1.72) |
| With non-relatives | 0.56 (0.35, 0.90) * | 0.60 (0.36, 1.00) | 0.81 (0.48, 1.37) |
| Chronic disease | |||
| No | Ref. | Ref. | Ref. |
| Yes | 1.06 (0.81, 1.39) | 1.00 (0.75, 1.33) | 1.14 (0.85, 1.54) |
| Insomnia drugs | |||
| No | Ref. | Ref. | Ref. |
| Yes | 1.39 (0.95, 2.03) | 1.06 (0.71, 1.58) | 1.18 (0.77, 1.81) |
| Referral and see psychologist | |||
| No | Ref. | Ref. | Ref. |
| Yes | 1.32 (0.92, 1.89) | 1.02 (0.72, 1.46) | 1.42 (1.00, 2.04) |
| Exercise habit per week (at least 1 time and ⩾30 min) | |||
| No | Ref. | Ref. | Ref. |
| Yes | 0.79 (0.63, 0.99) * | 0.81 (0.63, 1.03) | 0.97 (0.75, 1.26) |
| Frequency for alcohol consumption in a month | |||
| 2 days and below | Ref. | Ref. | Ref. |
| 3 days and above | 1.43 (1.05, 1.95) * | 1.39 (1.01, 1.94) * | 0.93 (0.66, 1.33) |
| Frequency for smoke in a month | |||
| 2 days and below | Ref. | Ref. | Ref. |
| 3 days and above | 1.15 (0.78, 1.68) | 1.09 (0.71, 1.65) | 1.35 (0.88, 2.07) |
| Body-mass index | |||
| Normal (18.5–23.9) | Ref. | Ref. | Ref. |
| Underweight | 0.93 (0.64, 1.35) | 1.06 (0.71, 1.58) | 0.91 (0.60, 1.40) |
| Overweight | 1.07 (0.82, 1.40) | 0.99 (0.74, 1.32) | 0.70 (0.52, 0.95) * |
| SSI pain-related items | |||
| Without pain | Ref. | Ref. | Ref. |
| With pain | 1.07 (0.73, 1.58) | 1.13 (0.76, 1.68) | 1.15 (0.76, 1.73) |
| CD-RISC | |||
| Weak resilience (⩽80) | Ref. | Ref. | Ref. |
| Greater resilience | 0.26 (0.19, 0.37) *** | 0.21 (0.14, 0.33) *** | 0.18 (0.11, 0.29) *** |
| Severity | |||
| Minimal (none) | NA | Ref. | Ref. |
| Mild (subthreshold) | Ref. | 2.15 (1.65, 2.81) *** | 2.98 (2.24, 3.97) *** |
| Moderate | 1.76 (1.31, 2.37) *** | 4.19 (2.80, 6.27) *** | 7.68 (5.11, 11.55) *** |
| Moderately severe and above | 2.98 (1.85, 4.79) *** | 7.62 (4.33, 13.40) *** | 10.71 (5.28, 21.73) *** |
Abbreviations: PHQ-9, Patient Health Questionnaire-9; GAD-7, Generalised Anxiety Disorder-7; ISI, Insomnia Severity Index; SSI, Somatic Symptoms Inventory; CD-RISC, Connor-Davidson Resilience Scale.
*p-values < 0.05; **p-values < 0.01; ***p-values < 0.001.
In terms of the demographic indicators, being married contributed a beneficial effect to all of depression, anxiety and insomnia. Participants with alcohol consumption (i.e. 3 days and above in a month) were more vulnerable to probable depression (OR = 1.43, 95% CI: 1.05, 1.95) and anxiety (OR = 1.39, 95% CI: 1.01, 1.94), whereas the association with suspected insomnia was not detected. In particular, exercising once per week and living with a non-relative were protective factors against depression. Having lived in Shenzhen for more than 10 years was associated with a greater risk for insomnia, whereas being overweight was protective against insomnia. Unadjusted and adjusted pooled estimates of probable depression, anxiety and suspected insomnia are available in the online supplementary materials.
Time-dependent change of main outcomes and behaviours during COVID-19 outbreak
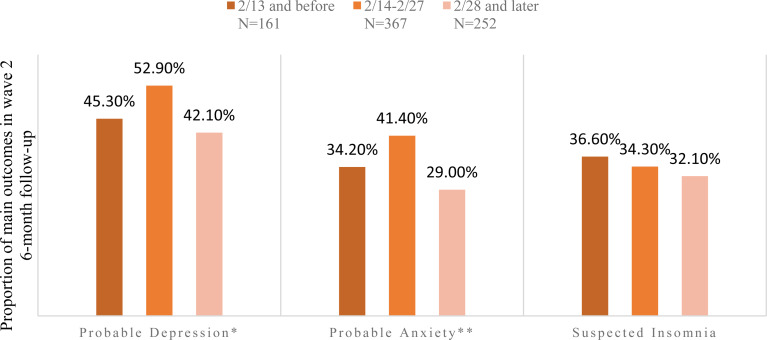
To characterise the effects of the COVID-19 pandemic on depression, anxiety and insomnia, we compared the proportion of participants meeting criteria for probable depression, anxiety and suspected insomnia at endpoint at the three different time intervals. Of the 780 participants who completed the study during the COVID-19 pandemic, 161 (20.6%), 367 (47.1%) and 252 (32.3%) were included in the peak, post-peak and remission plateau subgroups, respectively.
Baseline symptom severity did not differ between the three COVID-19-related subgroups (i.e. probable depression, χ2 = 3.86, p = 0.43; probable anxiety, χ2 = 2.01, p = 0.92; suspected insomnia, χ2 = 7.29, p = 0.30). Figure 1 showed that probable depression and anxiety significantly changed across the follow-up time intervals. The highest rates of probable depression and anxiety were detected between 14 and 27 February 2020, which was after the peak of the newly diagnosed cases per day. From 28 February 2020 and onwards, the rate of probable depression and anxiety dropped to the lowest rate at 43% and 29%, respectively. However, the rate of suspected insomnia was not significantly different across the time process of COVID-19.
Fig. 1.
Time-dependent change of probable depression, anxiety and suspected insomnia during COVID-19 outbreak. *p-values < 0.05. **p-values < 0.01.
Individuals reporting greater symptoms of COVID-19-related distress, as operationalised by the number of days worried about COVID-19 in the past week (i.e. more than 3 days), perceived influence of COVID-19 on current and future life (i.e. moderate to severe) and perceived risk of infection (i.e. moderate to high), were significantly more likely, than those reporting fewer symptoms of distress, to have probable depression, anxiety and/or insomnia. Focusing on the COVID-19-related behaviours (Fig. 2), all four items demonstrated an inverse linear relationship with time process, with the Pearson correlation coefficient of −0.163 (p < 0.001), −0.154 (p < 0.001), −0.136 (p < 0.001) and −0.155 (p < 0.001) in days focus on COVID-19, perceived influence on current life, perceived influence on future life and perceived risk of COVID-19 infection, respectively. Different from the main outcomes change, the highest rate of behaviours was detected in the first time period of 13 February 2020 and before. Participants who perceived moderate or severe influence on current life accounted for the highest rate among all behaviour items.
Fig. 2.
Time-dependent change of behaviours during COVID-19 outbreak. *p-values < 0.05. **p-values < 0.01. ***p-values < 0.001.
Discussion
Our prospective longitudinal study described the psychological impact of the COVID-19 pandemic on subthreshold depressive symptoms. The general remission of depression, anxiety and insomnia had been detected from baseline to 6-month follow-up, whereas wave 2 participants who completed the follow-up during the COVID-19 pandemic had significantly higher rates of probable depression, anxiety and insomnia relative to wave 1, after controlling for the sociodemographic characteristics, health status and behaviours and severity of baseline mental health outcomes. With the time process of COVID-19 outbreak in China, the highest rate of probable depression and anxiety had been found after the peak of newly diagnosed cases, and further decreased with the remission plateau. Similarly, the frequency and degree of COVID-19-related behaviours were also improving as the severity of the COVID-19 pandemic subsided.
COVID-19 and subthreshold depressive symptoms
The COVID-19 pandemic is having a profound effect on all aspects of society. We found that participants in our study had a relative higher proportion (47.8%) of probable depression during COVID-19 outbreak (wave 2), when compared to the general population in a previous study (30.3%) (Wang et al., 2020a). A survey conducted in southwestern China, near Wuhan, also demonstrated a relative low prevalence of depression and anxiety with 8.3% and 14.6%, respectively (Lei et al., 2020). Nevertheless, health care workers shared similar rates of probable depression, anxiety and suspected insomnia with our study, which were 50.4%, 44.6% and 34.0%, respectively (Lai et al., 2020). It could be hypothesised that the significant workload, as well as close contact with people who potentially have been infected by the virus, were also considered a susceptible population during the COVID-19 outbreak.
COVID-19 and stress
Epidemics act as a stressor during an outbreak and never affect all populations equally. For example, the individual effects of mental health stressors are moderated by personality and cognitive constructs. In our study, we found that resilience may play a more important protective role on depression, anxiety and insomnia. For example, it has been reported that the association between adverse childhood experiences and depression was stronger among individuals with low resilience compared to those with high resilience (Poole et al., 2017). Psychological models suggest that individual differences in the strength of the personality or schema features determine how stressors will be interpreted. Stress appraisals that represent threats or depletion in the core areas of self-worth may portend depressive symptoms (Hammen, 2005). A separate study also demonstrated that the SL genotype in serotonin transporter (5-HTTLPR) gene appeared resilient to depression in terms of cortisol and recent stress (Ancelin et al., 2017).
With respect to this large-scale pandemic and similar disasters, improving copying methods and mood resilience were an effective way to prevent mental health disorders. A randomised-controlled trial demonstrated that interventions targeting stress management, goal setting, cognitive reframing and meaning making significantly improved resilience and marginal effect to avoid depression in patients with cancer (Rosenberg et al., 2018). Second, stressor content can lead to various mental health outcomes. Chronic and unpredictable stress (defined as stress for more than 12 months) is a stronger predictor of depressive symptoms than acute stressors. Interpersonal ‘loss’ event was unique significance for depression, which included bereavement, separations, endings or threats of separation (Hammen, 2005). To explore the psychological impact of COVID-19, we not only needed to consider the susceptible population, but also consider the different interpretations of stress in varying populations.
The temporality of the epidemic may lead to various outcomes vis-a-vis mental health conditions. In our study, probable depression and anxiety fluctuated with the COVID-19 outbreak curve, but, overall, the increasing rate of mental health outcomes were consistent with a study that showed higher average levels of symptoms (stress, anxiety and depression) after the nationwide state of alert and stay-at-home order (Ozamiz-Etxebarria et al., 2020). The study suggested that individuals have difficulty assimilating and processing the current crisis. The late stage reduction trend was similar to a meta-analysis which showed that depression, anxiety and insomnia symptoms in post-illness stage were lower than acute stage in SARS and Middle East respiratory syndrome (MERS) epidemic among the population (Rogers et al., 2020). This study suggested that if a COVID-19 infection follows a similar course to that of SARS or MERS, most patients should recover without experiencing mental illness. In general, an increasing trend of depression and anxiety may only be specific to a time interval of an acute stimulation. Some positive effects in terms of psychological regulation and personal coping styles were worth noting for mental health improvement. In addition, these mental health symptoms levels can be expected to increase further as confinement and isolation are extended, in addition to the adverse events induced by epidemic. Hence, it would be useful to further evaluate mental health conditions over time (Brooks et al., 2020).
Target interventions
In our study, there were other factors that drew our attention to their effects on the mental health condition in the 6-month follow-up. Regular exercise may attenuate the probability of developing depression compared with people with non-regular exercise habits (adjusted OR = 0.79, 95% CI = 0.63–0.99). The result was consistent with a meta-analysis which indicated that higher levels of physical activity were related to lower odds of developing depression (adjusted OR = 0.83, 95% CI = 0.79–0.88) (Schuch et al., 2018). A randomised-controlled trial also demonstrated that the intervention of yoga plus regular care could significantly improve depression symptoms and score on CD-RISC, but not anxiety (Michael de Manincor et al., 2016). We also found that high frequency of alcohol consumption was harmful on probable depression and anxiety compared to low frequency.
The foregoing finding was in accordance with a study that indicated that hazardous drinking (a score⩾8 of Alcohol Use Disorder Identification Test) was associated with a higher risk of depression than non-hazardous alcohol consumption (risk ratio = 1.8, 95% CI = 1.4, 2.4) (Gemes et al., 2019). However, depending on the various definitions of alcohol consumption, depression is primarily related to drinking larger quantities per occasion and less related to volume and unrelated to drinking frequency; this effect is stronger for women than for men (Graham et al., 2007). Further studies need to identify the dose−response relationship between behaviours and mental health outcomes. Recommending a healthy lifestyle may afford a positive attitude to coping with adverse events.
Further research
Taken together, we draw the following views and provide some directions for further research: (1) more attention (e.g. resource allocation) could be paid to vulnerable and susceptible populations when an adverse event occurs. For example, expert resources including psychiatrists, psychologists and mental health hospital may target the people at severe risk actively (i.e. bereavement, COVID-19 infected/suspected cases and severe mental health symptoms). People with mild mental health symptoms could be monitored by social workers and receive psychoeducation from public media and service. It may be more cost-effective to allocate mental health resources in a reasonable way; (2) finding an appropriate way to improve mood resilience and coping methods under the stress may afford protections to prevent mental health disorders; (3) medical health workers may need to invest resources to mitigate the effects of a mental health outbreak during an epidemic and provided intervention promptly, as well as further attention on the following adverse events induced by epidemic; (4) mental health education may need to be provided, and may not only convey general information about mental health, but also recommend and conduct behaviours- and life-style-related intervention to prevent mental health problems.
Strengths and limitations
Major strengths of this study are its prospective design, the large representative community-based sample and the use of a clinically validated diagnostic interview to establish a wide range of mental disorders (Mini International Neuropsychiatric Interview). However, our study also has several methodological limitations that affect the inferences and interpretations of our data. First, we did not compare participants with mental health disorders to the general population in our study, wherein individuals without mental health disorders may have different reactions to mental health conditions on COVID-19. Second, our study only estimated the mental health impact until April 2020. Consequently, the effect of an economic recession, unemployment and concern about epidemic relapse after the epidemic may have a greater mental health impact on the general population and should be taken into consideration in future research.
In conclusion, COVID-19 plays an essential role to worsen mental health conditions, including depression, anxiety and insomnia. During the COVID-19 outbreak, mental health conditions and behaviours fluctuated with the epidemic time process, which may provide insight to mental health workers to conduct interventions to alleviate stress and anxiety. Furthermore, regular exercise and ways to improve resilience may be a feasible recommendation as a mental health prevention, whether or not an adverse event occurs.
Acknowledgements
The authors thank all participants of the study. The Depression Cohort in China (DCC) is conducted by the Sun Yat-Sen University and Shenzhen Nanshan Center for Chronic Disease Control in Shenzhen, China.
Availability of data and materials
Data for the Depression Cohort in China are available through the Sun Yat-Sen University. Contact Professor Lu for access approval.
Financial support
This study was supported by the National Natural Science Foundation of China (Grant No. 81761128030).
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S2045796021000044.
click here to view supplementary material
Conflict of interest
All other authors declare no competing interests.
References
- Ancelin ML, Scali J, Norton J, Ritchie K, Dupuy AM, Chaudieu I and Ryan J (2017) Heterogeneity in HPA axis dysregulation and serotonergic vulnerability to depression. Psychoneuroendocrinology 77, 90–94. [DOI] [PubMed] [Google Scholar]
- Bastien CH, Vallières A and Morin CM (2001) Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Medicine 2, 297–307. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya O, Schull M, Shojania K, Stergiopoulos V, Naglie G, Webster F, Brandao R, Mohammed T, Christian J, Hawker G, Wilson L and Levinson W (2016) Building Bridges to Integrate Care (BRIDGES): incubating health service innovation across the continuum of care for patients with multiple chronic conditions. Healthcare Quarterly 19, 60–66. [DOI] [PubMed] [Google Scholar]
- Brooks SK, Webster RK, Smith LE, Woodland L, Wessely S, Greenberg N and Rubin GJ (2020) The psychological impact of quarantine and how to reduce it: rapid review of the evidence. The Lancet 395, 912–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LS, Eaton WW, Gallo JJ, Nestadt G and Crum RM (2000) Empirical examination of current depression categories in a population-based study: symptoms, course, and risk factors. American Journal of Psychiatry 157, 573–580. [DOI] [PubMed] [Google Scholar]
- Connor KM and Davidson JR (2003) Development of a new resilience scale: the Connor-Davidson Resilience Scale (CD-RISC). Depression and Anxiety 18, 76–82. [DOI] [PubMed] [Google Scholar]
- Cuijpers P and Smit F (2004) Subthreshold depression as a risk indicator for major depressive disorder: a systematic review of prospective studies. Acta Psychiatrica Scandinavica 109, 325–331. [DOI] [PubMed] [Google Scholar]
- Gagnon C, Belanger L, Ivers H and Morin CM (2013) Validation of the Insomnia Severity Index in primary care. Journal of the American Board of Family Medicine 26, 701–710. [DOI] [PubMed] [Google Scholar]
- Gémes K, Forsell Y, Janszky, Laszlo KD, Lundin A, Ponce Dee LA, Mukamal KJ and Moller J (2019) Moderate alcohol consumption and depression − a longitudinal population-based study in Sweden. Acta Psychiatry Scandinavica 139, 526–535. [DOI] [PubMed] [Google Scholar]
- Goldstein DJ, Lu Y, Detke MJ, Hudson J, Iyengar S and Demitrack MA (2004) Effects of duloxetine on painful physical symptoms associated with depression. Psychosomatics 45, 17–28. [DOI] [PubMed] [Google Scholar]
- Graham K, Massak A, Demers A and Rehm J (2007) Does the association between alcohol consumption and depression depend on how they are measured? Alcoholism: Clinical and Experimental Research 31, 78–88. [DOI] [PubMed] [Google Scholar]
- Hammen C (2005) Stress and depression. Annual Review of Clinical Psychology 1, 293–319. [DOI] [PubMed] [Google Scholar]
- Kurt Kroenke RLS and Williams JBW (2001) The PHQ-9 validity of a brief depression severity measure. Journal of General Internal Medicine 16, 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J, Ma S, Wang Y, Cai Z, Hu J, Wei N, Wu J, Du H, Chen T, Li R, Tan H, Kang L, Yao L, Huang M, Wang H, Wang G, Liu Z and Hu S (2020) Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Network Open 3, e203976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YY, Stockings EA, Harris MG, Doi SAR, Page IS, Davidson SK and Barendregt JJ (2019) The risk of developing major depression among individuals with subthreshold depression: a systematic review and meta-analysis of longitudinal cohort studies. Psychological Medicine 49, 92–102. [DOI] [PubMed] [Google Scholar]
- Lei L, Huang X, Zhang S, Yang J, Yang L and Xu M (2020) Comparison of prevalence and associated factors of anxiety and depression among people affected by versus people unaffected by quarantine during the COVID-19 epidemic in southwestern China. Medical Science Monitor 26, e924609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Yang LL, Zhang CX, Xiang YT, Liu ZC, Hu SH and Zhang B (2020) Online mental health services in China during the COVID-19 outbreak. The Lancet Psychiatry 7, 228–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löwe BDO, Müller S, Brähler E, Schellberg D, Herzog W and Herzberg PY (2008) Validation and standardization of the Generalized Anxiety Disorder Screener (GAD-7) in the general population. Medical Care 46, 266–274. [DOI] [PubMed] [Google Scholar]
- McIntyre RS and Lee Y (2020a) Projected increases in suicide in Canada as a consequence of COVID-19. Psychiatry Research 290, 113104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre RS and Lee Y (2020b) Preventing suicide in the context of the COVID-19 pandemic. World Psychiatry 19, 250–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael De Manincor AB, Smith CA, Barr K, Schweickle M, Donoghoe LL, Bourchier S and Fahey P (2016) Individualized yoga for reducing depression and anxiety, and improving well-being: a randomized controlled trial. Depression and Anxiety 33, 816–828. [DOI] [PubMed] [Google Scholar]
- Ozamiz-Etxebarria N, Dosil-Santamaria M, Picaza-Gorrochategui M and Idoiaga-Mondragon N (2020) Stress, anxiety, and depression levels in the initial stage of the COVID-19 outbreak in a population sample in the northern Spain. Cadernos de Saude Publica 36, e00054020. [DOI] [PubMed] [Google Scholar]
- Poole JC, Dobson KS and Pusch D (2017) Childhood adversity and adult depression: the protective role of psychological resilience. Child Abuse & Neglect 64, 89–100. [DOI] [PubMed] [Google Scholar]
- Potloc Study (2020) Potloc Study: Canadian health workers share their insights from the front lines of the COVID-19 pandemic. Available at https://potloc.com/blog/en/potloc-study-canadian-health-workers-insights-front-lines-covid-19-pandemic/ (Accessed 16 April 2020).
- Rodríguez MR, Roberto N, Chatterji S and Ayuso-Mateos JL (2012) Definitions and factors associated with subthreshold depressive conditions: a systematic review. BMC Psychiatry 12, 181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JP, Chesney E, Oliver D, Pollak TA, Mcguire P, Fusar-Poli P, Zandi MS, Lewis G and David AS (2020) Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. The Lancet Psychiatry 7, 611–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg AR, Bradford MC, Mccauley E, Curtis JR, Wolfe J, Baker KS and Yi-Frazier JP (2018) Promoting resilience in adolescents and young adults with cancer: results from the PRISM randomized controlled trial. Cancer 124, 3909–3917. [DOI] [PubMed] [Google Scholar]
- Schuch FB, Vancampfort D, Firth J, Rosenbaumr S, Ward PB, Silva ES, Hallgren M, Ponce De Leon A, Dunn AL, Deslandes AC, Fleck MP, Carvalho AF and Stubbs B (2018) Physical activity and incident depression: a meta-analysis of prospective cohort studies. American Journal of Psychiatry 175, 631–648. [DOI] [PubMed] [Google Scholar]
- Su TP, Lien TC, Yang CY, Su YL, Wang JH, Tsai SL and Yin JC (2007) Prevalence of psychiatric morbidity and psychological adaptation of the nurses in a structured SARS caring unit during outbreak: a prospective and periodic assessment study in Taiwan. Journal of Psychiatry Research 41, 119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Pan R, Wan X, Tan Y, Xu L, Ho CS and Ho RC (2020a) Immediate psychological responses and associated factors during the initial stage of the 2019 Coronavirus Disease (COVID-19) epidemic among the general population in China. International Journal Environment Research and Public Health 17, 1705–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Pan R, Wan X, Tan Y, Xu L, McIntyre RS, Choo FN, Tran B, Ho R, Sharma VK and Ho C (2020b) A longitudinal study on the mental health of general population during the COVID-19 epidemic in China. Brain Behavior and Immunity 87, 40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Chen JH and Xu YF (2020) Patients with mental health disorders in the COVID-19 epidemic. The Lancet Psychiatry 7, e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XN and Zhang JX (2007) Factor analysis and psychometric evaluation of the connor-davidson resilience scale (cd-risc) with Chinese people. Social Behavior and Personality 35, 19–30. [Google Scholar]
- Zhang WR, Wang K, Yin L, Zhao WF, Xue Q, Peng M, Min BQ, Tian Q, Leng HX, Du JL, Chang H, Yang Y, Li W, Shangguan FF, Yan TY, Dong HQ, Han Y, Wang YP, Cosci F and Wang HX (2020) Mental health and psychosocial problems of medical health workers during the COVID-19 epidemic in China. Psychotherapy Psychosomatics 89, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S2045796021000044.
click here to view supplementary material
Data Availability Statement
Data for the Depression Cohort in China are available through the Sun Yat-Sen University. Contact Professor Lu for access approval.