Abstract
Waldenström macroglobulinemia (WM) is a distinct type of indolent lymphoplasmacytic lymphoma (LPL) with a high frequency of MYD88L265P mutation. Treatment for WM/LPL is highly variable in clinic and ibrutinib (a Bruton tyrosine kinase inhibitor, BTKi) has become a new treatment option for WM. To investigate the clinical impact of genetic alterations in WM, we assembled a large cohort of 219 WMs and 12 LPLs dividing into two subcohorts: a training cohort, patients sequenced by a same targeted 29-gene next-generation sequencing (NGS) panel, and a validation cohort, patients sequenced by allele specific-PCR or other targeted NGS panels. In both training and validation subcohorts, MYD88L265P and TP53 mutations showed favorable and adverse prognostic effects, respectively. CXCR4 nonsense/missense mutations (CXCR4NS/MS), cytogenetic complex karyotypes, and a family history of lymphoma/leukemia in first-degree relatives were associated with significantly worse clinical outcomes only or more in the validation subcohort. We further investigated the efficacy of various treatments and interaction with genetic factors in the entire cohort. Upfront dexamethasone usage was associated with poorer clinical outcomes in patients who received non-proteasome-containing chemotherapy as first-line treatment independent of genetic factors. Maintenance rituximab was associated with better survival. Ibrutinib/BTKi showed potential benefit in relapsed/refractory patients and patients without CXCR4NS/MS including those with TP53 mutations. In conclusion, genetic testing for MYD88L265P, TP53, and CXCR4 mutations and cytogenetic analysis provide important information for prognosis prediction and therapy selection. The findings in these study are valuable for improving treatment decisions on therapies available for WM/LPL patients with integration of NGS in clinic.
Keywords: Waldenström macroglobulinemia, MYD88, CXCR4, TP53, Cytogenetic karyotype, Ibrutinib
Abbreviations
- WM
Waldenström macroglobulinemia
- WHO
World Health Organization
- LPL
lymphoplasmacytic lymphoma
- IgM
immunoglobulin M
- WGS
whole-genome sequencing
- TIR
Toll/Interleukin-1 receptor
- DLBCL
diffuse large B-cell lymphoma
- BTK
Bruton's tyrosine kinase
- BTKi
BTK inhibitor
- B2M
beta-2-microglobulin
- LDH
lactate dehydrogenase
- ECOG
Eastern Cooperative Oncology Group
- AS-PCR
allele-specific polymerase chain reaction
- OS
overall survival
- PFS
progression-free survival
- TTT
time to treatment
- OS1
overall survival after first-line treatment
- PFS1
progression-free survival after first-line treatment
- IPSSWM
the International Prognostic Scoring System for WM
- NS/MS mutations
nonsense/missense mutation
- BR
bendamustine and rituximab
- DRC
dexamethasone, rituximab, and cyclophosphamide
- FCR
fludarabine, cyclophosphamide, and rituximab
- 2-CdA-CR
cladribine, cyclophosphamide, and rituximab
- R-CHOP
rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone, R-CVP, rituximab, cyclophosphamide, vincristine, and prednisone
- CPR
cyclophosphamide, prednisone, and rituximab
- CyBorD
cyclophosphamide, bortezomib, and dexamethasone
- PI
proteasome inhibitor
- BDR
bortezomib/Velcade®, dexamethasone, and rituximab
- BorR
bortezomib and rituximab
- BorD
bortezomib, and dexamethasone
- CaRD
carfilzomib, rituximab, and dexamethasone
- CR
complete response
- VGPR
very good partial response
- PR
partial response
Introduction
Waldenström macroglobulinemia (WM) is a rare indolent B-cell neoplasm of the elderly first described in 1944 by Jan Waldenström characterized by infiltration of small lymphocytes, plasma cells and plasmacytoid lymphocytes, predominantly in the bone marrow [1,2]. WM imposes clinical challenges in both diagnosis and treatment. The fourth edition of the World Health Organization (WHO) classification defined WM as a type of lymphoplasmacytic lymphoma (LPL) with an immunoglobulin M (IgM) paraprotein [2,3]. Approximately 75% of WM patients have symptoms at diagnosis. The most common symptoms are those caused by anemia as a result of the WM cell growth in the bone marrow, whereas hyperviscosity syndrome symptoms due to abnormal monoclonal IgM accumulation in the blood occurs in 10-30% patients, which can be life-threating and need immediate treatment [2]. However, there is no single standard treatment for symptomatic WM; with highly variable regimens currently used in clinic, no cure is available for WM [4]; benefit of treatment also need weigh over side effects of chemotherapy and targeted drugs. WM/LPL patients will eventually relapse, and a small subset of WM/LPL will transform to aggressive non-Hodgkin lymphoma [5,6].
WM usually arises sporadically, however nearly 20% of WM patients have at least one first-degree relative with WM or other B-cell lymphoma, suggesting a role of genetic alterations in the WM pathogenesis [7,8]. In addition, patients with WM have an increased risk of developing other cancers, both solid and hematologic malignancies [9]. It is not until 2012 through whole-genome sequencing (WGS) in 30 WM patients and Sanger sequencing in additional 27 WM/LPL patients, a distinct genetic characteristic of WM was discovered, the highly frequent missense MYD88 L265P mutation (>90%) [10]. Further analysis of the WGS results reported a 27% frequency of WHIM-like CXCR4 mutations in WM [11]. The MYD88L265P mutation is in the evolutionarily conserved beta-beta loop of the Toll/Interleukin-1 receptor (TIR) domain of MYD88 which recruits IRAK1 and IRAK4. Study in diffuse large B-cell lymphoma (DLBCL) demonstrated that MYD88L265P mutation results in spontaneous formation of the MYD88/IRAK complex (myddosome) and cytosolic myddosome aggregates, promoting cell survival through activation of the NF-κB pathway [12,13]. However, in WM, MYD88L265P mutation promotes NF-κB activation mainly by binding and phosphorylating the Bruton's tyrosine kinase (BTK) in the B-cell receptor pathway [10,14]. Ibrutinib, a BTK inhibitor (BTKi) and the first approved targeted agent alone or in combination with rituximab (anti-CD20 antibody) for WM [15], reduces the binding strength of BTK to MYD88L265P and impairs the NF-κB pathway. MYD88L256P mutation and wild-type CXCR4 status was associated with better prognosis and response to ibrutinib in a prospective study [16] in 2015 but not in phase 3 clinical trials in 2017 and 2018 [5,17]. Different from MYD88, more than 40 CXCR4 mutations (nonsense or frameshift) have been described in WM/LPL patients with S338X mutations the most frequent, and CXCR4 mutations have lower variant allele frequencies (mean, 35.2%) [18], suggesting clonal evolution in WM pathogenesis. CXCR4 mutation impairs the rapid internalization of CXCR4 upon binding to its ligand CXCL12, and the extended signaling triggers AKT and ERK1/2 signaling pathway leading to drug resistance to multiple therapies including ibrutinib [19], [20], [21], [22]. Other gene mutations in WM were not well studied likely due to their rarity. TP53 genetic alterations, including mutation, deletion, and copy-neutral loss of heterozygosity, were also reported in WM with low frequency [23], [24], [25]. TP53 mutations and deletion predict an unfavorable prognosis despite the low frequency in WM [23], [24], [25].
Recurrent chromosomal changes in WM are not well studied owing to technical difficulties. The most common cytogenetic abnormality is 6q deletion (harboring the PRDM1/BLIMP1 gene), which is present in 7% to 54% of patients with WM (cytogenetic analysis produced lower frequencies than fluorescence in situ hybridization [FISH]) and was suggested as a prognostic indicator, which remains controversial [26], [27], [28]. However, 6q deletion is not specific for WM and less common in extramedullary LPL [26,29]. Other aberrancies identified in WM by cytogenetic analysis or FISH include trisomy 4, 12, and 18 and deletions in 13q14 and 17p [23,30]. The optimal therapies for WM/LPL patients with cytogenetic alterations or unfavorable genetic mutations have not been established [31].
Molecular testing and implementation of next-generation sequencing (NGS) in clinic provide opportunities to gain knowledge and further improve the WM/LPL management based on genetic alterations. Here we reported the prognostic impact of frequent mutations, cytogenetic abnormalities, and family history and clinical outcome of various therapeutic agents and regimens in a large cohort of WM/LPL patients including 76 cases analyzed by NGS panels as a routine clinical workup.
Methods
Patients
We collected 219 patients with WM (with IgM) and 12 patients with LPL (5 patients with IgA and 7 patients with IgG) seen at The University of Texas MD Anderson Cancer Center, Baylor College of Medicine, and Duke University Medical Center in 2014–2019. The study was approved by the institutional review board of the participating institutions. The diagnosis was according to consensus guidelines outlined at the second International Workshop for WM [1] and has been confirmed based on histopathologic review by authors (Y.W., H.C.L., K.H.Y.). Patients with other low-grade lymphomas (marginal zone lymphoma, chronic lymphocytic leukemia [CLL], follicular lymphoma, hairy cell leukemia, or mantle cell lymphoma, n = 52) have been excluded.
The following clinicopathological parameters were collected and assessed: age, sex, hemoglobin, platelets, beta-2-microglobulin (B2M), quantitation of monoclonal IgM, IgG, and IgA, kappa/lambda light chain, percentage of bone marrow involvement, extramedullary involvement, lactate dehydrogenase (LDH), B-symptoms, Eastern Cooperative Oncology Group (ECOG) performance status score, WBC counts, lymphocyte percentage, CD5, CD10, Amyloid, family history, transformation to DLBCL, treatment regimens, and treatment response based the criteria from the sixth International Workshop for WM [32].
Molecular and genetic analyses
Routine clinical workup of targeted NGS was performed for 76 patients with several targeted gene somatic mutation analysis panels by the CLIA-certified molecular diagnostic laboratory in UT MD Anderson Cancer Center [33], [34], [35] in 2014–2019 (all except 4 cases were sequenced between June 2016 and March 2019), as well as sequencing of MYD88L265P with allele-specific polymerase chain reaction (AS-PCR) for 127 patients, CXCR4 mutations (codons 291-353) for 64 patients, and TP53 mutations (exons 4-9, codons 33-331) for 16 patients. For targeted NGS, genomic DNA extracted from the bone marrow aspirate was used for preparing sequencing libraries with molecular barcodes using the Agilent HaloPlex Target Enrichment System (Agilent Technologies), followed by bidirectional paired-end sequencing using the Miseq sequencer (Illumina Inc.). Illumina Experiment Manager, MiSeq Control Software, Real Time Analysis, Sequence Analysis Viewer, MiSeq Reporter, and Agilent SureCall were utilized for experimental setup and NGS data analysis. Although the NGS assay is capable of achieving sensitivity of 1%, the effective lower limit of detection of the assays used for clinical workup was determined to be 5% to 10% taking into consideration the depth of coverage and the ability to confirm low-level mutations using independent conventional platforms. With >250x depth of coverage, targeted mutation analysis included MYD88 exons 3-5 (codons 168-310; in 5 patients codons 10-50 and 41-184 were also covered), CXCR4 exon 2 (codons 6-353), and TP53 exons 2 (codons 1-25) and 4-11 (codons 33-394, a few codons were not covered).
The gene lists for the two clinical NGS panels that include MYD88 are shown below, which were selected based on the frequencies of mutations in WM and other types of indolent B-cell non-Hodgkin lymphoma [33]. In addition, a 50-gene panel, a 52-gene panel, and an 81-gene panel [34, 35] that include the TP53 gene and other genes were used for three cases, respectively.
Twenty-nine-gene panel (68 patients): ATM, BIRC3, BTK, CALR, CARD11, CD79A, CD79B, CHD2, CSMD3, CXCR4, DDX3X, EZH2, FAT1, FBXW7, KLHL6, LRP1B, MAPK1, MUC2, MYD88, NOTCH1, PLCG2, PLEKHG5, POT1, SF3B1, SPEN, TGM7, TP53, XPO1, and ZMYM3.
Twenty-eight-gene panel (5 patients): ABL1, ASXL1, BRAF, DNMT3A, EGFR, EZH2, FLT3, GATA1, GATA2, HRAS, IDH1, IDH2, KIT, KRAS, MDM2, IKZF2, JAK2, MLL, MPL, MYD88, NOTCH1, NPM1, NRAS, PTPN11, RUNX1, TET2, TP53, and WT1.
Cytogenetic analysis
Conventional cytogenetic analysis was performed as part of the clinical workup for bone marrow aspirate with standard methods in the clinical laboratories. At least 20 metaphase spreads were analyzed to identify chromosomal abnormalities according to the 2016 International System for Human Cytogenetic Nomenclature. A Complex karyotype is defined by presence of at least three chromosomal aberrations in at least two cells. TP53 deletion was identified by FISH.
Statistical analysis
Overall survival (OS) and progression-free survival (PFS) were calculated from date of diagnosis to date of death from any cause, the first disease progression, or last follow-up available in 230 patients. To evaluate the therapeutic effects of various regimens, OS1 and PFS1 were calculated from the date of primary treatment to OS/PFS events or last follow-up in treated symptomatic patients; time to treatment (TTT) was measured from the date of diagnosis to the starting date of primary treatment. TTT data were not available in 11 patients, and post-treatment PFS1/OS1 data were not available in 2 patients. For relapsed/refractory patients treated with ibrutinib, PFS for ibrutinib treatment was calculated from the date of ibrutinib therapy to disease progression/relapse.
Comparisons of features between 2 groups were performed with Fisher's exact test. Survival curves were generated with the Kaplan-Meier method and the log-rank test using GraphPad Prism 7.0. Multivariate survival analyses were performed by fitting Cox proportional hazards regression models. P values ≤0.05 were considered statistically significant.
Results
Clinicopathologic characteristics of the study cohort
The clinical features of the study cohort are summarized in Table 1. With a median follow-up duration of 48.6 months from the diagnosis (range, 1 month to 25.5 years) in 230 patients (one patient had no follow-up duration available), the estimated median PFS and OS duration was 5.8 years and 22.6 years, respectively. There was no difference in survival between WM (219 patients) and LPL (12 patients) cases. Sixty-eight patients were under observation (watch-and-wait) and not treated at the last follow-up, and 163 patients received 1 to 6 (median, 2) lines of therapies for symptomatic disease. Among patients who received watch-and-wait only, 5 patients died at 1 to 10 month, and the remaining 93% patients remained asymptomatic or progression-free with a median follow-up of 13.0 months (range, 1 month to 11.5 years). Among treated patients, 23 (14%) had died, and 73 (45%) patients had relapse/progression data with a median follow-up of 43.1 months (range, 3.2 months to 25.5 years). The International Prognostic Scoring System for WM (IPSSWM) scores [36] were determined for 110 treated patients according to age, hemoglobin, platelets, B2M, and serum IgM levels before treatment, including 30 low-risk patients (27.3%), 46 (41.8%) intermediate-risk patients, and 34 (30.9%) high-risk patients. The IPSSWM scores and four IPSSWM components (age, hemoglobin, platelets, B2M), as well as the following clinical factors, LDH, extramedullary involvement, B symptoms, ECOG, WBC counts, lymphocyte percentage, and Amyloid, were associated with significant prognostic effects (data not shown).
Table 1.
Clinicopathologic characteristics of patients with Waldernstrom macroglobulinemia or lymphoplasmacytic lymphoma in the study cohort.
| Characteristic | n | % | |
|---|---|---|---|
| Sex | Male | 145 | 62.8 |
| Female | 86 | 37.2 | |
| Age (median, 66 years; range, 30-91 years) | ≤ 65 years | 114 | 49.4 |
| > 65 years | 117 | 50.6 | |
| B-symptoms | No | 185 | 80.1 |
| Yes | 46 | 19.9 | |
| Serum LDH level | Normal | 193 | 86.5 |
| Elevated | 30 | 13.5 | |
| ECOG performance status | 0-1 | 203 | 87.9 |
| ≥ 2 | 28 | 12.1 | |
| Hemoglobin levela | ≤ 11.5 g/dL | 85 | 73.9 |
| > 11.5 g/dL | 30 | 26.1 | |
| Plateleta | ≤ 100,000/mcL | 22 | 19.3 |
| > 100,000/mcL | 92 | 80.7 | |
| β-2 microglobulina | ≤ 3 mg/L | 50 | 44.6 |
| > 3 mg/L | 62 | 55.4 | |
| Monoclonal IgM levela | ≤ 7 g/dL | 115 | 99.1 |
| > 7 g/dL | 1 | 0.9 | |
| IPSSWM risk groupa | Low (0-1) | 30 | 27.3 |
| Intermediate (2) | 46 | 41.8 | |
| High (3-4) | 34 | 30.9 | |
| Immunoglobulin* | IgM | 200 | 94.3 |
| IgA | 5 | 2.4 | |
| IgG | 7 | 3.3 | |
| Extramedullary involvement | No | 150 | 67.0 |
| Yes | 74 | 33.0 | |
| WBC counts | ≤ 8 K/µL | 154 | 73.0 |
| > 8 K/µL | 57 | 27.0 | |
| Lymphocytes | < 70% | 178 | 91.8 |
| ≥ 70% | 16 | 8.2 | |
| Amyloid stains | Negative | 44 | 84.6 |
| Positive | 8 | 15.4 | |
| CD5+ | Negative | 214 | 92.6 |
| Positive | 17 | 7.4 | |
| First-degree family history of blood cancer | No | 192 | 83.5 |
| Yes | 38 | 16.5 | |
| Transformation to DLBCL | No | 220 | 95.2 |
| Yes | 11 | 4.8 |
CR = complete response; ECOG = Eastern Cooperative Oncology Group; IPSS = International Prognostic Scoring System for Waldernstrom Macroglobulinemia; LDH = lactate dehydrogenase; MR = minimal response; PD = progression; PR = partial response; SD = stable disease; VGPR = very good partial response.
Note: Measurements at the first treatment; the cutoffs are according to the IPSSWM. Other clinicopathological features (sex, age, B-symptom, LDH, ECOG, WBC counts and lymphocytes) are data at the diagnosis and cutoffs were determined by prognostic analysis. IPSSWM were calculated for cases with available data (data were not always available for patients included in this retrospective study).
Mutational analysis in the training set and the validation set
Genetic testing with AS-PCR or NGS in clinic provides opportunity to gain more insight into the prognostic value of frequent mutations in WM. Considering the sensitivity difference between NGS and AS-PCR and that patients were not uniformly sequenced by AS-PCR or NGS in clinic, to avoid potential prognostic effects stemmed from these compounding factors, first we performed the mutation analysis only in the 68 cases uniformly assessed by a same 29-gene NGS panel (as a training set), and then analyzed in other cases assessed by AS-PCR and/or 4 different NGS panels (as an independent validation set). There were no significance differences in clinical features between the two subcohorts (Supplementary Table S1).
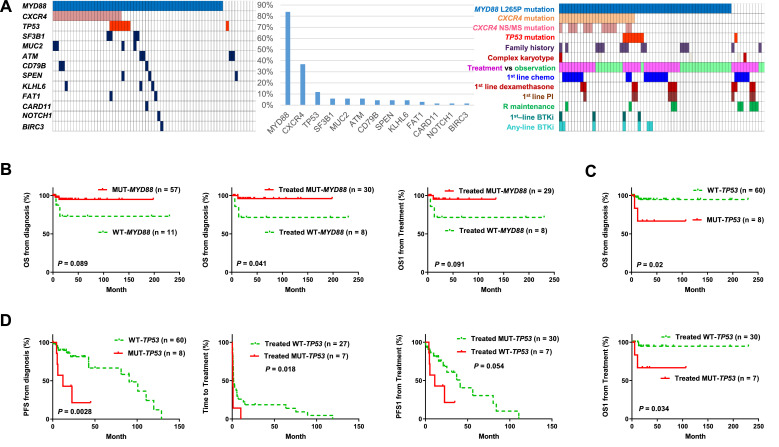
Fig. 1A shows the mutation frequency, case distribution of mutations, treatment vs observation, and major treatment factors for the training set. MYD88 was the most frequently mutated gene with a single missense variant p.L265P occurring in 83.8% of patients. Only one patient had a splice-altering mutation concurrent with the L265P mutation and another patient had a concurrent R230C mutation with <10% variant allele frequency. CXCR4 was the second most frequently mutated gene (frequency, 36.8%); the most prevalent variant was S338X (nonsense), followed by S341fs (frameshift) and R334X (nonsense) mutation in 17, 7, and 4 patients, respectively. The third frequently mutated gene was TP53 occurring in 11.8% patients. TP53 variants were heterogeneous although they were all localized in the DNA-binding domain (exons 5-8) (Table 2).
Fig. 1.
Mutational analysis in 68 patients with WM/LPL who were analyzed by NGS with a same 29-gene somatic mutation analysis panel (training set). (A) Case distribution of somatic mutations detected by the NGS panel, mutation frequencies, and case distribution of genetic and treatment factors in the training set. In the case distribution plots, each cell/box represents one patient. (B)MYD88 mutation (L265P) was associated with a trend of better OS in overall cases, a significantly better OS and a trend of better OS1 in treated symptomatic patients. (C-D)TP53 mutation was associated with significantly worse OS and PFS rates in overall patients and significantly shorter time-to-treatment and post-treatment OS1/PFS1 in treated patients. LPL, lymphoplasmacytic lymphoma; NGS, next-generation sequencing; WM, Waldenström macroglobulinemia.
Table 2.
Genetic alteration findings in the Waldenström macroglobulinemia/lymphoplasmacytic lymphoma study cohort.
| Genetic alteration | |
|---|---|
| MYD88 mutation | Missense mutations: R230C, L265P (n = 178) |
| CXCR4 mutation | Nonsense mutations: G332X, R334Xa (n = 4), G336Xa, S338Xa (n = 17), E343X; missense mutations: V114I, G335S; frameshift mutations: R322fsa, L326fsa, G332fs, R334fs, S338fs, V340fs, S341fs (n=7), T318fs |
| TP53 mutation | Nonsense mutation: W53X; missense mutations: Y205C, Y220C, Y236C, G245D, R282W (n = 2), T284P, M237I, D259Y; frameshift mutations: S99fs, G108fs |
|
Complex karyotype in cytogenetic analysis |
45,X,-Y,del(1)(p35),add(3)(q27),add(22)(p11.2)[8]/44,XY,idem,del(8)(q12q22),add (11)(q23),inv(11)(q21;q23),-15,add(17)(p11.2),-20,+mar[12]; 46,XX,inv(1)(p36.1q21),del(6)(q12),add(7)(q32)[7]/46,XX[10]; 46,XX,der(3)add(3)(p21)t(3;13)(q12;q12),del(7)(q32q34),del(13)(q12)[4]/46,XX[16]; 40-45,XY,-9,-12,-13,add(17)(p11.2),-20,+1-2mar[cp5]; 45-49,XX,+3,del(6)(q13q23),+1-2mar[cp7]/46,XX[13]; 48,XY,add(X)(p22),+4,t(7;9)(q11.2;p13),add(9)(p24),+18[5]/46,XY[15]; 46,X,-Y,+4,del(6)(q21q25)[2]/46,XY[20]; 45,XX,del(7)(q11.2),-15[1]/45,XX,del(7)(q11.2),der(11)t(11;12)(p15;q13),-12[2]/46,XX[17] |
Note: Not all complex karyotype data were available.
Previously reported CXCR4 mutations.
Prognostic analysis found that MYD88 mutation was associated with favorable OS with a marginal P value in 68 cases overall and significantly in 38 patients who received treatment (Fig. 1B); TP53 mutation was associated with significantly poorer OS/PFS in overall patients and shorter TTT/OS1/PFS1 in treated patients (Fig. 1C-D).
In the validation set (cases sequenced by either AS-PCR or a non-29-gene NGS panel), MYD88 (only L265P), CXCR4, and TP53 mutations were detected in 90.9% of 132 patients, 25.4% of 63 patients, and 22% of 18 patients sequenced, respectively (Fig. 2A). MYD88 mutation was associated with a significantly better OS and a trend of better PFS in overall 132 patients, a trend of favorable OS1 in treated 107 patients (which validated the results in the training set), and significantly longer TTT (Fig. 2B). Also validated is the significantly shorter TTT associated with TP53 mutation (Fig. 2C). Uniquely in the validation cohort, CXCR4 nonsense/missense (NS/MS) mutations were associated with significantly shorter TTT and post-treatment OS1 in treated patients (Fig. 2D).
Fig. 2.
Mutational analysis in patients sequenced by AS-PCR or a NGS panel different from the 29-gene somatic mutation analysis panel (validation set). (A) Mutation frequency of somatic mutations detected and case distribution of genetic and treatment factors in sequenced patients. In the case distribution plot, each cell/box represents one patient; cases with specific mutations and treatment are highlighted in corresponding colors; cases not assessed for mutations and other factors are filled with olive green color and diagonal stripes. (B)MYD88 mutation (L265P) was associated with a significantly better OS and a trend of better PFS in overall patients and significantly longer time-to-treatment and a nonsignificant trend of better post-treatment OS1 in treated patients. (C)TP53 mutation was associated with shorter time-to-treatment in treated patients with a border-line P value. (D)CXCR4 mutation (nonsense or missense) was associated with significantly shorter time-to-treatment and post-treatment OS1 in treated patients. (E) Complex karyotype was associated with significantly shorter time-to-treatment in treated patients in the validation subcohort.
We examined the clinicobiological features of CXCR4NS/MS patients which could be relevant for its different prognostic effects in the training and validation sets. Only in the validation set, CXCR4NS/MS mutations were associated with higher frequencies of complex karyotypes and platelet count ≤100k/mcL. In addition, CXCR4NS/MS patients in the validation set more frequently received a dexamethasone-containing first-line regimen (61.5%) than CXCR4NS/MS patients in the training set (7.7%) and CXCR4WT/FS patients in both cohorts (30% in the validation set and 14.3% in the training set)
Combining 2 independent subcohorts, MYD88 mutation was associated with significantly longer OS (P = 0.0098), PFS (P = 0.04), TTT (P = 0.0021), and OS1 (P = 0.021); TP53 mutations were associated with significantly shorter PFS (P = 0.046), TTT (P = 0.016), and treatment/symptomatic disease (all except one [91.7%] patients with TP53 mutation were treated, compared with the 54.7% in patients with wild-type TP53); and CXCR4NS/MS mutations were associated with significantly shorter TTT (P = 0.05). Only CXCR4NS/MS mutations were associated with IPSSWM risk groups: 93.3% of CXCR4NS/MS patients compared with 68.4% of CXCR4WT/FS patients had intermediate/high-risk IPSSWM scores (P = 0.051).
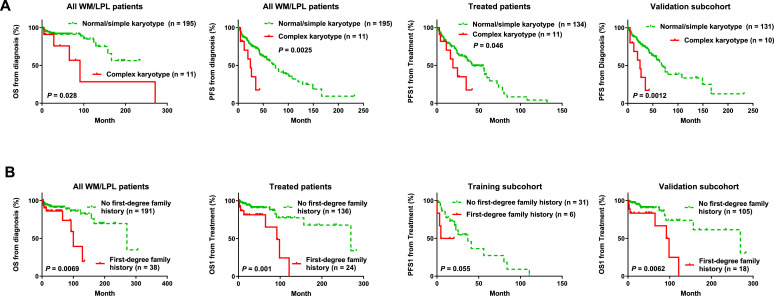
Conventional cytogenetic analysis and having a family history of blood cancer show significant prognostic impact
Conventional cytogenetic analysis was done in 207 patients: 11 patients (1 in the training subchort and 10 in the validation subcohort) had complex karyotypes (Table 2), 15 patients had a simple karyotype with one (12 patients) to two (3 patients) chromosomal abnormalities, and 181 patients had normal karyotype. Complex karyotypes were associated with significantly shorter OS, PFS, TTT and PFS1 in the entire cohort and the validation subcohort (Fig. 2E, Fig. 3A, and figures not shown). TP53 deletion was detected by FISH in 11 (all in the validation subcohort) of 115 patients (10%). No significant prognostic differences were observed between patients with and without TP53 deletion.
Fig. 3.
Survival analysis for cytogenetic karyotypes and family history in patients with WM/LPL. (A) Complex karyotype was associated with significantly poorer OS/PFS in the entire study cohort and significantly poorer post-treatment PFS1 in treated patients in the entire cohort and the validation subcohort. (B) WM/LPL patients whose first-degree relatives had lymphoma or leukemia incidence had significantly shorter OS and post-treatment OS1 than those without such family history in the entire study cohort, a poorer post-treatment PFS1 in the training subcohort, and a poorer post-treatment OS1 in the validation subchort.
Family history was also analyzed as a potential genetic factor. In the entire cohort, 38 patients (16.5%) had a record of family history of blood cancer in first-degree relatives. Presence of a family blood cancer history was associated with significantly shorter OS/OS1 in the entire cohort and the validation subcohort, and shorter PFS1 in the training subcohort (Fig. 3B).
BDR and dexamethasone usage in frontline treatment are associated with significantly worse survival
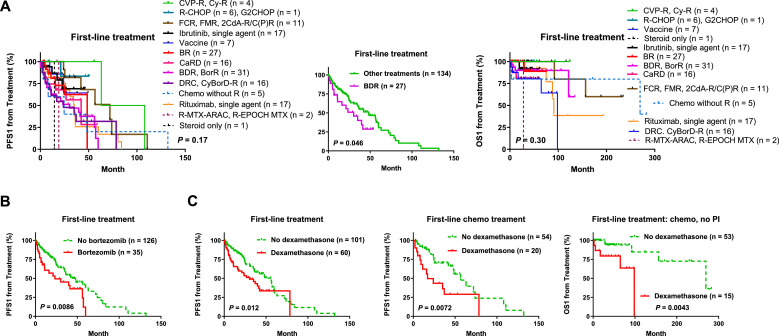
Consistent with the indolent but incurable nature of WM/LPL and lack of standard treatment in clinic [2], the regimens in this study cohort were highly variable (Fig. 4A), including (1), chemotherapy alone or with rituximab: BR, DRC (dexamethasone, rituximab, and cyclophosphamide), FCR (fludarabine, cyclophosphamide, and rituximab), 2-CdA-CR (cladribine, cyclophosphamide, and rituximab), R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone), R-CVP (rituximab, cyclophosphamide, vincristine, and prednisone), CPR (cyclophosphamide, prednisone, and rituximab), CyBorD (cyclophosphamide, bortezomib, and dexamethasone), and other less used chemotherapies; (2), proteasome inhibitor (PI)-based chemo-free therapies including BDR, BorR (bortezomib and rituximab), BorD, and CaRD (carfilzomib, rituximab, and dexamethasone); (3), single-agent rituximab/Rituxan® or other anti-CD20 antibodies including obinutuzumab, ublituximab, and ofatumumab (in only 4 cases); (4), single-agent ibrutinib or other BTKi (acalabrutinib in 5 cases); (5) immunomodulatory therapies with vaccines (as primary treatment in 7 cases), lenalidomide/Revlimid® (in first-line, maintenance, or salvage therapies in 5 cases), pomalidomide or thalidomide (in only 2 relapsed/refractory patients); (6) radiotherapy, stem cell transplantation; (7) combination regimens with targeted, chemo, and/or immunomodulatory agents. There were also cases changed regimens during treatment due to side effects. Primary/frontline regimens used in >10 patients (out of total 163 treated patients) included BDR, BR, rituximab alone, ibrutinib alone, CaRD, and DRC.
Fig. 4.
Therapeutic efficacy analysis for the diverse frontline regimens in treated patients with WM/LPL. (A) Comparison of post-treatment PFS1 and OS1 of patients receiving various frontline regimens. Frontline BDR (bortezomib, dexamethasone, and rituximab) was associated with a significantly poorer PFS1. (B) Bortezomib inclusion in frontline treatment analyzed as a prognostic factor was associated with a significantly poorer PFS1 in WM/LPL. (C) Dexamethasone usage in frontline treatment was associated with significantly worse post-treatment PFS1 rates in overall cohort and the subcohort with chemotherapy as frontline treament, and associated with a significantly worse OS1 in patients who received chemotherapy without proteasome inhibitor (PI) combination in frontline treatment.
Treatment response to primary treatment included complete response (CR, n = 20), very good partial response (VGPR, n = 18), partial response (PR, n = 66), minimal response (MR, n = 24), stable disease (n = 17), and progressive disease (n = 13) (response data were not available for 5 cases). No association between treatment response and survival outcome was observed.
To gain insight into the efficacy of different regimens, we first directly compared different frontline regimens in term of PFS1 and OS1 (Fig. 4A) in all symptomatic patients who received treatment. The pre-treatment TTT from diagnosis, which is not indicative of therapy efficacy, was also examined and compared between therapies (Supplementary Fig. S1A), and found patients treated in vaccine clinical trials had significantly longer TTT than other treated patients (P = 0.039, Supplementary Fig. S1B), which could be related to either the high frequency of low-risk IPSSWM scores in these patients (83% including 50% patients scored 0) or the vaccine production time. Frontline BDR regimen was associated with significantly shorter PFS1 (Fig. 4A); there was no difference in IPSSWM risk groups, age, or TTT between BDR-treated and other treated patients. No other frontline regimens showed significant associations with PFS1/OS1 effect by univariate survival analysis or multivariate analysis adjusting for clinical features.
Next, because most regimens were used in small numbers of cases and did not show significant advantage over other regimens regarding clinical outcome, we attempted to dissect the prognostic effect of single therapeutic agents composing the diverse regimens used in clinic, by comparing the survival of patients who received a specific agent in frontline treatment (regardless included in what combination regimens) with other treated patients (who did not receive that agent in frontline treatment). The following agents were dissected from frontline regimens and analyzed as a prognostic factor for OS1/PFS1 by univariate and multivariate survival analysis: anti-CD20 monoclonal antibodies (mainly rituximab), PI (bortezomib, carfilzomib), BTKi, chemotherapy, alkylators (bendamustine, cyclophosphamide, chlorambucil), purine nucleoside analogues (fludarabine, cladribine), corticosteroids (dexamethasone, prednisone), vaccines, immunomodulatory agents, radiotherapy, and stem cell transplantation.
Using this method, we found stratifying patients based on first-line usage of bortezomib (Fig. 4B, used in BDR, BorR, or CyBorD mostly) or dexamethasone (Fig. 4C, used in BDR, CaRD, DRC, CyBorD, or chemotherapies) in treatment showed significantly adverse effect on PFS1. Adjusting for clinical features using Cox regression models, bortezomib was a significant factor for poorer PFS1 (P = 0.032), and dexamethasone was a significant factor for poorer OS1 (P = 0.017) and PFS1 (P = 0.033). Fludarabine included in first-line treatment (only in six patients however) was associated with significantly better PFS1 in univariate analysis (P = 0.039, Supplementary Fig. S1C) but not in multivariate analysis with the adjustment of clinical parameters (P = 0.97). No other frontline agents showed significant effects on therapeutic outcome, although in the sub-cohort of patients who received rituximab-containing frontline therapies, combined cases receiving any type of chemotherapy as first-line treatment had a higher frequency of clinical responses (CR, VGPR, PR, or MR, 95.7% vs 69.8%) and a significantly better PFS1 (P = 0.031, Supplementary Fig. S1D).
To eliminate potential compounding effects arising from different regimens, we analyzed the effect of frontline bortezomib and dexamethasone in subcohorts treated with similar regimens, including camparisons in patients treated with BorR versus single-agent rituximab, patients treated with BDR (with dexamethasone) versus BorR (without dexamethasone), patients treated with DRC (with dexamethasone) versus RC/CP/CP-R/R-CVP (without dexamethasone), patients treated with chemotherapy, patients treated with chemo-free therapies, patients who received bortezomib in first-line treatment and those who did not (for dexamethasone analysis only). Significant adverse prognostic effect was shown in the comparison of DRC versus CP/CP-R/R-CVP, but not in the comparisons of BDR versus BorR, nor BorR versus R (Supplementary Fig. S2A). However, dexamethasone was associated with significantly worse survival in patients without bortezomib or a PI in first-line treatment (Supplementary Fig. S2B). In patients receiving chemotherapy as first-line treatment, first-line dexamethasone usage was associated with significantly shorter PFS1 (Fig. 4C) and OS1 (Supplementary Fig. S2C) despite the similar TTT (P = 0.77). The adverse effects of dexamethasone remained significant after the exclusion of patients who received a PI in first-line treatment (Fig. 4C, Supplementary Fig. S2B). In treated patients who never received chemotherapy (these patients received BRD, CaRD, rituximab/ibrutinib as single-agent or in combination, BorR, or vaccines as first-line treatment), frontline dexamethasone was also associated with shorter OS1 (P = 0.017) and TTT (border-line P =0.056) (Supplementary Fig. S2C). However, in patients who did not receive chemotherapy in first-line (received BRD, CaRD, BorR, single-agent rituximab, ibrutinib, or only steroid as first-line treatment) but received chemotherapy in later-line treatment, the adverse impact of first-line dexamethasone was not observed (P = 0.84 for PFS1, P = 0.35 for OS1). These later-line-chemo patients had significantly longer TTT thanfirst-line-chemo patients and chemo-free patients (Supplementary Fig. S2D); the non-dexamethasone group of later-line-chemo patients had a higher frequency of TP53 deletion (non-significant however. In patients evaluated by FISH, 4 of 6 non-dexamethasone patients versus 0 of 3 dexamethasone patients had TP53 deletion; P = 0.17); and TP53 deletion was associated with a significantly poorer OS1 in these later-line-chemo patients (Supplementary Fig. S2D). Together, these results suggested that frontline dexamethasone indicated unfavorable prognosis in patients who received non-PI-containing chemotherapy as first-line treatment.
Benefit of rituximab maintenance therapy and ibrutinib in relapsed/refractory patients
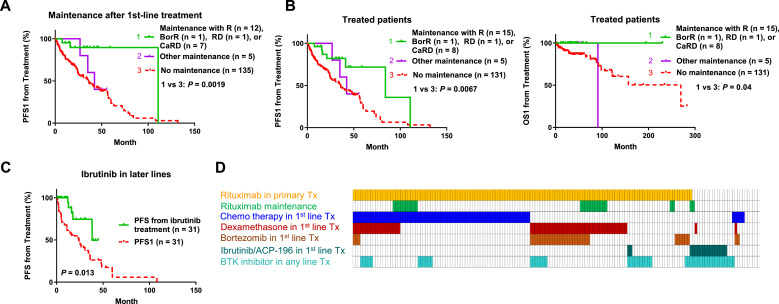
Maintenance regimen after frontline therapy was given to 21 patients after achieving a clinical response with rituximab alone or in combination and to 5 patients with other types of regimens. Only rituximab-containing maintenance was associated with significant better PFS1 (Fig. 5A). Maintenance with single-agent rituximab or CaRD therapy was also given to 4 patients after a non-first-line therapy. Adding these 4 patients, the maintenance-rituximab group showed significantly better PFS1 and OS1 (Fig. 5B) and similar TTT compared with patients without maintenance-rituximab. We further excluded patients with stable/progressive disease after first-line treatment from the non-maintenance group, and still found that the maintenance-rituximab group had significantly better PFS1 (P = 0.0061 for PFS1 and P = 0.060 for superior OS1; Supplementary Fig. S3A).
Fig. 5.
Therapeutic analysis in treated patients with WM/LPL. (A) Maintenance therapy with either rituximab (R) alone or R-containing regimens after first-line treatment was associated with a significantly better post-treatment PFS1. (B) Rituximab-based maintenance therapy after any-line treatment, but not other type of maintenance therapies, was associated with significantly better PFS1 and OS1. (C) Ibrutinib-based treatment in the relapsed/refractory setting was associated with a significantly better PFS after ibrutinib treatment compared with the PFS after first-line treatment. (D) Case distribution plot for various regimens in treated symptomatic patients.
In the relapsed/refractory setting, ibrutinib treatment showed potential benefit: ibrutinib was given to 31 relapsed/refractory patients, and their PFS after the ibrutinib treatment was significantly longer than their PFS1 (Fig. 5C).
Case distribution for treatment factors is shown in in Fig. 5D. In multivariate analysis, a Cox model incorporating various treatment factors and clinical parameters was used, which showed that dexamethasone (but not bortezomib) usage in frontline treatment was an independent prognostic factor for poorer OS1 (hazard ratio, 5.51, 95% confidence interval, 1.5-17.74, P = 0.009) and maintenance rituximab was an independent prognostic factor for better PFS1 (hazard ratio, 0.14, 95% confidence interval, 0.033–0.6, P = 0.008, Table 3).
Table 3.
Multivariate survival analysis for regimens and genetic factors in the study cohort.
| OS1 From Treatment |
PFS1 From Treatment |
||||||
|---|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | P | HR | 95% CI | P | |
| Regression model for Tx factors in treated patients | |||||||
| Age >65 years | 3.97 | 1.32–11.94 | 0.014 | 1.56 | 0.82–2.96 | 0.18 | |
| Hemoglobin >11.5 g/dL | 0.26 | 0.031–2.18 | 0.21 | 0.63 | 0.26–1.57 | 0.32 | |
| Platelets >100 × 109/L | 1.17 | 0.28–4.81 | 0.72 | 0.77 | 0.36–1.68 | 0.52 | |
| B2M >3mg/L | 6.09 | 1.28–28.9 | 0.023 | 1.43 | 0.74–2.78 | 0.29 | |
| Dexamethasone in frontline Tx | 5.51 | 1.50–17.74 | 0.009 | 1.61 | 0.77–3.36 | 0.21 | |
| Chemotherapy in frontline Tx | 1.61 | 0.50–5.23 | 0.43 | 0.59 | 0.29–1.20 | 0.14 | |
| Bortezomib in frontline Tx | 0.79 | 0.18–3.45 | 0.76 | 1.14 | 0.46–2.83 | 0.78 | |
| Rituximab maintenance | <0.001 | - | 0.97 | 0. 14 | 0.033–0.60 | 0.008 | |
| Regression model for genetic and Tx factors in treated patients | |||||||
| MYD88 mutation | 0.54 | 0.026–11.3 | 0.69 | 0.009 | <0.001–0.25 | 0.006 | |
| CXCR4 non-FS mutation | 4.26 | 0.52–35.1 | 0.18 | 1.39 | 0.21–9.08 | 0.73 | |
| TP53 mutation | 6.31 | 0.81–49.4 | 0.079 | 5.31 | 1.12–25.2 | 0.035 | |
| Family history | 1.03 | 0.10–10.4 | 0.98 | 3.67 | 1.15–11.7 | 0.028 | |
| Complex karyotype | 4.34 | 0.57–33.1 | 0.16 | 2.25 | 0.22–23.2 | 0.50 | |
| Dexamethasone in frontline Tx | 9.10 | 0.60–138.2 | 0.11 | 0.18 | 0.024–1.33 | 0.092 | |
| Rituximab maintenance | <0.001 | - | 0.98 | 0.005 | <0.001–0.12 | 0.001 | |
| BTKi in any line Tx | 0.20 | 0.009–4.29 | 0.30 | 1.37 | 0.14–13.87 | 0.79 | |
|
OS From Diagnosis |
PFS From Diagnosis |
||||||
| Variable | HR | 95% CI | P | HR | 95% CI | P | |
| Regression model for genetic and Tx factors in all patients | |||||||
| MYD88 mutation | 0.073 | 0.004–1.48 | 0.088 | 0.13 | 0.029–0.56 | 0.007 | |
| CXCR4 non-FS mutation | 10.12 | 0.38–273.1 | 0.17 | 7.54 | 0.68–83.8 | 0.16 | |
| TP53 mutation | 10.4 | 1.19–88.3 | 0.035 | 2.41 | 0.73–7.91 | 0.15 | |
| Family history | 2.91 | 0.35–24.1 | 0.32 | 4.73 | 1.10–20.44 | 0.037 | |
| Complex karyotype | 60.45 | 0.50–790.7 | 0.094 | 0.72 | 0.054–9.58 | 0.80 | |
| Chemotherapy in frontline Tx | 0.18 | 0.006–5.04 | 0.31 | 0.59 | 0.29–1.20 | 0.14 | |
| Dexamethasone in frontline Tx | 1.90 | 0.27–13.40 | 0.52 | 2.60 | 0.92–7.35 | 0.071 | |
| Rituximab maintenance | <0.001 | - | 0.98 | 0.20 | 0.041–0.98 | 0.048 | |
| BTKi in any line Tx | 0.084 | 0.003–2.71 | 0.16 | 1.41 | 0.38–5.33 | 0.60 | |
BTKi = Bruton's tyrosine kinase inhibitor; CI = confidence interval; FS mutation = frameshift mutation; HR = hazard ratio; NS/MS = nonsense or missense mutation; OS = overall survival; PFS = progression-free survival; PI = proteasome inhibitor; Tx = treatment.
Note: Boldface indicates statistically significance of P values.
Treatments for patients with unfavorable genetic factors
We examined the efficacy of therapies in patients with adverse genetic factors, including WT-MYD88, MUT-TP53, MUT-CXCR4 (NS/MS), complex karyotype, and family history. Maintenance rituximab was associated with significantly better PFS1 in MUT-TP53 patients (Fig. 6A) and strong trends of better PFS1 and OS1 in WT-MYD88 patients (Supplementary Fig. S3B). The benefit of maintenance rituximab was not particular for these patients, and also showed significantly better PFS1 in patients with MUT-MYD88 and WT-TP53 patients (Supplementary Fig. S3C).
Fig. 6.
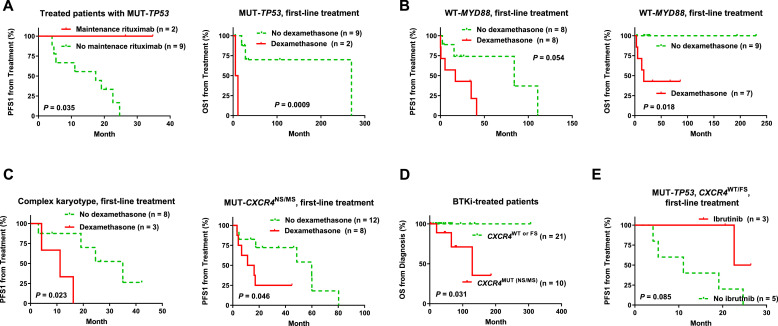
Therapeutic analysis in WM/LPL patients with unfavorable genetic factors. (A-B) Frontline dexamethasone was associated with significantly poorer post-treatment PFS1/OS1 in patients with TP53 mutation or with wild-type MYD88. (C) Frontline dexamethasone was associated with significantly poorer post-treatment PFS1 in patients with complex cytogenetic karyotype or CXCR4 mutation (nonsense or missense). (D) In patients treated with BTK inhibitors (regardless of line of the treatment), CXCR4 mutation (nonsense or missense) was associated with significantly worse OS. (E) In patients with TP53 mutation but not CXCR4 nonsense/missense mutation, single-agent ibrutinib as first-line treatment was associated with a trend of better PFS1 after treatment.
Frontline dexamethasone usage was associated with significantly shorter OS1 (and trends of shorter PFS1) in MUT-TP53 patients and WT-MYD88 patients (Fig. 6A-B, Supplementary Fig. S4A), and significantly shorter PFS1 but not OS1 in patients with complex karyotypes or CXCR4NS/MS mutations (Fig. 6C). The unfavorable prognostic effect of first-line dexamethasone was independent of these genetic factors (Supplementary Fig. S4A-D).
Ibrutinib or BTKi treatment did not show any benefit over other types of treatment for patients with MUT-CXCR4, and CXCR4NS/MS mutations were associated with significantly shorter OS (and a marginal shorter OS1) in patients receiving ibrutinib or any BTKi treatment (Fig. 6D). Conversely, only in patients without CXCR4NS/MS mutations, ibrutinib or BTKi treatment (any-line) was associated with trends of better OS1 and OS (P = 0.093 and 0.099, respectively; Supplementary Fig. S4E). In MUT-TP53 patients without CXCR4 NS/MS mutation, 3 patients received ibrutinib (all in the frontline setting), and ibrutinib treatment was associated with trends of better PFS and PFS1 (P = 0.085, Fig. 6E, Supplementary Fig. S4A).
As genetic factors and treatment interacted with each other in affecting clinical outcome, multivariate analysis was performed using Cox regression models incorporating prognostic genetic, treatment, and clinical factors in all patients and treated patients. Results showed that TP53 mutation and having a family history of blood cancer were independent unfavorable factors for OS or PFS/PFS1, whereas MYD88 mutation and maintenance rituximab were independent favorable factors for PFS/PFS1 (Table 3).
Discussion
WM represents a unique lymphoid malignancy with an almost unifying somatic point mutation in the MYD88 gene. Despite the molecular insights gained in the era of precision medicine, WM/LPL remains incurable and the regimens are highly diverse in clinic. Whether certain treatment is better than other treatments for symptomatic patients with adverse genetic factors is largely unknown. To gain new molecular and prognostic insights, in the current study we comprehensively evaluated the impact of genetic factors and various treatments on clinical outcomes by univariate and multivariate analysis in a large cohort of WM/LPL patients, as well as clinical utility of a 29-gene NGS panel, adding valuable data to this rare disease.
MYD88L265P is thought to distinguish WM/LPL patients with higher bone marrow disease involvement, serum IgM levels, and symptomatic disease [37] and serve as a predictive marker for patients receiving ibrutinib [14,16]. However, later studies did not show such association [5,17,38]; ibrutinib only partially inhibits Toll-like receptor signaling [39]; and ibrutinib resistance can be acquired either by CXCR4 mutation in a subset of MYD88L265P cases [19,22] or by upregulation of BCL-2 and AKT [40]. In our study cohort, the frequency of MYD88L265P mutation was 88.5%, higher than the 67%-79% [31,38,41], comparable with the 86% [42], and lower than the 93%-100% [43,44] reported by previous studies using AS-PCR and Sanger sequencing. MYD88L256P mutation was a favorable prognostic factor in treated patients in this study, but had no impact on ibrutinib efficacy, which could either related to the small case numbers or other factors significantly affecting the efficacy of ibrutinib. In patients with WT-MYD88, mutated genes included ATM, TP53 (consistent with a previous study in 18 patients with WT-MYD88 [45]), TET2, PTPN11, and SPEN.
Different from the single MYD88L265P mutation, CXCR4 mutations were diverse in this study, with a total frequency of 31.0%, comparable to the 29.1% detected by Sanger sequencing [37], 27% by WGS [11], 28% by targeted NGS [5], 24.5%-26.4% by targeted NGS and Sanger sequencing [18], 38% [44] and 43% [46] by AS-PCR and Sanger sequencing in previous studies. All CXCR4 mutated patients harbored MYD88L265P, in line with the notion that CXCR4 mutations were acquired after MYD88L265P in the disease course [18,46]. CXCR4NS/MS patients had significantly worse TTT and OS1 than CXCR4WT/FS patients in the validation cohort (but not the training cohort) and worse OS among all patients who received BTKi treatment. However, no significant survival difference was identified between CXCR4WT and CXCR4FS or overall CXCR4 mutations in our cohort, not supporting an earlier function study showing hyperactivation of AKT1 and MAPK1 in CXCR4FS cells [19] but is consistent with two previous studies [37,44]. CXCR4 nonsense mutations are gain-of-function mutations leading to higher responsiveness to CXCL12/SDF-1a [18], and were associated with complex karyotypes in our validation cohort, whereas all CXCR4FS patients had a normal/simple karyotype, which may be relevant for the prognostic impact of CXCR4 mutations. Complex karyotype was associated with significantly worse clinical outcome despite the small case numbers, consistent with our previous study in another independent WM cohort of 312 patients [47], suggesting cytogenetic analysis is necessary in routine screening for high-risk patients.
As another adverse genetic factor in this study, TP53 mutation was associated with symptomatic disease, and predicted significantly shorter TTT and PFS in overall studied cases. Nearly all TP53 mutations in our study were missense mutations located in the DNA-binding domain [48]. The prevalence of TP53 mutation in WM was 14.0% in our cohort, higher than the reported 7.3% by Sanger sequencing and ultradeep-targeted NGS [24], 7% by WGS [11], and 2.2% by a most recent study using targeted NGS in other cohorts [25], suggesting heterogeneity existed in WM/LPL.
Previous studies suggested LAPTM5c403t and HCLS1g496a mutations and chromosomes 1q and 4q were relevant for familial WM predisposition [7,49,50]. Familial WM was less responsive to rituximab-containing regimen but more sensitive to bortezomib-containing regimens compared with sporadic WM [51]. In our cohort, patients whose first-degree relatives had incidence of lymphoma/leukemia had poorer OS/OS1 regardless of whether they received bortezomib-containing regimens or not. However, this prognostic significance was lost in patients treated with frontline-ibrutinib or any-line BTKi.
Whether the efficacy of certain treatment differs in patients with particular genetic alterations is of interest to physicians. To gain insight into the efficacy of various treatments in WM, we first directly compared various frontline treatments in all symptomatic WM/LPL patients, and then “isolated” each therapeutic agent from the highly variable regimens and analyzed as a factor for prognostic associations. We found frontline dexamethasone usage is an adverse prognostic factor in patients who received a certain type of non-PI-containing chemotherapy as first-line treatment. However, we could not tell whether the observed prognostic effect was caused by dexamethasone-induced immunosuppression or specific WM symptoms that indicated dexamethasone usage and were not included in IPSSWM and our multivariate analysis. A recent functional study showed that administration of dexamethasone or prednisolone had negative impact on therapeutic outcomes and antitumor immunity [52]. However, oppositely an earlier preclinical study showed that dexamethasone potentiated ibrutinib's effects on antiproliferation, apoptosis, and DNA damage reduction in CLL cells in vitro [53]. In clinical studies, high-dose dexamethasone was associated with serious infection in relapsed/refractory CLL [54] and with CMV antigenemia in indolent B-cell lymphoma (including one LPL patient) and mantle cell lymphoma [55]. The adverse impact of dexamethasone was independently of MYD88/TP53/CXCR4 mutations and cytogenetic karyotype complexity and remained in subcohorts with a same genetic background in our study. However, the significance was lost in a multivariate analysis that included all genetic and treatment factors, which could be attributable to the small number of cases with all genetic/treatment data available.
Whether rituximab maintenance therapy is needed is controversial in WM [4]. Consistent with two previous studies [56,57], in our cohort receiving maintenance regimen with rituximab alone or in combination was associated with significantly prolonged survival in WM/LPL patients including those with prognostic unfavorable TP53 mutations. However, in a phase III clinical trial, 2-year rituximab maintenance after first-line treatment with BR in 109 WM patients did not show significant better OS/PFS than 109 patients without maintenance [58]. Compared with this study, our results are limited by retrospective analysis, small number of maintenance cases, maintenance with single-agent rituximab or rituximab-containing combinational therapies, and non-uniform first-line treatment (maintenance after BR, DRC, CaRD, BDR, FND, single-agent ibrutinib, or single-agent rituximab) although the efficacy of various regimens was often very similar in the study cohort. However, the inconsistency of data outside the clinical trials warrants further investigation on the role of maintenance rituximab in WM/LPL.
Ibrutinib used in the relapse/refractory setting prolonged PFS after ibrutinib than their PFS1, consistent with previous clinical trials [5,16,17]. When treatment efficacy was examined in a “controlled” genetic background, ibrutinib and BTKi usage (as primary or salvage therapy) was associated with trends of better OS/OS1 in patients without CXCR4NS/MS mutations and PFS/PFS1 in patients with TP53 mutation but without CXCR4NS/MS mutations, suggesting the direct involvement of CXCR4 signaling in BTKi mechanism of action [59] (Supplementary Figure S5). In a previous study in relapsed/refractory CLL treated with ibrutinib, complex karyotype but not del(17p) predicted poor clinical outcome [60]. Altogether, for WM/LPL patients with TP53 mutation, our findings suggest ibrutinib but not dexamethasone-containing regimen is optimal as frontline treatment. For patients with CXCR4NS/MS mutations, ibrutinib/BTKi may be not able to inhibit surface expression and prosurvival function of CXCR4 and the chemotaxis of tumor cells as shown in a CLL mouse model with wild-type CXCR4 [61]. Novel therapies targeting CXCR4, MYD88L265P signaling [19,62], AKT1, MAP2K1/MAPK1 [19], Nampt [63], HCK [20], deubiquitinating enzymes [64], or XPO1 (selinexor) [65] are worth investigation in WM/LPL.
In summary (Supplementary Figure S5), in a large cohort of WM/LPL patients, MYD88 mutation is a favorable prognostic genetic factor, whereas TP53 mutation and familial predisposition are unfavorable factors. The adverse prognostic effect of CXCR4NS/MS mutations in WM/LPL was not robust and the association of CXCR4NS/MS mutations with complex karyotypes and other factors may be involved in the adverse effect. Maintenance rituximab and ibrutinib/BTKi treatment but not upfront dexamethasone usage are favorable for WM/LPL patients including those with adverse genetic factors, however BTKi is not optimal for patients with CXCR4NS/MS mutations. These data add knowledge regarding how to use genetic factors to guide treatment options and disease monitoring in WM/LPL management.
Ethics approval and consent to participate
The study was approved by as being of minimal to no risk or as exempt by the institutional review board.
Consent for publication
Not applicable.
Availability of data and materials
The datasets generated and used in this study are available from the corresponding author after garnering institutional approval and enacting appropriate data sharing agreements.
Declaration of Competing Interest
Robert Z. Orlowski has received honoraria from or held membership on an entity's board of directors or advisory committees for Amgen, Janssen, Bristol-Myers Squibb, Kite Pharma, Celgene, Ionis Pharmaceuticals, Legend Biotech, Molecular Partners, Sanofi-Aventis, Servier, Takeda, and Pharmaceuticals North America; and received research funding from Amgen, BioTheryX, and Spectrum Pharmaceuticals. Hans Chulhee Lee declares consulting fees from Amgen, Celgene, Genentech, GlaxoKlineSmith, Janssen, Sanofi, and Takeda and research funding from Amgen, Celgene, Daiichi Sankyo, GlaxoKlineSmith, Janssen, Regeneron, and Takeda. All other authors declare no conflicts of interest.
Funding
This study was supported by the National Institutes of Health/National Cancer Institute (grants R01CA233490, R01CA138688, and R01CA187415 to K.H.Y. and Y.L.), the International Waldenstrom’s Macroglobulinemia Foundation to Y.L., The University of Texas MD Anderson Cancer Center Institutional Research and Development Fund, the Gundersen Lutheran Medical Foundation, the Hagemeister Lymphoma Foundation, and the University Cancer Foundation via the Sister Institution Network Fund at The University of Texas MD Anderson Cancer Center. This work was also partially supported by the National Cancer Institute and National Institutes of Health grants P50CA136411 and P50CA142509 to R.Z.O and the MD Anderson Cancer Center Support Grant. K.H.Y. also received research support from Roche Molecular System, Gilead Sciences Pharmaceutical, Seattle Genetics, Dai Sanyo Pharmaceutical, Adaptive Biotechnology, and HTG Molecular Diagnostics.
Author contributions
H.C.L. and K.H.Y. designed the study. Y.W., V.L.G., Z.Y.X.-M., D.S., and K.H.Y. conducted the research. Y.W., V.L.G., Z.Y.X.-M., D.S., S.K.T., D.M.W., FZ, XF, M.D., Y.L., M.Z., F.B.H., R.Z.O., H.C.L., and K.H.Y. contributed vital new reagents, resources, technology, analytical tools, and clinical and follow-up data with the approval of the institutional review boards. Y.W., Z.Y.X.-M., H.C.L., and K.H.Y. wrote the manuscript. All authors contributed vital strategies, participated in discussions, provided scientific input, and approved the manuscript.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.neo.2021.02.002.
Appendix. Supplementary materials
References
- 1.Owen R.G., Treon S.P., Al-Katib A., Fonseca R., Greipp P.R., McMaster M.L., Morra E., Pangalis G.A., San Miguel J.F., Branagan A.R. Clinicopathological definition of Waldenstrom's macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom's Macroglobulinemia. Semin Oncol. 2003;30(2):110–115. doi: 10.1053/sonc.2003.50082. [DOI] [PubMed] [Google Scholar]
- 2.Ansell S.M., Kyle R.A., Reeder C.B., Fonseca R., Mikhael J.R., Morice W.G., Bergsagel P.L., Buadi F.K., Colgan J.P., Dingli D. Diagnosis and management of Waldenström macroglobulinemia: Mayo stratification of macroglobulinemia and risk-adapted therapy (mSMART) guidelines. Mayo Clin Proc. 2010;85(9):824–833. doi: 10.4065/mcp.2010.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swerdlow S.H., Campo E., Pileri S.A., Harris N.L., Stein H., Siebert R., Advani R., Ghielmini M., Salles G.A., Zelenetz A.D. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kapoor P., Ansell S.M., Fonseca R., Chanan-Khan A., Kyle R.A., Kumar S.K., Mikhael J.R., Witzig T.E., Mauermann M., Dispenzieri A. Diagnosis and Management of Waldenström Macroglobulinemia: Mayo Stratification of Macroglobulinemia and Risk-Adapted Therapy (mSMART) Guidelines 2016. JAMA Oncol. 2017;3(9):1257–1265. doi: 10.1001/jamaoncol.2016.5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dimopoulos M.A., Trotman J., Tedeschi A., Matous J.V., Macdonald D., Tam C., Tournilhac O., Ma S., Oriol A., Heffner L.T. Ibrutinib for patients with rituximab-refractory Waldenstrom's macroglobulinaemia (iNNOVATE): an open-label substudy of an international, multicentre, phase 3 trial. Lancet Oncol. 2017;18(2):241–250. doi: 10.1016/S1470-2045(16)30632-5. [DOI] [PubMed] [Google Scholar]
- 6.Dimopoulos M.A., Tedeschi A., Trotman J., García-Sanz R., Macdonald D., Leblond V., Mahe B., Herbaux C., Tam C., Orsucci L. Phase 3 Trial of Ibrutinib plus Rituximab in Waldenström's Macroglobulinemia. New Engl J Med. 2018;378(25):2399–2410. doi: 10.1056/NEJMoa1802917. [DOI] [PubMed] [Google Scholar]
- 7.Treon S.P., Hunter Z.R., Aggarwal A., Ewen E.P., Masota S., Lee C., Santos D.D., Hatjiharissi E., Xu L., Leleu X. Characterization of familial Waldenstrom's macroglobulinemia. Ann Oncol. 2006;17(3):488–494. doi: 10.1093/annonc/mdj111. [DOI] [PubMed] [Google Scholar]
- 8.Kapoor P., Paludo J., Ansell S.M. Waldenstrom Macroglobulinemia: Familial Predisposition and the Role of Genomics in Prognosis and Treatment Selection. Curr Treat Options Oncol. 2016;17(3):16. doi: 10.1007/s11864-016-0391-7. [DOI] [PubMed] [Google Scholar]
- 9.Varettoni M., Tedeschi A., Arcaini L., Pascutto C., Vismara E., Orlandi E., Ricci F., Corso A., Greco A., Mangiacavalli S. Risk of second cancers in Waldenstrom macroglobulinemia. Ann Oncol. 2012;23(2):411–415. doi: 10.1093/annonc/mdr119. [DOI] [PubMed] [Google Scholar]
- 10.Treon S.P., Xu L., Yang G., Zhou Y., Liu X., Cao Y., Sheehy P., Manning R.J., Patterson C.J., Tripsas C. MYD88 L265P somatic mutation in Waldenstrom's macroglobulinemia. N Engl J Med. 2012;367(9):826–833. doi: 10.1056/NEJMoa1200710. [DOI] [PubMed] [Google Scholar]
- 11.Hunter Z.R., Xu L., Yang G., Zhou Y., Liu X., Cao Y., Manning R.J., Tripsas C., Patterson C.J., Sheehy P. The genomic landscape of Waldenstrom macroglobulinemia is characterized by highly recurring MYD88 and WHIM-like CXCR4 mutations, and small somatic deletions associated with B-cell lymphomagenesis. Blood. 2014;123(11):1637–1646. doi: 10.1182/blood-2013-09-525808. [DOI] [PubMed] [Google Scholar]
- 12.Avbelj M., Wolz O.O., Fekonja O., Bencina M., Repic M., Mavri J., Kruger J., Scharfe C., Delmiro Garcia M., Panter G. Activation of lymphoma-associated MyD88 mutations via allostery-induced TIR-domain oligomerization. Blood. 2014;124(26):3896–3904. doi: 10.1182/blood-2014-05-573188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ngo V.N., Young R.M., Schmitz R., Jhavar S., Xiao W., Lim K.H., Kohlhammer H., Xu W., Yang Y., Zhao H. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470(7332):115–119. doi: 10.1038/nature09671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang G., Zhou Y., Liu X., Xu L., Cao Y., Manning R.J., Patterson C.J., Buhrlage S.J., Gray N., Tai Y.T. A mutation in MYD88 (L265P) supports the survival of lymphoplasmacytic cells by activation of Bruton tyrosine kinase in Waldenstrom macroglobulinemia. Blood. 2013;122(7):1222–1232. doi: 10.1182/blood-2012-12-475111. [DOI] [PubMed] [Google Scholar]
- 15.Raedler L.A. Imbruvica (Ibrutinib), First-in-Class Bruton's Tyrosine Kinase Inhibitor, Receives Expanded Indications for Patients with Relapsed Chronic Lymphocytic Leukemia. Am Health Drug Benefits. 2015;8:66–69. (Spec Feature) [PMC free article] [PubMed] [Google Scholar]
- 16.Treon S.P., Tripsas C.K., Meid K., Warren D., Varma G., Green R., Argyropoulos K.V., Yang G., Cao Y., Xu L. Ibrutinib in previously treated Waldenstrom's macroglobulinemia. New Engl J Med. 2015;372(15):1430–1440. doi: 10.1056/NEJMoa1501548. [DOI] [PubMed] [Google Scholar]
- 17.Dimopoulos M.A., Tedeschi A., Trotman J., Garcia-Sanz R., Macdonald D., Leblond V., Mahe B., Herbaux C., Tam C., Orsucci L. Phase 3 Trial of Ibrutinib plus Rituximab in Waldenstrom's Macroglobulinemia. New Engl J Med. 2018;378(25):2399–2410. doi: 10.1056/NEJMoa1802917. [DOI] [PubMed] [Google Scholar]
- 18.Poulain S., Roumier C., Venet-Caillault A., Figeac M., Herbaux C., Marot G., Doye E., Bertrand E., Geffroy S., Lepretre F. Genomic Landscape of CXCR4 Mutations in Waldenstrom Macroglobulinemia. Clin Cancer Res. 2016;22(6):1480–1488. doi: 10.1158/1078-0432.CCR-15-0646. [DOI] [PubMed] [Google Scholar]
- 19.Cao Y., Hunter Z.R., Liu X., Xu L., Yang G., Chen J., Tsakmaklis N., Kanan S., Castillo J.J., Treon S.P. CXCR4 WHIM-like frameshift and nonsense mutations promote ibrutinib resistance but do not supplant MYD88(L265P) -directed survival signalling in Waldenstrom macroglobulinaemia cells. Br J Haematol. 2015;168(5):701–707. doi: 10.1111/bjh.13200. [DOI] [PubMed] [Google Scholar]
- 20.Hunter Z.R., Yang G., Xu L., Liu X., Castillo J.J., Treon S.P. Genomics, Signaling, and Treatment of Waldenstrom Macroglobulinemia. J Clin Oncol. 2017;35(9):994–1001. doi: 10.1200/JCO.2016.71.0814. [DOI] [PubMed] [Google Scholar]
- 21.Cao Y., Hunter Z.R., Liu X., Xu L., Yang G., Chen J., Patterson C.J., Tsakmaklis N., Kanan S., Rodig S. The WHIM-like CXCR4(S338X) somatic mutation activates AKT and ERK, and promotes resistance to ibrutinib and other agents used in the treatment of Waldenstrom's Macroglobulinemia. Leukemia. 2015;29(1):169–176. doi: 10.1038/leu.2014.187. [DOI] [PubMed] [Google Scholar]
- 22.Treon S.P., Gustine J., Meid K., Yang G., Xu L., Liu X., Demos M., Kofides A., Tsakmaklis N., Chen J.G. Ibrutinib Monotherapy in Symptomatic, Treatment-Naive Patients With Waldenstrom Macroglobulinemia. J Clin Oncol. 2018;36(27):2755–2761. doi: 10.1200/JCO.2018.78.6426. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen-Khac F., Lambert J., Chapiro E., Grelier A., Mould S., Barin C., Daudignon A., Gachard N., Struski S., Henry C. Chromosomal aberrations and their prognostic value in a series of 174 untreated patients with Waldenstrom's macroglobulinemia. Haematologica. 2013;98(4):649–654. doi: 10.3324/haematol.2012.070458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poulain S., Roumier C., Bertrand E., Renneville A., Caillault-Venet A., Doye E., Geffroy S., Sebda S., Nibourel O., Nudel M. TP53 Mutation and Its Prognostic Significance in Waldenstrom's Macroglobulinemia. Clin Cancer Res. 2017;23(20):6325–6335. doi: 10.1158/1078-0432.CCR-17-0007. [DOI] [PubMed] [Google Scholar]
- 25.Gustine J.N., Tsakmaklis N., Demos M.G., Kofides A., Chen J.G., Liu X., Munshi M., Guerrera M.L., Chan G.G., Patterson C.J. TP53 mutations are associated with mutated MYD88 and CXCR4, and confer an adverse outcome in Waldenstrom macroglobulinaemia. Br J Haematol. 2019;184(2):242–245. doi: 10.1111/bjh.15560. [DOI] [PubMed] [Google Scholar]
- 26.Schop R.F., Kuehl W.M., Van Wier S.A., Ahmann G.J., Price-Troska T., Bailey R.J., Jalal S.M., Qi Y., Kyle R.A., Greipp P.R. Waldenstrom macroglobulinemia neoplastic cells lack immunoglobulin heavy chain locus translocations but have frequent 6q deletions. Blood. 2002;100(8):2996–3001. doi: 10.1182/blood.V100.8.2996. [DOI] [PubMed] [Google Scholar]
- 27.Ocio E.M., Schop R.F., Gonzalez B., Van Wier S.A., Hernandez-Rivas J.M., Gutierrez N.C., Garcia-Sanz R., Moro M.J., Aguilera C., Hernandez J. 6q deletion in Waldenstrom macroglobulinemia is associated with features of adverse prognosis. Br J Haematol. 2007;136(1):80–86. doi: 10.1111/j.1365-2141.2006.06389.x. [DOI] [PubMed] [Google Scholar]
- 28.Chang H., Qi C., Trieu Y., Jiang A., Young K.H., Chesney A., Jani P., Wang C., Reece D., Chen C. Prognostic relevance of 6q deletion in Waldenstrom's macroglobulinemia: a multicenter study. Clin Lymphoma Myeloma. 2009;9(1):36–38. doi: 10.3816/CLM.2009.n.008. [DOI] [PubMed] [Google Scholar]
- 29.Cook J.R., Aguilera N.I., Reshmi S., Huang X., Yu Z., Gollin S.M., Abbondanzo S.L., Swerdlow S.H. Deletion 6q is not a characteristic marker of nodal lymphoplasmacytic lymphoma. Cancer Genet Cytogenet. 2005;162(1):85–88. doi: 10.1016/j.cancergencyto.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 30.Rivera A.I., Li M.M., Beltran G., Krause J.R. Trisomy 4 as the sole cytogenetic abnormality in a Waldenstrom macroglobulinemia. Cancer Genet Cytogenet. 2002;133(2):172–173. doi: 10.1016/s0165-4608(01)00577-5. [DOI] [PubMed] [Google Scholar]
- 31.Paludo J., Abeykoon J.P., Shreders A., Ansell S.M., Kumar S., Ailawadhi S., King R.L., Koehler A.B., Reeder C.B., Buadi F.K. Bendamustine and rituximab (BR) versus dexamethasone, rituximab, and cyclophosphamide (DRC) in patients with Waldenstrom macroglobulinemia. Ann Hematol. 2018;97(8):1417–1425. doi: 10.1007/s00277-018-3311-z. [DOI] [PubMed] [Google Scholar]
- 32.Owen R.G., Kyle R.A., Stone M.J., Rawstron A.C., Leblond V., Merlini G., Garcia-Sanz R., Ocio E.M., Morra E., Morel P. Response assessment in Waldenstrom macroglobulinaemia: update from the VIth International Workshop. Br J Haematol. 2013;160(2):171–176. doi: 10.1111/bjh.12102. [DOI] [PubMed] [Google Scholar]
- 33.Hu B., Patel K.P., Chen H.C., Wang X., Wang F., Luthra R., Routbort M.J., Kanagal-Shamanna R., Medeiros L.J., Yin C.C. Routine sequencing in CLL has prognostic implications and provides new insight into pathogenesis and targeted treatments. Br J Haematol. 2019;185(5):852–864. doi: 10.1111/bjh.15877. [DOI] [PubMed] [Google Scholar]
- 34.Chen Z., Ok C.Y., Wang W., Goswami M., Tang G., Routbort M., Jorgensen J.L., Medeiros L.J., Wang S.A. Low-Grade Myelodysplastic Syndromes With Preserved CD34+ B-Cell Precursors (CD34+ Hematogones) Cytometry B Clin Cytom. 2020;98(1):36–42. doi: 10.1002/cyto.b.21830. [DOI] [PubMed] [Google Scholar]
- 35.Ok C.Y., Loghavi S., Sui D., Wei P., Kanagal-Shamanna R., Yin C.C., Zuo Z., Routbort M.J., Tang G., Tang Z. Persistent IDH1/2 mutations in remission can predict relapse in patients with acute myeloid leukemia. Haematologica. 2019;104(2):305–311. doi: 10.3324/haematol.2018.191148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morel P., Duhamel A., Gobbi P., Dimopoulos M.A., Dhodapkar M.V., McCoy J., Crowley J., Ocio E.M., Garcia-Sanz R., Treon S.P. International prognostic scoring system for Waldenstrom macroglobulinemia. Blood. 2009;113(18):4163–4170. doi: 10.1182/blood-2008-08-174961. [DOI] [PubMed] [Google Scholar]
- 37.Treon S.P., Cao Y., Xu L., Yang G., Liu X., Hunter Z.R. Somatic mutations in MYD88 and CXCR4 are determinants of clinical presentation and overall survival in Waldenstrom macroglobulinemia. Blood. 2014;123(18):2791–2796. doi: 10.1182/blood-2014-01-550905. [DOI] [PubMed] [Google Scholar]
- 38.Abeykoon J.P., Paludo J., King R.L., Ansell S.M., Gertz M.A., LaPlant B.R., Halvorson A.E., Gonsalves W.I., Dingli D., Fang H. MYD88 mutation status does not impact overall survival in Waldenstrom macroglobulinemia. Am J Hematol. 2018;93(2):187–194. doi: 10.1002/ajh.24955. [DOI] [PubMed] [Google Scholar]
- 39.Dadashian E.L., McAuley E.M., Liu D., Shaffer A.L., 3rd, Young R.M., Iyer J.R., Kruhlak M.J., Staudt L.M., Wiestner A., Herman S.E.M. TLR Signaling Is Activated in Lymph Node-Resident CLL Cells and Is Only Partially Inhibited by Ibrutinib. Cancer Res. 2019;79(2):360–371. doi: 10.1158/0008-5472.CAN-18-0781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paulus A., Akhtar S., Yousaf H., Manna A., Paulus S.M., Bashir Y., Caulfield T.R., Kuranz-Blake M., Chitta K., Wang X. Waldenstrom macroglobulinemia cells devoid of BTK(C481S) or CXCR4(WHIM-like) mutations acquire resistance to ibrutinib through upregulation of Bcl-2 and AKT resulting in vulnerability towards venetoclax or MK2206 treatment. Blood Cancer J. 2017;7(5):e565. doi: 10.1038/bcj.2017.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gachard N., Parrens M., Soubeyran I., Petit B., Marfak A., Rizzo D., Devesa M., Delage-Corre M., Coste V., Laforet M.P. IGHV gene features and MYD88 L265P mutation separate the three marginal zone lymphoma entities and Waldenstrom macroglobulinemia/lymphoplasmacytic lymphomas. Leukemia. 2013;27(1):183–189. doi: 10.1038/leu.2012.257. [DOI] [PubMed] [Google Scholar]
- 42.Jimenez C., Sebastian E., Chillon M.C., Giraldo P., Mariano Hernandez J., Escalante F., Gonzalez-Lopez T.J., Aguilera C., de Coca A.G., Murillo I. MYD88 L265P is a marker highly characteristic of, but not restricted to, Waldenstrom's macroglobulinemia. Leukemia. 2013;27(8):1722–1728. doi: 10.1038/leu.2013.62. [DOI] [PubMed] [Google Scholar]
- 43.Xu L., Hunter Z.R., Yang G., Zhou Y., Cao Y., Liu X., Morra E., Trojani A., Greco A., Arcaini L. MYD88 L265P in Waldenstrom macroglobulinemia, immunoglobulin M monoclonal gammopathy, and other B-cell lymphoproliferative disorders using conventional and quantitative allele-specific polymerase chain reaction. Blood. 2013;121(11):2051–2058. doi: 10.1182/blood-2012-09-454355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Castillo J.J., Xu L., Gustine J.N., Keezer A., Meid K., Dubeau T.E., Liu X., Demos M.G., Kofides A., Tsakmaklis N. CXCR4 mutation subtypes impact response and survival outcomes in patients with Waldenstrom macroglobulinaemia treated with ibrutinib. Br J Haematol. 2019;187(3):356–363. doi: 10.1111/bjh.16088. [DOI] [PubMed] [Google Scholar]
- 45.Hunter Z.R., Xu L., Tsakmaklis N., Demos M.G., Kofides A., Jimenez C., Chan G.G., Chen J., Liu X., Munshi M. Insights into the genomic landscape of MYD88 wild-type Waldenstrom macroglobulinemia. Blood Adv. 2018;2(21):2937–2946. doi: 10.1182/bloodadvances.2018022962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu L., Hunter Z.R., Tsakmaklis N., Cao Y., Yang G., Chen J., Liu X., Kanan S., Castillo J.J., Tai Y.T. Clonal architecture of CXCR4 WHIM-like mutations in Waldenstrom Macroglobulinaemia. Br J Haematol. 2016;172(5):735–744. doi: 10.1111/bjh.13897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cao X., Ye Q., Orlowski R.Z., Wang X., Loghavi S., Tu M., Thomas S.K., Shan J., Li S., Qazilbash M. Waldenström macroglobulinemia with extramedullary involvement at initial diagnosis portends a poorer prognosis. J Hematol Oncol. 2015;8:74. doi: 10.1186/s13045-015-0172-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu-Monette Z.Y., Wu L., Visco C., Tai Y.C., Tzankov A., Liu W.M., Montes-Moreno S., Dybkaer K., Chiu A., Orazi A. Mutational profile and prognostic significance of TP53 in diffuse large B-cell lymphoma patients treated with R-CHOP: report from an International DLBCL Rituximab-CHOP Consortium Program Study. Blood. 2012;120(19):3986–3996. doi: 10.1182/blood-2012-05-433334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roccaro A.M., Sacco A., Shi J., Chiarini M., Perilla-Glen A., Manier S., Glavey S., Aljawai Y., Mishima Y., Kawano Y. Exome sequencing reveals recurrent germ line variants in patients with familial Waldenstrom macroglobulinemia. Blood. 2016;127(21):2598–2606. doi: 10.1182/blood-2015-11-680199. [DOI] [PubMed] [Google Scholar]
- 50.McMaster M.L., Goldin L.R., Bai Y., Ter-Minassian M., Boehringer S., Giambarresi T.R., Vasquez L.G., Tucker M.A. Genomewide linkage screen for Waldenstrom macroglobulinemia susceptibility loci in high-risk families. Am J Hum Genet. 2006;79(4):695–701. doi: 10.1086/507687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Treon S.P., Tripsas C., Hanzis C., Ioakimidis L., Patterson C.J., Manning R.J., Sheehy P., Turnbull B., Hunter Z.R. Familial disease predisposition impacts treatment outcome in patients with Waldenstrom macroglobulinemia. Clin Lymphoma Myeloma Leuk. 2012;12(6):433–437. doi: 10.1016/j.clml.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 52.Yang H., Xia L., Chen J., Zhang S., Martin V., Li Q., Lin S., Chen J., Calmette J., Lu M. Stress-glucocorticoid-TSC22D3 axis compromises therapy-induced antitumor immunity. Nat Med. 2019;25(9):1428–1441. doi: 10.1038/s41591-019-0566-4. [DOI] [PubMed] [Google Scholar]
- 53.Manzoni D., Catallo R., Chebel A., Baseggio L., Michallet A.S., Roualdes O., Magaud J.P., Salles G., Ffrench M. The ibrutinib B-cell proliferation inhibition is potentiated in vitro by dexamethasone: Application to chronic lymphocytic leukemia. Leuk Res. 2016;47:1–7. doi: 10.1016/j.leukres.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 54.Smolej L., Doubek M., Panovská A., Simkovič M., Brychtová Y., Belada D., Motyčková M., Mayer J. Rituximab in combination with high-dose dexamethasone for the treatment of relapsed/refractory chronic lymphocytic leukemia. Leuk Res. 2012;36(10):1278–1282. doi: 10.1016/j.leukres.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 55.Matsumoto Y., Kobayashi T., Shimura Y., Kawata E., Nagoshi H., Ohshiro M., Sugitani M., Shimura K., Iwai T., Fuchida S.I. Combined rituximab, bendamustine, and dexamethasone chemotherapy for relapsed or refractory indolent B-cell non-Hodgkin lymphoma and mantle cell lymphoma: a multicenter phase II study. Int J Hematol. 2019;110(1):77–85. doi: 10.1007/s12185-019-02650-w. [DOI] [PubMed] [Google Scholar]
- 56.Treon S.P., Hanzis C., Manning R.J., Ioakimidis L., Patterson C.J., Hunter Z.R., Sheehy P., Turnbull B. Maintenance Rituximab is associated with improved clinical outcome in rituximab naive patients with Waldenstrom Macroglobulinaemia who respond to a rituximab-containing regimen. Br J Haematol. 2011;154(3):357–362. doi: 10.1111/j.1365-2141.2011.08750.x. [DOI] [PubMed] [Google Scholar]
- 57.Castillo J.J., Gustine J.N., Meid K., Dubeau T.E., Severns P., Xu L., Yang G., Hunter Z.R., Treon S.P. Response and survival for primary therapy combination regimens and maintenance rituximab in Waldenstrom macroglobulinaemia. Br J Haematol. 2018;181(1):77–85. doi: 10.1111/bjh.15148. [DOI] [PubMed] [Google Scholar]
- 58.Rummel M.J., Lerchenmüller C., Hensel M., Goerner M., Buske C., Schulz H., Schmidt B., Kojouharoff G., Lange E., Willenbacher W. Two Years Rituximab Maintenance Vs. Observation after First Line Treatment with Bendamustine Plus Rituximab (B-R) in Patients with Waldenström's Macroglobulinemia (MW): Results of a Prospective, Randomized, Multicenter Phase 3 Study (the StiL NHL7-2008 MAINTAIN trial) Blood. 2019;134(Supplement_1):343. - [Google Scholar]
- 59.de Rooij M.F., Kuil A., Geest C.R., Eldering E., Chang B.Y., Buggy J.J., Pals S.T., Spaargaren M. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood. 2012;119(11):2590–2594. doi: 10.1182/blood-2011-11-390989. [DOI] [PubMed] [Google Scholar]
- 60.Thompson P.A., O'Brien S.M., Wierda W.G., Ferrajoli A., Stingo F., Smith S.C., Burger J.A., Estrov Z., Jain N., Kantarjian H.M. Complex karyotype is a stronger predictor than del(17p) for an inferior outcome in relapsed or refractory chronic lymphocytic leukemia patients treated with ibrutinib-based regimens. Cancer. 2015;121(20):3612–3621. doi: 10.1002/cncr.29566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen S.S., Chang B.Y., Chang S., Tong T., Ham S., Sherry B., Burger J.A., Rai K.R., Chiorazzi N. BTK inhibition results in impaired CXCR4 chemokine receptor surface expression, signaling and function in chronic lymphocytic leukemia. Leukemia. 2016;30(4):833–843. doi: 10.1038/leu.2015.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu X., Li W., Deng Q., Liu H., Wang X., Hu H., Cao Y., Xu-Monette Z.Y., Li L., Zhang M. MYD88 L265P Elicits Mutation-specific Ubiquitination to Drive NF-κB Activation and Lymphomagenesis. Blood. 2020 doi: 10.1182/blood.2020004918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cea M., Cagnetta A., Acharya C., Acharya P., Tai Y.T., Yang C., Lovera D., Soncini D., Miglino M., Fraternali-Orcioni G. Dual NAMPT and BTK Targeting Leads to Synergistic Killing of Waldenstrom Macroglobulinemia Cells Regardless of MYD88 and CXCR4 Somatic Mutation Status. Clin Cancer Res. 2016;22(24):6099–6109. doi: 10.1158/1078-0432.CCR-16-0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paulus A., Akhtar S., Caulfield T.R., Samuel K., Yousaf H., Bashir Y., Paulus S.M., Tran D., Hudec R., Cogen D. Coinhibition of the deubiquitinating enzymes, USP14 and UCHL5, with VLX1570 is lethal to ibrutinib- or bortezomib-resistant Waldenstrom macroglobulinemia tumor cells. Blood Cancer J. 2016;6(11):e492. doi: 10.1038/bcj.2016.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen C., Siegel D., Gutierrez M., Jacoby M., Hofmeister C.C., Gabrail N., Baz R., Mau-Sorensen M., Berdeja J.G., Savona M. Safety and efficacy of selinexor in relapsed or refractory multiple myeloma and Waldenstrom macroglobulinemia. Blood. 2018;131(8):855–863. doi: 10.1182/blood-2017-08-797886. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and used in this study are available from the corresponding author after garnering institutional approval and enacting appropriate data sharing agreements.